Abstract
As part of the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) Trial, a prospective multicenter cohort of 221 children and adolescents with systemic lupus erythematosus (SLE) (mean age 15.7 years, 83% female) underwent baseline measurement of markers of cardiovascular risk, including fasting levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), lipoprotein A (Lpa), homocysteine and high-sensitivity C-reactive protein (hs-CRP). A cross-sectional analysis of the baseline laboratory values and clinical characteristics of this cohort was performed. Univariable relationships between the cardiovascular markers of interest and clinical variables were assessed, followed by multivariable linear regression modeling. Mean levels of LDL, HDL, Lpa, TG, hs-CRP and homo-cysteine were in the normal or borderline ranges. In multivariable analysis, increased Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), prednisone dose, and hypertension (HTN) were independently associated with higher LDL levels. Higher hs-CRP and creatinine clearance were independently related to lower HDL levels. Higher body mass index (BMI), prednisone dose, and homocysteine levels were independently associated with higher TG levels. Only Hispanic or non-White status predicted higher Lpa levels. Proteinuria, higher TG and lower creatinine clearance were independently associated with higher homocysteine levels, while use of multivitamin with folate predicted lower homocysteine levels. Higher BMI, lower HDL, and longer SLE disease duration, but not SLEDAI, were independently associated with higher hs-CRP levels. The R2 for these models ranged from 7% to 23%. SLE disease activity as measured by the SLEDAI was associated only with higher LDL levels and not with hs-CRP. Markers of renal injury (HTN, proteinuria, and creatinine clearance) were independently associated with levels of LDL, HDL, and homocysteine, highlighting the importance of renal status in the cardiovascular health of children and adolescents with SLE. Future longitudinal analysis of the APPLE cohort is needed to further examine these relationships.
Keywords: atherosclerosis, cardiovascular, lipid, pediatric, SLE, systemic lupus erythematosus
Introduction
Premature atherosclerosis is a well-recognized complication of systemic lupus erythematosus (SLE). Although a focus of intense research, the pathophysiology underlying this association remains incompletely understood. Medication side effects, chronic inflammation, autoantibodies, hypertension, renal disease, and abnormal lipid profiles likely contribute to the enhanced cardiovascular risk evident in SLE.1 Given their lifelong exposure to atherogenic risk factors, children and adolescents with SLE are at particularly high risk for developing premature atherosclerosis and are therefore ideal candidates for primary prevention. However, optimal primary prevention strategies for children and adolescents with SLE are not yet evidence-based. Several markers of atherosclerotic risk have been extensively investigated as targets for primary and secondary prevention in the general population, and efforts are underway to define optimal prevention strategies for SLE patients.
In large-scale observational studies of the general population, abnormal levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (TG) are clearly linked with heightened risk of atherosclerotic events, and well established guidelines for primary and secondary prevention, including those published by the National Cholesterol Education Program (NCEP), are widely accepted.2 High rates of abnormal HDL and LDL levels have also been described in adult and pediatric SLE cohorts.3,4 Across the age spectrum, active SLE has been associated with low HDL levels.4,5 Although lowering LDL and increasing HDL decrease atherosclerotic events in the general population, at least 50% of vascular events occur in the absence of overt dyslipidemia.6 Thus, there is great interest in the development of novel markers of atherosclerotic risk to complement the prognostic capabilities of LDL, HDL, and TG.
Among newer candidate markers for athero-sclerotic risk, high-sensitivity C-reactive protein (hs-CRP) has stimulated the most interest. Elevated levels of hs-CRP independently predict myocardial infarction, peripheral arterial disease, and sudden cardiac death in large-scale adult epidemiologic studies, adding prognostic benefit beyond LDL and the traditional Framingham risk score.7 Potential pro-atherogenic mechanisms of hs-CRP include enhanced expression of vascular adhesion molecules, impaired fibrinolysis, and endothelial dysfunction.7 Among adults with SLE in a large epidemiologic study, hs-CRP was associated with cardiovascular mortality.8 One case–control study showed no difference in hs-CRP levels in children with SLE compared with healthy controls.9 Normal, borderline, and elevated levels of hs-CRP have been defined for the general adult population, but not for children and adolescents or for individuals with SLE.10 Despite the chronic inflammation associated with SLE, levels of hs-CRP are not uniformly elevated in active disease. Currently, there are no specific guidelines for hs-CRP screening or recommended interventions to lower hs-CRP in the general adult or pediatric populations.
Several epidemiologic studies have established lipoprotein A (Lpa) as an independent cardiovascular risk factor.11,12 Lpa is a lipid moiety consisting of an LDL-like particle, linked by its apolipoprotein B 100 component to a molecule of apolipoprotein a. Among lipid-lowering therapies, only niacin appears to lower Lpa levels significantly; however, no randomized trial data show that lowering Lpa levels prevents cardiovascular morbidity.13 No guidelines for routine Lpa screening or intervention have been established. While this has not been studied extensively, adult SLE cohorts have shown elevated Lpa levels compared with healthy controls and higher Lpa levels with increased disease activity.14 However, in a small pediatric SLE cohort study, Lpa levels did not differ from controls.15 Published data from the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) trial showed that higher Lpa levels at baseline were associated with thicker carotid intima media.16
Elevated homocysteine levels are associated with endothelial dysfunction, platelet activation, and accelerated LDL oxidation.17 In the general population, elevated homocysteine levels are associated with increased risk of cardiovascular disease (CVD).18 While it is known that supplementation with vitamins B6, B12, and folic acid reduces homocysteine levels, clinical trials have failed to show that lowering homocysteine levels improves cardiovascular outcomes in the general population.19 Among adults with SLE, higher homocysteine levels are associated with atherosclerosis progression, arterial stiffness, left ventricular hypertrophy, and coronary artery calcification.20–23 Among children, small studies have suggested that homocysteine levels are higher in pediatric SLE than in healthy controls. One study in pediatric SLE patients found no correlation with endothelial dysfunction.15
In this study, we investigate the levels and clinical associations of laboratory markers of cardiovascular risk – LDL, HDL, TG, Lpa, homocysteine, and hs-CRP – in a large and diverse pediatric SLE cohort enrolled in the APPLE trial. In approaching this exploratory analysis, our hypothesis was that children and adolescents with SLE would have abnormal levels of LDL, HDL, TG, Lpa, homocysteine, and hs-CRP. We anticipated that abnormalities of LDL, HDL, Lpa, and hs-CRP would be influenced by SLE disease activity and that prednisone dose would be related to levels of LDL and TG.
Patients and methods
Patient population: baseline APPLE cohort
A prospective multicenter cohort of 221 children and adolescents with SLE from 21 sites in North America underwent measurement of fasting LDL, HDL, TG, Lpa, homocysteine, and hs-CRP as part of the APPLE Trial. The APPLE Trial is a double-blind randomized placebo-controlled study designed to determine the efficacy and safety of atorvastatin in preventing the progression of atherosclerosis in children and adolescents with SLE. The primary endpoint is the rate of progression of mean carotid intimal thickness (CIMT) in the common carotid artery over 3 years. Subjects are randomized to receive either placebo or atorvastatin (10 or 20 mg daily depending upon weight). Prescription of multivitamin (MVI) with folate, hydroxychloroquine, and low-dose aspirin was recommended for all subjects, as were dietary recommendations and risk factor counseling. Enrollment was completed in 2006, with study results expected in 2010.
All subjects met the ACR 1997 revised diagnostic criteria for SLE.24 Full inclusion and exclusion criteria for the APPLE trial are as previously published.16 Inclusion criteria included weight ≥ 25 kg, outpatient status, and age between 10 and 21 years, and patients were excluded for active nephrotic syndrome, myositis, liver disease, renal insufficiency, or hypercholesterolemia warranting treatment (total cholesterol > 350 mg/dl) at enrollment.
Clinical variables of interest
The APPLE baseline assessment included demographics, a history and physical examination performed by a pediatric rheumatologist, chart review, and subject and parent questionnaires. Variables of interest for this analysis were SLE clinical and laboratory parameters, non-lupus and lupus-related medical history, family history, current and prior medication use, traditional risk factors for atherosclerosis, and SLE disease activity. Race and ethnicity were self-reported.
SLE disease activity and damage were assessed using the modified SELENA Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)25 and Systemic Lupus International Collaborating Clinics/ACR Damage Index (SDI)26 For these analyses, the total SLEDAI score was treated as a continuous variable. SDI scores were analyzed as a dichotomous variable with subjects grouped as a score of 0 (no damage) or a score of ≥ 1 (damage present). Proteinuria was defined as spot urine protein/creatinine ratio ≥ 0.5 or timed urine with protein > 500 mg/24 h. A history of glomerulonephritis referred to current or prior history of nephritis or prior history of nephrotic syndrome. Similarly, a history of hypertension referred to the treating clinician's report of current or historical hypertension. Creatinine clearance was calculated using the Schwartz formula.27 Leukopenia was defined as a white blood cell count < 3000/mm3. A family history of CVD was defined as subject-reported cerebrovascular accident, myocardial infarction, angina, or atherosclerosis in a parent or grandparent. A family history of hyperlipidemia was defined as subject-reported hyperlipidemia in a parent or grandparent. Current oral glucocorticoid doses were weight-adjusted and recorded as prednisone equivalent in mg/kg/d. For the purposes of this analysis, use of immunosuppressant medications and non-steroidal anti-inflammatory drugs (NSAIDs) referred to current usage.
Laboratory assessment
The following tests were obtained following a 12-h fast and were measured in a central laboratory: total cholesterol, TG, HDL, apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), Lpa, homocysteine, and hs-CRP. All other laboratory assessments were performed locally. Total cholesterol and TG were measured by Roche Enzymatic assay. HDL was measured by Roche Enzymatic with MnCl precipitation. LDL was calculated using the Friedewald equation.28 ApoA1 and ApoB were measured using Dade-Behring Immunoturbimetric Nephelometry. Lpa and hs-CRP were measured using Polymedco Immunoturbimetric Assay. Homocysteine was measured using high performance liquid chromatography.
Statistical methods
The characteristics of the study sample were summarized using descriptive statistics, with dichotomous or ordinal data presented as percentages and continuous data as medians with interquartile ranges or as means with standard deviations (SD). Differences between groups were assessed using either the χ2 test for dichotomous data or the non-parametric Wilcoxon test for continuous data.
Using cross-sectional baseline data from the APPLE trial, we first investigated potential risk factors and their univariable relationships with each of the six outcomes of interest (baseline levels of LDL, HDL, TG, Lpa, homocysteine, and hs-CRP). The following potential predictors were considered: age, gender, Hispanic or non-White status, BMI, duration of SLE, SDI, current immunosuppressive use, current NSAID use, current prednisone dose, creatinine clearance, proteinuria, history of glomerulonephritis, history of hypertension, family history of CVD, and family history of hyperlipidemia. Lipid values (total cholesterol, HDL, LDL, TG, ApoA1, ApoB, and Lpa) were also studied for potential relationships with hs-CRP and homocysteine models. Given the anticipated high correlation, relationships between HDL, LDL, TG, ApoA1, and ApoB were not assessed. Homocysteine and hs-CRP were considered potential predictors for HDL, LDL, TG, and Lpa in the multivariable models. Hydroxychloroquine use was not included as a potential predictor in these models because 96% of subjects were taking this medication. Information on duration of hydroxychloroquine use was not available. Use of MVI was considered in the homocysteine analyses, as vitamins B6, B12, and folate are known to impact homocysteine levels.
Separate univariable analyses using linear regression models were performed, examining the relationship between each predictor and each of the six outcomes. Residual plots of the regression models were used to assess the linear models. Scatter plots with overlay of locally weighted scatterplot smoothing (LOESS) were created to examine the relationships between continuous variables and outcome variables.29 Histograms of each of the outcome variables according to presence or absence of categorical predictors were produced to assess the relationship between categorical predictors and outcome variables. When the relationship between the continuous predictor and the outcome variable was not linear, appropriate transformations or piecewise linear regression models were pursued to better understand the relationship between variables. Upon examination of the distributions of the continuous variables, hs-CRP, TG, and Lpa and hs-CRP were log-transformed to achieve a more normal distribution before proceeding to multivariable linear regression.
As this was an exploratory analysis, potential predictors with p values <0.15 in univariable analysis were included in the multivariable linear regression models. Forward, backward, and stepwise regression model building procedures were used to derive the final regression model for each of the six outcomes. We assessed each model with co-linearity diagnostics. Bonferroni adjustments for multiple comparisons were not performed due to the exploratory nature of our analyses. Statistical associations were considered significant in the final models if p values were <0.05. All statistical calculations were performed using SAS 8.2 and SAS Enterprise Guide 4.0 (SAS Enterprise Institute, Cary, NC).
Results
A summary of baseline characteristics of the APPLE cohort is depicted in Table 1. Of the 221 enrolled subjects, mean age was 15.7 years (range 10.1–21.7 years), 83% were female, and 65% were Hispanic or non-White. Subjects had mean disease duration of 31 months (median 24 months, interquartile range 8 to 46 months) and had mild disease activity with a mean SLEDAI score of 4.6 (range 0 to 20, median 4.0, interquartile range 2 to 6). As summarized in Table 1, mean LDL was 86.4 mg/dl (normal < 110 mg/dl); mean HDL was 46.3 mg/dl (normal > 45 mg/dl); mean TG levels were 114 mg/dl (normal < 90, borderline 90–129 mg/dl); and mean Lpa levels were 23.1 mg/dl (normal < 30 mg/dl).30,31 Mean levels of homocysteine (7.5 μmol/l) were between the 75th and 95th percentiles for US children aged 3–19 years, and mean hs-CRP level (3.6 mg/l) in this cohort represented the 90th percentile for US children aged 3–19 years.32 As shown in Figure 1, the most commonly detected abnormalities were elevated TG and Lpa levels.
Table 1.
Baseline characteristics of the APPLE subjects
| Variable | Mean (SD) or n (%) | Total N |
|---|---|---|
| Age (years) | 15.7 (2.6) | 221 |
| Female | 184 (83.3%) | 221 |
| Weight (kg) | 62.0 (17.2) | 221 |
| Body mass index | 24.4 (5.3) | 221 |
| Duration of SLE (months) | 31.2 (28.5) | 220 |
| Hispanic | 54 (24.4%) | 221 |
| Racea | ||
| White | 114 (51.6%) | 221 |
| African American | 59 (26.7%) | 221 |
| Asian | 23 (10.4%) | 221 |
| American Indian | 6 (2.7%) | 221 |
| Native Hawaiian | 5 (2.3%) | 221 |
| Other | 30 (13.6%) | 221 |
| Hispanic or non-White (Hispanic ethnicity or non-White race) | 144 (65.2%) | 221 |
| Hx. smoking (self-report) | 7 (3.2%) | 221 |
| Prednisone dose (mg/kg/d) | 0.19 (0.19) | 218 |
| Family Hx. cardiovascular disease | 79 (37.4%) | 211 |
| Creatinine clearance (ml/tnin/m2) | 139.4 (33.0) | 216 |
| Current proteinuria | 56 (25.5%) | 220 |
| Current leukopenia | 22 (9.9%) | 221 |
| SLEDAI total | 4.6 (4.2) | 221 |
| SDI > 0 | 59 (26.7%) | 221 |
| Hx. renal abnormalities | ||
| Hx. hypertension | 73 (34.1%) | 214 |
| Hx. nephrotic syndrome | 38 (17.4%) | 219 |
| Hx. nephritis | 79 (36.1%) | 219 |
| Other | 20 (10.5%) | 190 |
| Current medications | ||
| Corticosteroids | 181 (81.9%) | 221 |
| Cyclophosphamide | 26 (11.8%) | 221 |
| Mycophenolate | 53 (24.0%) | 221 |
| Azathioprine | 30 (13.6%) | 221 |
| Methotrexate | 29 (13.1%) | 221 |
| ACE inhibitors | 54 (24.4%) | 221 |
| NSAID | 68 (30.8%) | 221 |
| Hydroxychloroquine | 203 (96%) | 211 |
| Multivitamin with folate | 156 (70.6%) | 211 |
| Total cholesterol (mg/dl) | 155.1 (38.0) | 211 |
| HDL cholesterol (mg/dl) | 46.3 (12.8) | 211 |
| LDL cholesterol (mg/dl) | 86.4 (31.4) | 210 |
| Triglycerides (mg/dl) | 114.0 (66.4) | 211 |
| Homocysteine (μmol/1) | 7.5 (3.1) | 207 |
| Lipoprotein a (mg/dl) | 23.1 (26.8) | 206 |
| hs-CRP (mg/1) | 3.6 (13.9) | 202 |
| Apo-A1 (mg/dl) | 138.2 (25.3) | 206 |
| Apo-B (mg/dl) | 83.6 (25.3) | 206 |
Some subjects self-reported more than one race.
ACE: angiotensin converting enzyme, Apo-A1: apolipoprotein A1, Apo-B: apolipoprotein B, HDL: high-density lipoprotein, LDL: low-density lipoprotein, hs-CRP: high-sensitivity C-reactive protein, Hx: history of, SLE: systemic lupus erythematosus, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index, SDI: Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.
Figure 1.
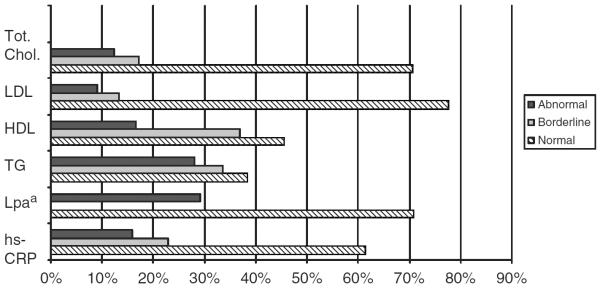
Proportion of APPLE subjects with normal, abnormal or borderline levels of laboratory markers of cardiovascular risk. aAdapted from references Pearson et al.,10 NCEP,30 and Obisesan.31 aThere is no accepted borderline range for lipoprotein A. HDL: high-density lipoprotein, hs-CRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein, Lpa: Lipoprotein A, TG: triglycerides, Tot. Chol: total cholesterol.
LDL
As shown in Table 2, univariable analysis suggested that the following factors were associated with higher LDL levels: Hispanic or non-White ethnicity, history of glomerulonephritis, history of hypertension, higher SLEDAI score, proteinuria, higher prednisone dose, higher homocysteine levels, and current use of cyclophosphamide, mycophenolate mofetil, or an ACE-inhibitor. Methotrexate use and family history of CVD were associated with lower LDL levels. Complete univariable modeling results are shown in Table 3. Multivariable modeling showed independent relationships of increased LDL levels with increased SLEDAI, increased prednisone dose, and history of hypertension.
Table 2.
Univanable relationships between clinical variables and laboratory markers of cardiovascular risk in the baseline APPLE cohort
| LDL |
HDL |
Triglycerides (log) |
Lpa (log) |
hs-CRP (log) |
Homocysteine |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable name | Slope (SE) | p Value | Slope (SE) | p Value | Slope (SE) | p Value | Slope (SE) | p Value | Slope (SE) | p Value | Slope (SE) | p Value |
| Age (years) | 0.398 (0.815) | 0.626 | 0.189 (0.332) | 0.568 | −0.004 (0.012) | 0.692 | 0.023 (0.032) | 0.465 | 0.048 (0.040) | 0.237 | 0.172 (0.080) | 0.033 |
| Female | 1.582 (5.693) | 0.781 | 4.100 (2.299) | 0.076 | 0.080 (0.086) | 0.350 | −0.228 (0.219) | 0.299 | −0.135 (0.277) | 0.626 | −1.045 (0.562) | 0.064 |
| Hispanic or non-White status | 10.387 (4.470) | 0.021 | 1.707 (1.837) | 0.354 | −0.056 (0.068) | 0.411 | 0.668 (0.170) | <0.001 | 0.341 (0.224) | 0.129 | 0.512 (0.453) | 0.259 |
| BMI | −0.039 (0.421) | 0.927 | −0.434 (0.169) | 0.011 | 0.016 (0.006) | 0.012 | 0.006 (0.017) | 0.737 | 0.062 (0.004) | 0.001 | 0.069 (0.042) | 0.107 |
| Duration of SLE (months) | 0.062 (0.078) | 0425 | 0.012 (0.032) | 0.710 | −0.001 (0.001) | 0.228 | 0.003 (0.003) | 0.260 | 0.013 (0.004) | 0.001 | −0.005 (0.008) | 0.491 |
| Hx glomerulonephritis | 9.571 (4.400) | 0.031 | 0.702 (1.805) | 0.698 | 0.139 (0.066) | 0.036 | 0.049 (0.172) | 0.775 | −0.039 (0.219) | 0.858 | 0.488 (0.443) | 0.313 |
| Hx hypertension | 12.148 (4.676) | 0.010 | 0.427 (1.906) | 0.840 | 0.136 (0.070) | 0.055 | 0.074 (0.185) | 0.688 | −0.047 (0.231) | 0.840 | 0.508 (0.475) | 0.286 |
| Family Hx CVD | −8.330 (4.547) | 0.068 | −0.074 (1.900) | 0.969 | −0.030 (0.070) | 0.665 | 0.167 (0.180) | 0.353 | −0.376 (0.227) | 0.099 | −0.448 (0.466) | 0.337 |
| Family Hx. hyperlipidemia | −2.135 (4.628) | 0.645 | −2.061 (1.901) | 0.280 | −0.072 (0.069) | 0.301 | 0.014 (0.179) | 0.938 | −0.157 (0.231) | 0.497 | −0.063 (0.468) | 0.893 |
| SLEDAI | 1.636 (0.505) | 0.001 | −0.154 (0.210) | 0.466 | 0.020 (0.008) | 0.011 | 0.027 (0.020) | 0.174 | 0.026 (0.025) | 0.314 | 0.136 (0.051) | 0.008 |
| SDI > 0 | 0.122 (4.906) | 0.980 | 0.503 (1.995) | 0.801 | −0.042 (0.074) | 0.571 | 0.339 (0.198) | 0.075 | 0.379 (0.238) | 0.113 | −0.240 (0.488) | 0.624 |
| Proteinuria | 12.955 (4.952) | 0.010 | 0.846 (2.019) | 0.006 | 0.165 (0.075) | 0.028 | 0.135 (0.195) | 0.488 | 0.110 (0.246) | 0.654 | 1.417 (0.492) | 0.004 |
| Creatinine clearance (ml/min/m2) | 0.044 (0.066) | 0.507 | −0.073 (0.026) | 0.006 | <0.001 (0.001) | 0.814 | 0.003 (0.003) | 0.325 | 0.006 (0.003) | 0.063 | −0.031 (0.006) | <0.001 |
| Leukopenia | 0.263 (0.560) | 0.639 | 0.011 (0.321) | 0.961 | 0.014 (0.093) | 0.093 | 0.006 (0.022) | 0.790 | 0.046 (0.027) | 0.094 | 0.091 (0.054) | 0.095 |
| Prednisone dose (mg/kg/d) | 36.109 (11.282) | 0.002 | 9.541 (4.652) | 0.042 | 0.675 (0.168) | <0.001 | 0.186 (0.449) | 0.679 | −0.734 (0.564) | 0.195 | 1.714 (1.146) | 0.136 |
| ACE inhibitor | 8.990 (2.507) | 0.085 | 2.999 (2.144) | 0.165 | 0.070 (0.077) | 0.363 | 0.140 (0.203) | 0.492 | 0.049 (0.254) | 0.849 | 0.239 (0.509) | 0.638 |
| NSAID current | −2.745 (4.689) | 0.559 | −1.184 (1.906) | 0.535 | 0.013 (0.071) | 0.857 | 0.139 (0.182) | 0.445 | 0.474 (0.231) | 0.041 | 0.075 (0.470) | 0.873 |
| MVI with folate current | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | −1.539 (0.457) | 0.001 |
| Methotrexate current | −10.838 (6.337) | 0.089 | −0.212 (2.596) | 0.935 | −0.150 (0.077) | 0.118 | 0.188 (0.254) | 0.459 | −0.090 (0.320) | 0.779 | −0.610 (0.653) | 0.352 |
| Azathioprine current | −0.134 (6.288) | 0.983 | 2.139 (2.554) | 0.403 | −0.052 (0.095) | 0.586 | 0.145 (0.242) | 0.551 | 0.442 (0.304) | 0.147 | 0.208 (0.625) | 0.739 |
| Cyclophosphamide current | 13.098 (6.758) | 0.054 | −0.552 (2.725) | 0.840 | 0.217 (0.100) | 0.031 | −0.132 (0.263) | 0.616 | −0.019 (0.331) | 0.954 | 1.095 (0.674) | 0.106 |
| Mycophenolate mofetil current | 9.448 (5.124) | 0.067 | −1.502 (2.099) | 0.466 | −0.150 (0.096) | 0.118 | 0.096 (0.201) | 0.632 | −0.108 (0.255) | 0.673 | 0.453 (0.513) | 0.378 |
| LDL (mg/dl) | NI | NI | NI | NI | NI | NI | NI | NI | −0.002 (0.003) | 0.572 | 0.011 (0.007) | 0.116 |
| HDL (mg/dl) | NI | NI | NI | NI | NI | NI | NI | NI | −0.032 (0.008) | <0.001 | −0.017 (0.017) | 0.317 |
| Log TG (mg/dl) | NI | NI | NI | NI | NI | NI | NI | NI | 0.291 (0.232) | 0.212 | 1.573 (0.462) | 0.001 |
| Log hs-CRP (mg/l) | −0.824 (1.456) | 0.572 | −2.321 (0.576) | <0.001 | 0.027 (0.021) | 0.212 | 0.082 (0.056) | 0.147 | NI | NI | 0.182 (0.143) | 0.203 |
| Homocysteine (mg/dl) | 1.102 (0.698) | 0.116 | −0.288 (0.287) | 0.317 | 0.034 (0.010) | 0.001 | −0.020 (0.027) | 0.474 | 0.044 (0.035) | 0.293 | NI | NI |
| Log Lpa(mg/dl) | NI | NI | NI | NI | NI | NI | NI | NI | 0.128 (0.088) | 0.147 | −0.128 (0.179) | 0.474 |
| ApoA1 | NI | NI | NI | NI | NI | NI | NI | NI | −0.015 (0.004) | <0.001 | 0.000 (0.009) | 0.979 |
| ApoB | NI | NI | NI | NI | NI | NI | NI | NI | 0.000 (0.004) | 0.954 | 0.017 (0.008) | 0.049 |
BMI: body mass index, CVD: cardiovascular disease, HDL: high-density lipoprotein, hs-CRP: high sensitivity C-reactive protein, Hx: history of, LDL: low-density lipoprotein, Lpa: lipoprotein A, NI: not included in univariable analysis due to predicted collinearity, SE: standard error, SDI: Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index, SLE: systemic lupus erythematosus, TG: triglycerides.
Table 3.
Multivanable adjusted relationships between clinical variables and laboratory markers of cardiovascular risk in the baseline APPLE cohort
| Outcome | Variable | Slope (SE) | p Value | Adjusted R2 |
|---|---|---|---|---|
| LDL | Hx HTN | 10.968 (4.572) | 0.017 | 0.114 |
| SLEDAI | 1.187 (0.518) | 0.023 | ||
| Prednisone dose | 33.648 (11.581) | 0.004 | ||
| HDL | Creatinine clearance | −0.058 (0.026) | 0.030 | 0.103 |
| Log hs-CRP | −2.258 (0.583) | <0.001 | ||
| TG | BMI | 0.018 (0.006) | 0.003 | 0.167 |
| Prednisone dose | 0.775 (0.160) | <0.001 | ||
| Homocysteine | 0.025 (0.010) | 0.009 | ||
| Lpa | Hispanic or non-White status | 0.668 (0.170) | <0.001 | 0.070 |
| Homocysteine | Proteinuria | 1.132 (0.464) | 0.016 | 0.229 |
| Creatinine clearance | −0.029 (0.006) | <0.001 | ||
| Log TG | 1.401 (0.440) | 0.002 | ||
| MVI with folate | −1.511 (0.424) | <0.001 | ||
| hs-CRP | BMI | 0.042 (0.020) | 0.038 | 0.170 |
| Duration of SLE | 0.013 0.003) | 0.003 | ||
| Current NSAID use | 0.472 (0.215) | 0.029 | ||
| HDL | −0.029(0.008) | <0.001 |
BMI: body mass index, HDL: high-density lipoprotein, hs-CRP: high-sensitivity C-reactive protein, Lpa: lipo-protein a, HTN: hypertension, Hx: history of, MVI: multivitamin, NSAID: non-steroidal anti-inflammatory drug, SE: standard error, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index, TG: triglyceride.
HDL
Univariable analysis revealed that male gender, higher BMI, hs-CRP and creatinine clearance all predicted lower HDL levels, while higher prednisone dose was associated with higher HDL. The final multivariable model showed that only higher hs-CRP and higher creatinine clearance were independently associated with lower HDL levels.
TG
Univariable analysis suggested that the following risk factors were independently associated with higher TG levels: proteinuria, history of glomerulonephritis, leukopenia, history of hypertension, current use of cyclophosphamide or mycophenolate mofetil, higher prednisone dose, homocysteine, SLEDAI score, and BMI. Current use of methotrexate predicted lower TG levels. The final multivariable indicated that higher BMI, prednisone dose, and homocysteine levels were independently associated with higher TG levels.
Lpa
For Lpa, univariable analysis showed that Hispanic or non-White status, higher hs-CRP and SDI score were risk factors for higher Lpa; however, multivariable modeling showed a significant relationship only between Hispanic or non-White status and Lpa.
hs-CRP
Hispanic or non-White status, BMI, duration of SLE, history of glomerulonephritis, SDI score, higher creatinine clearance, lower HDL or apoA1, NSAID use, and higher Lpa levels were independently related to higher hs-CRP levels. A family history of CVD was associated with lower levels of hs-CRP. In multivariable modeling, current NSAID use, higher BMI, lower HDL, and longer SLE disease duration predicted higher hs-CRP levels.
Homocysteine
Age, male gender, higher BMI, SLEDAI, proteinuria, leukopenia, lower creatinine clearance, higher prednisone dose, current use of cyclophosphamide, and higher TG, LDL, and apoB levels were independently related to higher homocysteine levels in univariable analysis. Use of MVI with folate was associated with lower homocysteine levels. In multivariable analysis, proteinuria, higher TG and lower creatinine clearance were independently associated with higher homocysteine levels, while use of MVI with folate predicted lower homocysteine levels.
Additional analyses
To further explore the relationship between hs-CRP and NSAIDs, additional analysis was performed, which revealed that the relationship between NSAID use and hs-CRP was strongest at two sites. When these two sites were removed from the multivariable model, the relationship between NSAID use and hs-CRP was no longer significant, but none of the other observed relationships changed. We also compared those taking NSAIDS (n = 68) with those not taking NSAIDs (n = 153). This analysis revealed only two statistically significant differences between NSAID users and non-users: NSAID users were less likely to have a personal history of hypertension (39% vs 24%, p = 0.033) and less likely to have a family history of hyperlipidemia (33% vs 50%, p = 0.019). As arthritis is a common indication for NSAID use, the relationship between arthritis and hs-CRP was further explored. On univariable analysis, the presence of arthritis (identified as present on the SLEDAI) was not independently associated with hs-CRP (slope 0.606; SE 0.394; p = 0.126), and adding arthritis to the hs-CRP model did not change the model results.
Discussion
The majority of APPLE subjects had normal or borderline values of LDL, HDL, TG, and Lpa according to definitions developed for the general pediatric and adolescent population in the United States. Optimal levels of hs-CRP and homocysteine are not well defined for pediatric populations, and hs-CRP guidelines are extrapolated from recommendations developed for the general adult population. Screening benchmarks developed for the general population may not be appropriate for children and adolescents with SLE, a group whose high atherogenic potential may warrant a more aggressive prevention strategy, similar to that used for people with diabetes. The exclusion of patients with a family history of familial hypercholesterolemia or total cholesterol >350 mg/dl, nephrotic syndrome, and significant renal insufficiency likely resulted in a sample with lower rates of dyslipidemia than in other pediatric and adult SLE cohorts. In addition, all subjects were required to take hydroxychloroquine, which favorably impacts lipid profiles in adults and children with SLE.33,34
Chronic steroid use has conventionally been viewed as pro-atherogenic; however, in epidemiologic studies of adults with SLE, individuals treated with lower steroids (and other immunosuppressives) actually had higher carotid plaque burden.23 In the baseline APPLE cohort, higher prednisone was associated with higher LDL and triglyceride levels. Given the cross-sectional nature of the data, we were not able to assess cumulative steroid exposure, which is likely important in cardiovascular risk and will be a focus of longitudinal data analysis once the APPLE trial is complete.
While this analysis corroborated several known associations – including the relationships between Lpa and Hispanic or non-White status; between BMI, triglycerides, and hs-CRP; between triglycerides and homocysteine; and between HDL and hs-CRP – other observations were novel. Higher SLE disease activity, as measured by the SLEDAI, was associated with higher LDL levels in this cohort. We did not find a significant relationship between higher disease activity and lower HDL levels, as frequently reported in the literature.34–36 This difference may be explained by the exclusion of individuals with nephrotic syndrome and significant baseline hypercholesterolemia or the effects of treatment with glucocorticoids (although an independent relationship between HDL and prednisone use was not found in multivariable analysis). In addition, APPLE subjects had low SLE disease activity at baseline, which may have limited the ability to identify effects of active disease on lipid profiles.
The association between NSAID use and higher levels of hs-CRP was surprising and intriguing, particularly given the association between NSAID use and cardiovascular disease. However, this relationship proved to exist primarily at two of 21 sites, and thus is of unclear significance and may represent a spurious finding in the setting of multiple comparisons. The baseline APPLE data did not include information concerning the relative cyclo-oxygenase-selectivity, dose, or frequency of NSAID use. No previous studies have addressed NSAID use and its relationship with hs-CRP in the setting of SLE.
In this cohort, hs-CRP and SLE disease activity did not show a significant association with each other, but longer disease duration did predict higher hs-CRP. The existing literature demonstrates mixed results regarding the relationship between SLE disease activity and hs-CRP, bringing into question the utility of this laboratory measure as a marker of disease activity.37–42 Two large studies have evaluated hs-CRP in adults with SLE. Bertoli et al. studied 588 subjects with SLE in the LUMINA cohort and found that hs-CRP was associated with disease activity (as measured by Systemic Lupus Activity Measure-Revised (SLAM-R).37 Lee et al. examined 610 patients with SLE and found in multivariable analysis that higher SELENA-SLEDAI scores predicted higher hs-CRP, but duration of SLE was not an independent predictor of hs-CRP.38 Other studies have not demonstrated any relationship between hs-CRP and SLE disease activity.40–42 Antibodies to C-reactive protein (not measured in the APPLE cohort) can lower hs-CRP levels in SLE and may represent an important confounder.43
Even in the absence of frank renal insufficiency and nephrotic syndrome, evidence of renal injury (proteinuria, creatinine clearance, and history of hypertension) was significantly associated with markers of cardiovascular risk, especially HDL and homocysteine levels. Lower creatinine clearance predicted higher levels of homocysteine and HDL. These seemingly disparate results may in part be due to the known relationship between hyper-glomerulofiltration and markers of the metabolic syndrome (including low HDL) in the setting of chronic kidney disease.44 Proteinuria was associated with higher levels of homocysteine, consistent with findings in the general population, chronic kidney disease and diabetes.45,46 In the general population, even small decrements of renal function are associated with significant increases in cardiovascular morbidity.47 Taken together, these results suggest that even modest renal injury is related to homocysteine and HDL concentrations, and highlight the importance of renal status in cardiovascular health for children and adolescents with SLE.
There are several limitations to this study, including its cross-sectional nature. The sample size was powered to detect change in the primary outcome of the APPLE trial, progression rate of CIMT; thus it may not have had sufficient power to identify meaningful associations between clinical features and markers of cardiovascular risk. In addition, we performed many comparisons in these analyses, and our chances of finding some statistically significant relationships were high. Thus, the results of this exploratory analysis must be interpreted with caution and confirmed in future prospective studies. Despite these limitations, these results are derived from the largest, most diverse pediatric SLE cohort in which fasting LDL, HDL, Lpa, hs-CRP, TG and homocysteine have been measured. The potential relationships reported here are hypothesis-generating and need to be confirmed by future, longitudinal studies of the APPLE and other cohorts. As in any clinical trial, there is risk of selection bias, limiting the generalizability of the APPLE cohort to pediatric lupus patients as a whole. As examples of this selection bias, some obese patients were excluded due to inability to obtain high-quality CIMT images. Patients with nephrotic syndrome and significant renal insufficiency were also excluded. As in all studies, historical variables are subject to recall bias. Race and ethnicity are highly complex, and any categorization introduces bias and limitations. In this study, race and ethnicity were separately self-reported. Given the small sample size, we elected to limit analysis to the largest groups, non-Hispanic Whites, Hispanics, and non-Whites.
The presented models explain only 7–23% of the variability in these markers of cardiovascular risk, suggesting that other important measured or unmeasured risk factors were not identified in the current study. Few studies in adult or pediatric SLE have examined LDL, HDL, TG, Lpa, homocysteine, or hs-CRP individually as outcomes using multivariable linear regression models, and therefore it is difficult to compare the fit of our models with others in the literature. Silverman et al. described models with significantly higher model adjusted R2 values for HDL (0.577) and TG (0.629); however, these analyses involved a more homogeneous sample of recently diagnosed and untreated pediatric SLE patients, suggesting that treatment effects and variations in disease activity likely influence these markers of cardiovascular risk.35
Taken together, the results of these analyses suggest that low SLE disease activity and preserved renal function may correlate with improvements in markers of cardiovascular risk, particularly LDL, HDL, and homocysteine. Because cardiovascular screening recommendations developed for the general population may not be adequate or optimal for children and adults with SLE, further research is needed to determine which markers of cardiovascular risk reliably predict cardiovascular events in children and adolescents with SLE.
Acknowledgments
Funding This work was supported by the NIH (NIAMS contract N01-AR-2-2265) and in part by the Duke Edna and Fred L Mandel, Jr Center for Hypertension and Atherosclerosis Research. Dr Ardoin's participation was supported by the American College of Rheumatology's Physician Scientist Development Award. Pfizer contributed the study drug to the APPLE trial, and Dr Schanberg has received consulting fees from Pfizer (less than US$10,000). Dr. Singer's participation was supported by CTSA NIH UL1RR024989.
References
- 1.Westerweel PE, Luyten RK, Koomans HA, Derksen RH, Verhaar MC. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1384–1396. doi: 10.1002/art.22568. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 4.Ilowite NT, Samuel P, Ginzler E, Jacobson MS. Dyslipoproteinemia in pediatric systemic lupus erythematosus. Arthritis Rheum. 1988;31:859–863. doi: 10.1002/art.1780310706. [DOI] [PubMed] [Google Scholar]
- 5.Kashef S, Ghaedian MM, Rajaee A, Ghaderi A. Dyslipoproteinemia during the active course of systemic lupus erythematosus in association with anti-double-stranded DNA (anti-dsDNA) antibodies. Rheumatol Int. 2007;27:235–241. doi: 10.1007/s00296-006-0195-3. [DOI] [PubMed] [Google Scholar]
- 6.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 7.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- 8.Toloza SM, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–3957. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 9.Bowser CS, Kumar S, Salciccioli L, et al. Absence of Chlamydia pneumoniae and signs of atherosclerotic cardiovascular disease in adolescents with systemic lupus erythematosus. Pediatr Cardiol. 2008;29:545–551. doi: 10.1007/s00246-007-9131-x. [DOI] [PubMed] [Google Scholar]
- 10.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 11.Craig WY, Neveux LM, Palomaki GE, Cleveland MM, Haddow JE. Lipoprotein(a) as a risk factor for ischemic heart disease: metaanalysis of prospective studies. Clin Chem. 1998;44:2301–2306. [PubMed] [Google Scholar]
- 12.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke. 2007;38:1959–1966. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 13.McCormack PL, Keating GM. Prolonged-release nicotinic acid: a review of its use in the treatment of dyslipidaemia. Drugs. 2005;65:2719–2740. doi: 10.2165/00003495-200565180-00014. [DOI] [PubMed] [Google Scholar]
- 14.Sari RA, Polat MF, Taysi S, Bakan E, Capoglu I. Serum lipoprotein(a) level and its clinical significance in patients with systemic lupus erythematosus. Clin Rheumatol. 2002;21:520–524. doi: 10.1007/s100670200127. [DOI] [PubMed] [Google Scholar]
- 15.Soep JB, Mietus-Snyder M, Malloy MJ, Witztum JL, von Scheven E. Assessment of atherosclerotic risk factors and endothelial function in children and young adults with pediatric-onset systemic lupus erythematosus. Arthritis Rheum. 2004;51:451–457. doi: 10.1002/art.20392. [DOI] [PubMed] [Google Scholar]
- 16.Schanberg LE, Sandborg C, Barnhart HX, et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: Risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 2009;60:1496–1507. doi: 10.1002/art.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143–150. doi: 10.3132/dvdr.2007.033. [DOI] [PubMed] [Google Scholar]
- 18.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 19.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 20.Pieretti J, Roman MJ, Devereux RB, et al. Systemic lupus erythematosus predicts increased left ventricular mass. Circulation. 2007;116:419–426. doi: 10.1161/CIRCULATIONAHA.106.673319. [DOI] [PubMed] [Google Scholar]
- 21.Tso TK, Huang HY, Chang CK, Huang WN. A positive correlation between homocysteine and brachial-ankle pulse wave velocity in patients with systemic lupus erythematosus. Clin Rheumatol. 2006;25:285–290. doi: 10.1007/s10067-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 22.Von Feldt JM, Scalzi LV, Cucchiara AJ, et al. Homocysteine levels and disease duration independently correlate with coronary artery calcification in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2220–2227. doi: 10.1002/art.21967. [DOI] [PubMed] [Google Scholar]
- 23.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 25.Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Amer Statistical Assoc. 1979;74:829. [Google Scholar]
- 30.National Cholesterol Education Program (NCEP) highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 31.Obisesan TO, Aliyu MH, Adediran AS, Bond V, Maxwell CJ, Rotimi CN. Correlates of serum lipoprotein (A) in children and adolescents in the United States. The third National Health Nutrition and Examination Survey (NHANES-III) Lipids Health Dis. 2004;3:29. doi: 10.1186/1476-511X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 33.Kavanaugh A, Adams-Huet B, Jain R, Denke M, McFarlin J. Hydroxychloroquine Effects on Lipoprotein Profiles (the HELP trial): A Double-Blind, Randomized, Placebo-Controlled, Pilot Study In Patients With Systemic Lupus Erythematosus. J Clin Rheumatol. 1997;3:3–8. [PubMed] [Google Scholar]
- 34.Ilowite NT, Copperman N, Leicht T, Kwong T, Jacobson MS. Effects of dietary modification and fish oil supplementation on dyslipoproteinemia in pediatric systemic lupus erythematosus. J Rheumatol. 1995;22:1347–1351. [PubMed] [Google Scholar]
- 35.Tyrrell PN, Beyene J, Benseler SM, Sarkissian T, Silverman ED. Predictors of lipid abnormalities in children with new-onset systemic lupus erythematosus. J Rheumatol. 2007;34:2112–2119. [PubMed] [Google Scholar]
- 36.Hodis HN, Quismorio FP, Jr, Wickham E, Blankenhorn DH. The lipid, lipoprotein, and apolipoprotein effects of hydroxychloroquine in patients with systemic lupus erythematosus. J Rheumatol. 1993;20:661–665. [PubMed] [Google Scholar]
- 37.Bertoli AM, Vila LM, Reveille JD, Alarcon GS LUMINA Study Group Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): LXI. Value of C-reactive protein as a marker of disease activity and damage. J Rheumatol. 2008;35:2355–2358. doi: 10.3899/jrheum.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SS, Singh S, Magder LS, Petri M. Predictors of high sensitivity C-reactive protein levels in patients with systemic lupus erythematosus. Lupus. 2008;17:114–123. doi: 10.1177/0961203307085878. [DOI] [PubMed] [Google Scholar]
- 39.Inoue T, Kanayama Y, Katoh N, et al. Significance of C-reactive protein elevation in the pretreatment stage of systemic lupus erythematosus. Scand J Rheumatol. 1981;10:222–224. doi: 10.3109/03009748109095302. [DOI] [PubMed] [Google Scholar]
- 40.Bertouch JV, Roberts-Thompson PJ, Feng PH, Bradley J. C-reactive protein and serological indices of disease activity in systemic lupus erythematosus. Ann Rheum Dis. 1983;42:655–658. doi: 10.1136/ard.42.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linares LF, Gomez-Reino JJ, Carreira PE, Morillas L, Ibero I. C-reactive protein (CRP) levels in systemic lupus erythematosus (SLE) Clin Rheumatol. 1986;5:66–69. doi: 10.1007/BF02030970. [DOI] [PubMed] [Google Scholar]
- 42.Suh CH, Chun HY, Ye YM, Park HS. Unresponsiveness of C-reactive protein in the non-infectious inflammation of systemic lupus erythematosus is associated with interleukin 6. Clin Immunol. 2006;119:291–296. doi: 10.1016/j.clim.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Shoenfeld Y, Szyper-Kravitz M, Witte T, et al. Autoantibodies against protective molecules–C1q, C-reactive protein, serum amyloid P, mannose-binding lectin, and apolipoprotein A1: prevalence in systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1108:227–239. doi: 10.1196/annals.1422.025. [DOI] [PubMed] [Google Scholar]
- 44.Tomaszewski M, Charchar FJ, Maric C, et al. Glomerular hyper-filtration: a new marker of metabolic risk. Kidney Int. 2007;71:816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 45.Seshadri N, Messerli AW, Acharya N, et al. Relation of homocysteine and C-reactive protein to urinary albumin loss. Am J Cardiol. 2004;93:926–928. doi: 10.1016/j.amjcard.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 46.Vermeulen EG, Rauwerda JA, van den Berg M, et al. Homocysteine-lowering treatment with folic acid plus vitamin B6 lowers urinary albumin excretion but not plasma markers of endothelial function or C-reactive protein: further analysis of secondary end-points of a randomized clinical trial. Eur J Clin Invest. 2003;33:209–215. doi: 10.1046/j.1365-2362.2003.01135.x. [DOI] [PubMed] [Google Scholar]
- 47.Mann JF, Gerstein HC, Dulau-Florea I, Lonn E. Cardiovascular risk in patients with mild renal insufficiency. Kidney Int Suppl. 2003;84:S192–6. doi: 10.1046/j.1523-1755.63.s84.27.x. [DOI] [PubMed] [Google Scholar]


