Abstract
Tannerella forsythia is an important pathogen in periodontal disease. Previously, we showed that sialidase activity is key to its utilisation of sialic acid from a range of human glycoproteins for biofilm growth and initial adhesion. Removal of terminal sialic acid residues often exposes β-linked glucosamine or galactosamine which may also be important adhesive molecules. In turn, these residues are often removed by a group of enzymes known as β-hexosaminidases. We show here that T. forsythia has the ability to cleave glucosamine and galactosamine from model substrates and that this activity can be inhibited by the hexosaminidase inhibitor PugNAc (O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino N-phenyl carbamate). We now demonstrate for the first time that β-hexosaminidase activity plays a role in biofilm growth on glycoprotein coated surfaces since biofilm growth and initial cell adhesion is inhibited by PugNAc. In contrast, adhesion to siallo-protein coated surfaces is unaltered by PugNAc in the absence of sialidase activity (using a sialidase deficient mutant) or surprisingly on the clinically relevant substrates saliva or serum. These data indicate that β-hexosaminidase activity has a significant role in biofilm formation in combination with sialidase activity in the biofilm lifestyle of T. forsythia.
Keywords: beta-hexosaminidase, periodontal pathogens, Tannerella forsythia
Tannerella forsythia (TF), alongside Treponema denticola and Porphyromonas gingivalis is a member of the “Red complex” oral bacteria that are strongly associated with periodontal disease (Socransky et al. 1998). Bacteria linked with periodontal disease are also associated with systemic disease such as endocarditis, atherosclerosis and pre-term birth (Tanner et al. 2006). Recent work in our laboratories have highlighted that the T. forsythia NanH sialidase is important in interactions with human gingival epithelial cells (Honma et al. 2011) and a range of sialloglycoproteins (Roy et al. 2011).
While the role of sialidase in T. forsythia adhesion and nutrition seems well established, like many members of the Bacteroidetes group (e.g. B. fragilis, B. thetaiotomicron)(Xu & Gordon 2003; Sonnenburg et al. 2005), T. forsythia possesses a plethora of glycosidase and sugar focussed metabolic capabilities (Braham & Moncla 1992; Moncla et al. 1990; Maiden et al. 1996). One example is the ability to cleave β-linked sugars such as galactosamine and glucosamine from glycoproteins by the action of β-hexosaminidase enzymes (Hughes et al. 2003). This is supported by the presence of at least three putative β-hexosaminidase encoding genes in the T. forsythia genome (TF0036, TF2925 and TF0014), The cloning and purification of TF2925 has previously been reported and its N-acetyl-β-D-glucosaminidase activity documented (Hughes et al. 2003). In contrast, TF0036 lies in the sialic acid operon (Roy et al. 2010; Thompson et al. 2009) and is annotated as a potential β-hexosaminidase enzyme (a collective term for enzymes able to cleave β-linked galactose and glucose residues)(Slámová et al. 2010), while nothing is known of TF0014. The activity of these enzymes may be of relevance in T. forsythia biofillm formation in vivo since removal of terminal sialic acid residues often results in the exposure of galactose or glucose moieties and these may play either adhesive or nutritional roles (Spiro & Bhoyroo 1974; Salyers et al. 1988).
In this study, we aimed to determine whether the T. forsythia β-hexosaminidase enzymatic activity plays a role in biofilm formation and initial attachment to sialoglycoproteins or desialylated versions such as asialofetuin.
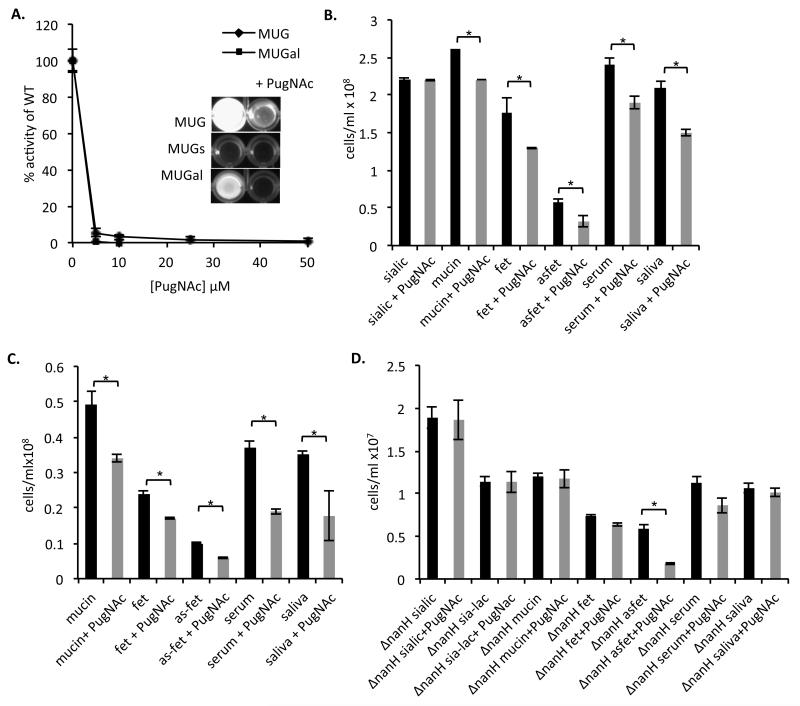
Firstly, we aimed to confirm the ability of plate grown T. forsythia (on Fastidious Anaerobe agar (Lab M) (5% oxalated horse blood with 0.05% NAM)) to cleave β-linked glucosamine and galactosamine residues using the 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-glucopyranoside (MUG), 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-galactopyranoside (MU-Gal) and a sulphated MUG version named 4-methylumbelliferyl-2-acetamido-2-deoxy-6-sulfo-β-D-glucopyranoside (MUGs). Assays were performed using 1×107 bacterial cells per well in a reaction mixture containing 1.5mM substrate in 50mM sodium citrate buffer, pH 4.5, for 2h at 37°C before the fluorescence intensity was measured using a BMG Labtech micro-plate reader (λexcitation = 355nm, λmission = 430±10nm). It is clear that T. forsythia can cleave both MUG and MUGal to release free umbelliferyl (as detected by fluorescence at 405nm) while the sulphated form was left uncleaved (Fig1A. inset).
Figure 1.
Effect of PugNAc on whole-cell hexosaminidase activity
Five-day-old T. forsythia cells were harvested, washed with PBS, and adjusted to a cell density at A600 of 0.05. The hexosaminidase substrates, 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-glucopyranoside (MUG), 4-methylumbelliferyl-2-acetamido-2-deoxy-6-sulfo-β-D-glucopyranoside (MUGs) and 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-galactopyranoside (MUGal) were then added to a final concentration of 1.5mM. The inhibitor PugNAc (O-(2-Acetamido-2-deoxy-D-glucopyranosylidene) amino N-phenyl carbamate) was added at the concentrations shown. Reactions were allowed to proceed for 2h before measurement at a fixed excitation wavelength of 355nm and the emission measured at 430±10nm in a fluorimeter. Assays were performed in triplicate, and the readings are presented as a percentage of the cell activity without any PugNAC added; standard error is shown. (Inset) Photograph of end-point assay in the presence of 25μM PugNAc.
(B) Biofilm growth of T. forsythia on glycoprotein coated surfaces. Biofilm growth was assessed for the wild-type strain in the absence (black) or presence of PugNAc (25μM) (grey). Biofilms were set-up in triplicate and wells supplemented with 6 mM sialic acid (sialic), 6mM sialyl-lactose (sia-lac) or in coated wells with 6mM mucin, fetuin and a-sialo fetuin, 2mg/mL human serum and saliva with and without the addition of 25μM PugNAc to the TSB-medium as indicated at the time of inoculation. Glycoproteins were coated on the 96-well plate overnight at 4°C and washed before inoculation with T. forsythia to a final OD600 of 0.05. Data are number of cells after harvesting from wells after 4 days growth with standard deviations. Difference between mean data in this experiment were evaluated by t-test, with p<0.05 being taken as the level of significance (* statistically significant).
(C) Initial adhesion growth was compared as above with the wild-type strain of T. forsythia without and with 25mM PugNAc except that plates were only incubated for 3h before harvesting of bacteria and enumeration.
(D) Initial adhesion of the ΔnanH mutant of T. forsythia in the presence and absence of 25mM PugNAc.
To assess the role of these enzyme activities in biofilm formation of this pathogen, a potent inhibitor was required in the absence of a suite of mutants in this genetically recalcitrant organism.
One candidate identified was O-(2-Acetamido-2-deoxy-D-glucopyranosylidene) amino N-phenyl carbamate, abbreviated as PugNAc (Haltiwanger et al. 1998) which was originally identified as an inhibitor of β-N-glucosaminidases of animal, plant and fungal enzymes (Horsch et al. 1991). First we tested whether PugNAc was able to inhibit whole cell β-hexosaminidase activity of T. forsythia, as described above. Increasing concentrations of PugNAc (5–50μM) strongly inhibited this activity with 25μM reducing activity to <2%. In addition, since access to underlying glucosamine or galactosamine residues is T. forsythia sialidase dependent we established that PugNAc has no inhibitory activity on whole cell sialidase activity (data not shown).
To investigate the influence of this glycosidase capability on biofilm formation of T. forsythia on glycoprotein coated surfaces we performed biofilm growth experiments essentially as described previously but with the inclusion of PugNAc (25μM) in the medium at the time of inoculation (Roy et al., 2011). Briefly, T. forsythia (ATCC 43037) was grown for five days on Fastidious Anaerobe agar plates supplemented with 5% horse blood containing 10μg/mL NAM before colonies were harvested and washed twice in fresh 10% trypticase soya broth (TSB, Oxoid) supplemented with 2% yeast extract (YE, Sigma-Aldrich), 1mg/mL hemin, 1mg/mL menadione, Sigma-Aldrich) and resuspended to an OD600 of 0.05 prior to inoculation into uncoated polystyrene wells (Greiner). For biofilm growth with sialic acid (6mM) as a growth factor this was included in the media (in uncoated wells) while experiments with glycoproteins were performed with inoculation of bacteria into wells that had been pre-coated overnight with the relevant glycoprotein (bovine submaxillary mucin (Type I-S), fetuin, asialofetuin, all 6mM), saliva (2μg/mL) or serum (2μg/mL) in PBS at 4°C before washing with PBS to remove excess proteins and unbound bacteria. These were incubated anaerobically at 37°C for five days. The cell number in mature biofilms was assessed as described previously (Roy et al., 2011); briefly, after five days, culture medium was removed, samples were washed twice in sterile PBS, followed by rigorous resuspension of biofilm cells by pipetting in PBS before counting microscopically using a Helber counting chamber (Hawksley) under a phase-contrast lens (×400 magnification). The effectiveness of the method was assessed by determining the number of residual cells attached (usually 0.01%) by crystal violet staining (Pham et al. 2010). Experiments were performed in triplicate wells on three independent occasions and compared by Student’ s t-test, representative data sets are shown in all cases.
In the presence of PugNAc, there was a small but statistically significant reduction in biofilm growth on the sialylated proteins mucin and fetuin (15 and 30%, respectively, p< 0.05) and on saliva and serum (20 and 30%, respectively, p< 0.05) while the weak growth on asialofetuin was reduced more markedly (~two-fold) (Figure 1B). As expected there was no effect on growth of biofilms using sialic acid as the growth substrate, indicating that PugNAc does not affect sialic acid metabolism or uptake.
We then assessed the effect of inhibiting whole cell β-hexosaminidase activity on initial adhesion to the glycoproteins. These assays were performed essentially as the biofilm growth experiments except that they were only incubated for 4h at 37°C. Figure 1C illustrates that the inclusion of PugNAc reduces initial adhesion at a similar, if slightly stronger (1.3–2-fold), level to the five-day biofilm growth experiments.
While these data are not as striking as the reduction in biofilm growth with the sialidase inhibitor (oseltamivir or siastatin B) or a ΔnanH mutant (2–5-fold) (Roy et al., 2011) they do indicate that T. forsythia β-hexosaminidase activity does influence its ability to form biofilms on sialyl- and asialyl-glycoproteins. The reason that these effects are not more striking may be that the removal of sialic acid by sialidases is a rate-limiting step resulting in only a marginal inhibitory effect. Alternatively, neither N-acetyl-galactosamine or N-acetyl-glucosamine are effective growth substrates for T. forsythia (Wyss 1989), meaning that this data reflects alterations in initial adhesion only. In contrast to the sialidase data, there is a reduction in adhesion using both sialyated fetuin and its desialylated form, asialofetuin (Figure 1B) This is expected since the sialic acid has already been removed from this substrate to expose β-galactosamine as the terminal moiety.
Our data clearly indicate that in addition to our previous finding that sialic acid and sialidase activity are key to biofilm growth and adhesion to siallo-glycoprotein coated surfaces, the underlying sub-terminal hexose sugars are also important (Roy et al 2011). However, it is clear that the ability to remove sialic acid is the key step in this process, i.e. the inability to remove sialic acid should result in PugNAc having no effect in the absence of sialidase activity. To test this hypothesis, we repeated the adhesion experiments in the presence of PugNAc but using our sialidase deficient ΔnanH mutant strain of T. forsythia (N.B. β-hexosaminidase activity of the ΔnanH mutant is identical to wild-type (data not shown)). As shown in Figure 1D, adhesion of this mutant to glycoprotein-coated surfaces was unaltered by addition of PugNAc on any of the siallo-glycoproteins (mucin, fetuin) but was reduced 3.3-fold on asialofetuin (where the galactose and glucose residues are exposed). Surprisingly, there was no significant reduction in adhesion to serum or saliva, suggesting that initial interaction with sialic acid might be crucial for initial adhesion of T. forsythia to glycoprotein coated surfaces, i.e. this interaction may be of higher affinity than with underlying sugars
The role of β-hexosaminidases in bacterial biofilm formation is not well characterised except in a few cases such as in S. pneumoniae where a lytB β-glucosaminidase mutant displays reduced biofilm forming capacity, however this may be involved in autolysis making it difficult to decipher whether this is a direct effect (Moscoso et al. 2006). In the oral context Aggregatibacter actinomycetemconcomitans possesses a β-N-glucosaminidase called Dispersin B that is involved in the release of individual cells from the biofilm matrix (Kaplan et al. 2004). There is also evidence that some bacterial enzymes, such as the Spy1600 enzyme from S. pyogenes, may alter host cell function since it can trim glycans from human proteins (Sheldon et al. 2006). In the case of T. forsythia it is possible that the β-hexosaminidase activity has effects not only on interactions with saliva-coated surfaces, but also interactions with host epithelial cells but this has yet to be tested. In addition, these enzymes may also influence interactions with other bacteria in terms of nutrition as release of glucose or galactose sugars may act as growth substrates for cohabiting bacteria. In summary, this is the first study to demonstrate a role for β-hexosaminidase activity in biofilm formation is novel of a periodontal pathogen and sheds new light on the factors at work in the biofilm lifestyle of T. forsythia.
Acknowledgements
This work was funded by grants from the Royal Society, Dunhill Medical Trust and British Oral and Dental Research Trust to GS and by a University of Sheffield Oral Disease cluster studentship and Wellcome Trust Value in People Fellowship to SR. The work in AS laboratory was funded by U.S. Public Health Grant DE014749.
References
- Braham PH, Moncla BJ. Rapid presumptive identification and further characterization of Bacteroides forsythus. Journal of clinical microbiology. 1992;30:649–654. doi: 10.1128/jcm.30.3.649-654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J.Biol.Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. PM:9452489. [DOI] [PubMed] [Google Scholar]
- Honma K, Mishima E, Sharma A. Role of Tannerella forsythia NanH sialidase in epithelial cell attachment. Infect.Immun. 2011;79:393–401. doi: 10.1128/IAI.00629-10. PM:21078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch M, Hoesch L, Vasella A, Rast DM. [Accessed December 8, 2011];N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-N-acetylglucosaminidase. European journal of biochemistry / FEBS. 1991 197:815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. http://www.ncbi.nlm.nih.gov/pubmed/2029909. [DOI] [PubMed] [Google Scholar]
- Hughes CV, Malki G, Loo CY, Tanner AC, Ganeshkumar N. Cloning and expression of alpha-D-glucosidase and N-acetyl-beta-glucosaminidase from the periodontal pathogen, Tannerella forsythensis (Bacteroides forsythus) Oral Microbiol.Immunol. 2003;18:309–312. doi: 10.1034/j.1399-302x.2003.00091.x. PM:12930523. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Velliyagounder K, Rohde H, Mack D, Johannes K, Ragunath C, Knobloch JK, Ramasubbu N. Genes Involved in the Synthesis and Degradation of Matrix Polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae Biofilms Genes Involved in the Synthesis and Degradation of Matrix Polysaccharide in Actinobacillus actino. Biofilms. 2004 doi: 10.1128/JB.186.24.8213-8220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden MF, Tanner A, Macuch PJ. Rapid characterization of periodontal bacterial isolates by using fluorogenic substrate tests. J.Clin.Microbiol. 1996;34:376–384. doi: 10.1128/jcm.34.2.376-384.1996. PM:8789019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla BJ, Braham P, Hillier SL. [Accessed July 28, 2011];Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. Journal of clinical microbiology. 1990 28:422–425. doi: 10.1128/jcm.28.3.422-425.1990. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=269635&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso M, García E, López R, Garci E. Biofilm Formation by Streptococcus pneumoniae : Role of Choline , Extracellular DNA , and Capsular Polysaccharide in Microbial Accretion Biofilm Formation by Streptococcus pneumoniae : Role of Choline , Extracellular DNA , and Capsular Polysaccharide in M. 2006 doi: 10.1128/JB.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TK, Roy S, Noirel J, Douglas I, Wright PC, Stafford GP. [Accessed July 4, 2011];A quantitative proteomic analysis of biofilm adaptation by the periodontal pathogen Tannerella forsythia. Proteomics. 2010 10:3130–3141. doi: 10.1002/pmic.200900448. [DOI] [PubMed] [Google Scholar]
- Roy S, Douglas CWI, Stafford GP. [Accessed July 12, 2011];A novel sialic acid utilization and uptake system in the periodontal pathogen Tannerella forsythia. Journal of bacteriology. 2010 192:2285–2293. doi: 10.1128/JB.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Honma Kiyonobu, Douglas I, Sharma Ashu, Stafford G. [Accessed September 6, 2011];Role of sialidase in glycoprotein utilisation by Tannerella forsythia. Microbiology (Reading, England) 2011 157:3195–3202. doi: 10.1099/mic.0.052498-0. http://www.ncbi.nlm.nih.gov/pubmed/21885482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, Pajeau M, Mccarthyt RE. Importance of Mucopolysaccharides as Substrates for Bacteroides thetaiotaomicron Growing in Intestinal Tracts of Exgermfree Mice. Society. 1988;54:1970–1976. doi: 10.1128/aem.54.8.1970-1976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon WL, Macauley MS, Taylor EJ, Robinson CE, Charnock SJ, Davies GJ, Vocadlo DJ, Black GW. Functional analysis of a group A streptococcal glycoside hydrolase Spy1600 from family 84 reveals it is a β-N-acetylglucosaminidase and not a hyaluronidase. Society. 2006;247:241–247. doi: 10.1042/BJ20060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slámová K, Bojarová P, Petrásková L, Kren V. [Accessed October 28, 2011];β-N-acetylhexosaminidase: what’ s in a name…? Biotechnology advances. 2010 28:682–693. doi: 10.1016/j.biotechadv.2010.04.004. http://www.ncbi.nlm.nih.gov/pubmed/20438826. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee a D, Cugini M a, Smith C, Kent RL. Microbial complexes in subgingival plaque. Journal of clinical periodontology. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu Jian, Leip DD, Chen C-H, Westover BP, Weatherford J, Buhler JD, Gordon Jeffrey I. [Accessed July 1, 2011];Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science (New York, N.Y.) 2005 307:1955–1959. doi: 10.1126/science.1109051. http://www.ncbi.nlm.nih.gov/pubmed/15790854. [DOI] [PubMed] [Google Scholar]
- Spiro RG, Bhoyroo VD. Structure of the O-glycosidically linked carbohydrate units of fetuin. J.Biol.Chem. 1974;249:5704–5717. PM:4137945. [PubMed] [Google Scholar]
- Tanner ACR, Izard J, Moore H. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontology. 2006;2000;42:88–113. doi: 10.1111/j.1600-0757.2006.00184.x. http://www.ncbi.nlm.nih.gov/pubmed/16930308. [DOI] [PubMed] [Google Scholar]
- Thompson H, Homer K a, Rao S, Booth V, Hosie AHF. [Accessed July 28, 2011];An orthologue of Bacteroides fragilis NanH is the principal sialidase in Tannerella forsythia. J.Bacteriol. 2009 191:3623–3628. doi: 10.1128/JB.01618-08. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2681896&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss C. Dependence of proliferation of Bacteroides forsythus on exogenous N-acetylmuramic acid. InfectImmun. 1989;57:1757–1759. doi: 10.1128/iai.57.6.1757-1759.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. PM:12923294. [DOI] [PMC free article] [PubMed] [Google Scholar]