Summary
Chronic infection with hepatitis C virus (HCV) is a major global health problem—there are approximately 120–130 million chronic infections worldwide. Since discovery of HCV 24 y ago, there has been a relentless effort to develop successful antiviral therapies. Studies of interferon- □(IFN)-based therapies have helped define treatment parameters, and these treatment strategies have cured a substantial percentage of patients. However, IFN must be injected, and there are problems with tolerability, adherence, and incomplete response in a large percentage of patients. New drug candidates designed to target the virus or the host have recently been introduced at an unprecedented pace. In phase I–III studies, these agents have exceeded expectations and achieved rates of response previously not thought possible. We are therefore entering a new era of therapy for HCV infection and interferon independence.
Keywords: DAA, NS3/4A protease inhibitor, nucleoside/nucleotide, non-nucleoside
HCV Structure and Life Cycle
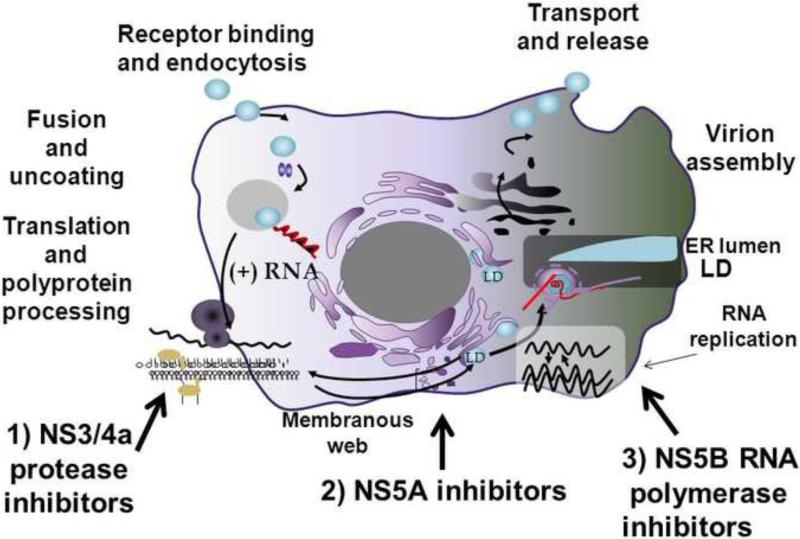
HCV has a positive-stranded RNA genome with a single long open-reading frame that is translated into a large polyprotein and processed by host and viral proteases into structural and nonstructural (NS) proteins.1 All steps in the HCV life cycle can be considered vulnerable to pharmacologic intervention, including entry, translation, RNA replication, assembly, and export of progeny viruses (Fig. 1). Viral enzymes and proteins involved in essential functions of the HCV lifecycle are the most common targets for new drugs which are now called direct-acting antiviral agents (DAAs). Host cellular proteins that are essential for the viral life cycle may also be responsive to antiviral intervention and these compounds are termed host-targeted antiviral agents (HTA). This review will focus on those DAAs in advanced clinical development that are moving HCV treatment paradigms to IFN-free regimens. These include NS3/4A protease inhibitors (PI), nucleoside/nucleotide and non-nucleoside inhibitors (NI and NNI respectively) of the RNA-dependent RNA polymerase (RdRp), and NS5A inhibitors (Fig. 1).
Figure 1.
Therapeutic Targets of the HCV Replication Cycle. DAA viral target sites in advanced clinical development are numbered 1-3.
ER, endoplasmic reticulum; LD, luminal domain
Interferon and Ribavirin
Type I interferon-α (IFN) is the prototypical HTA and was the first effective antiviral agent used against chronic HCV infection even before the viral species was identified.2 IFN monotherapy achieved only low rates of sustained virologic responses (SVR), however, later combination regimens of pegylated IFN and ribavirin (RBV) led to SVR rates of nearly 50% in patients entered into large clinical trials.3 Numerous trials during the IFN era established working guidelines for optimal treatment outcomes and the concept of response guided management for patients with different host and viral variables emerged. Although these principles are solid and continue to guide new DAA drug development, new IFN-free regimens have re-defined treatment intervals and some response parameters.
Type I IFN exerts primary antiviral activities against both RNA and DNA viruses through numerous interferon-stimulated gene (ISG) products which antagonize HCV replication and within hours decrease serum viral load. Different ISGs target both host and viral processes resulting in a broad antiviral attack.4 In response, HCV evolved highly efficient mechanisms to evade host IFN signaling at key pathogen recognition points that limit the antiviral effectiveness of IFN-based therapies and impair the host's ability to resolve acute infection.5
IFN gene expression is variable at the cellular level and in some patients ISG expression is diminished.4 Those patients with reduced ISG expression appear more sensitive to exogenous IFN therapy. IFN sensitivity is significantly associated with single nucleotide polymorphisms (SNP) located in the interleukin 28B (IL28B) gene promoter region. Genotype for the most commonly used SNP, rs12979860 (CC, CT or TT), is strongly associated with treatment outcome, with CC patients showing highest rates of SVR as compared to patients with a T allele.6 Interestingly, in chronically infected persons, the responsive CC genotype is inversely correlated with ISG expression 7.
RBV has limited direct antiviral effects when given alone, yet in combination with IFN promotes a major increase in SVR by accelerating the long term decline of HCV RNA8. However, the primary mechanism whereby RBV enhances SVR in patients is not clear. Pharmacologically, RBV is a synthetic guanosine analog, but the drug shows multiple other intracellular drug actions such as promotion of viral mutagenesis, enhancement of ISG expression, and immunomodulatory behavior.9
Antiviral treatment response
Interferon therapy initially defined patient response parameters. SVR was defined as HCV becoming undetectable in blood during treatment and remaining so 24 weeks after the end of treatment, as assessed using a sensitive viral detection method, (LOD: ≤10-15 IU/mL) Responder/relapsers (RR) achieve undetectable HCV RNA during therapy, yet virus re-appears post treatment. Partial responders (PR) show more than a 2 log drop by week 12 without loss of detectable virus; and null responders (NR) do not achieve a 2 log drop in viral load by week 12. While this terminology has persisted into the DAA era, more prognostic, shorter-term response intervals are now emerging that reflect the more rapid kinetic decline of virus seen with DAAs. Recent data indicate that undetectable HCV RNA at 12 weeks post treatment is highly reliable to predict SVR.10
SVR is considered curative with presumed clearance of all HCV-infected cells in the patient's body. Viral kinetic modeling studies performed on patient blood samples established two viral disappearance rates during IFN treatment that are necessary to achieve a SVR.11 (i) a rapid phase I decline in circulating HCV RNA indicating inhibition of viral production, and (ii) a significant second phase decline in circulating HCV RNA that is steep enough to ensure complete eradication of virus from liver cells during the treatment time. Otherwise infected cells remain and rapidly restore infection after treatment is completed. PR and NR have decreased phase I slopes, resulting from sub-optimal drug dosing, and/or insufficient host response. Early modeling data with DAAs suggest that the more effective the drug, the earlier HCV RNA can be eliminated from infected cells resulting in overall shorter treatment duration.12 Understanding of DAA viral clearance kinetics will be important for drug selection for IFN-free regimens.
Classes and Mechanisms of DAA (Table 1)
Table 1.
DAA Characteristics used in Major IFN-free trials.
| Characteristic | Protease inhibitors | Nucleos(t)ide Polymerase inhibitors | Nonnucleoside Polymerase inhibitors | NS5A inhibitors |
|---|---|---|---|---|
| Potency | High; variable among genotypes | Moderate-high; consistent across genotype, subtype | Variable; variable among genotypes | High; multiple among genotypes |
| Barrier to Resistance | Low gen 1a < 1b | Very High; gen 1a = 1b | Very Low gen 1a < 1b | Low gen 1a < 1b |
| Drug Interaction Potential | High | Low | Variable | Low to moderate |
| Toxicity | Rash Anemia ↑Bilirubin | Mitochondrial Nuclear interactions (ART, RBV) | Variable | Variable |
| Pharmacokinetics | Variable; QD to TID | QD | Variable; QD to TID | QD |
| Agents approved or in late stage clinical trials |
1Telaprevir 1Boceprevir 2Simeprevir 3Faldepravir 3Asunaprevir 3ABT/450/r 3Danoprevir/r |
2Sofosbuvir 4Mericitabine 4VX-135 (ALS-2200) |
3BI-207127 3BMS-791325 3ABT-072 3ABT-333 |
3Daclatasvir 3GS-5885 3ABT-267 |
| Comments | 2nd generation PIs: better barrier, pan-genotypic | Single target active site | Allosteric; many targets | Multiple antiviral MOA |
Currently approved for use
Submitted to FDA [April, 2013]
Current phase III or in phase III planning
Current phase II
Protease Inhibitors (PI)
The NS3/4A protease complex cleaves all NS proteins from the polyprotein downstream from the NS2-3 junction. The protease domain comprises the amino-terminal third of NS3 and must be complexed with the NS4A fragment for optimal proteolytic activity and subcellular location. The catalytic active site is long and shallow as compared to other serine-activated proteases, which initially made design of PIs challenging.
The first generation PIs telaprevir and boceprevir contain ketoamide moieties that tightly “trap” the catalytic serine of the active site irreversibly.13 These genotype 1 specific drugs cannot be used as monotherapy because of rapid emergence of resistant variants and were approved only for use with IFN/RBV. Second-wave, first-generation PIs such as simeprevir, (genotypes 1, 2, 4 and 6) 14 danoprevir, (genotypes 1,4, and 6) and others 15 have improved resistance profiles in addition to expanded genotype sensitivities.
Nucleos(t)ide Polymerase Inhibitors (NI)
NIs are analogues of natural RdRp substrates and are incorporated into the RNA chain at the highly conserved enzyme active site and abruptly terminate replication. In vitro and in vivo, NIs demonstrate broad genotypic anti-viral activity with high barrier to resistance.16 Because of high resistance barrier, NIs are a prudent choice to serve as a “backbone” to IFN-free DAA regimens. NI development was initially hampered by problems with candidate drug toxicity.17, 18 However, promising drugs such as sofosbuvir are now in phase III testing with others in development (Table 1).19.
Non-nucleoside Polymerase Inhibitors (NNI)
NNIs bind to one of four allosteric sites (Thumb I and II; Palm I and II) on the polymerase, thus causing decreased enzymatic performance. These drugs generally have a low genetic barrier to resistance 16 and are genotype or subtype dependent. In spite of these shortcomings, phase II trials of NNIs in combination with other DAAs have yielded promising results as discussed below. NNIs may be an effective combination with protease inhibitors because there is no cross-resistance with common PI variants.20
NS5A Inhibitors
NS5A is a non-enzymatic RNA-binding phosphoprotein required for RNA replication and assembly of infectious particles. The protein is an attractive drug target because it has no known human homologues. In HCV replicon cells, inhibition of NS5A disrupts formation of new replication complexes.21 A number of NS5A inhibitors have been developed (Table 1) and are currently in early to mid-phase clinical testing. Daclatasvir (DCV) is the most advanced NS5A inhibitor showing activity in the picomolar range, broad genotypic sensitivity, and once daily administration. However, in spite of robust activity, 22 the drug has a low resistance barrier to subtype 1a and selected mutants have good in vitro and in vivo fitness in the presence of the compound.23
Drug pharmacology and resistance
Resistance is an ongoing concern for all DAAs because of untoward effects on treatment outcome. DAA resistance arises during treatment because there is selection pressure for viral variants with amino acid substitutions at the drug target that are less susceptible to drug inhibition .24 Recent work has shown that drug-resistant variants do not arise as new viral mutations, but preexist constitutively as minor groups within the patient's quasispecies population, generated by the error prone RdRp. The variants have reduced replicative capacity but increased drug resistance as compared to wild type virus and survive to fill the vacant replication space. Secondary mutations can theoretically restore the fitness of the resistant virus in vivo, allowing replication to reach near-baseline levels and even to persist after drug withdrawal. 24
In the host, viral resistance is influenced by three major factors.24 (i) The genetic barrier to resistance, defined as the number of amino acid substitutions needed for a viral variant to acquire full drug resistance. If only a single substitution is needed for drug resistance, then the drug is considered to have a low genetic barrier to resistance. If three or more substitutions are needed then the drug has a high genetic barrier. (ii) The in vivo fitness of the variant population, defined as its ability to survive and grow in the replicative environment. A selected resistant variant must have the capacity to propagate in order to fill in the replication space left vacant by the susceptible “wild-type“ virus during drug exposure. Thus, a highly resistant but poorly fit virus will be less clinically significant than a less resistant but fitter virus that can replicate efficiently in the presence of the drug. (iii) Drug exposure, defined as the drug concentration achieved in the relevant cellular compartment in vivo relative to the IC50-IC90/EC50-EC90s (where IC50 or 90 = 50 or 90% maximal inhibitor concentration and EC50 or 90 = 50 or 90% maximal effective concentration) of resistant variants. If drug levels achieved in vivo are far above these IC/EC values, then resistant variants will be efficiently inhibited, even if they are far less sensitive than the wild-type virus in vitro.
All of the major classes of DAAs have been shown to select resistant viral species in vitro. Clinically, PI monotherapy selects resistant viral populations within a few days or weeks, depending on the level of drug exposure. The first generation PIs, share cross-resistance in vitro with substitutions (in order of increasing in vitro resistance) that include: V36A/M/C, T54A/S, R155K/T/Q, V36A/M+R155K/T, A156V, and V36A/M+A156V/T.25, 26 Subtype 1a has a lower genetic barrier to PI resistance than does 1b. Newer second-generation PIs such as MK-5172 have pan-genotype activity and increased resistance to most viral variants that arise from first-generation PIs.27, 28
The highly conserved active site of the RdRp results in a high drug resistance barrier. Early in vitro studies showed that drug-induced mutations in the polymerase active site result in moderate loss of antiviral activity, but with a costly reduction in replicative capacity for the virus.29 Thus, mutations are seldom seen in vivo.30 In contrast, non-competitive inhibition of the polymerase by NNIs results in low barriers to resistance because viral mutations at allosteric sites are easily accommodated by the virus without dramatic loss of fitness. Fortunately, there is a general lack of cross-resistance of NNI specific for different allosteric sites.29
Viral inhibition with NS5A inhibitors leads to several resistance mutations in at least two locations on the NS5A protein. Current NS5A inhibitors in development, such as DCV and GS-5885, have a lower resistance barrier for genotype 1a than for genotype 1b.23 Multiple potential mutation sites were shown in genotype 1a patients after GS-5885 administration while genotype 1b patients showed only a single focused site.31
Although the appearance of resistance mutations during antiviral therapy always generates concern, it is not known whether mutant HCV promote greater disease activity. Resistant variants diminish after discontinuation of the failed DAA and it is not known whether these patients would be harder to treat with other DAAs. Consequently, retreatment of resistant patients with another DAA agent is reasonable, provided the new drug is not less potent and does not share cross-resistance with the initial drug. Lessons learned from HIV and recent data obtained with DAA IFN-free trials indicate that clinical resistance can be managed with prudent drug combinations.
The path to interferon freedom
Seminal phase 3 clinical trials with telaprevir and boceprevir provided the first proof of concept that DAAs, added to an IFN/RBV regimen, could increase SVR and shorten treatment duration. Either PI in combination with IFN/RBV boosted SVR rates nearly two fold in genotype 1 therapy-naïve patients after response-guided therapy.32-34 Experienced RR and NR patients also showed improved SVR with triple therapies as compared to conventional combination therapy (69-88% and 33-59% respectively). 35-37
The advent of triple therapy revealed new patient barriers further complicating an already rigorous regimen of IFN/RBV. Both telaprevir and boceprevir have low tolerability and a high pill burden contributing to suboptimal treatment adherence. Because these are additive side effects to IFN/RBV, an unacceptable percentage of patients cannot tolerate triple therapy or are simply unwilling to undergo IFN-based regimens because of poor quality of life and anticipation for IFN-free protocols.
Development of IFN-free DAA regimens
Experimental treatment of chimpanzees provided the first evidence that SVR could be achieved without IFN using a combination of NI (MK-0608), and PI (MK-7009).38 The INFORM-1 trial provided the first evidence in humans that DAAs from different drug classes can overcome drug resistance, at least in the short-term. In naïve and IFN NR patients, Gane et al, tested antiviral activities of danoprevir, a macrocyclic PI plus the NI mericitabine.19 Patients received 14 days of DAA therapy before starting IFN/RBV at intervals up to 14 days later. During this IFN-free period there was a biphasic decline of HCV RNA levels and no evidence of emergent resistance; thus opening the door for longer treatment interventions with IFN-sparing regimens.
Proof of concept that patients could achieve SVR without IFN or RBV employed the NS5A inhibitor, DCV, and a PI, asunaprevir, in 11 prior NRs 39 (table 3). Two genotype 1b patients and 2 of 9 genotype 1a patients experienced SVR after 24 weeks of treatment, which was well tolerated. Overall, 4 of 11 difficult-to-treat patients were cured with an all-oral DAA regimen, while in contrast, 10 additional subjects who received quadruple therapy with DCV and asunaprevir plus IFN/RBV all achieved SVR.39 PI resistant mutations were primarily associated with treatment failure, though the NS5A resistance variant (Q30E) was also detected at relapse. This study also documented that genotype 1 subtype can influence the outcome of DAA regimens, quite similar to phase III findings from triple therapy protocols.32, 33 Persons with genotype 1a showed inferior rates of response as compared to those with genotype 1b, due to a more frequent selection of variants bearing amino acid substitutions in both the NS5A and NS3 protease regions.
Table 3.
Two drug trial +/− RBV
| Drug and Trial name | RBV | # Patients / genotype / experience | Treatment Time (wks) | SVR/Total | % SVR | Reference |
|---|---|---|---|---|---|---|
| SOF (NI) + DCV (NS5A) | 0 | 15 / 1 / naive | SOF × one wk then SOF + DCV × 23 wks | 15/15 | 100% | 45Sulkowski et al. 2013 |
| 0 | 14 / 1 / naive | 24 | 14/14 | 100% | ||
| + | 15/ 1 / naive | 24 | 15/15 | 100% | ||
| 0 | 16 / 2,3 / naive | SOF × one wk then SOF + DCV / 23 wks | 14/16 | 88% | ||
| 0 | 14 / 2,3 / naive | 24 | 14/14 | 100% | ||
| + | 14 / 2,3 / naive | 24 | 12/14 | 86% | ||
| + | 20/1 /null | 24 | 20/20 | 100 | ||
| 0 | 21/1 /null | 24 | 21/21 | 100 | ||
| Asunaprevir (PI) + Daclatasvir (NS5A) | 0 | 2 / 1b / null | 24 | 2/2 1b ) | 100% 1b | 39Lok et al 2012 |
| 9 / 1a / null | 2/9 1a | 22% 1a | ||||
| 0 | 10 / 1b / null | 24 | 9/10 | 90 | 40Chayama 2012 | |
| 0 | 18 / 1b / null | 24 | 15/18 | 83% SVR12 | 51Lok et al 2012 | |
| Faldaprevir (PI) + B1207127 (NNI) SOUND-C2 | + | 314 / 1 / naive | 16 | 48/73 | 66% | 52Zeuzem et al 2012 |
| + | 28 | 47/67 | 70% | |||
| + | 40 | 40/58 | 69% | |||
| + | 28 | 54/75 | 72% | |||
| 0 | 28 | 18/41 | 44% | |||
| ABT 450/R (PI) + ABT-333 (NNI) CO-PILOT | + | 19 / 1 / naïve [250 ABT-450] | 12 | 18/19 | 95% | 47Poordad et al. 2013 |
| 14 / 1 / naïve [150 ABT-450] | 13/14 | 93% | ||||
| 17 / 1 / null [150 ABT-450 | 8/17 | 47% | ||||
| ABT 450R (PI) + ABT-072 (NNI) PILOT | + | 11/ 1 / naïve | 12 | 10/11 | 91% | 53Lawitz et al 2012 |
| Telaprevir (PI) + VX-222 (NNI) +/− IFN/RBV ZENITH | + | 23 / 1a / naïve | 12 | 5/5 1ER | 100% | 48Jacobson et al. 2012 |
| + | 23 / 1b / naive | 12 | 4/6 ER | 67% |
ER = Early responders [patients without detectable virus at 2 or 8 weeks after start of DAA only regimen treated with oral therapy only 48]
Further support for the efficacy of oral DCV/asunaprevir came from a Japanese study of 10 prior NRs infected with genotype 1b.40 SVR with 24 weeks of treatment even without RBV was 90%, suggesting that combination of two potent DAA drugs (one with a low barrier to resistance, the other one with a high barrier for this subtype) can suffice to produce SVR (table 3).
With the initial proof of concept that HCV can be successfully treated without interferon, clinical research on a global scale has focused on DAA treatment parameters for optimal responses. Successful strategies include combinations of different classes of DAAs that have sufficient antiviral potency with non-overlapping resistance profiles. Whether it is advantageous to have one drug with a high barrier to viral resistance remains unsettled. IFN-free studies are best understood if viewed from the perspective of one, two, or three drug combinations used together with or without RBV (Tables 2, 3 and 4, respectively).
Table 2.
One drug trials +/− RBV
| Drug and Trial name | RBV | # Patients / genotype / experience | Treatment Time (wks) | 2SVR/Total | SVR (%) | Reference |
|---|---|---|---|---|---|---|
| Phase II | ||||||
| 1SOF ELECTRON | + | 10 / 2,3 / naive | 12 | 10/10 | 100% | 41Gane et al 2013 |
| 0 | 10 / 2,3 / naive | 12 | 6/10 | 60% | ||
| + | 25 / 1 / naive | 12 | 22/25 | 88% | ||
| + | 10 / 1 / null | 12 | 1/10 | 10% | ||
| SOF SPARE | Full: 1.2g or Half: 0.6g daily |
60 / 1 / naive [82% AA/ 72% geno 1a] | 24 | 10/10 Full RBV | 100% | 42Osinusi et al 2013 and CROI 2013 Abstract #157LB |
| 17/24 Full RBV | 68% SVR12 | |||||
| 12/22 half RBV | 48% SVR12 | |||||
| Phase III | ||||||
| SOF POSITRON | + | 207 / 2,3 / naive | 12 | 161/207 | 78% SVR12 | 43Jacobson et al. 2013 |
| SOF FISSON | + | 256 / 2,3 / naive | 12 | 170/253 | 67% SVR12 | 44Lawitz et al. 2013 |
| SOF FUSION | + | 103 / 2,3 / null | 12 | 50/100 | 50% SVR12 | 43Jacobson et al 2013 |
| 98 / 2, 3 / null | 16 | 69/95 | 73% SVR12 | |||
SOF = sofosbuvir
SVR = SVR at 24 wks unless indicated otherwise.
Table 4.
Three drug trials +/− RBV
| Drug and Trial name | RBV | Patients (genotype 11) | Time (wks) | SVR12/ # patients2 | (%) SVR | Reference | |
|---|---|---|---|---|---|---|---|
| SVR12 | SVR24 | ||||||
| ABT 450/r (PI) ABT 267 (NS5A) ABT 333 (NNI) AVIATOR. | Naïve | 49Kowdley et al. 2013 | |||||
| + | 80 | 8 | 71/80 | 89% | 88% | ||
| + | 79 | 12 | 78/79 | 99% | 96% | ||
| 0 | 79 | 12 | 71/79 | 90% | 87% | ||
| + No ABT267 | 41 | 12 | 35/41 | 85% | 83% | ||
| + No ABT333 | 79 | 12 | 72/79 | 91% | 89% | ||
| + | 80 | 24 | 74/80 | 93% | 90% | ||
| Prior Null responders | |||||||
| + | 45 | 12 | 42/45 | 93% | 93% | ||
| + No ABT333 | 45 | 12 | 39/44 | 89% | 89% | ||
| + | 43 | 24 | 23/24 | 98% | 95% | ||
| Drug and Trial name | RBV | Patients/genotype/experience | Time (wks) | SVR/#pts | SVR | Reference | |
| DCV (NS5A) Asunaprevir (PI) BMS-791325 (NNI) | 0 | 16 /1 /naïve 75mg BMS 791325 | 24 | 15/16 | 94% SVR24 | 50Everson et al. 2013 | |
| 16 /1 / naïve 75 mg BMS-791325 | 12 | 15/16 | 94% SVR36 | ||||
| 16/ 1 /naïve 150 mg BMS-791325 | 24 | 15/16 | 94% SVR4 | ||||
| 18/1 /naïve 150 mg BMS-791325 | 12 | 16/18 | 8 9% SVR12 | ||||
Overall, 66% patients were genotype 1a and within groups, 1a and 1b well-mixed.
Patients dosed with drug.
One DAA with or without RBV
Studies of a single NI given alone or in combination with RBV (ELECTRON) have provided exciting IFN-free treatment data (Table 2). 35 naïve patients were treated with once daily sofosbuvir (SOF) and RBV for 12 weeks without IFN.41 Ten genotype 2/3 and 25 genotype 1 patients achieved SVR rates of 100% and 84% respectively. The high percentage SVR in the genotype 1 patients occurred in spite of the fact that 88% were subtype 1a and over half had unfavorable IL28B T allele.
Another 10 genotype 2/3 naïve patients were treated with SOF monotherapy and 6 achieved SVR. In contrast, only 1 of 10 genotype 1 prior NRs achieved SVR and treatment failure was entirely due to viral relapse since all patients cleared virus on therapy.41 80% of relapsers had unfavorable IL28B alleles and 90% were viral subtype 1a.
Although the significance of ELECTRON is limited due to small sample sizes, the study made it clear that a potent NI in combination with RBV can produce SVR without IFN. These results also emphasized the importance of RBV, since only 6/10 genotype 2/3 infected persons achieved SVR without RBV as compared to 10/10 with RBV. ELECTRON also showed that retreating prior NRs with IFN-free regimen can still result in a high viral relapse rate in spite of the fact that most patients cleared virus on oral therapy. This implies that longer treatment durations may be more efficacious in these patients.41
In the SPARE trial, SOF in combination with full or half dosage RBV was given to 60 “hard to treat” naïve patients for 24 weeks.42 The majority of subjects (70%) were viral subtype 1a, male (62%), AA (83%), non-CC IL28 genotype (80%), BMI > 30 (48%), and nearly two thirds had advanced liver disease (stage F3 or F4) with high viral loads. In the first group, 10 patients with early hepatic fibrosis scores were given SOF with full dosage ribavirin (RBV). In the second group, 50 patients with all stages of fibrosis received SOF with only half dosage RBV. All patients cleared virus by end of treatment and 100% of patients in the first group, achieved SVR12. In the second group, 17/24 (68%) and 12/22 ( 48%) of those patients receiving high and low dosage RBV respectively achieved SVR12. These findings indicated that important variables for IFN-based therapy, such as fibrosis scores and ribavirin, are also important for DAA only protocols. Whether known negative treatment parameters can be overcome with more complicated multi-drug regimens remains to be shown in “hard to treat” patients.
Results of three important phase III studies of SOF in combination with RBV in genotype 2,3 patients recently were recently reported (Table 2). POSITRON tested whether SOF/RBV could be successfully used in a difficult population of patients who were unwilling to take, or intolerant to IFN. After 12 weeks of SOF/RBV, 78% (161/207) achieved SVR12 with no SAEs and a benign side effect profile as compared to IFN/RBV therapy.43
In FISSION, naïve patients were randomized to receive either 24 weeks of IFN/RBV (n = 243) or 12 weeks of SOF/RBV (n = 256) to directly compare both regimens. Both groups achieved an identical SVR rate of 67% indicating that the all oral regimen is not inferior to the current standard of care for genotype 2/3 patients.44
In FUSION, the length of treatment of SOF/RBV was compared between two groups of patients who were prior RR to INF/RBV. Oral therapy was given for 12 (n = 103) or 16 (n = 98) weeks (Table 2). Patients treated 16 weeks (n = 98) showed higher rate of SVR (73%) as compared to those treated 12 weeks (50%) (n = 103).43 In all the phase III studies, adverse drug events have been relatively minor and far fewer patients discontinued treatment because of an adverse event of SOF/RBV as compared to IFN/RBV. All in all, POSITRON, FISSION and FUSION are key phase III trials that hopefully will facilitate approval of SOF in 2013.
Two DAAs with or without RBV
Early two-drug DAA protocols suggest that it is advantageous to have one DAA with a high resistance barrier (Table 3). In a recent combination of SOF with the NS5A inhibitor DCV, three groups each of naïve genotype 1 or genotype 2/3 patients were given 23-24 weeks of both DAAs with or without RBV. In subtype 1a/1b and genotype 2,3 patients SVR was 100% and 86-100% respectively.45 Although there were small patient numbers in each group, there were no significant differences in treatment outcomes due to RBV, genotype or other parameters. A later “add-on” of two groups of genotype 1, prior NRs, treated with SOF + DCV, also showed 100% SVR regardless of ribavirin.45 Although direct comparisons with other DAA combinations and “hard to treat” populations are needed, it appears that a NI and NS5A inhibitor is a potent treatment combination.
SOUND-C2 evaluated a second wave, first-generation PI, faldaprevir (BI201335) and a NNI, BI207127, with or without RBV.46 This study included 314 genotype 1 naïve patients who were treated for 16-24 weeks. While there was no difference in SVR rates in groups as a function of treatment length, there was a marked effect of RBV on SVR (69% vs 44% with and without RBV, respectively). This study also documented differences in response patterns according to subtype 1a and 1b as well as IL28B allele status. Response rates in patients with subtype 1a were influenced by IL28B genotype with CC patients demonstrating significantly higher SVRs than those having T allele. In contrast, subtype 1b subjects showed high SVR rates irrespective of IL28B genotype. These data are in contrast to those of SOF and DCV noted above which showed no effect of RBV, IL28B genotype, or 1a/1b subtype on outcome. The CO-PILOT and PILOT phase II studies separately assessed the effects of two different NNIs combined with the same PI on HCV genotype 1 patients.47CO-PILOT included 50 patients of which 33 naïve subjects were randomized to two different daily dosages of the PI ABT-450/r (ritonavir boosted) together with the NNI, ABT-333 and RBV. Seventeen treatment experienced patients also received ABT-450/r, ABT-333 and RBV. A majority of both naïve and NR patients were subtype 1a (table 3). While SVR12 was achieved in 94% of treatment-naïve patients at either dosage of ABT-450 only 47% of prior NRs achieved SVR, most likely because all NRs contained the IL28B T haplotype. In the PILOT study ABT-450/r and the NNI ABT-072 plus RBV were given to 11 treatment naïve genotype 1 patients (8 = 1a), all with IL28B CC, and 9 of 11 achieved SVR24.
Another all-oral two DAA combination plus RBV was tested in the ZENITH trial.48 Telaprevir and the NNI VX-222 together with RBV were given to genotype 1a or 1b naïve patients for 12 weeks, and if HCV was detectable at 2 or 8 weeks after start of therapy, patients were given an additional 24 weeks of IFN/RBV. Of those patients with no detectable virus at 2 or 8 weeks, (11 of 46 total patients treated), 9 (82%) went on to achieve SVR12. In spite of the fact that early responders represented only 24% of all the patients treated in the study, the data also verified that early viral clearance is a useful indicator for eventual SVR.
Finally, considering all of the two DAA +/− RBV trials (Table 3), there was a noticeable performance difference between regimens containing a high resistance barrier NI, as compared to other combinations, although the numbers of patients treated were small. This was especially apparent for prior NR and “hard to treat” patient cohorts. Whether this high performance is a NI class effect will need clarification.
Three DAAs with or without RBV
The encouraging results of the CO-PILOT and PILOT studies gave rise to the phase II study, AVIATOR, the most comprehensive three DAA trial to date (Table 4).49 The PI ABT-450/r was combined with the NS5A inhibitor ABT-267 and the NNI ABT-333 with and without RBV for durations of 8, 12, or 24 weeks. The large study included 438 treatment naïve and 133 prior NRs of which 66% had genotype1a infection and over 70% were non-CC IL28B. SVR12 and SVR24 rates were comparable and all were 90% or greater for naïve as well as prior NRs for patients receiving all four drugs for 12 or 24 weeks. Furthermore, there was little, if any differences in SVR rates when data were tested for variables of RBV use, non-CC IL28B genotype or viral 1a subtype in either null or treatment naïve groups.
A similar three DAA combination showed as impressive SVR rates as the AVIATOR study, but was conducted without RBV.50 Asunaprevir, the NNI BMS-791325, and DCV were given to two groups of 16 genotype 1 patients for 24 or 12 weeks. The patient population was well-represented for difficult to treat IL28B non-CC genotypes (72%), AA (25%) and a majority were subtype 1a. SVR24 was 94% for those receiving 12 weeks of therapy. Two additional groups of genotype 1 patients were treated for 24 or 12 weeks with twice the dosage of BMS-791325 and achieved 94% SVR4 and 89% SVR12 respectively.50 In both three–drug studies, combinations were well-tolerated with only minor constitutional side effects and no serious adverse events. Altogether, the triple DAA combination trials clearly demonstrated that multiple drugs can potentially overcome the necessity for RBV, as well as the adverse impact of unfavorable parameters such as IL28B non-C allele, subtype 1a, and prior NR on the likelihood of SVR.
Persistent NRs and special populations of patients
Even with new, impressive IFN-free regimens treatment of groups such as NRs, cirrhotics, HIV co-infections, and the immunosuppressed will remain challenging. Randomized testing of DAAs in these groups as compared to “uncomplicated” patients will likely follow initial approval of DAA regimens. Another point is whether patients with IFN insensitivity, clearly important for IFN combinations, will remain a significant factor for DAA only regimens. Some DAAs, such as PIs can restore endogenous IFN signaling pathways that are impaired by viral proteins such as NS3/4A and restore IFN sensitivity.5 Restoration of IFN sensitivity with DAAs might facilitate resolution of HCV acute infection, immunological based therapies, and immunization.
Epidemiological and clinical impact
The most obvious beneficiaries of IFN sparing regimens are persons who are intolerant or insensitive to IFN and available information suggests the impact will be considerable. In addition, as HCV treatment becomes safer and less complicated it will change patient and provider perceptions of disease leading to expanded testing. Simpler regimens will allow treatment in unconventional settings such as public health clinics or developing countries. The degree to which these trends occur will determine the net impact of IFN-free treatments worldwide.
Conclusions
With the advent of oral regimens that obviate IFN, it is clear that we have entered the final, long awaited chapter of HCV therapeutics. IFN-free therapy is a major milestone in liver disease that not only eliminates the IFN side effects barrier but it expands patient feasibility and eligibility for treatment. We can anticipate a number of new DAA combinations in the coming years that will grow from the present exciting preliminary data and lead to true interferon independence.
Acknowledgments
Research Support
Supported by Merit Review grant from the Veterans Affairs BX000159 and the Doriann Foundation for hepatitis research, University of Iowa (WNS). K24 DK070528 (KES). NIH DK78772, DA33541 (RTC). NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 R000064. (DRN).
List of abbreviations
- DAA
direct acting antiviral agent
- DCV
daclatasvir
- HCV
hepatitis C virus
- HTA
host targeted agent
- IFN
Interferon
- ISG
Interferon stimulated genes
- LOD
Limit of detection
- PR
partial responder
- Peg/INF
Pegylated interferon
- PI
protease inhibitor
- NR
null responder
- NI
nucleoside inhibitor
- NNI
non-nucleoside inhibitor
- RR
responder relapser
- RBV
ribavirin
- RdRp
RNA dependent RNA polymerase
- SOF
sofosbuvir
- SVR
sustained virological response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Warren Schmidt: study design and concept, data aquisition and interpretation, manuscript draft and revisions.
David Nelson: study concept, manuscript analysis and intellectual content, manuscript revision.
Jean-Michel Pawlotsky: study concept, manuscript analysis and intellectual content, manuscript revision.
Kenneth Sherman: study concept, manuscript analysis and intellectual content, manuscript revision.
David L. Thomas: study concept, manuscript analysis and intellectual content, manuscript revision.
Raymond T. Chung: study design and concept, data aquisition and interpretation, and manuscript revisions.
Presented in part as summary for American Association for the Study of Liver Disease, Single Topic Conference on Direct Acting Antiviral Agents, Atlanta GA March 15-16, 2012.
Conflict of Interest.
Warren N. Schmidt has served as advisor for Gilead and Merck David R. Nelson has received research grant support from Abbott, BI, BMS, Genentech, Gilead, Merck, Vertex
Jean-Michel Pawlotsky has received research grants from Gilead. He has served as an advisor for Abbott, Abbvie, Achillion, Anadys, Biotica, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, Idenix, Inhibitex, Janssen-Cilag, Madaus-Rottapharm, Merck, Novartis, Pfizer, and Roche.
Kenneth E. Sherman (within 12 months): Research support (to institution) from Anadys, Abbott, BMS, Boehringer-Ingelheim, Genentech, Gilead, Merck, Novartis, Vertex and Advisory Board/Consultant to Janssen (Tibotec), Merck, Roche Molecular Systems, Kadmon, MedPace David L. Thomas has received research grants from Merck and Gilead. He has served as an advisor for Gilead, Merck and Jansen.
Raymond T. Chung has received research support from Gilead, Vertex, Merck, and Mass Biologics. He has served as a consultant to Enanta and Idenix.
References
- 1.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–8. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575–8. doi: 10.1056/NEJM198612183152503. [DOI] [PubMed] [Google Scholar]
- 3.Rosen HR. Chronic Hepatitis C Infection. New England Journal of Medicine. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 4.Sarasin-Filipowicz M, Oakeley EJ, Duong FHT, et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285:22741–7. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B Polymorphism Improves Viral Kinetics and Is the Strongest Pretreatment Predictor of Sustained Virologic Response in Genotype 1 Hepatitis C Virus. Gastroenterology. 2010;139:120–U178. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Urban TJ, Thompson AJ, Bradrick SS, et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010;52:1888–96. doi: 10.1002/hep.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann E, Lee JH, Marinos G, et al. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology. 2003;37:1351–1358. doi: 10.1053/jhep.2003.50218. [DOI] [PubMed] [Google Scholar]
- 9.Jain MK, Zoellner C. Role of ribavirin in HCV treatment response: now and in the future. Expert Opin Pharmacother. 2010;11:673–83. doi: 10.1517/14656560903580001. [DOI] [PubMed] [Google Scholar]
- 10.Martinot-Peignoux M, Stern C, Maylin S, et al. Twelve Weeks Posttreatment Follow-up Is as Relevant as 24 Weeks to Determine the Sustained Virologic Response in Patients with Hepatitis C Virus Receiving Pegylated Interferon and Ribavirin. Hepatology. 2010;51:1122–1126. doi: 10.1002/hep.23444. [DOI] [PubMed] [Google Scholar]
- 11.Dahari H, Guedj J, Perelson AS, et al. Hepatitis C Viral Kinetics in the Era of Direct Acting Antiviral Agents and IL28B. Curr Hepat Rep. 2011;10:214–227. doi: 10.1007/s11901-011-0101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adiwijaya BS, Hare B, Caron PR, et al. Rapid decrease of wild-type hepatitis C virus on telaprevir treatment. Antiviral Therapy. 2009;14:591–5. [PubMed] [Google Scholar]
- 13.Njoroge FG, Chen KX, Shih NY, et al. Challenges in modern drug discovery: A case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection. Accounts of Chemical Research. 2008;41:50–59. doi: 10.1021/ar700109k. [DOI] [PubMed] [Google Scholar]
- 14.Moreno C, Berg T, Tanwandee T, et al. Antiviral activity of TMC435 monotherapy in patients infected with HCV genotypes 2-6: TMC435-C202, a phase ha, open-label study. Journal of Hepatology. 2012;56:1247–1253. doi: 10.1016/j.jhep.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Everson G, Cooper C, Hezode C, et al. Rapid and Sustained Achievement of Undetectable Hcv Rna during Treatment with Ritonavir-Boosted Danoprevir/Peg-Ifn Alpha-2a/Rbv in Hcv Genotype 1 or 4 Patients: Dauphine Week 12 Interim Analysis. Journal of Hepatology. 2012;56:S466–S466. [Google Scholar]
- 16.Sarrazin C, Zeuzem S. Resistance to Direct Antiviral Agents in Patients With Hepatitis C Virus Infection. Gastroenterology. 2010;138:447–462. doi: 10.1053/j.gastro.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 17.Nelson DR, Zeuzem S, Andreone P, et al. Balapiravir plus peginterferon alfa-2a (40KD)/ribavirin in a randomized trial of hepatitis C genotype 1 patients. Annals of Hepatology. 2012;11:15–31. [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien C, Godofsky E, Rodriguez-Torres M, et al. Randomized trial of valopicitabine (NM283), alone or with peg-interferon, vs. retreatment with peg-interferon plus ribavirin (pegifn/RBV) in hepatitis C patients with previous non-response to pegifn/RBV: First interim results. Hepatology. 2005;42:234a–234a. [Google Scholar]
- 19.Gane EJ, Roberts SK, Stedman CAM, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467–1475. doi: 10.1016/S0140-6736(10)61384-0. [DOI] [PubMed] [Google Scholar]
- 20.Susser S, Welsch C, Wang YL, et al. Characterization of Resistance to the Protease Inhibitor Boceprevir in Hepatitis C Virus-Infected Patients. Hepatology. 2009;50:1709–1718. doi: 10.1002/hep.23192. [DOI] [PubMed] [Google Scholar]
- 21.Targett-Adams P, Graham EJ, Middleton J, et al. Small molecules targeting hepatitis C virus-encoded NS5A cause subcellular redistribution of their target: insights into compound modes of action. Journal of Virology. 2011;85:6353–68. doi: 10.1128/JVI.00215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–U108. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CF, Huang HC, Valera L, et al. Hepatitis C Virus RNA Elimination and Development of Resistance in Replicon Cells Treated with BMS-790052. Antimicrobial Agents and Chemotherapy. 2012;56:1350–1358. doi: 10.1128/AAC.05977-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlotsky JM. Treatment Failure and Resistance with Direct-Acting Antiviral Drugs Against Hepatitis C Virus. Hepatology. 2011;53:1742–1751. doi: 10.1002/hep.24262. [DOI] [PubMed] [Google Scholar]
- 25.Kieffer TL, Sarrazin C, Miller JS, et al. Telaprevir and pegylated interferon-alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology. 2007;46:631–639. doi: 10.1002/hep.21781. [DOI] [PubMed] [Google Scholar]
- 26.Sarrazin C, Kieffer TL, Bartels D, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767–1777. doi: 10.1053/j.gastro.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Brainard DM, Petry A, Van Dyck K, et al. Safety and Antiviral Activity of Mk-5172, a Novel Hcv N53/4a Protease Inhibitor with Potent Activity against Known Resistance Mutants, in Genotype 1 and 3 Hcv-Infected Patients. Hepatology. 2010;52:706a–707a. [Google Scholar]
- 28.Clark VC, Peter JA, Nelson DR. New therapeutic strategies in HCV: second-generation protease inhibitors. Liver Int. 2013;33(Suppl 1):80–4. doi: 10.1111/liv.12061. [DOI] [PubMed] [Google Scholar]
- 29.Powdrill MH, Bernatchez JA, Gotte M. Inhibitors of the Hepatitis C Virus RNA-Dependent RNA Polymerase NS5B. Viruses. 2010;2:2169–95. doi: 10.3390/v2102169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll SS, Ludmerer S, Handt L, et al. Robust Antiviral Efficacy upon Administration of a Nucleoside Analog to Hepatitis C Virus-Infected Chimpanzees. Antimicrob Agents Chemother. 2009;53:926–934. doi: 10.1128/AAC.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawitz EJ, Gruener D, Hill JM, et al. A phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. Journal of Hepatology. 2012;57:24–31. doi: 10.1016/j.jhep.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 33.Poordad F, McCone J, Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–24. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 36.Muir AJ, Poordad FF, McHutchison JG, et al. Retreatment with telaprevir combination therapy in hepatitis C patients with well-characterized prior treatment response. Hepatology. 2011;54:1538–46. doi: 10.1002/hep.24549. [DOI] [PubMed] [Google Scholar]
- 37.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen DB, Davies ME, Handt L, et al. Sustained viral response in a hepatitis C virus- infected chimpanzee via a combination of direct-acting antiviral agents. Antimicrob Agents Chemother. 2011;55:937–9. doi: 10.1128/AAC.00990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–24. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 40.Chayama K, Takahashi S, Toyota J, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–748. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 41.Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 42.Osinusi A, Heytens L, Lee YJ, et al. High Efficacy Of GS-7977 In Combination With Low or Full dose Ribavirin for 24 weeks In Difficult To Treat HCV Infected Genotype 1 Patients : Interim Analysis From The SPARE Trial. Hepatology. 2012;56:1518–1518. [Google Scholar]
- 43.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 44.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. N Engl J Med. 2013 doi: 10.1056/NEJMc1307641. [DOI] [PubMed] [Google Scholar]
- 45.Sulkowski MS, Gardiner D,F, Rodriguez-Torres M, et al. Sustained virological response with daclatasvir plus sofosbuvir+/− ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC). Journal of Hepatology. 2013;58:S567–S577. [Google Scholar]
- 46.Zeuzem S, Soriano V, Asselah T, et al. Svr4 and Svr12 with an Interferon-Free Regimen of Bi201335 and Bi207127, +/− Ribavirin, in Treatment-Naive Patients with Chronic Genotype-1 Hcv Infection: Interim Results of Sound-C2. Journal of Hepatology. 2012;56:S45–S45. [Google Scholar]
- 47.Poordad F, Lawitz E, Kowdley KV, et al. Exploratory Study of Oral Combination Antiviral Therapy for Hepatitis C. New England Journal of Medicine. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson IM, Sulkowski MS, Gane EJ, et al. VX-222, Telaprevir and Ribavirin in Treatment-Naive Patients with Genotype 1 Chronic Hepatitis C: Results of the ZENITH Study Interferon-Free Regimen. Hepatology. 2012;56:308a–308a. [Google Scholar]
- 49.Kowdley KV LE, Poordad F, et al. Safety and efficacy of interferon-free regimens of ABT-450/r, ABT-267, ABT-333 +/− ribavirin in patients with chronic HCV GT1 infection: Results from the AVIATOR study. Journal of Hepatology. 2013;58:S2. [Google Scholar]
- 50.Everson G, Sims KD, Rodriguez-Torres M, et al. Interim analysis of an interferon (IFN)- and ribavirin (RBV)-free regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 in treatment-naive, hepatitis C virus genotype 1-infected patients. Journal of Hepatology. 2013;58:S567–S577. [Google Scholar]
- 51.Lok AS, Gardiner DF, Hezode C, et al. Sustained Virologic Response in Chronic HCV Genotype (GT) 1-Infected Null Responders With Combination of Daclatasvir (DCV; NS5A Inhibitor) and Asunaprevir (ASV; NS3 Inhibitor) With or Without Peginterferon Alfa-2a/Ribavirin (PEG/RBV). Hepatology. 2012;56:230a–231a. [Google Scholar]
- 52.Zeuzem S, Soriano V, Asselah T, et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor BI 201335 and the non-nucleoside NS5B inhibitor BI 207127 +/− ribavirin (R): Final results of SOUND-C2 and predictors of response. Hepatology. 2012;56:308a–309a. [Google Scholar]
- 53.Lawitz E, Poordad F, Kowdley KV, et al. A 12-Week Interferon-Free Regimen of Abt-450/R, Abt-072, and Ribavirin Was Well Tolerated and Achieved Sustained Virologic Response in 91% Treatment-Naive Hcv Il28b-Cc Genotype-1-Infected Subjects. Journal of Hepatology. 2012;56:S7–S7. [Google Scholar]