Abstract
Research suggests that cognitive and behavioral therapies produce significant benefits over medications alone in the treatment of severe, nonpsychotic major depression or primary psychotic disorders such as schizophrenia. However, previous research has not demonstrated the efficacy of psychotherapy for major depression with psychotic features. In this initial treatment development study, we conducted an open trial of a new behavioral intervention that combines elements of Behavioral Activation and Acceptance and Commitment Therapy for depression and psychosis. Fourteen patients with major depressive disorder with psychotic features were provided with up to six months of Acceptance-based Depression and Psychosis Therapy (ADAPT) in combination with pharmacotherapy. Patients reported a high degree of treatment credibility and acceptability. Results showed that patients achieved clinically significant and sustained improvements through post-treatment follow-up in depressive and psychotic symptoms, as well as psychosocial functioning. In addition, the processes targeted by the intervention (e.g., acceptance, mindfulness, values) improved significantly over the course of treatment, and changes in processes were correlated with changes in symptoms. Results suggest that ADAPT combined with pharmacotherapy is a promising treatment approach for psychotic depression that should be tested in a future randomized trial.
Keywords: psychotic depression, acceptance and commitment therapy, behavioral activation, treatment development
INTRODUCTION
Psychotic Depression
Research estimates that hallucinations or delusions are present in 5–25% of depressed individuals (Coryell, Pfohl, & Zimmerman, 1984; Gaudiano, Dalrymple, & Zimmerman, 2009; Johnson, Horwath, & Weissman, 1991; Ohayon & Schatzberg, 2002). Patients with psychotic depression tend to have increased depression severity, psychomotor disturbance, psychiatric comorbidity, personality dysfunction, suicidality, and social/work impairment (Gaudiano et al., 2009; Gaudiano, Young, Chelminski, & Zimmerman, 2008; Gaudiano & Zimmerman, 2010; Lattuada, Serretti, Cusin, Gasperini, & Smeraldi, 1999; Serretti, Lattuada, Cusin, Gasperini, & Smeraldi, 1999; Thakur, Hays, & Kishnan, 1999). Compared to nonpsychotic depression, patients with psychotic depression tend to exhibit a longer duration of illness and more frequent hospitalizations and relapses (Coryell, et al., 1996; Goldberg & Harrow, 2004; Lykouras, et al., 1986; Tohen, Hennen, Zarate, Baldessarini, & al., 2000).
Somatic Treatments for Psychotic Depression
Patients with psychotic depression have been reported in some trials to show a poorer response to antidepressant medications alone (Brown, Frances, Kocsis, & Mann, 1982; Schatzberg, 2003). Additional research suggests that antidepressant plus antipsychotic medications may be superior to drug monotherapy (Meyers, et al., 2009). However, other studies have failed to find a consistent advantage for combined medications (Wijkstra, Lijmer, Balk, Geddes, & Nolen, 2006). Many individuals with psychosis who are treated with pharmacotherapy still exhibit significant symptoms and functional impairment that places them at risk for relapse and rehospitalization (Curson, Patel, Liddle, & Barnes, 1988; Rothschild & Duval, 2003; Tarrier, Barrowclough, & Bamrah, 1991). Evidence also suggests that electroconvulsive therapy (ECT) may be more effective during acute illness episodes than medication treatment (Parker, Roy, Hadzi-Pavlovic, & Pedic, 1992). However, ECT possesses several disadvantages that have limited its utility in clinical practice, including high relapse rates, patient unacceptability, cognitive and other adverse effects, and limited availability in many areas (Vega, Mortimer, & Tyson, 2000).
Combined Treatments for Severe Depression and Psychotic Disorders
Research shows that cognitive behavior therapy (CBT) is as effective as antidepressant medications alone for moderate to severe depression (DeRubeis, et al., 2005). Meta-analyses have demonstrated that combined pharmacotherapy plus CBT is more effective than either alone, particularly for chronically or severely depressed patients (Friedman, et al., 2004; Pampallona, Bollini, Tibaldi, Kupelnick, & Munizza, 2004; Thase, et al., 1997). Furthermore, meta-analyses have shown that CBT is more efficacious than comparison conditions for the treatment of positive and negative symptoms of psychosis, with effects in the medium to large range depending on the control condition (ES = .37–.91) (Gould, Mueser, Bolton, Mays, & Goff, 2001; Pilling, et al., 2002; Rector & Beck, 2001; Tarrier & Wykes, 2004). However, we are unaware of any published trials conducted specifically in samples with psychotic depression. Some previous trials of CBT have included small numbers of patients with psychotic mood disorders, suggesting the feasibility of treatment development for this population (Gaudiano, Beevers, & Miller, 2005; Gaudiano, Miller, & Herbert, 2007; Jackson, et al., 1998; Mueser, et al., 2008; Trower, et al., 2004).
Newer Behavioral Treatments for Depression and Psychosis
Behavioral Activation (BA) (Martell, Addis, & Jacobson, 2001) and Acceptance and Commitment Therapy (ACT) (Hayes, Strosahl, & Wilson, 2012) both ascribe to a contextualist philosophy, which “views psychological events as ongoing actions of the whole organism interacting in and with historically and situationally defined contexts” (Hayes, Luoma, Bond, Masuda, & Lillis, 2006, p. 4). BA involves the identification of an individual’s behavioral avoidance patterns via functional analysis (i.e, examining antecedents and consequences of behavior) and the development of a goal-oriented plan for changing these behavioral deficits using a stepwise process. The historical roots of BA stem from the early work of Lewinsohn (Lewinsohn & Graf, 1973) using pleasant-events scheduling, but the approach has been elaborated over time to focus more on reducing avoidance to target depression rather than producing pleasurable feelings per se (Martell et al., 2001). A randomized controlled trial (Dimidjian, et al., 2006) demonstrated that for patients with moderate to severe depression, BA and antidepressant treatment produced similar improvements and both were superior to cognitive therapy alone. Metaanalysis suggests that BA is as effective as traditional CBT for depression (Cuijpers, van Straten, & Warmerdam, 2007) and the treatment currently is listed as an empirically-supported treatment for depression (Society of Clinical Psychology, n.d.-c).
ACT (Hayes et al., 2012) is another promising newer behavioral approach. Overall, the goal of ACT is to promote increased psychological flexibility through acceptance of unavoidable distress, cultivation of a mindful outlook (i.e., awareness of mental events as products of the mind rather than literal truths) to counteract excessive entanglement with cognitions, and clarification of personal values linked to behavioral goals. To foster greater psychological flexibility, ACT employs a variety of strategies, including metaphors and stories to communicate key treatment concepts and experiential exercises to increase a person’s willingness to contact the internal distress that often accompanies behavior change efforts.
Meta-analyses suggest that ACT is efficacious for a variety of disorders with small to large effect size differences (ES = .18–.96) depending on the control condition utilized (Hayes et al., 2006; Levin & Hayes, 2009; Öst, 2008; Powers, Zum Vörde Sive Vörding, & Emmelkamp, 2009). Controlled trials demonstrate that ACT is similar in efficacy to traditional CBT but may work through different mechanisms (Hayes et al., 2006). Currently, ACT is recognized as an empirically-supported treatment for depression (Society of Clinical Psychology, n.d.-a) and psychosis (Society of Clinical Psychology, n.d.-b). Two controlled pilot trials have demonstrated that ACT is more efficacious than treatment as usual in the treatment of hospitalized patients with psychosis. Results showed greater reductions in the ACT condition in terms of symptom severity, disability, distress, psychotic symptom believability, and rehospitalization rates at 4 and 12 month follow-up (Bach & Hayes, 2002; Bach, Hayes, & Gallop, 2012; Gaudiano & Herbert, 2006). ACT also was found to produce greater improvements compared to TAU in the subset of inpatients with psychotic depression (Gaudiano et al., 2007). Consistent with the proposed mechanisms of ACT, improvements in the degree of believability of psychotic symptoms (i.e., how real or true the experience is perceived to be) statistically mediated the effects of treatment condition on distress related to psychotic symptoms at discharge (Gaudiano, Herbert, & Hayes, 2010) and rehospitalization rates at follow-up (Bach, Gaudiano, Hayes, & Herbert, in press). In a recent trial in an outpatient sample, White et al. (2011) used ACT to treat emotional dysfunction following a psychotic episode and found greater improvements in mood symptoms and reduced crisis calls at follow-up in ACT compared to treatment as usual.
Experiential Avoidance in Depression and Psychosis
ACT and BA posit that experiential avoidance is an important factor in the development and maintenance of psychopathology. Experiential avoidance involves excessive negative evaluation of internal experiences (e.g., thoughts, emotions, sensations) and an unwillingness to experience them, which results in efforts to control or escape them that can interfere with functioning (Hayes, et al., 2004). Results from clinical and non-clinical samples demonstrate that experiential avoidance is strongly correlated with various forms of psychopathology, including depression (Hayes et al., 2004; Kashdan, Barrios, Forsyth, & Steger, 2006; Tull, Gratz, Salters, & Roemer, 2004). The negative effects of avoidance-based coping also have been implicated in psychosis (Escher, Delespaul, Romme, Buiks, & Van Os, 2003; Falloon & Talbot, 1981; Farhall & Gehrke, 1997; Tait, Birchwood, & Trower, 2004; Udachina, et al., 2009). For example, Shawyer et al. (2007) investigated coping with command hallucinations in patients with psychotic-spectrum disorders. Results showed that greater acceptance of voices was associated with lower depression, greater quality of life, and greater confidence in resisting command hallucinations. Both BA and ACT promote behavioral activation and psychological flexibility to increase contact with previously avoided external and internal stimuli that tend to inhibit functioning.
Current Study
In summary, multiple sources of data suggest the potential utility of psychosocial interventions for treating psychotic depression: 1) combined pharmacotherapy and psychotherapy produces benefits over pharmacotherapy alone in the treatment of severe depression, 2) CBT is incrementally efficacious in the treatment of schizophrenia and other psychotic disorders compared to medications alone, 3) some psychotherapy trials have included a small number of patients with psychotic depression, suggesting the feasibility of treating this subpopulation, and 4) an acceptance-based behavioral approach shows considerable promise for treating patients with depression or psychosis. Thus, the aim of the current study was to develop a specialized psychotherapeutic intervention for use in combination with pharmacotherapy for psychotic depression. As an initial step of treatment development, we developed a treatment manual and tested its preliminary effects in an open trial of patients with psychotic depression.
METHOD
Acceptance-Based Depression and Psychosis Therapy
Overview
Acceptance-based Depression and Psychosis Therapy (ADAPT) was developed specifically for patients experiencing severe depression with co-occurring hallucinations or delusions. ADAPT is an integration of both BA (Martell et al., 2001) and ACT (Hayes et al., 2012) approaches. The general aim of ADAPT is to improve functioning by implementing acceptance and mindfulness-based coping strategies to help individuals work toward behavioral activation goals that are consistent with their core values. The therapy is designed to be delivered in weekly individual sessions for up to six months.
Phase 1: Rapport Building (Sessions 1–3)
Session 1 focuses on building rapport and developing a therapeutic alliance. The therapist mainly reviews current symptoms and recent history. The therapist helps the patient to explore unsuccessful past attempts to cope with symptoms, highlighting those focused on avoiding or struggling with symptoms and orienting the patient toward “workability” as a guide to choosing ways of coping with problems. The general treatment rationale consistent with ACT and BA is explained in Session 2. Sessions 2–3 focus on reviewing the Valued Living Questionnaire (see Method; Wilson, Sandoz, Kitchens, & Roberts, 2010) to introduce the concept of values in ACT. Next, short-term behavioral treatment goals linked with the patient's values are elicited by the therapist to guide future behavioral activation efforts. Discrepancies between values and behaviors are highlighted and linked with distress/symptoms. Relevant metaphors and stories are incorporated where appropriate. For example, the Polygraph Metaphor (Hayes et al., 2012) can be used to highlight that excessive attempts to control symptoms can paradoxically increase their frequency.
Phase 2: Behavioral Activation (Sessions 4–10)
Behavioral activation strategies are introduced in the second phase of treatment to help patients resume their normal activities and pursue their goals. First, patients are taught to monitor their mood and activities for a 1 week period. The therapist highlights examples of how activity level and mood affect each other. Next, the therapist explains the role of avoidance in influencing mood using the TRAP model: Trigger, Response, Avoidance Pattern (Martell et al., 2001). Both behavioral (e.g., withdrawal) and experiential (e.g., rumination) forms of avoidance and their negative consequences are highlighted by the therapist. Patients are then taught to use the TRAP model to monitor their avoidance patterns prior to attempting to change them. Next, the therapist teaches patients how to develop a behavioral activation plan using the TRAC model: Trigger, Response, Alternative Coping (Martell et al., 2001). The therapist helps patients to break down goals into smaller, more manageable steps. Throughout this phase, the therapist encourages patients to increase their willingness to experience unwanted thoughts and feelings in the service of valued behavioral goals. Behavioral activation homework assignments are given between sessions and worksheets are provided for patients to record their progress and summarize session content.
Phase 3: Acceptance and Mindfulness (Sessions 11–20)
In this phase, the behavioral activation strategies taught in the previous phase are implemented each week with therapist guidance. In addition, the therapist more formally introduces the six components of ACT to facilitate behavioral activation. Patients are taught to defuse from negative thoughts, increase their willingness to experience distress, practice mindfulness techniques (e.g., sitting meditation), contact a stable part of the self that is separate from transient mental events (i.e., self-as-context), clarify values, and commit to making valued behavioral changes. These principles are applied to depressive as well as psychotic symptoms as needed and are described in detail elsewhere (Bach, Gaudiano, Pankey, Herbert, & Hayes, 2006; Gaudiano, 2010, in press). Examples of ACT metaphors commonly used during this phase include: Tug of War, Passengers on the Bus, Dirty vs Clean Discomfort, Bubble in the Road, Chessboard, and Path Up the Mountain (Hayes et al., 2012). ACT experiential exercises include: Fingercuffs, Vocal Repetition ("Milk, Milk, Milk"), Contents on Cards, Take your Mind for a Walk, Leaves in the Stream Meditation, Observer Exercise, and Eulogy/Epitaph (Hayes et al., 2012). The therapist chooses metaphors and exercises that are most relevant and applicable to the patient. In addition, a family member is invited to one of the sessions when appropriate. In these joint meetings, the therapist provides psychoeducation and discusses the goals of the treatment program and how the person can be supportive of this process.
Phase 4: Relapse Prevention (Sessions 21–24)
The last few sessions are focused on treatment transition/termination. One session focuses on preventing relapse such as identifying warning signs of relapse (e.g., increased withdrawal and isolation). The therapist also reviews patient progress, focusing particularly on improved functioning and quality of life rather than symptom reduction per se. In another session, values are again reviewed and new longer-term goals consistent with these values are developed. The final session focuses on wrapping up loose ends and ensuring that the patient has a clear post-ADAPT treatment plan based on clinical need and patient preferences.
Medication Treatment
All patients also received pharmacotherapy provided by a community treatment provider, which typically involved the prescription of an antidepressant medication, as well as antipsychotic and other medications as appropriate. Pharmacotherapy was unrestricted, and the specific choice of medications and schedule of contacts were determined by the provider and patient. If a patient did not have a medication provider at study entry, an appropriate referral was provided. Releases of information were obtained so that study staff could consult with community prescribers to coordinate care.
Participants
Participants were recruited from a Northeastern U.S., free-standing psychiatric hospital and met the following criteria: 1) DSM-IV diagnosis of major depressive disorder, severe with psychotic features according to the SCID; 2) over age 18; 3) ability to speak and read English sufficiently to complete study procedures; and 4) receiving concurrent pharmacotherapy. Exclusion criteria were: 1) bipolar disorder, 2) psychotic disorder (e.g., schizoaffective disorder), 3) current alcohol or drug dependence (except nicotine) according to the SCID, or 4) concurrent community psychotherapy.
Measures
To aid in our treatment development efforts, patients completed a broad array of measures assessing diagnosis, psychiatric symptoms, psychosocial functioning, medication adherence, treatment received, treatment acceptability/satisfaction, and process changes (e.g., acceptance and mindfulness).
Diagnostic
At baseline, the Structured Clinical Interview of DSM-IV (SCID-I) was administered at baseline to determine Axis I diagnosis (First, Spitzer, M, & Williams, 2002). The Structured Interview for DSM-IV Personality Disorders (SID-P) is a reliable and valid interview that was administered at mid-treatment for diagnosing Axis II disorders (First, Spitzer, Gibbon, & Williams, 1997).
Interviewer-rated outcomes
Primary symptom severity outcomes were assessed with the following measures. The Quick Inventory of Depressive Symptomatology Clinician Rating (QIDS-C) is a 16-item scale that is reliable and valid for assessing depression severity (Rush, et al., 2006). The Brief Psychiatric Rating Scale (BPRS) 18-item version (Overall & Gorham, 1962, 1988) is a widely used scale with demonstrated reliability and validity for assessing a variety of psychiatric symptoms. We report the BPRS Total, as well as Psychosis (thought disorder) and Mood (affect) subscale scores as previously derived by factor analysis (Mueser, Curran, & McHugo, 1997).
Self-report outcomes
Patients also completed multiple self-report measures to measure changes in symptoms and functioning. The Peters Delusions Inventory-21 Item (PDI-21) (Peters, Joseph, Day, & Garety, 2004) is a 21-item dimensional measure that is reliable and valid for assessing delusion proneness. Launay-Slade Hallucinations Scale-Revised (LSHS-R) (Morrison, Wells, & Nothard, 2000) is a 21-item dimensional measure that is reliable and valid for assessing hallucinatory proneness. The World Health Organization Disability Assessment Schedule (WHODAS-II) (Epping-Jordan & Bedirhan Ustun, 2001) is a 36-item self-report measure that has evidence of reliability and validity for assessing various aspects of disability, including activity limitations (communication, mobility, self care) and participation in society (interpersonal, work, general).
Process measures
Additional self-report measures were administered to assess changes in processes targeted by the intervention. The Acceptance and Action Questionnaire-II (AAQ-II) is a 7-item validated self-report measure in which patients rate the degree of agreement with statements related to experiential avoidance and psychological inflexibility (Bond, et al., 2011; Hayes et al., 2004). The Behavioral Activation for Depression Scale (BADS) is a 29-item validated self-report measure of activation and withdrawal related to depression (Kanter, Mulick, Busch, Berlin, & Martell, 2006). The Valued Living Questionnaire (VLQ) is a reliable and valid measure that assesses consistency between a person’s individual values and his/her daily activities (Wilson et al., 2010). Ratings of importance and consistency are multiplied for each of 10 valued domains and summed to provide a total score. The Cognitive and Affective Mindfulness Scale (CAMS) is a 12-item validated scale that assesses mindfulness and was designed for treatment research (Feldman, Hayes, Kumar, Greeson, & Laurenceau, 2007).
Treatment-related measures
Given the treatment development aim of the project, measures were administered to assess treatment received, expectancies, and satisfaction. The number of medication doses missed per month was assessed using the Medication Compliance Questionnaire (MCQ) (Lam, et al., 1996). The Credibility and Expectancy Scale (CES) is a widely-used measure of patients’ expectancies for improvement from treatment (Devilly & Borkovec, 2000). It was completed following presentation of the treatment rationale in session two. The Client Satisfaction Questionnaire-8 (CSQ-8) is an 8-item scale that yields a total score that reflects respondents' satisfaction with services, which was completed at the end of treatment (Larsen, Attkisson, Hargreaves, & Nguyen, 1979). A post-treatment interview was administered consisting of open-ended questions (e.g., “What did you find most/least helpful about the treatment?) to obtain additional feedback about the intervention.
Procedure
Charts of newly admitted patients were routinely screened by a research assistant based on study criteria after obtaining a Protected Health Information waiver from the Institutional Review Board (IRB). Approval was obtained from the treating physician to approach the patient. If the patient was potentially interested in participating, the nature, purpose, risks, and benefits of the study were explained and informed consent was obtained using procedures approved by the IRB.
Primary assessments were conducted at baseline, mid-treatment (3 months), post-treatment (6 months), and follow-up (9 months). The QIDS-C and BPRS also were administered early in treatment (1 month following baseline) to measure early symptom change. Interviewers were trained to administer study measures by reviewing didactic materials, practicing administration with confederates, rating previously recorded patient assessments, and administering measures with patients while being supervised until achieving acceptable interrater reliability (> .80). Interviewers also received ongoing review and supervision throughout the study to prevent "drift." Training on the BPRS was conducted according to "gold standard" procedures described by Ventura et al. (1993), which involved rating 6 standardized patient video recordings and achieving acceptable levels of interrater reliability (> .80). In addition to receiving study treatment free of charge, patients were compensated ($25) for completing each of the assessments.
Statistical Analyses
Results are reported for completers only and intention-to-treat (ITT) samples to examine the consistency of results. The last observation was carried forward for drop outs/missing data to provide a more conservative estimate of change. Repeated measures analyses of variance (ANOVAs) were conducted to examine changes over time on continuous measures. Within-subjects effect sizes were reported based on Cohen's d statistic. Examining absolute change over the course of treatment does not take into account the degree to which the gains can be attributed to the intervention itself, in contrast to factors such as regression to the mean and measurement error. The Reliable Change Index (RCI) takes into account the reliability of an assessment instrument to determine if treatment gains exceed the error attributable to measurement (Jacobson & Truax, 1991). Internal consistency reliability estimates used for calculating RCIs were .85 for the QIDS-C (Trivedi, et al., 2004) and .80 for the BPRS (Long & Brekke, 1999). Also, remission (i.e., no significant symptoms) was defined as a QIDS-C score of < 6 and scores of < 4 on BPRS psychotic symptom items (Gitlin, et al., 2001). For exploratory purposes, Pearson correlations were conducted using pre- to post-treatment residualized gain scores between process and outcome measures to determine whether changes in symptoms were related to changes in processes targeted by the intervention. Residualized change scores control for error related to repeated measurement by standardizing scores and accounting for the correlations between time points (Steketee & Chambless, 1992).
RESULTS
Description of Sample
Participants were 14 patients with a mean age of 49.6 years old (range 26–70, SD = 12.9). A total of 86% (n = 12) were female, 93% were White (n = 13), 7% were African-American (n = 1), 21.4% were Hispanic (n = 3), and 50% were married (n = 7). A total of 64% (n = 9) were recruited from inpatient, 21% (n = 3) from partial/day hospital, and 14% (n = 2) from outpatient. Chronicity and severity in the sample was high. The mean number of lifetime inpatient hospitalizations in the sample was 2 (SD = 1.6). A total of 86% (n = 12) had recurrent major depression, 79% (n = 11) reported 4 or more lifetime depressive episodes, and the average age of onset of first depressive episode was 22.3 years (SD = 12.1). A total of 71% (n = 10) had a comorbid anxiety disorder and 29% (n = 4) had a personality disorder. Regarding psychotic symptoms, 64% (n = 9) reported a history of hallucinations and 86% (n = 12) had a history of delusions.
Treatment Received
Two patients (14%) started but dropped out of treatment prematurely. In addition, one patient ended study participation at the 3-month point in consultation with the therapist, citing satisfactory improvement in symptoms and inability to continue due to competing responsibilities. Therefore, 11 out of 14 patients (79%) had complete treatment data available.
Patients received an average of 21 (SD = 3.6) sessions. One patient completed the last several sessions over the telephone after an injury left this person homebound. All patients were prescribed an antidepressant and 86% (n = 12) were prescribed antipsychotic medication during the study. According to the MCQ, 93% (n = 13/14) of the sample on average reported missing no more than one to two doses by mid-treatment and 73% (n = 8/11) reported missing no more than one or two doses by post-treatment. One patient was voluntarily rehospitalized during the treatment phase following medication self-discontinuation leading to psychosis and one patient was admitted to partial hospital during the follow-up period due to increased depression. All patients (100%) continued to receive pharmacotherapy and 21% (n = 3) received additional community psychotherapy from post-treatment to follow-up.
Interviewer-Rated Outcomes
Completers
For interviewer-rated measures, a 5-way repeated-measures ANOVA of QIDS-C scores indicated a significant time effect (F = 29.59, dfs = 4, 40, p < .001), with depression severity showing a large effect size decrease from baseline through follow-up (d = 2.58). Results also showed significant time effects for the BPRS Total (F = 36.95, dfs = 4, 40, p < .001), Psychosis (F = 20.20, dfs = 4, 40, p < .001), and Mood (F = 22.91, dfs = 4, 40, p < .001) Subscales. Overall psychiatric symptoms (d = 3.33), psychotic symptoms (d = 2.43), and mood symptoms (d = 2.99) showed large effect size improvements over time. See Table 1.
Table 1.
Interviewer-Rated Measures
| Measures | Completers n = 11 |
Intent-to-Treat n = 14 |
|---|---|---|
| QIDS-C | ||
| Baseline | 20.7 (3.4) | 21.1 (3.3) |
| 1 Mo. | 10.4 (5.7) | 11.7 (5.9) |
| Mid-Treatment | 7.3 (4.2) | 8.6 (4.9) |
| Post-Treatment | 7.2 (5.1) | 8.5 (5.6) |
| 3 Mo. Follow-Up | 6.8 (6.8) | 8.2 (6.8) |
| BPRS-Total | ||
| Baseline | 48.0 (8.2) | 48.5 (7.3) |
| 1 Mo. | 32.7 (7.7) | 34.0 (8.7) |
| Mid-Treatment | 26.7 (9.0) | 30.0 (10.2) |
| Post-Treatment | 25.5 (7.2) | 28.2 (8.6) |
| 3 Mo. Follow-Up | 24.3 (5.8) | 27.3 (8.0) |
| BPRS-Psychosis | ||
| Baseline | 12.3 (3.9) | 12.2 (3.7) |
| 1 Mo. | 7.2 (2.5) | 7.0 (2.7) |
| Mid-Treatment | 5.8 (3.1) | 6.4 (3.0) |
| Post-Treatment | 5.0 (1.6) | 5.7 (2.2) |
| 3 Mo. Follow-Up | 5.1 (1.5) | 5.8 (2.1) |
| BPRS-Mood | ||
| Baseline | 21.5 (4.5) | 21.6 (4.0) |
| 1 Mo. | 13.9 (4.7) | 14.7 (4.7) |
| Mid-Treatment | 9.5 (3.9) | 11.2 (5.1) |
| Post-Treatment | 10.0 (5.1) | 11.5 (5.7) |
| 3 Mo. Follow-Up | 9.3 (3.6) | 11.0 (5.0) |
Note. BPRS = Brief Psychiatric Rating Scale; QIDS-C = Quick Inventory of Depressive Symptomatology-Clinican Rated.
ITT
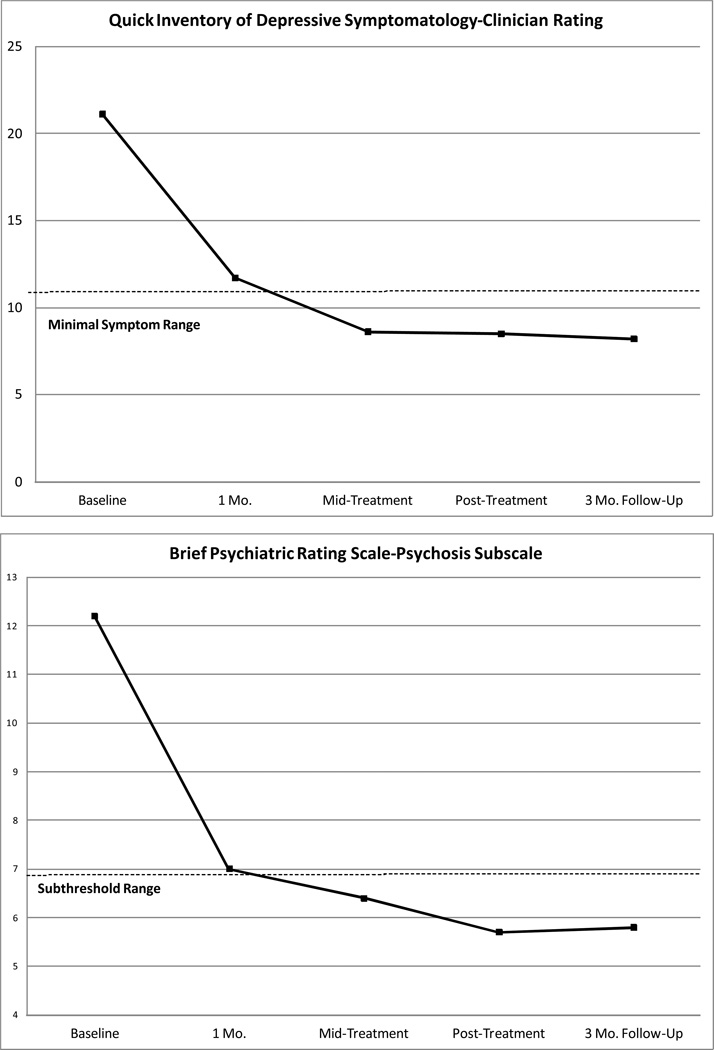
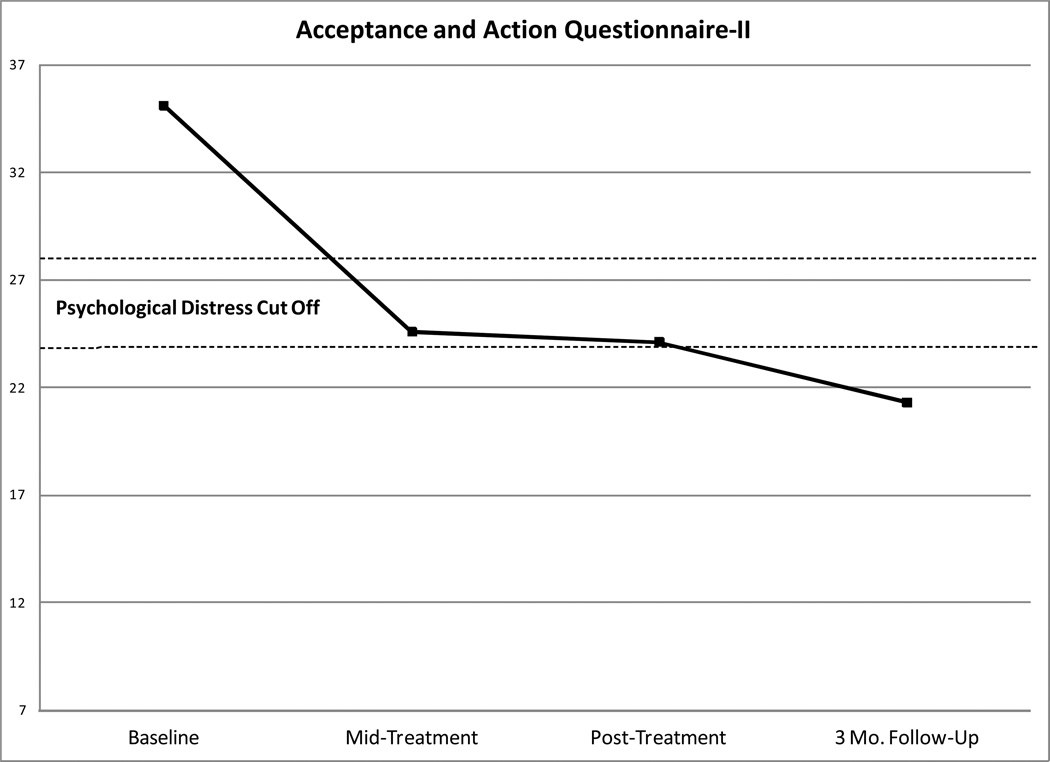
A repeated-measures ANOVA of QIDS-C scores demonstrated a significant time effect (F = 33.83, dfs = 4, 52, p < .001), with depression severity showing a large effect size decrease from baseline through follow-up (d = 2.41). Fig. Panel 1 shows that by mid-treatment, average QIDS-C scores were in the minimal symptoms range (< 11) (Trivedi et al., 2004) on this instrument. Results also showed significant time effects for the BPRS Total (F = 30.01, dfs = 4, 52, p < .001), Psychosis (F = 18.56, dfs = 4, 52, p < .001), and Mood (F = 20.23, dfs = 4, 54, p < .001) Subscales. Overall psychiatric symptoms (d = 2.77), psychotic symptoms (d = 2.13), and mood symptoms (d = 2.34) showed large effect size improvements over time. Fig. Panel 1 also shows that by mid-treatment, average BPRS-Psychosis scores were in the subthreshold range (< 4 on psychotic symptom items) (Gitlin et al., 2001).
Fig. Panel 1.
Changes Over Time on Symptom and Process Measures in the Intent-to-Treat Sample
Self-Report Measures
Completers
For 4-way repeated-measures ANOVAs conducted on self-report outcomes, results showed significant time effects for the PDI-21 (F = 4.26, dfs = 3, 30, p < .05), LSHS-R (F = 4.11, dfs = 3, 30, p < .05), and WHODAS-II (F = 10.45, dfs = 3, 30, p < .001). Self-reported delusional proneness (d = 0.77) and functional impairment (d = 1.64) showed large effect size decreases, and hallucination proneness (d = 0.51) showed a moderate effect size decrease from baseline through follow-up. Results also showed significant time effects for all the process measures: AAQ-II (F = 14.53, dfs = 3, 30, p < .001), BADS (F = 10.27, dfs = 3, 30, p < .001), VLQ (F = 5.27, dfs = 3, 30, p < .01), and CAMS-R (F = 12.44, dfs = 3, 30, p < .001). Consistent with the processes targeted by the intervention, psychological inflexibility (d = 2.25), behavioral activation (d = 1.74), value-behavior consistency (d = 1.08), and mindfulness (d = 1.70) showed large effect size improvements from baseline through follow-up. See Table 2.
Table 2.
Self-Report Measures
| Measures | Completers n = 11 |
Intent-to-Treat n = 14 |
|---|---|---|
| PDI-21 | ||
| Baseline | 8.3 (5.8) | 7.4 (5.4) |
| Mid-Treatment | 5.5 (3.9) | 5.2 (3.6) |
| Post-Treatment | 5.2 (4.6) | 4.9 (4.2) |
| 3 Mo. Follow-Up | 4.3 (4.5) | 4.2 (4.1) |
| LSHS-R | ||
| Baseline | 33.6 (12.0) | 30.9 (11.9) |
| Mid-Treatment | 26.9 (13.2) | 25.7 (12.5) |
| Post-Treatment | 26.1 (13.3) | 25.1 (12.5) |
| 3 Mo. Follow-Up | 26.6 (15.2) | 25.5 (14.1) |
| WHODAS-II | ||
| Baseline | 50.8 (18.7) | 50.4 (18.3) |
| Mid-Treatment | 29.0 (20.7) | 28.0 (20.2) |
| Post-Treatment | 28.0 (20.4) | 27.2 (19.8) |
| 3 Mo. Follow-Up | 20.7 (18.1) | 21.4 (18.0) |
| AAQ-II | ||
| Baseline | 36.5 (7.7) | 35.1 (7.5) |
| Mid-Treatment | 23.3 (8.5) | 24.6 (8.9) |
| Post-Treatment | 22.6 (10.7) | 24.1 (10.6) |
| 3 Mo. Follow-Up | 19.1 (7.8) | 21.3 (9.1) |
| BADS | ||
| Baseline | 101.2 (28.6) | 104.0 (26.7) |
| Mid-Treatment | 151.2 (23.8) | 141.4 (27.7) |
| Post-Treatment | 140.5 (35.7) | 139.1 (29.8) |
| 3 Mo. Follow-Up | 152.3 (30.1) | 148.4 (27.6) |
| VLQ | ||
| Baseline | 45.5 (23.4) | 41.3 (22.3) |
| Mid-Treatment | 61.1 (22.4) | 52.7 (26.5) |
| Post-Treatment | 66.5 (20.2) | 57.0 (26.7) |
| 3 Mo. Follow-Up | 69.3 (20.8) | 59.1 (27.9) |
| CAMS-R | ||
| Baseline | 23.3 (8.8) | 23.3 (7.8) |
| Mid-Treatment | 36.5 (5.9) | 33.5 (8.2) |
| Post-Treatment | 36.3 (7.6) | 33.4 (9.1) |
| 3 Mo. Follow-Up | 36.5 (6.6) | 33.6 (8.6) |
Note. BPRS = Brief Psychiatric Rating Scale; PDI-21 = Peters Delusion Inventory-21 Item; LSHS-R = Launay-Slade Hallucinations Scale-Revised; WHODAS-II = World Health Organization Disability Assessment Scale-II; AAQ-II = Acceptance and Action Questionnaire-II; VLQ - Valued Living Questionnaire; BADS = Behavioral Activation in Depression Scale; CAMS-R = Cognitive and Affective Mindfulness Scale-Revised.
ITT
For 4-way repeated-measures ANOVAs conducted on self-report outcomes, results showed significant time effects for the PDI-21 (F = 3.96, dfs = 3, 39, p < .05), LSHS-R (F = 3.32, dfs = 3, 39, p < .05), and WHODAS-II (F = 14.40, dfs = 3, 39, p < .001). Self-reported delusion proneness (d = 0.67) and functional impairment (d = 1.60) showed large effect size decreases and hallucination proneness (d = 0.41) showed a moderate effect size decrease from baseline through follow-up. Results also showed significant time effects for all the process measures: AAQ-II (F = 10.58, dfs = 3, 39, p < .001), BADS (F = 9.41, dfs = 3, 39, p < .001), VLQ (F = 4.18, dfs = 3, 39, p < .05), and CAMS-R (F = 9.39, dfs = 3, 39, p < .001). Psychological inflexibility (d = 1.65), behavioral activation (d = 1.60), value-behavior consistency (d = 0.70), and mindfulness (d = 1.25) showed large effect size improvements from baseline through follow-up. Fig. Panel 1 also shows that mean scores on the AAQ-II were below the cut off range (24–28) (Bond et al., 2011) indicative of psychological distress by follow-up.
Clinically Significant Change
Completers
A total of 100% (n = 11) met criteria for reliable change on the QIDS-C and 82% (n = 9) met criteria for reliable change on the BPRS Psychosis Subscale from baseline to post-treatment. A total of 91% (n = 10) met criteria for reliable change criteria on the QIDS-C and 82% (n = 9) met criteria for reliable change on the BPRS Psychosis Subscale from baseline to follow-up. No patients showed reliable deterioration in symptoms. Using the most stringent criteria possible, 36% (n = 4) of patients were in complete remission for depressive and psychotic symptoms at post-treatment and 55% (n = 6) were in remission at follow-up.
ITT
A total of 100% (n = 14) met criteria for reliable change on the QIDS-C and 79% (n = 11) met criteria for reliable change on the BPRS Psychosis Subscale from baseline to post-treatment. A total of 93% (n = 13) met reliable change criteria on the QIDS-C and 79% (n = 11) met criteria for reliable change on the BPRS Psychosis Subscale from baseline to follow-up. No patients showed reliable deterioration in symptoms. A total of 28% (n = 4) of patients were in complete remission for both depressive and psychotic symptoms at post-treatment and 43% (n = 6) were in remission at follow-up.
Relationship between Outcome and Process Changes
Residualized change scores were computed from pre- to post-treatment for process (AAQ-II, VLQ, BADS, and CAMS) and symptom measures (QIDS-C, BPRS-Psychosis) in the ITT sample to maximize power (see Table 3). Consistent with the proposed mechanisms of the intervention, decreases in depressive symptoms were associated with decreases in psychotic symptoms and increases in psychological flexibility, mindfulness, and valued living. Decreases in psychological inflexibility were associated with increases in behavioral activation and mindfulness. Increases in mindfulness also were associated with increases in valued living.
Table 3.
Residualized Pre- to Post-Treatment Change Score Correlations between Outcome and Process Measures in the Intent-to-Treat Sample (n = 14)
| BPRS-Psychosis | AAQ-II | BADS | CAMS-R | VLQ | |
|---|---|---|---|---|---|
| QIDS-C | .66** | .55* | − 50 | −.69** | −.59* |
| BPRS-Psychosis | -- | −.17 | − 16 | −.45 | −.48 |
| AAQ-II | -- | -- | −77*** | −.86*** | −.29 |
| BADS | -- | -- | -- | .74** | .35 |
| CAMS-R | -- | -- | -- | -- | .56* |
p < .05
p < .01
p < .001.
QIDS-C = Quick Inventory of Depressive Symptomatology-Clinican Rating, BPRS-Psychosis = Brief Psychiatric Rating Scale-Psychosis Subscale, AAQ-II = Acceptance and Action Questionnaire-II, BADS = Behavioral Activation for Depression Scale, CAMS-R = Cognitive and Affective Mindfulness Scale-Revised.
Treatment Credibility/Satisfaction/Acceptability
Results from the CES administered after the explanation of the treatment rationale in Session 2 indicated a high degree of credibility and expectancy for change. On a 1–9 point scale, mean ratings were high for how logical the treatment sounded (M = 8.3, SD = 0.8), how successful patients anticipated treatment would be in reducing their symptoms (M = 7.8, SD = 1.0), and how confident patients would be in recommending the treatment to a friend (M = 8.2, SD = 1.0). In addition, treatment acceptability and satisfaction was high at post-treatment. The average total score on the CSQ-8 was 30.5 (SD = 1.3) out of a possible score of 32.
Finally, participants provided additional feedback about the treatment in response to open-ended questions administered at post-treatment. Comments in response to the question "What aspects of the therapy did you find most beneficial?" included:
"Dealing with everyday problems."
"[The therapist's] way of explaining and visual aids, they gave me a clearer understanding."
"Goal setting, homework"
"Being able to talk to someone about my problems."
"Recognizing certain habits with rumination, how to redirect thoughts, seemed to help me understand how I feel."
"Mindfulness and taking walks…Doesn't take my mind off it but you've gotten yourself out of the house."
"Seeing thoughts and experiences in a different way; more accepting than before."
"Writing everything down, what you've done, mood."
Comments in response to the question "What aspects of the therapy did you find difficult or unhelpful?" included:
"Sometimes it was difficult to discuss certain subjects but overall, talking about these things was helpful."
"Difficulty within myself taking the skills and using them, but they were well worth using."
"Difficult questionnaires, repeated questions."
Comments in response to the question "What did you get out of doing therapy?" included:
"I feel more comfortable with who I am… I developed better ways to handle situations or life problems."
"I learned to look at things more lightly and not be so serious; to take things for what they are."
"Instead of isolating and giving in, I push to accomplish even little tasks, push myself to get up and have a routine."
"I feel a lot stronger. I can cope a lot better with situations in day-to-day life and they don't drain me like they might have before."
"I used to hold on to anger and pain and self-guilt, but I'm not being so hard on myself and feel a lot more peaceful."
"The whole mindfulness thing… I sit back when my mind races and put the thought on a leaf; do it so often."
"I learned how to cope, stay on track."
DISCUSSION
To our knowledge, this is the first study to demonstrate the feasibility, credibility, acceptability, and potential efficacy of psychotherapy in conjunction with pharmacotherapy developed specifically for patients with psychotic depression. Patients showed significant improvements on a wide array of outcome measures. Results indicated that patients on average demonstrated large and sustained reductions in depressive (d = 2.41) and psychotic symptoms (d = 2.13) following an acute episode and maintenance of treatment gains through the follow-up period. Patients also demonstrated significant improvements in psychosocial functioning over time. To put the obtained effect sizes in some context, they appear to compare favorably with previous trials of CBT for depression in similar samples. In a combined dataset consisting of two clinical trials of psychotherapy plus pharmacotherapy provided for six months following hospitalization conducted in our research group (Gaudiano et al., 2005), patients with nonpsychotic depression demonstrated a pre-post effect size improvement of d = 2.27 (n = 105). In contrast, the subsample of patients in these trials with psychotic depression (n = 14) only demonstrated a pre-post effect size of d = 1.49 (n = 14). The present trial results are encouraging and suggest that adapting combined treatment specifically for psychotic depression may be able to maximize outcomes.
Furthermore, processes specifically targeted by ADAPT also showed large gains, including in the areas of psychological flexibility, valued living, behavioral activation, and mindfulness. Changes in processes targeted by the treatment were correlated with changes in depressive symptoms in expected directions. Patients also reported high degrees of credibility and satisfaction with ADAPT. Feedback about the intervention generally was very positive. In this very severe and high risk group of patients, the vast majority showed clinically significant changes in symptoms and 55% of completers were in remission for depression and psychosis through follow-up.
Comparison with Traditional Cognitive and Behavioral Approaches
ADAPT is based on an ACT approach (Hayes et al., 2012). Traditional CBT (e.g., Beckian cognitive therapy) focuses on the active disputation and modification of dysfunctional beliefs to decrease their frequency and believability. Newer approaches, such as ACT, focus on cultivating mindfulness and psychological acceptance to promote behavior change without directly seeking to change symptom frequency or content. Patients are taught to decrease their use of counterproductive strategies aimed at controlling unpleasant private experiences, while noticing in a nonjudgmental fashion the occurrence of thoughts, feelings, and sensations without treating them as “real” or “true.” Accepting does not imply “giving in” to symptoms, but instead recognizes that thoughts and feelings are transient mental events that can be treated and experienced in this way. These strategies are applied to hallucinations and delusions as well as depression.
ADAPT also integrates strategies from BA (Martell et al., 2001) within an acceptance and mindfulness context. Many of the behavioral activation techniques used in ADAPT are similar to those used in the standard treatment (e.g., TRAP/TRAC), but modifications are made more in the way that the strategies are conceptualized and implemented. For example, there not a focus in ADAPT on using BA strategies to decrease depression itself. Furthermore, BA is presented in the context of acceptance rather than change of uncomfortable internal experiences. In addition, activation activities are chosen and explicitly linked with values clarification work in an ongoing and evolving process. These modifications are designed to improve motivation for change and to encourage task persistence in the face of unavoidable obstacles. During the latter part of treatment, there is an increasing and explicit focus on ACT’s six core processes (acceptance, defusion, self-as-context, present moment awareness, values, committed action) to extend and expand upon the BA framework. These core processes are applied to increasingly complex and multi-faceted life goals (e.g., improving interpersonal relationships).
Primary Treatment Principles
More broadly, ADAPT has several primary treatment principles. First, acceptance is modeled by the therapist during sessions. The therapist emphasizes through example that distressing experiences can be discussed in an open, straightforward, and nonthreatening fashion. Symptoms of depression or psychosis are conceptualized as understandable human problems and reactions to life. Research on ACT for psychosis suggests that working with psychotic symptoms in this more indirect fashion can paradoxically lead to changes in the believability of symptoms, perhaps through the reduction of avoidance-based coping and the adoption of a mindful outlook which allows these problems to resolve on their own accord as psychological stress is reduced (Bach et al., in press; Gaudiano et al., 2010). Second, ADAPT emphasizes changing functioning rather than changing symptoms directly. Of course, symptom reduction is often a desirable and achievable outcome of successful treatment and was demonstrated in the current trial, but the value of reduced symptoms is linked to its association with improved functioning. One reason for this is that sometimes an overemphasis on reducing symptoms paradoxically increases the frequency and distress related to the very symptoms the person is trying to control, especially in clinical populations with chronic or recurring symptoms (Wenzlaff & Bates, 1998). Finally, ADAPT is designed to be a flexible and personalized approach with its focus on functionally-derived behavioral activation plans and individualized values work. ACT and BA principles are applied in a transdiagnostic fashion and modifications (e.g., slowing down the pace of treatment, using relatable stories and exercises, and incorporating visual aids and worksheets) are made to ensure patient engagement with treatment regardless of level of impairment.
Special Clinical Considerations
ADAPT is designed to be delivered in combination with pharmacotherapy given the current evidence to support medications for psychotic depression. In ADAPT, medication is viewed as an intervention that can serve various functions, such as helping to decrease the person’s symptoms to a more manageable level so that he/she can better implement valued behavioral changes. The “workability” of medication is emphasized as the way to judge the usefulness and appropriateness of the treatment. Suffering is not viewed as necessary. If suffering can be eased through the use of medications, the therapist supports this if it fits with the person’s core values. However, most research shows that medications only provide partial benefits to patients with psychotic depression (Rothschild & Duval, 2003). Thus, ADAPT is used to target additional symptoms and functional goal attainment which requires further intervention. Implementing behavioral activation and acceptance strategies may produce a temporary increase in symptoms, similar to what happens when exposure therapies are implemented in patients with anxiety disorders. If the pharmacotherapist is not aware of this, he/she may interpret the temporary increase in distress reported by the patient as a signal that medications need to be increased or changed (e.g., adding a benzodiazapine). Therefore, the therapist should educate the prescriber about the treatment model and what to expect to minimize future confusion.
Patients with psychotic depression are two to five times more likely to have a history of suicide attempts (Johnson et al., 1991). Ongoing assessment and monitoring of suicidality constitutes an important component of any treatment. Furthermore, the therapist and patient should collaborate to develop a mutually agreed upon safety plan detailing actions to be taken in response to increased suicidal ideation. In addition, nonadherence to medication and other treatments is a frequent problem in psychotic depression (Rothschild, 1996). The therapist should routinely monitor the patient’s level of adherence to treatment (e.g., missed doses and appointments; homework noncompliance). For example, therapist can help the patient problem solve issues surrounding medication management and promote closer consultation with the treating psychiatrist in the context of improving values-action consistency. Also, it is important to consider potential cognitive deficits and limitations, which frequently are problems in psychotic depression (Vega et al., 2000). The therapist can help mitigate this issue by focusing more on concrete behavioral goals and strategies when dealing with severely ill patients. The therapist can further tailor treatments for patients with cognitive limitations (due to medication side effects, the disorder itself, or both) by explaining treatment concepts using simpler language (e.g., ACT’s use of metaphors and stories), reviewing session information frequently, and providing visual aids and patient handouts/workbooks to supplement the discussion.
In general, patients with psychotic depression tend to be characterized by multiple problems at the same time and to exhibit a more chronic course with multiple relapses. Thus, the therapist needs to tailor the treatment to the presenting problems of the individual patient. The BAT and ACT approaches are designed to target principles of behavior change that have been found to cut across different categories of psychopathology (e.g., mood, anxiety, psychosis) and thus are more easily adaptable to the multiproblem patient. Further, patients with psychotic depression may experience periods of acute illness that can make it difficult to proceed with the therapy. During these periods, the therapist can collaborate with other providers to assess and monitor the patient to determine the need for hospitalization. During hospitalization, the patient can keep in contact with the patient by phone and plan to resume following discharge to promote continuity of care.
In general, we found the intervention to be generally well-tolerated by patients and supported by other treatment providers. However, we recommend caution when using formal, intensive meditation techniques with acutely ill psychotic patients as some case reports suggest that intensive meditation may exacerbate psychosis (Sethi & Bhargava, 2003). ACT employs a number of nonmeditation-based exercises that foster mindfulness using alternative techniques (Bach et al., 2006).
Finally, we recommend tailoring therapy to patient severity so as not to overwhelm the individual with treatment goals and strategies. The notion of acceptance can be overwhelming and often needs to be “titrated” such that small periods of acceptance-based coping are implemented first and the patient is able to build these skills slowly over time. The patient should be met wherever he/she is and the therapist needs to have good communication with the person to gauge how quickly or slowly to proceed. The values clarification work can also be overwhelming to some patients in acute distress. Instead of focusing on the entire values assessment, one value that the patient is able and willing to work on can be addressed. Values work can be balanced with other interventions designed to improve the person’s ability to tolerate distress as they move forward with their goals. Many depressed patients as they are recovering tend to set unrealistic and overly ambitious goals to achieve. BAT recommends helping patients break down complex goals into manageable steps that can be achieved more successfully.
Limitations
As this is the first step of a treatment development project, certain limitations should be considered. First, the provision of concurrent pharmacotherapy makes it more difficult to discern the amount of improvement attributable to ADAPT specifically. However, it is important to consider that patients in the study were not medication naive and most were recruited after an index hospitalization, which suggests that pharmacotherapy was not providing sufficient benefits alone. The next step in treatment development is to conduct a randomized controlled pilot study to examine the effects of pharmacotherapy alone versus pharmacotherapy plus ADAPT to better understand the unique contribution of the intervention as part of treatment. In addition, ADAPT combines components from ACT and BA; therefore, single-subject experimental designs would be useful to examine the timing of different intervention components and their relationship to outcomes. Treatment was delivered by the lead author to develop and test the initial ADAPT treatment protocol. Future research will be needed to determine treatment fidelity when the treatment is administered by multiple therapists. Furthermore, the effects of treatment should be investigated in a more diverse group of patients, including more males and ethnic/minority patients, and also over a more extended follow-up period.
Although one would expect symptoms to decrease in general following hospitalization, patients with psychotic depression tend to experience high rates of symptom recurrence, relapse, and rehospitalization (Rothschild & Duval, 2003). For example, our previous research showed that many patients with psychotic depression treated with non-specialized psychotherapy and pharmacotherapy following hospitalization tended to remain severely ill even at post-treatment (Gaudiano et al., 2005). Patients receiving ADAPT following hospitalization tended to show continued and sustained improvement over at least a 9 month post-hospital period with minimal evidence of relapse or recurrence.
Conclusion
Despite these caveats, results of the current feasibility trial suggest that ADAPT is a promising treatment option for patients experiencing psychotic symptoms in the context of a depressive episode, but will require further controlled research to determine its specific efficacy. Changes in mindfulness, behavioral activation, valued living, and psychological flexibility not typically targeted by pharmacotherapy were associated with symptomatic improvement. Future research should examine whether these changes are specific to ADAPT or if they also occur in patients receiving pharmacotherapy alone. Findings from the current study are consistent with previous research showing that behavioral and acceptance-based approaches to treating severe mental illnesses such as psychotic depression are acceptable, safe, and potentially beneficial to patients
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (K23 MH076937) awarded to Dr. Gaudiano.
Biography
Brandon A. Gaudiano, Ph.D. is a research psychologist at Butler Hospital's Psychosocial Research Program. He also is assistant professor of research in the Department of Psychiatry and Human Behavior at the Warren Alpert Medical School of Brown University. Dr. Gaudiano's research interests include developing novel psychosocial treatments for patients severe mental illness and acceptance/mindfulness-based approaches.
Kathryn Nowlan, B.A. graduated with a bachelor of arts degree in psychology from Rowan University. Currently, she is a clinical research coordinator in the Department of Psychiatry at the University of North Carolina and is working on a therapy research study for couples where one partner has anorexia nervosa. Previously, she worked as a research assistant in the Psychosocial Research Program at Brown University/Butler Hospital. Her research interests include the bi-directionality between psychopathology and interpersonal relationship functioning, and couples treatment research.
Lily A. Brown, M.A. is currently pursuing her doctoral degree in clinical psychology and the psychology of learning and behavior at the University of California, Los Angeles. Her research interests include the etiology and treatment of anxiety and mood disorders.
Gary Epstein-Lubow, M.D. is an assistant professor of psychiatry and human behavior and assistant professor of health services, policy and practice at the Alpert Medical School of Brown University. He also is an attending psychiatrist and the assistant unit chief for geriatrics at Butler Hospital. His research interests include the prevention of unnecessary hospitalizations and improved coordination of clinical services for family caregivers.
Ivan W. Miller, Ph.D. is a professor of psychiatry and human behavior at the Alpert Medical School of Brown University and director of the Psychosocial Research Program at Butler Hospital in Providence, Rhode Island. His research interests include the assessment and treatment of mood disorders, with specific interests in the treatment of suicidal patients and in family approaches.
REFERENCES
- Bach P, Gaudiano B, Pankey J, Herbert JD, Hayes SC. Acceptance, mindfulness, values, and psychosis: Applying Acceptance and Commitment Therapy (ACT) to the chronically mentally ill. In: Baer RA, editor. Mindfulness-based treatment approaches: Clinician’s guide to evidence base and applications. San Diego, CA: Academic Press; 2006. pp. 93–116. [Google Scholar]
- Bach P, Gaudiano BA, Hayes SC, Herbert JD. Acceptance and Commitment Therapy for psychosis: Intent to treat hospitalization outcome and mediation by believability. Psychosis. in press. [Google Scholar]
- Bach P, Hayes SC. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2002;70:1129–1139. doi: 10.1037//0022-006x.70.5.1129. [DOI] [PubMed] [Google Scholar]
- Bach P, Hayes SC, Gallop R. Long-term effects of brief acceptance and commitment therapy for psychosis. Behavior Modification. 2012;36:165–181. doi: 10.1177/0145445511427193. [DOI] [PubMed] [Google Scholar]
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, Waltz T, Zettle RD. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: a revised measure of psychological inflexibility and experiential avoidance. Behavior Therapy. 2011;42:676–688. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Brown R, Frances A, Kocsis J, Mann J. Psychotic vs. nonpsychotic depression: comparison of treatment response. Journal of Nervous and Mental Disease. 1982;170:635–637. doi: 10.1097/00005053-198210000-00008. [DOI] [PubMed] [Google Scholar]
- Coryell W, Leon A, Winokur G, Endicott J, Keller M, Akiskal H, Solomon S. Importance of psychotic features to long-term course in major depressive disorder. American Journal of Psychiatry. 1996;153:483–489. doi: 10.1176/ajp.153.4.483. [DOI] [PubMed] [Google Scholar]
- Coryell W, Pfohl B, Zimmerman M. The clinical and neuroendocrine features of psychotic depression. Journal of Nervous and Mental Disease. 1984;172:521–528. doi: 10.1097/00005053-198409000-00002. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clinical Psychology Review. 2007;27:318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Curson D, Patel M, Liddle P, Barnes T. Psychiatric comorbidity of a long stay hospital population with chronic schizophrenia and implications for future community care. British Medical Journal. 1988;297:819–822. doi: 10.1136/bmj.297.6652.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Gallop R, McGlinchey JB, Markley DK, Gollan JK, Atkins DC, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan J, Bedirhan Ustun T. The WHODAS II: Leveling the playing field for all disorders. WHO Bulletin of Mental Health. 2001;6:5. [Google Scholar]
- Escher S, Delespaul P, Romme M, Buiks A, Van Os J. Coping defence and depression in adolescents hearing voices. Journal of Mental Health. 2003;12:91–99. [Google Scholar]
- Falloon IRH, Talbot RE. Persistent auditory hallucinations: Coping mechanisms and implications for management. Psychological Medicine. 1981;11:329–339. doi: 10.1017/s0033291700052144. [DOI] [PubMed] [Google Scholar]
- Farhall J, Gehrke M. Coping with hallucinations: Exploring stress and coping framework. British Journal of Clinical Psychology. 1997;36(Pt 2):259–261. doi: 10.1111/j.2044-8260.1997.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Feldman G, Hayes AM, Kumar S, Greeson J, Laurenceau J-P. Mindfulness and Emotion Regulation: The Development and Initial Validation of the Cognitive and Affective Mindfulness Scale-Revised (CAMS-R) Journal of Psychopathology and Behavioral Assessment. 2007;29:177–190. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Personality Disorders, SCID-II. Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- First M, Spitzer R, M G, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. SCID-I/P. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Friedman M, Detweiler-Bedell J, Leventhal H, Horne R, Keitner G, Miller I. Combined psychotherapy and pharmacotherapy for the treatment of major depressive disorder. Clinical Psychology: Science and Practice. 2004;11:47–68. [Google Scholar]
- Gaudiano BA. A new psychological treatment for paranoia and other psychotic symptoms: Acceptance and commitment therapy. In: Columbus AM, editor. Advances in Psychology Research. Vol. 68. Hauppauge, NY: Nova Science; 2010. pp. 157–176. [Google Scholar]
- Gaudiano BA. Brief acceptance and commitment therapy for the acute treatment of hospitalized patients with psychosis. In: Steel C, editor. Cognitive behaviour therapy for schizophrenia: Evidence based interventions and future directions. Hoboken, NJ: Wiley & Sons; in press. [Google Scholar]
- Gaudiano BA, Beevers CG, Miller IW. Differential response to combined treatment in patients with psychotic versus nonpsychotic Major Depression. Journal of Nervous and Mental Disease. 2005;193:625–628. doi: 10.1097/01.nmd.0000177791.33649.69. [DOI] [PubMed] [Google Scholar]
- Gaudiano BA, Dalrymple KL, Zimmerman M. Prevalence and clinical characteristics of psychotic versus nonpsychotic major depression in a general psychiatric outpatient clinic. Depression and Anxiety. 2009;26:54–64. doi: 10.1002/da.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudiano BA, Herbert JD. Acute treatment of inpatients with psychotic symptoms using Acceptance and Commitment Therapy: Pilot results. Behavior Research and Therapy. 2006;44:415–437. doi: 10.1016/j.brat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Gaudiano BA, Herbert JD, Hayes SC. Is it the symptom or the relation to it? Investigating potential mediators of change in acceptance and commitment therapy for psychosis. Behavior Therapy. 2010;41:543–554. doi: 10.1016/j.beth.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudiano BA, Miller IW, Herbert JD. The treatment of psychotic major depression: is there a role for adjunctive psychotherapy. Psychotherapy and Psychosomatics. 2007;76:271–277. doi: 10.1159/000104703. [DOI] [PubMed] [Google Scholar]
- Gaudiano BA, Young D, Chelminski I, Zimmerman M. Depressive symptom profiles and severity patterns in outpatients with psychotic versus nonpsychotic major depression. Comprehensive Psychiatry. 2008;49:421–429. doi: 10.1016/j.comppsych.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudiano BA, Zimmerman M. Does comorbid posttraumatic stress disorder affect the severity and course of psychotic major depressive disorder? Journal of Clinical Psychiatry. 2010;71:442–450. doi: 10.4088/JCP.08m04794gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fogelson DL, Bartzokis G, Aravagiri M. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. American Journal of Psychiatry. 2001;158:1835–1842. doi: 10.1176/appi.ajp.158.11.1835. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Harrow M. Consistency of remission and outcome in bipolar and unipolar mood disorders: a 10-year prospective follow-up. Journal of Affective Disorders. 2004;81:123–131. doi: 10.1016/S0165-0327(03)00161-7. [DOI] [PubMed] [Google Scholar]
- Gould RA, Mueser KT, Bolton E, Mays V, Goff D. Cognitive therapy for psychosis in schizophrenia: an effect size analysis. Schizophrenia Research. 2001;48:335–342. doi: 10.1016/s0920-9964(00)00145-6. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: Model, processes and outcomes. Behavior Research and Therapy. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: The process and practice of mindful change. 2nd ed. New York: Guilford; 2012. [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG, Bissett RT, Pistorello J, Toarmino D, Polusny MA, Dykstra TA, Batten SV, Bergan J, Stewart SH, Zvolensky MJ, Eifert GH, Bond FW, Forsyth JP, Karekla M, McCurry SM. Measuring experiential avoidance: A preliminary test of a working model. The Psychological Record. 2004;54:553–578. [Google Scholar]
- Jackson H, McGorry P, Edwards J, Hulbert C, Henry L, Francey S, Maude D, Cocks J, Power P, Harrigan S, Dudgeon P. Cognitively-oriented psychotherapy for early psychosis (COPE). Preliminary results. British Journal of Psychiatry. 1998;172:93–100. [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Johnson J, Horwath E, Weissman M. The validity of major depression with psychotic features based on a community sample. Archives of General Psychiatry. 1991;48:1075–1081. doi: 10.1001/archpsyc.1991.01810360039006. [DOI] [PubMed] [Google Scholar]
- Kanter JW, Mulick PS, Busch AMB, Berlin KS, Martell CR. The Behavioral Activation for Depression Scale (BADS): Psychometric Properties and Factor Structure. Journal of Psychopathology and Behavioral Assessment. 2006;29:191–202. [Google Scholar]
- Kashdan TB, Barrios V, Forsyth JP, Steger MF. Experiential avoidance as a generalized psychological vulnerability: comparisons with coping and emotion regulation strategies. Behavior Research and Therapy. 2006;44:1301–1320. doi: 10.1016/j.brat.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lam D, Bright J, Jones S, Hayward P, Schuck N, Chisholm D, Sham P. Cognitive therapy for bipolar illness: A pilot study of relapse prevention. Cognitive Therapy and Research. 1996;24:503–520. [Google Scholar]
- Larsen D, Attkisson C, Hargreaves W, Nguyen T. Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- Lattuada E, Serretti A, Cusin C, Gasperini M, Smeraldi E. Symptomatologic analysis of psychotic and non-psychosis depression. Journal of Affective Disorders. 1999;54:183–187. doi: 10.1016/s0165-0327(98)00141-4. [DOI] [PubMed] [Google Scholar]
- Levin M, Hayes SC. Is acceptance and commitment therapy superior to established treatment comparisons? Psychotherapy and Psychosomatics. 2009;78:380. doi: 10.1159/000235979. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Graf M. Pleasant activities and depression. Journal of Consulting and Clinical Psychology. 1973;41:261–268. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- Long JD, Brekke JS. Longitudinal factor structure of the Brief Psychiatric Rating Scale. Psychological Assessment. 1999;11:498–506. [Google Scholar]
- Lykouras E, Malliares D, Christodoulou G, Moussas G, Christodoulou D, Tzonou A. Delusional depression: phenomenology and response to treatment. Psychopathology. 1986;19:157–164. doi: 10.1159/000284441. [DOI] [PubMed] [Google Scholar]
- Martell C, Addis M, Jacobson N. Depression in context: Strategies for guided action. New York: Guilford; 2001. [Google Scholar]
- Meyers BS, Flint AJ, Rothschild AJ, Mulsant BH, Whyte EM, Peasley-Miklus C, Papademetriou E, Leon AC, Heo M. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD) Archives of General Psychiatry. 2009;66:838–847. doi: 10.1001/archgenpsychiatry.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AP, Wells A, Nothard S. Cognitive factors in predisposition to auditory and visual hallucinations. British Journal of Clinical Psychology. 2000;39:67–78. doi: 10.1348/014466500163112. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Curran PJ, McHugo GJ. Factor structure of the Brief Psychiatric Ratiing Scale in schizophrenia. Psychological Assessment. 1997;9:196–204. [Google Scholar]
- Mueser KT, Rosenberg SD, Xie H, Jankowski MK, Bolton EE, Lu W, Hamblen JL, Rosenberg HJ, McHugo GJ, Wolfe R. A randomized controlled trial of cognitive-behavioral treatment for posttraumatic stress disorder in severe mental illness. Journal of Consulting and Clinical Psychology. 2008;76:259–271. doi: 10.1037/0022-006X.76.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M, Schatzberg A. Prevalence of depressive episodes with psychotic features in the general population. American Journal of Psychiatry. 2002;159:1855–1861. doi: 10.1176/appi.ajp.159.11.1855. [DOI] [PubMed] [Google Scholar]
- Öst LG. Efficacy of the third wave of behavioral therapies: A systematic review and meta-analysis. Behav Res Ther. 2008;46:296–321. doi: 10.1016/j.brat.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): Recent developments in ascertainment and scaling. [Review] Psychopharmacology Bulletin. 1988;24:97–99. [PubMed] [Google Scholar]
- Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Archives of General Psychiatry. 2004;61:714–719. doi: 10.1001/archpsyc.61.7.714. [DOI] [PubMed] [Google Scholar]
- Parker G, Roy K, Hadzi-Pavlovic D, Pedic F. Psychotic (delusional) depression: a meta-analysis of physical treatments. Journal of Affective Disorders. 1992;24:17–24. doi: 10.1016/0165-0327(92)90056-c. [DOI] [PubMed] [Google Scholar]
- Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophrenia Bulletin. 2004;30:1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Orbach G, Morgan C. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychological Medicine. 2002;32:763–782. doi: 10.1017/s0033291702005895. [DOI] [PubMed] [Google Scholar]
- Powers MB, Zum Vörde Sive Vörding MB, Emmelkamp PM. Acceptance and Commitment Therapy: A meta-analytic review. Psychotherapy and Psychosomatics. 2009;78:73–80. doi: 10.1159/000190790. [DOI] [PubMed] [Google Scholar]
- Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. Journal of Nervous and Mental Disease. 2001;189:278–287. doi: 10.1097/00005053-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ. Management of psychotic, treatment-resistant depression. Psychiatric Clinics of North America. 1996;19:237–252. doi: 10.1016/s0193-953x(05)70286-0. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ, Duval SE. How long should patients with psychotic depression stay on the antipsychotic medication? Journal of Clinical Psychiatry. 2003;64:390–396. doi: 10.4088/jcp.v64n0405. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Ibrahim HM, Trivedi MH, Biggs MM, Shores-Wilson K, Crismon ML, Toprac MG, Kashner TM. Comparison of self-report and clinician ratings on two inventories of depressive symptomatology. Psychiatric Services. 2006;57:829–837. doi: 10.1176/ps.2006.57.6.829. [DOI] [PubMed] [Google Scholar]
- Schatzberg A. New approaches to managing psychotic depression. Journal of Clinical Psychiatry. 2003;64:19–23. [PubMed] [Google Scholar]
- Serretti A, Lattuada E, Cusin C, Gasperini M, Smeraldi E. Clinical and demographic features of psychotic and nonpsychotic depression. Comprehensive Psychiatry. 1999;40:358–362. doi: 10.1016/s0010-440x(99)90141-4. [DOI] [PubMed] [Google Scholar]
- Sethi S, Bhargava SC. Relationship of meditation and psychosis: case studies. Australian and New Zealand Journal of Psychiatry. 2003;37:382. doi: 10.1046/j.1440-1614.2003.11721.x. [DOI] [PubMed] [Google Scholar]
- Shawyer F, Ratcliff K, Mackinnon A, Farhall J, Hayes SC, Copolov D. The voices acceptance and action scale (VAAS): Pilot data. Journal of Clinical Psychology. 2007;63:593–606. doi: 10.1002/jclp.20366. [DOI] [PubMed] [Google Scholar]
- Society of Clinical Psychology (n.d.-a) Acceptance and Commitment Therapy for depression. Retrieved April 9, 2012, from http://www.div12.org/PsychologicalTreatments/treatments/depression_acceptance.html.
- Society of Clinical Psychology (n.d.-b) Acceptance and commitment therapy for psychosis Retrieved September. Retrieved September 17, 2012, from http://www.div12.org/PsychologicalTreatments/treatments/schizophrenia_acceptance.html.
- Society of Clinical Psychology (n.d.-c) Behavior therapy/behavioral activation for depression. Retrieved April 9, 2012, from http://www.div12.org/PsychologicalTreatments/treatments/depression_behavior.html.
- Steketee GS, Chambless DL. Methodological issues in the prediction of treatment outcome. Clinical Psychology Review. 1992;12:387–400. [Google Scholar]
- Tait L, Birchwood M, Trower P. Adapting to the challenge of psychosis: Personal resilience and the use of sealing-over (avoidant) coping strategies. British Journal of Psychiatry. 2004;185:410–415. doi: 10.1192/bjp.185.5.410. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Barrowclough C, Bamrah J. Prodromal signs of relapse in schizophrenia. Social Psychiatry and Psychiatric Epidemiology. 1991;26:157–161. doi: 10.1007/BF00795207. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Wykes T. Is there evidence that cognitive behaviour therapy is an effective treatment for schizophrenia? A cautious or cautionary tale. Behavior Research and Therapy. 2004;42:1377–1401. doi: 10.1016/j.brat.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Thakur M, Hays J, Kishnan K. Clinical, demographic and social characteristics of psychotic depression. Psychiatry Research. 1999;86:99–106. doi: 10.1016/s0165-1781(99)00030-x. [DOI] [PubMed] [Google Scholar]
- Thase ME, Greenhouse JB, Frank E, Reynolds CF, Pilkonis PA, Hurley K, Grochocinski V, Kupfer DJ., 3rd Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Archives of General Psychiatry. 1997;54:1009–1015. doi: 10.1001/archpsyc.1997.01830230043006. [DOI] [PubMed] [Google Scholar]
- Tohen M, Hennen J, Zarate C, Baldessarini R&ale. Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. American Journal of Psychiatry. 2000;157:220–228. doi: 10.1176/appi.ajp.157.2.220. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological Medicine. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Trower P, Birchwood M, Meaden A, Byrne S, Nelson A, Ross K. Cognitive therapy for command hallucinations: randomised controlled trial. British Journal of Psychiatry. 2004;184:312–320. doi: 10.1192/bjp.184.4.312. [DOI] [PubMed] [Google Scholar]
- Tull MT, Gratz KL, Salters K, Roemer L. The role of experiential avoidance in posttraumatic stress symptoms and symptoms of depression, anxiety, and somatization. Journal of Nervous and Mental Disease. 2004;192:754–761. doi: 10.1097/01.nmd.0000144694.30121.89. [DOI] [PubMed] [Google Scholar]
- Udachina A, Thewissen V, Myin-Germeys I, Fitzpatrick S, O'Kane A, Bentall RP. Understanding the relationships between self-esteem, experiential avoidance, and paranoia: structural equation modelling and experience sampling studies. [Comparative Study]. Journal of Nervous and Mental Disease. 2009;197:661–668. doi: 10.1097/NMD.0b013e3181b3b2ef. [DOI] [PubMed] [Google Scholar]
- Vega J, Mortimer A, Tyson P. Somatic treatment of psychotic depression: review and recommendations for practice. Journal of Clinical Psychopharmacology. 2000;20:504–519. doi: 10.1097/00004714-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterien K, Liberman RP, Green MF, Shaner A. Brief psychiatric rating scale (BPRS): Expanded version, Ver. 4.0. Los Angeles: UCLA Department of Psychiatry and Biobehavioral Sciences; 1993. [Google Scholar]
- Wenzlaff RM, Bates DE. Unmasking a cognitive vulnerability to depression: how lapses in mental control reveal depressive thinking. Journal of Personality and Social Psychology. 1998;75:1559–1571. doi: 10.1037//0022-3514.75.6.1559. [DOI] [PubMed] [Google Scholar]
- White R, Gumley A, McTaggart J, Rattrie L, McConville D, Cleare S, Mitchell G. A feasibility study of Acceptance and Commitment Therapy for emotional dysfunction following psychosis. Behavior Research and Therapy. 2011;49:901–907. doi: 10.1016/j.brat.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wijkstra J, Lijmer J, Balk FJ, Geddes JR, Nolen WA. Pharmacological treatment for unipolar psychotic depression: Systematic review and meta-analysis. British Journal of Psychiatry. 2006;188:410–415. doi: 10.1192/bjp.bp.105.010470. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Sandoz EK, Kitchens J, Roberts ME. The Valued Living Questionnaire: Defining and measuring valued action within a behavioral framework. The Psychological Record. 2010;60:249–272. [Google Scholar]