Abstract
Digeneans are endoparasitic flatworms with complex life cycles including one or two intermediate hosts (first of which is always a mollusk) and a vertebrate definitive host. Digeneans may harbor intracellular endosymbiotic bacteria belonging to the genus Neorickettsia (order Rickettsiales, family Anaplasmataceae). Some Neorickettsia are able to invade cells of the digenean's vertebrate host and are known to cause diseases of wildlife and humans. In this study we report the results of screening 771 digenean samples for Neorickettsia collected from various vertebrates in terrestrial, freshwater, brackish, and marine habitats in the United States, China and Australia. Neorickettsia were detected using a newly designed real-time PCR protocol targeting a 152 bp fragment of the heat shock protein coding gene, GroEL, and verified with nested PCR and sequencing of a 1371 bp long region of 16S rRNA. Eight isolates of Neorickettsia have been obtained. Sequence comparison and phylogenetic analysis demonstrated that 7 of these isolates, provisionally named Neorickettsia sp. 1–7 (obtained from allocreadiid Crepidostomum affine, haploporids Saccocoelioides beauforti and Saccocoelioides lizae, faustulid Bacciger sprenti, deropegid Deropegus aspina, a lecithodendriid, and a pleurogenid) represent new genotypes and one (obtained from Metagonimoides oregonensis) was identical to a published sequence of Neorickettsia known as SF agent. All digenean species reported in this study represent new host records. Three of the 6 digenean families (Haploporidae, Pleurogenidae, and Faustulidae) are also reported for the first time as hosts of Neorickettsia. We have detected Neorickettsia in digeneans from China and Australia for the first time based on PCR and sequencing evidence. Our findings suggest that further surveys from broader geographic regions and wider selection of digenean taxa are likely to reveal new Neorickettsia lineages as well as new digenean host associations.
Introduction
Neorickettsia (family Anaplasmataceae) is a genus containing obligate intracellular endosymbionts of digeneans (Platyhelminthes, Digenea). Although relatively small, this genus has received significant attention in the recent years, with a rapid increase in the number of known species level lineages in this group [1], [2], [3], [4], [5]. Neorickettsia are vertically transmitted through all stages of complex digenean life cycles. Additionally, they are capable of being horizontally transmitted to the vertebrate hosts of the digenean, both human and wildlife, where they can cause disease [1], [4], [6], [7], [8], [9]. These diseases are potentially debilitating, e.g., Sennetsu fever in humans (Neorickettsia sennetsu), or even fatal, e.g., salmon dog poisoning (Neorickettsia helminthoeca), and Potomac horse fever (Neorickettsia risticii) [4].
Neorickettsia have now been reported from several countries and all continents including Antarctica [4], [5]. However, a majority of the records are based almost exclusively on immunological detection of Neorickettsia in vertebrate hosts, mainly horses. It should be noted that positive immunological tests of horses could be a result of either actual infection or a previous vaccination against Neorickettsia risticii (causative agent of Potomac horse fever). Therefore, immunological results alone without PCR-based confirmation need to be considered with caution [10], [11]. At the same time, screening of digeneans for Neorickettsia has been limited, which prevents understanding of the actual diversity of these bacteria and potential sources of infection of vertebrate animals including humans. Only 21–22 digenean species, in most cases identified only to genus or family level, have been previously confirmed as hosts of Neorickettsia [3], [4], [12], [13], [14], [15]. Records resulting from PCR detection of neorickettsial DNA are known only from North and South America, eastern Asia, and Antarctica. However, most of these records are based on Neorickettsia detected in vertebrate host tissues (horses, dogs, humans, fish). As posited by Vaughan et al. [4], future studies focusing on screening digenean extracts for Neorickettsia will reveal additional host associations of these bacteria and new pathways of their circulation in nature. This has already been demonstrated in a recent study by Tkach et al. [3] who found 4 species level genetic lineages of Neorickettsia in 7 species of digeneans belonging to 7 different families.
Currently, there are 3 named species and 10 not formally named genotypes of Neorickettsia that are likely to represent additional species based on levels of 16S rRNA sequence divergence [2], [3], [4], [5], [16], [17], [18], [19]. Of these 13 known species/genotypes of Neorickettsia, 6 have been found in North America. Circulation of digenean hosts of known Neorickettsia occurs in either freshwater (Rainbow trout agent, Catfish agent 1, Catfish agent 2), freshwater/terrestrial (N. helminthoeca, N. risticii, Elokomin Fluke Fever agent, Diplostomum agent), or fully terrestrial (N. risticii) environments [3], [4], [12], [13], [20], [21], [22]. Presently, however, there are no records of Neorickettsia from digeneans having fully marine life cycles. Presence of Neorickettsia in tissues of marine fishes in Antarctica and the Gulf of Mexico [5], [23] may suggest a marine circulation pathway, however, these authors did not screen any digeneans.
In this study, we used real-time PCR-based detection methods to study the diversity of Neorickettsia in previously understudied or unstudied regions. To accomplish this goal, we screened for Neorickettsia numerous DNA extracts from an extensive collection of adult digeneans collected from various vertebrates as well as snail hosts in the United States (several states), Australia, and China. As a result, we have found 7 new genetic lineages of Neorickettsia some of which may potentially represent new species. All of our records represent new digenean host associations. Our findings also expand the range of circulation pathways known for Neorickettsia. We have for the first time detected Neorickettsia from Australia and China using PCR-based detection and DNA sequencing. We have also conducted a molecular phylogenetic analysis in order to estimate interrelationships among the newly discovered genotypes with previously known named species and not yet named lineages of Neorickettsia.
Materials and Methods
Ethics statement
Digeneans were collected from marine and freshwater fishes, amphibians, reptiles, birds, mammals, and invertebrates from 2009 through 2012 from multiple localities in China, Australia, Argentina, Costa Rica, and several states in the USA (Mississippi, Louisiana, Florida, Oregon, North Dakota, Minnesota). An IACUC protocol 10100105 was issued by the University of Southern Mississippi for collecting and humane euthanasia of wild animals in Mississippi, Florida, and Louisiana. Birds were collected by shotgun in accordance to the state and federal permits, raccoons were live trapped and euthanized in the field by firearm (.22 caliber rifle) in accordance to issued permits, and amphibians and reptiles were collected by hand and humanly euthanized by immersion in a solution of chlorobutanol (chloretone) in water in accordance with the issued permits and IACUC protocol. IACUC protocols were not required for invertebrate collecting in Oregon, North Dakota, and Minnesota or for fish. Fish were purchased dead from food markets in Argentina, Costa Rica, and China. In Australia, fish were caught by cast net by one of the authors (EP) from around Townsville (Queensland), Darwin (Northern Territory) and Broome (Western Australia). Fish were placed on ice after capture, and if necessary were sacrificed with the use of a 250 mg/l bath of Tricaine methane sulfonate (MS-222) in accordance with the issued permits.
Sample collections
All digenean samples were collected over a period of 7 years as parts of several independent projects dealing with parasite biodiversity and systematics. Thus, the source of DNA available for Neorickettsia screening was opportunistic, but as inclusive as possible. Required scientific collecting permits were obtained in all cases. The Florida Fish and Wildlife Conservation Commission issued the permits LSSC-11-00074 and FNW-13-05(renewal) that allowed for the collection of invertebrates, amphibians, reptiles, and mammals as well as freshwater fishes in Florida. The Mississippi Department of Wildlife Fisheries and Parks issued a permit 0123131 for the collection of invertebrates, amphibians, reptiles, mammals, and freshwater and marine fishes in Mississippi, and the Louisiana Wildlife and Fisheries issued a permit LNHP-13-017 also for the collection of the above mentioned animals in Louisiana. Additionally, a federal permit (MB681207-0) was issued by the US Fish and wildlife service for collection of reptiles, mammals, birds and freshwater fish in Mississippi and Louisiana. Three separate permits were issued in Australia for the collection of fish; Western Australia (Government of Western Australia, Department of Fisheries, SPA-01-10); Northern Territory (Northern Territory Government, Department of Resources, S17/2932); and Queensland (Department of Primary Industries and Fisheries 133621). Only abundant commercial fish species were purchased from local markets and fishermen in China, Argentina, and Costa Rica, and therefore, permits were not required. Additionally, aquatic snails were collected from North Dakota, Minnesota, and Oregon. Snails were identified using Burch's “North American Freshwater snails”[24], and also did not require permits. We do not provide a complete list of screened digenean species because in the case of Neorickettsia a negative result does not necessarily mean that a certain species of digenean cannot be a host for Neorickettsia. As discussed by Tkach et al. [3] digeneans do not have any known co-dependency with their Neorickettsia endosymbionts and therefore, different individuals of the same digenean species may or may not be infected.
Sample processing
Live worms were rinsed in saline, examined briefly, killed with hot water, and fixed in 70% ethanol that allowed for both morphological examination and molecular study. When numerous specimens were found, some were fixed in 95% ethanol for molecular work. Fixed worms were stained in aqueous alum carmine, Mayer's hematoxylin, or Van Cleave's hematoxylin; dehydrated in a graded ethanol series; cleared in clove oil (carmine and Van Cleave's) or methyl salicylate (Mayer's); and mounted permanently in Damar Gum for morphological identification.
Genomic DNA was extracted from individual adult worms and pooled larval stages either using the Qiagen DNAeasy tissue kit (Qiagen, Inc., Valencia, California) following the manufacturer's instructions or the guanidine thiocyanate method according to Tkach and Pawlowski [25].
Molecular screening
DNA extracts were first tested for the presence of Neorickettsia using a real-time PCR protocol designed and extensively tested by one of the authors (SEG). Five microliters of each DNA extract were used. The real-time PCR amplified a 152-bp portion of the 3′ end of the heat shock protein coding gene, GroEL. The primer pair (designed by SEG) used is listed in Table 1. Samples that tested positive with real-time PCR were verified using a substantially modified nested PCR protocol initially described by Barlough et al.[26]. Five microliters of each DNA extract were used for the first PCR reaction and 1 µl of the first PCR product was used for the nested PCR. A 1470 bp long fragment of the 16S rRNA gene was first amplified using the primer pair listed in Table 1,designed by SEG. The nested PCR step amplified a 1371-bp fragment using internal primers also listed in Table 1. The same nested PCR primers were used in sequencing reactions along with internal forward and internal reverse primers (Table 1), designed by SEG.
Table 1. PCR Primers used in the study.
| Reaction type | Primer | Sequence (5′-3′) |
| Real-time | groel-1500F | ATAGATCCAGCKAAGGTAGTGCGTGT |
| groel-1620R | TTCCACCCATGCCACCACCAGGCATCATTG | |
| 1st round PCR (Neorickettsia) | n16S-25F | TCAGAACGAACGCTAGCGGT |
| n16S-1500R | AAAGGAGGTAATCCAGCCGCAGGTTCAC | |
| Nested PCR/sequencing (Neorickettsia) | n16s-50F | TAGGCTTAACACATGCAAGTCGAACG |
| n16S-1400R | CGGTTAGCTCACTAGCTTCGAGTAA | |
| Sequencing (Neorickettsia) | 16S-n900F | GACTCGCACAAGCGGTGGAGTAT |
| 16S-n900R | ATACTCCACCGCTTGTGCGAGTC | |
| PCR/sequencing (digenean) | digl2 | AAGCATATCACTAAGCGG |
| 1500R | GCTATCCTGAGGGAAACTTCG | |
| Sequencing (digenean) | 300F | CAAGTACCGTGAGGGAAAGTTG |
| 900F | CCGTCTTGAAACACGGACCAAG | |
| 300R | CAACTTTCCCTCACGGTACTTG | |
| ECD2 | CTTGGTCCGTGTTTCAAGACGGG |
The real-time PCR reactions were run on a Bio-Rad CFX96 Touch real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) using iTaq universal sybr green supermix (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. A two-step program was used with a denaturation temperature of 95°C for 3 seconds, followed by an annealing and extension temperature of 64°C for 25 seconds and 36 cycles. In addition, a melt curve was run starting from 72°C and increasing at 0.2°C increments every 5 seconds until reaching 87°C. The nested PCR reactions were run on EP Gradient thermocycler (Eppendorf, Hauppauge, NY) using Quick load OneTaq mastermix (New England Biolabs, Ipswich, MA) according to the manufacturer's instructions. Annealing temperature of 56°C and 40 cycles were used in both first and nested PCRs. DNA of N. sennetsu used as a positive control was graciously provided by Dr. Sabine Dittrich (Lao Oxford Mahosot Wellcome Trust Research Unit). Pure water was used for negative controls in both real-time and nested PCRs.
Digenean host identification
Adult digeneans were identified based on their morphology using stained whole mounts. Identification to generic level was done using the three-volume “Keys to the Trematoda” [27] [28] [29] with subsequent species identification done using numerous original descriptions and revision articles. Neorickettsia positive cercariae or metacercariae were identified to the lowest possible taxonomic level using a partial sequence of the nuclear large ribosomal subunit gene (28S). Digenean DNA was amplified from the extracted DNA by PCR using the primer pair listed in Table 1. The same PCR primers and additional internal primers were used for sequencing (Table 1).
DNA sequencing
PCR amplicons of both Neorickettsia and digeneans were purified using the Zymo DNA Clean & Concentrator -5(Zymo Research, Irvine, CA) or ExoSap PCR clean-up enzymatic kit from Affimetrix (Santa Clara, CA) according to the manufacturer's instructions. The PCR products were cycle-sequenced using ABI BigDye chemistry, ethanol precipitated, and run on an ABI Prism 3100 automated capillary sequencer. Contiguous sequences of Neorickettsia and digeneans were assembled using Sequencher ver. 4.2 (GeneCodes Corp., Ann Arbor, MI) and submitted to GenBank under accession numbers KF661342-KF661351 and KF878083- KF878084.
Phylogenetic analysis
Newly obtained sequences (Table 2 and Fig. 1) and sequences of neorickettsiae from GenBank were used in the phylogenetic analysis. The new sequences and sequences obtained from GenBank were initially aligned with the aid of ClustalW as implemented in the BioEdit program, version 7.0.1 [30]. The alignments were manually refined in MacClade, version 4 [31].
Table 2. Neorickettsia genotypes, natural hosts, types of life cycles, life cycle stage, geographic origins, definitive and intermediate hosts.
| Neorickettsia species | Digenean family | Digenean genus and species | Life cycle stage | Host (this study) | Definitive host | 1st/2nd intermediate hosts | Life cycle | Locality |
| Neorickettsia sp. 1 (KF661342) | Allocreadiidae | Crepidostomum affine | Adult | Mooneye (Hiodon tergisus) | Fishes | Unknown/aquatic arthropod | aquatic (freshwater) | Pearl River, Mississippi |
| Neorickettsia sp. 2 (KF661343) | Haploporidae | Saccocoelioides beauforti | Adult | Striped mullet (Mugil cephalus) | Fishes | unknown | aquatic (brackish/marine) | Cedar Key, Florida |
| Neorickettsia sp. 3 (KF661344) | Pleurogenidae | Unknown | Metacercariae | Crayfish (Procambarus sp.) | Mammals and amphibians | Aquatic snail/crustacean | aquatic/terrestrial (freshwater) | Williford Springs Florida |
| SF agent (KF661347) | Heterophyidae | Metagonimoides oregonensis | Adult | Raccoon (Procyon lotor) | Mammals | Aquatic snail/amphibian | aquatic/terrestrial (freshwater) | Williford Springs Florida |
| Neorickettsia sp. 4 (KF661345) | Haploporidae | Saccocoelioides lizae | Adult | Striped mullet (Mugil cephalus) | Fishes | Unknown | aquatic (brackish/marine) | Daya Bay, Guangdong, China |
| Neorickettsia sp. 5 (KF661346) | Faustulidae | Bacciger sprenti | Adult | Spotbanded scat (Selenotoca multifasciata) | Fishes | Unknown | aquatic (brackish/marine) | Eli Creek, Queensland, Australia |
| Neorickettsia sp. 6 (KF878083) | Lecithodendriidae | Prosthodendrium sp. | Cercariae | Stream snail (Juga yrekaensis) | Bats and birds | Aquatic snail/aquatic arthropod | aquatic/terrestrial (freshwater) | Dunn Forest, Corvallis, Oregon |
| Neorickettsia sp. 7 (KF878084) | Derogenidae | Deropegus aspina | Cercariae | Stream snail (Juga yrekaensis) | Fishes and amphibians | Aquatic snail/aquatic arthropod | aquatic/terrestrial (freshwater) | Dunn Forest, Corvallis, Oregon |
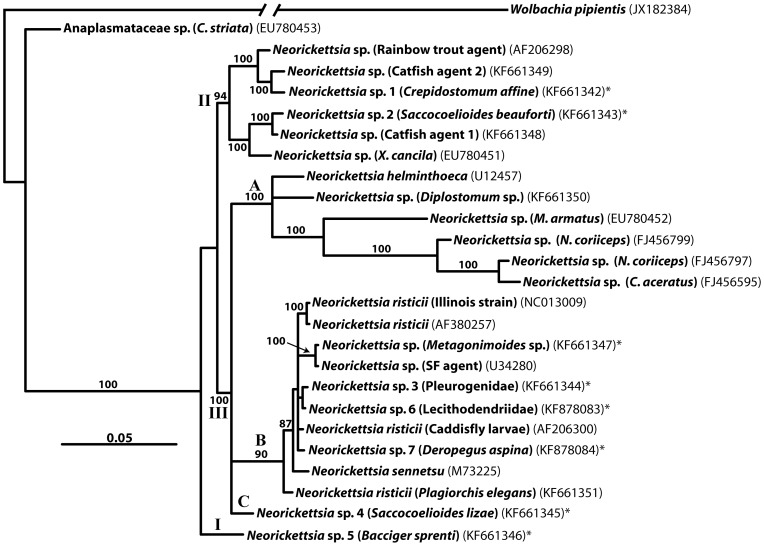
Figure 1. Phylogenetic relationships among 25 taxa of bacterial endosymbionts in the family Anaplasmataceae resulting from Bayesian analysis.
Phylogenetic relationships among 25 taxa of bacterial endosymbionts in the family Anaplasmataceae resulting from Bayesian analysis (1,500,000 generations) of partial sequences of 16S rDNA gene. Posterior probabilities greater than 80% are shown above internodes. Roman numerals (I, II, II) represent the different clades within the “Neorickettsia clade” and letters (A, B, C) correspond to the subclades within clade III. An asterisk (*) at the end of a taxon name indicates a new genotype of Neorickettsia discovered in this study. GenBank numbers are given here for all taxa.
Phylogenetic analysis was carried out using Bayesian inference (BI) as implemented in the MrBayes program, version 2.01 [32] with the following nucleotide substitution parameters: lset nst = 6, rates = invgamma, ngammacat = 4, that correspond to a general time reversible (GTR) model including estimates of the proportion of invariant sites (I) and gamma (G) distributed among-site rate variation. Posterior probabilities were approximated over 1,500,000 generations for both data-sets, log-likelihood scores plotted and only the final 75% of trees were used to produce the consensus trees by setting the “burnin” parameters at 375,000 generations. The GTR+I+G model was used for the analysis based off of the results obtained from jModelTest, version 0.1.1 [33], [34].
Results
A total of 775 digenean samples were screened for Neorickettsia. A list of digenean families screened and the number of extracts corresponding to each family are provided in Table 3. Screening revealed 8 different genetic lineages of Neorickettsia in 8 digenean species belonging to 7 different families (Table 2). Neorickettsia infections were detected in the following collecting sites: Pearl River, Mississippi (30°20′36″N, 89°38′03″W); Cedar Key, Florida (29°08′13″N, 83°02′36″W); Williford Springs, Florida (30°26′20.40″N, 85°32′50.77″W and 30° 26′21.95″N, 85°32′42.26″W); Daya Bay, Guangdong, China (22°43′N 114°32′E); Eli Creek, Queensland, Australia (25°15′45″S, 152°48′28″E). We have detected Neorickettsia from digeneans for the first time from the Australian continent and China as well as from Oregon and Florida. One of the forms discovered in our study is identical to the SF agent (Table 2). Sequences of the 7 other forms clearly differed from all other known forms of Neorickettsia, therefore, potentially representing new species.
Table 3. List of digenean families screened for Neorickettsia and number of individual extracts associated with each family.
| Digenean Family | Number of extracts | Digenean Family | Number of extracts | Digenean Family | Number of extracts |
| Acanthocolpidae | 2 | Fellodistomoidae | 3 | Paramphistomidae | 2 |
| Allocreadiidae | 40 | Gorgoderidae | 33 | Philophthalmidae | 5 |
| Apocreadiidae | 46 | Haploporidae | 227 | Pleurogenidae | 4 |
| Atracotrematidae | 4 | Haplosplanchnidae | 17 | Pronocephalidae | 4 |
| Azygiidae | 13 | Hemiuridae | 6 | Psilostomidae | 4 |
| Bivesculidae | 8 | Heterophyidae | 5 | Renicolidae | 2 |
| Bucephalidae | 54 | Lecithodendriidae | 17 | Rhopaliidae | 2 |
| Clinostomatidae | 3 | Lepocreadiidae | 43 | Spirorchiidae | 8 |
| Cryptogonimidae | 20 | Lissorchiidae | 8 | Telorchiidae | 4 |
| Cyathocotylidae | 2 | Macroderoididae | 23 | Troglotrematidae | 8 |
| Derogenidae | 6 | Microphallidae | 28 | Zoogonidae | 3 |
| Diplostomatidae | 16 | Monorchiidae | 17 | Unidentified | 30 |
| Echinostomatidae | 1 | Notocotylidae | 1 | ||
| Eucotylidae | 1 | Opecoelidae | 22 | ||
| Faustulidae | 22 | Opisthorchiidae | 7 |
The phylogentic analysis was run using a dataset including 25 ingroup taxa and Wolbachia pipientis (a member of Anaplasmataceae endosymbiotic in insects and filariid nematodes) as an outgroup. The alignment included a total of 1,258 sites, of which 1,249 could be aligned unambiguously. Positions that could not be aligned unambiguously were excluded from the analysis. Bayesian analysis produced a tree where all Neorickettsia sequences clearly fall into a well-defined clade, with a 100% support (Figs. 1 and 2).
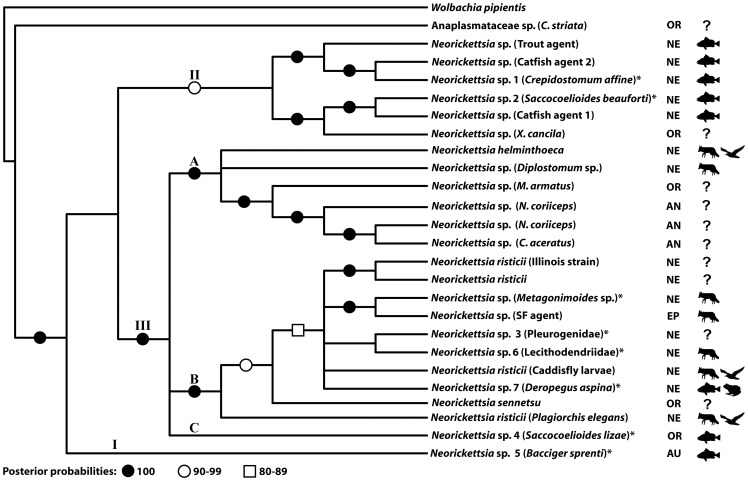
Figure 2. Phylogenetic tree resulting from Bayesian analysis (1,500,000 generations) of partial sequences of 16S rDNA gene.
Phylogenetic tree resulting from Bayesian analysis (1,500,000 generations) of partial sequences of 16S rDNA gene. Posterior probabilities greater than 80% are shown. Roman numerals (I, II, II) represent the different clades within the “Neorickettsia clade” and letters (A, B, C) correspond to the subclades within clade III. An asterisk (*) at the end of a taxon name indicates a new genotype of Neorickettsia discovered in this study. Digenean vertebrate definitive host groups (fishes, mammals and birds) are indicated by symbols. Zoogeographic regions are shown as the following; NE (nearctic), EP (eastern palearctic), OR (oriental), AU (australian), and AN (antarctic).
The “Neorickettsia clade” includes three major, well supported clades (Figs. 1 and 2). Clade I is represented by a single sequence from Bacciger sprenti in Australia. Clade II includes 6 sequences, all of them representing yet unnamed lineages/species of Neorickettsia found in either digeneans parasitic in fish or in fish tissues. Two of these forms are represented by our newly sequenced genotypes, Neorickettsia sp. 1 (from Crepidostomum affine in Mississippi) and Neorickettsia sp. 2 (from Saccocoeliodes beauforti from Florida). All internal sub-clades of clade II are well resolved and 100% supported. Five lineages of this group are from North America while one is from Southeast Asia.
The major clade III contains a majority of the Neorickettsia species, including all three currently recognized named species of these bacteria, namely N. helminthoeca, N. risticii, and N.sennetsu (Figs. 1 and 2). This large clade is split into three sub-clades indicated as A, B, C on Figures 1, 2. Sub-clade A contains a diverse assemblage of lineages that includes N. helminthoeca, Neorickettsia sp. from a Diplostomum sp. published by Tkach et al. [3] as separate branches, and a well resolved cluster containing one form from Southeast Asia and three genotypes from the Antarctic [5]. All digenean hosts of the members of this sub-clade are parasitic in fish at least at some phase of their life cycle. Sub-clade B is 100% supported, however, internal interrelationships of its constituent taxa are less resolved than most of the topologies elsewhere in the tree. This sub-clade incorporates 4 Neorickettsia forms discovered in this study. The basal taxon in this sub-clade is the form reported as Neorickettsia risticii by Tkach et al. [3]. The closest derived taxon to it is N. sennetsu, the agent causing human disease in Southeast Asia. The remaining lineages of the sub-clade B form a polytomy that includes several lineages of N. risticii and the SF agent. Sub-clade C contains only our newly sequenced form from China. All species/genotypes of Neorickettsia in this clade are associated with digeneans who use mammals and/or birds as their definitive hosts.
Discussion
The newly developed real-time PCR protocol has provided for a quick and sensitive way of screening large collections of digenean DNA extracts for Neorickettsia. Removing the necessity of running a nested PCR and screening the results with gel electrophoreses for every extract has greatly reduced the amount of time required to screen large collections of DNA extracts. The elimination of the use of post-PCR handling steps in real-time PCR also reduces the likelihood of false positive results caused by contamination. Although real-time PCR using TaqMan probes has been used in the detection of N. risticii from snails and horses [35], the technique was not used for a broad screening of digeneans for Neorickettsia and there was no published protocol of “regular” real-time PCR using sybr green or another dye as an alternative to the TaqMan probes. Another advantage of the real-time PCR protocol is the ability to run melting curve analysis, which is particularly useful for differentiating potential non-specific binding of primers. It was of great importance for our study since we used relatively generic primers targeting all Neorickettsia species and genotypes. The combination of real-time PCR, nested PCR, and sequencing allows for a very robust and accurate screening.
Our screening has revealed 8 different genotypes of Neorickettsia: a new genotype 1 from Mississippi, new genotypes 2, 3, and SF agent from Florida, new genotypes 6 and 7 from Oregon, a new genotype 4 from China, and a new genotype 5 from Australia (Table 2). Our findings represent the first records of Neorickettsia in digeneans in Australia, China, and two states (Oregon and Florida) in the USA. All 8 digenean species detected as hosts of Neorickettsia in our study (Table 2) represent new host associations for Neorickettsia, although Pusterla et al. [13] detected Neorickettsia within a species of Deropegus from California, neither the species of Deropegus or Neorickettsia were identified or sequenced. Members of the families Haploporidae, Pleurogenidae, and Faustulidae have not been previously reported as hosts of Neorickettsia.
Sampling of digenean DNA extracts for Neorickettsia was mostly opportunistic. As stated in the Materials and Methods, digeneans were collected for various projects prior to our study and were not initially intended for detecting Neorickettsia. This explains the unevenness in the distribution of screened samples among digenean families. Digeneans were not targeted based on their likelihood of harboring the bacterial endosymbionts because very little is currently known about the evolutionary associations among Neorickettsia and their digenean hosts. Therefore, we screened as many samples and as diverse digenean taxa as possible. Our study is the first geographically and taxonomically broad screening of digeneans for the presence of Neorickettsia. Its purpose is to provide baseline data for future more focused studies within certain digenean families or those parasitic in certain hosts.
Our study has revealed a new circulation pathway of Neorickettsia in the natural environment. Until now, Neorickettsia have been found in digeneans with entirely freshwater, freshwater/terrestrial or entirely terrestrial life cycles [3], [4], but not in digeneans with completely marine life cycles. Reports of Neorickettsia in tissues of notothenioid fishes (Notothenia coriiceps and Chaenocephalus aceratus) in Antarctica [5] and mullet Mugil cephalus in the Gulf of Mexico [23] may suggest a marine circulation pathway, however, neither of these studies screened any digeneans. We have found three different forms of Neorickettsia (genotypes 2, 4, and 5) from three digenean species with completely marine life cycles, namely Saccocoeliodes beauforti, Saccocoelioides lizae, and Bacciger sprenti. This suggests that Neorickettsia can be encountered in almost every type of environment suitable for digenean life cycles.
One of our Neorickettsia genotypes obtained from Metagonimoides oregonensis in Florida fully matched the sequence of SF agent. SF agent was initially found in metacercariae of the heterophyid Stellantchasmus falcatus [36] infecting grey mullet in Japan [13], [37]. Our finding is the first record of SF agent outside Japan. Both S. falcatus and M. oregonensis belong to the family Heterophyidae, therefore, there is a probability that SF agent could be found in other members of this large digenean family, members of which are parasitic primarily in fish-eating birds and mammals including humans. SF agent causes mild clinical symptoms in experimentally infected mice and dogs, but not monkeys or humans [8], [11], [38], [39], [40], [41]. Thus, it cannot be excluded that it may cause yet unknown disease in wildlife.
Our phylogenetic analysis has produced a tree with strong branch support for most topologies (Figs. 1 and 2). Our data corroborate the conclusion by Seng et al. [2] who suggested that their sequence obtained from Channa striata most likely represents a separate genus of Anaplasmataceae. On the other hand, the Anaplasmataceae sp. from Mastacembelus armatus considered by Seng et al. [2] to be another new genus, clearly falls into the clade II of the “Neorickettsia clade”(Figs 1, 2). Neorickettsia sp. 5 genotype is of particular interest because it represents a separate clade of Neorickettsia with unresolved affinities to other members of the genus. Denser sampling of digeneans from both marine and freshwater habitats in Australia is necessary to see whether Neorickettsia sp. 5 is a unique, divergent form or a member of a larger lineage that includes additional, yet undiscovered, taxa. An additional systematically relevant result of the present phylogenetic analysis is the position of the N. risticii strain from Plagiorchis elegans. This form was initially identified by Tkach et al. [3] as N. risticii based on the comparison of much shorter DNA sequences and a phylogenetic analysis using those shorter sequences and fewer taxa. In the analysis by Tkach et al. [3] this genotype appeared in a polytomy that included 4 different isolates of N. risticii and N. sennetsu. The present analysis based on longer sequences and greater number of taxa places “N. risticii” from P. elegans as the basal lineage in sub-clade B of clade III. It is separated from the remaining taxa in this clade by one of the recognized distinct species, N. sennetsu. Thus, this genotype likely represents a different, novel species of Neorickettsia which needs to be better characterized and differentiated from formally named taxa.
The least resolved part of the tree is the polytomy in sub-clade B of clade III that includes several genotypes occupying a derived position in relation to N. sennetsu (Figs. 1, 2). The relationships between these genotypes are likely to be clarified with the inclusion of additional genes in future analyses. The last systematic consideration stemming from our analysis is the unresolved position of N. helminthoeca in relation to other members of the sub-clade A of clade III. Unfortunately, the only 16S sequence of N. helminthoeca available in the GenBank is of poor quality and contains numerous unresolved/problematic positions. We believe that obtaining new high quality sequences of N. helminthoeca will help to clarify its affinities with the other species/genotypes of Neorickettsia.
Some of the clades in the phylogenetic tree show close associations among Neorickettsia and the definitive hosts of digeneans in which they were found. For instance, the strongly supported clade II notably comprises neorickettsiae obtained from digeneans parasitic only in fish, mostly in North America. This clade shows the most clear association with a group of definitive hosts of digeneans. Another group that demonstrates a distinct pattern of associations with definitive hosts of digeneans is the sub-clade B of clade III. Digenean hosts of neorickettsiae in this sub-clade are parasitic in either birds or mammals with the exception of few cases where the vertebrate hosts of digeneans are unknown and Neorickettsia sp. 7 found in Deropegus aspina, a digenean that use fish and amphibians as a definitive host (Fig. 2). N. sennetsu, a causative agent of the human disease Sennetsu fever in Southeast Asia, is one of the species with yet unknown digenean hosts. Nevertheless, the phylogenetic tree topology allows us to hypothesize that it should be a digenean that uses either mammals or birds as a definitive host.
Patterns of geographic distribution of most of the digenean species harboring Neorickettsia in this study are varying and in some cases little is known. Crepidostomum affine was recently described by Tkach et al. [42] from Hiodon tergisus collected from Pearl River and Pascagoula River drainages in Mississippi, USA. Based on a molecular comparison to other species in the genus by Tkach et al. [42], C. affine is likely endemic to these river basins in Mississippi. Therefore, we hypothesize that the distribution of Neorickettsia sp. 1 is likely to be limited to that of C. affine.
Mugil cephalus, the host of two digenean species harboring Neorickettsia in this study (Table 2), is a globally distributed marine fish. However, these two digenean species, Saccocoelioides beauforti and Saccocoelioides lizae, are much more restricted in their distribution than their fish host. One of them, Saccocoelioides beauforti has only been reported from the southeastern United States (North Carolina, Louisiana, Mississippi, and Alabama) [43], [44]. The other species, Saccocoelioides lizae has only been found off the cost of South-eastern China [45]. Based on the combined distribution data of these digeneans and their fish host, it is likely that Neorickettsia sp. 2 may be found in other areas along the coast of the southeastern United States and that Neorickettsia sp. 4 may be found in other costal countries in Southeast Asia.
Deropegus aspina is only known from the Pacific Northwest of the United States in salmonid fishes, including Salmo clarki, S. gairdneri, Oncorhynchis kisutch, O. tshawytscha, and a frog, Rana boyli, [46]. Therefore, we hypothesize that Neorickettsia sp. 7 is limited in its distribution to the Pacific coast of the USA and Canada.
Bacciger sprenti (family Faustulidae) carrying Neorickettsia in our study was obtained from fish Selenotoca multifasciata (Table 2). This digenean species was originally identified from the intestine of a Mugil sp. in Australia [47]. However, later research by Cribb et al. [48] only found it in Selenotoca multifasciata (13 individuals were infected) and not in 11 individuals of Mugil cephalus or 49 individuals of Mugil georgii. This led Cribb et al. [48] to speculate that an error was made in the recording of the host fish species in the original description by Bray [47]. This is in concordance with available data on host associations of other faustulid trematodes, which are not known to be shared between scatophagid and mugilid fishes [48]. Currently Bacciger sprenti has only been reported from marine fishes in Australian coastal waters, therefore, we can assume Neorickettsia sp. 5 is a genotype unique to Australia.
Currently known distribution of Neorickettsia is very geographically uneven and is clearly associated with areas where most studies have taken place. The majority of genotypes were found in the USA and several countries in southeastern and eastern Asia [4]. Even after our discovery of Neorickettsia in Australia, there is no well-documented information supported by PCR/sequence data from Africa, Europe, most of South America, most of Asia, and nearly all island countries [4]. We believe that this situation does not reflect the true distribution of Neorickettsia and rather reflects insufficient knowledge due to the lack of broad screening efforts. With more than 18,000 described species of digeneans [49], there is a potential for many more species/genotypes of Neorickettsia to be found and characterized.
Acknowledgments
We are grateful to Dr. Sabine Dittrich (Lao Oxford Mahosot Welcome Trust Research Unit) for kindly providing us with positive control for our PCR reactions, and Kaylyn Patitucci (University of North Dakota) and Dr. Xuejuan Ding (South China Normal University) for their help collecting digeneans. The authors are also grateful to Dr. Richard Heard (University of Southern Mississippi) for the provision of microscopy and sampling equipment.
Funding Statement
This work was supported in part by the grant R15AI092622 from the National Institutes of Health, USA, and US Fish and Wildlife Service/Mississippi Department of Marine Resources MSCIAP MS.R.798 Award M10AF20151. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Headley SA, Scorpio DG, Vidotto O, Dumler JS (2011) Neorickettsia helminthoeca and salmon poisoning disease: a review. Vet J 187: 165–173. [DOI] [PubMed] [Google Scholar]
- 2. Seng P, Rolain JM, Raoult D, Brouqui P (2009) Detection of new Anaplasmataceae in the digestive tract of fish from southeast Asia. Clin Microbiol Infect 15: 88–90. [DOI] [PubMed] [Google Scholar]
- 3. Tkach VV, Schroeder JA, Greiman SE, Vaughan JA (2012) New genetic lineages, host associations and circulation pathways of Neorickettsia endosymbionts of digeneans. Acta Parasitol 57: 285–292. [DOI] [PubMed] [Google Scholar]
- 4. Vaughan JA, Tkach VV, Greiman SE (2012) Neorickettsial endosymbionts of the Digenea: diversity, transmission and distribution. Adv Parasitol 79: 253–297. [DOI] [PubMed] [Google Scholar]
- 5. Ward NL, Steven B, Penn K, Methe BA, Detrich WH (2009) Characterization of the intestinal microbiota of two Antarctic notothenioid fish species. Extremophiles 13: 679–685. [DOI] [PubMed] [Google Scholar]
- 6. Madigan JE, Pusterla N (2000) Ehrlichial diseases. Vet Clin North Am Equine Pract 16: 487–499. [DOI] [PubMed] [Google Scholar]
- 7. Newton P, Rolain JM, Rasachack B, Mayxay M, Vathanatham K, et al. (2009) Sennetsu neorickettsiosis: a probable fish-borne cause of fever rediscovered in Laos. Am J Trop Med Hyg 81: 190–194. [PMC free article] [PubMed] [Google Scholar]
- 8. Rikihisa Y (1991) The tribe Ehrlichia and ehrlichial diseases. Clin Microbiol Rev 4: 286–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker DH, Dumler JS (1996) Emergence of the Ehrlichioses as human health problems. Emerg Infect Dis 2: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madigan JE, Rikihisa Y, Palmer JE, DeRock E, Mott J (1995) Evidence for a high rate of false-positive results with the indirect fluorescent antibody test for Ehrlichia risticii antibody in horses. J Am Vet Med Assoc 207: 1448–1453. [PubMed] [Google Scholar]
- 11. Mott J, Rikihisa Y, Zhang Y, Reed SM, Yu CY (1997) Comparison of PCR and culture to the indirect fluorescent-antibody test for diagnosis of Potomac horse fever. J Clin Microbiol 35: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coimbra HS, Schuch LFD, Veitenheimer-Mende IL, Meireles MCA (2005) Neorickettsia (Ehrlichia) risticii no sul do Brasil: Heleobia spp. (Mollusca: Hydrobiidae) e Parapleurolophocecous cercariae (Trematoda: Digenea) como possíveis vetores. Arq Inst Biol 72: 325–329. [Google Scholar]
- 13. Fukuda T, Yamamoto S (1981) Neorickettsia-like organism isolated from metacercaria of a fluke, Stellantchasmus falcatus . Jpn J Med Sci Biol 34: 103–107. [DOI] [PubMed] [Google Scholar]
- 14. Gibson KE, Rikihisa Y, Zhang C, Martin C (2005) Neorickettsia risticii is vertically transmitted in the trematode Acanthatrium oregonense and horizontally transmitted to bats. Environ Microbiol 7: 203–212. [DOI] [PubMed] [Google Scholar]
- 15. Pusterla N, Johnson E, Chae J, DeRock E, Willis M, et al. (2000) Molecular detection of an Ehrlichia-like agent in rainbow trout (Oncorhynchus mykiss) from Northern California. Vet Parasitol 92: 199–207. [DOI] [PubMed] [Google Scholar]
- 16. Chapin EA (1926) A new genus and species of trematode, the probable cause of salmon-poisoning in dogs. North Am Vet 7: 42–43. [Google Scholar]
- 17. Donham CR (1925) So-called salmon poisoning of dogs. Science 61: 341. [DOI] [PubMed] [Google Scholar]
- 18. Fukuda T, Kitao T, Keida Y (1954) Studies on the causative agent of “Hyuganetsu” disease. I. Isolation of the agent and its inoculation trial in human beings. Med Biol 32: 200–209. [Google Scholar]
- 19. Rikihisa Y, Perry BD (1985) Causative ehrlichial organisms in Potomac Horse Fever. Infect Immun 49: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farrell RK, Leader RW, Johnston SD (1973) Differentiation from salmon poisoning disease and Elokomin fluke disease fever: studies with the black bear (Ursus americanus). Am J Vet Res 34: 919–922. [PubMed] [Google Scholar]
- 21. Philip CB, Hadlow WJ, Hughes LE (1953) Neorickettsia helmintheca, a new rickettsia-like disease agent of dogs in western United States transmitted by a helminth. Rome: International Congress of Microbiology Rept. Proc., 6th Congress Vol. II: 256–257. [Google Scholar]
- 22. Pusterla N, Johnson EM, Chae JS, Madigan JE (2003) Digenetic trematodes, Acanthatrium sp. and Lecithodendrium sp., as vectors of Neorickettsia risticii, the agent of Potomac horse fever. J Helminthol 77: 335–339. [DOI] [PubMed] [Google Scholar]
- 23. Larsen A, Tao Z, Bullard SA, Arias CR (2013) Diversity of the skin microbiota of fishes: evidence for host species specificity. FEMS Microbiol Ecol 85: 483–494. [DOI] [PubMed] [Google Scholar]
- 24.Burch JB (1989) North American freshwater snails. Hamburg, Michigan: Malacological Publications. 365 p. [Google Scholar]
- 25. Tkach VV, Pawlowski J (1999) A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitol 44: 147–148. [Google Scholar]
- 26. Barlough JE, Rikihisa Y, Madigan JE (1997) Nested polymerase chain reaction for detection of Ehrlichia risticii genomic DNA in infected horses. Vet Parasitol 68: 367–373. [DOI] [PubMed] [Google Scholar]
- 27.Gibson DI, Jones A, Bray RA (Eds.) (2002) Keys to the Trematoda, Volume 1. New York: CABI publishing. 544p. [Google Scholar]
- 28.Gibson DI, Jones A, Bray RA (Eds.) (2005) Keys to the Trematoda, Volume 2. New York: CABI publishing. 745p. [Google Scholar]
- 29.Gibson DI, Jones A, Bray RA (Eds.) (2008) Keys to the Trematoda, Volume 3. New York: CABI publishing. 848p. [Google Scholar]
- 30. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- 31.Maddison DR, Maddison WP (2005) MacClade 4: Analysis of phylogeny and character evolution. Version 4.08a. Available: http://macclade.org. [DOI] [PubMed]
- 32. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 33. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 34. Posada D (2008) jModelTest: Phylogenetic Model Averaging. Mol Biol Evol 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 35. Pusterla N, Leutenegger CM, Sigrist B, Chae JS, Lutz H, et al. (2000) Detection and quantitation of Ehrlichia risticii genomic DNA in infected horses and snails by real-time PCR. Vet Parasitol 90: 129–135. [DOI] [PubMed] [Google Scholar]
- 36. Katsuta I (1931) Studies on Formosan trematodes whose intermediate hosts are brackish water fishes. I. Stellantchasmus falcatus n.sp. with mullet as its vector. Taiwan Igakkai Zasshi 30: 1404–1417. [Google Scholar]
- 37. Fukuda T, Sasahara T, Kitao T (1973) Causative agent of “Hyuganetsu” disease. 11. Characteristics of rickettsia-like organisms isolated from metacercaria of Stellantchasmus falcatus . Kansenshogaku Zasshi 47: 474–482. [DOI] [PubMed] [Google Scholar]
- 38. Hirai K (1966) Study of infectious mononucleosis in Kumamoto Prefecture. 1. Isolation of a Rickettsia sennetsu -like organism from patients with epidemic glandular fever. Kumamoto Igakkai Zasshi 40: 1159–1173. [PubMed] [Google Scholar]
- 39. Shishido A, Honjo S, Suganuma M, Ohtaki S, Hikita M, et al. (1965) Studies on infectious mononucleosis induced in the monkey by experimental infection with Rickettsia sennetsu. 1. Clinical observations and etiological investigations. Jpn J Med Sci Biol 18: 73–83. [DOI] [PubMed] [Google Scholar]
- 40.Tachibana N (1986) Sennetsu fever: the disease, diagnosis, and treatment. In: Microbiology-1986. Washington, D.C.: American Society for Microbiology. 205–208. [Google Scholar]
- 41. Wen B, Rikihisa Y, Yamamoto S, Kawabata N, Fuerst PA (1996) Characterization of the SF agent, and Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol 46: 149–154. [DOI] [PubMed] [Google Scholar]
- 42. Tkach VV, Curran SS, Jeffrey AB, Overstreet RM (2012) A new species of Crepidostomum (Digenea: Allocreadiidae) from Hiodon tergisus in Mississippi and molecular comparisn with three congeners. J Parasitol 99: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 43. Hunter WS, Thomas LJ (1961) A new species of Saccocoelium (Trematoda, Haploporidae) from Beaufort, N. C. Trans Am Microsc Soc 80: 176–179. [Google Scholar]
- 44. Overstreet RM (1971) Some adult digenic trematodes in striped mullet from the northern Gulf of Mexico. J Parasitol 57: 967–974. [PubMed] [Google Scholar]
- 45. Liu SF (2002) One new species of Saccocoelioides (Digenea: Haploporidae) from Liza carinatus in Taiwan Strait. J Oceanogr 21: 37–44. [Google Scholar]
- 46. McCauley JE, Pratt I (1961) A new genus Deropegus with a redescription of D. aspina (Ingles, 1936) nov. comb. Trans Am Microsc Soc 55: 373–377. [Google Scholar]
- 47. Bray RA (1982) Two new species of Baccifer Nicoll, 1914 (Digenea: Fellodistomidae) from mullet in Australia. J Nat Hist 16: 23–29. [Google Scholar]
- 48. Cribb TH, Anderson GR, Bray RA (1999) Faustulid trematodes (Digenea) from marine fishes of Australia. Syst Parasitol 44: 119–138. [DOI] [PubMed] [Google Scholar]
- 49.Cribb TH, Bray RA, Littlewood DTJ, Pichelin SP, Herniou EA (2001) The Digenea. In: Interrelationships of the Platyhelminthes. London: Taylor and Francis 168–185. [Google Scholar]