1 Introduction
Kinesin and dynein walking on microtubules are the two main drivers of long-distance intracellular transport in a huge variety of systems, from neurons to melanophores. These motors, however, are oppositely directed, with (most) kinesin driving cargos towards the plus-ends of microtubules, while dynein drives cargos towards the minus-ends.1 There are only two types of dynein, cytoplasmic and axonemal, with only cytoplasmic dynein being used for organelle transport.2 In this review, when we use the term dynein, we are referring to cytoplasmic dynein. Dynein is generally associated with a large multi-subunit complex, dynactin, in vivo, which appears to be necessary for many types of transport.3 Kinesins make up a large family of motors involved in organelle transport, ranging from conventional kinesin (kinesin-1), which is a typical processive, plusended directed kinesin, to NCD, a non-processive, minus-end directed kinesin.4 In addition to dynein and kinesin, there is a third motor, myosin, which walks on actin. Oftentimes, myosin is also present on the cargo and the cargo is made to switch between microtubules and actin; the latter is often for final placement of the cargo.5
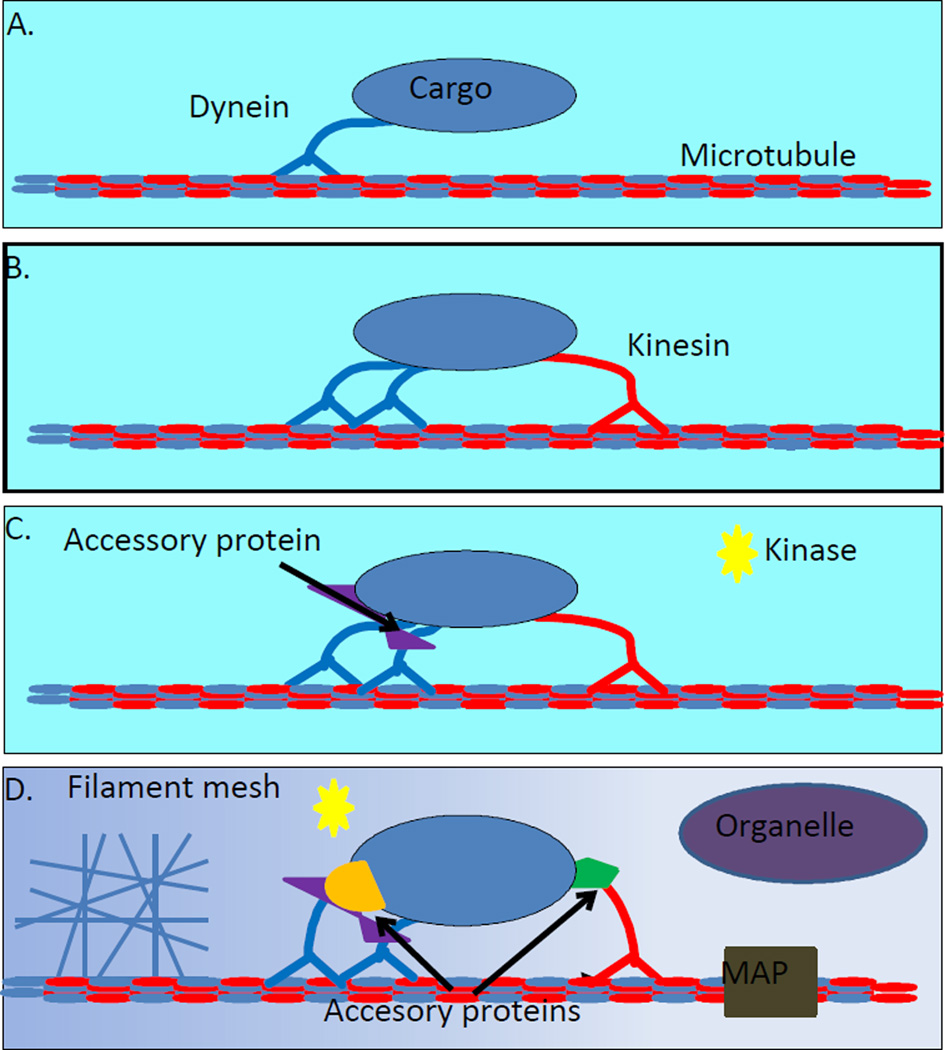
In this review we will describe experimental systems at multiple levels of complexity, including: single motor type in vitro assays, multimotor in vitro assays, purified organelle in vitro assays, and finally, in vivo cellular assays (Fig. 1). This spread of experiments allows an unprecedented view of the transport complex, as kinesin and dynein can be observed with differing components of the transport complex, i.e., different levels of accessory proteins, and in different environments. By combining measurements at all these levels of complexity, the ability to parse out the function of parts of the transport complex, and reconstitute it in vitro, becomes a real possibility.
Figure 1.
Molecular motor interactions at different levels of complexity. A. The simplest level of complexity is a single motor with a cargo or label attached, and a microtubule track, in an in vitro environment. This has been the predominant type of experiment in the study of molecular motors. It has revealed their stepping behavior, stall force, and other characteristics. However it has little to say about motor-motor interactions. B. Complexity can be increased by adding extra motors, either multiple kinesins, dyneins or kinesin-and-dynein. This is the most basic way to study motor-motor interactions, and has been used to study the cooperativity of groups motors. Knowing the absolute number of each motor can be difficult. C. Adding in accessory proteins and parts of the transport complex, like dynactin, is the next level of complexity. How accessory proteins and signaling molecules (like cAMP or a kinase) modulate kinesin-dynein interactions can be studied in this system. D. The living cell is the most complex system in which to study motor-motor interactions. Cellular gradients, accessory proteins, Microtubule Associated Proteins (MAPs), organelles, and filament meshes are just a few of the things present that could affect transport. This complexity makes it very difficult to isolate specific causes of transport behavior, but also allows the study of motor-motor interactions in their native settings.
In addition, new techniques, from in vivo optical trapping, to high-resolution imaging, will be discussed. They allow the detailed examination of all these systems in multiple domains: force, orientation, position, and velocity, amongst others. These techniques will allow the development and testing of the theoretical models that describe intracellular transport and multimotor interactions. The paper is organized such, that after reading the first section for an overview, each section can be read more-or-less independently.
1.1 Kinesin & Dynein Interaction: Tug-of-War vs. Coordinated Model
An initial question is why are multimotor models needed? After all, a single motor type is all that is needed for transport in one direction. Most motors appear to be recruited to cargos by specific binding factors, so the cell can control the presence of motors on a specific cargo.6 However, it is known that in many systems, both kinesins and dyneins are simultaneously present on cargo.7 Oftentimes, seemingly erratic up-and-back behavior is observed.8 How multiple motors, and different motor-types, interact and are regulated is fundamental to understanding intracellular transport. (For excellent reviews covering intracellular transport, see 1,5,9.)
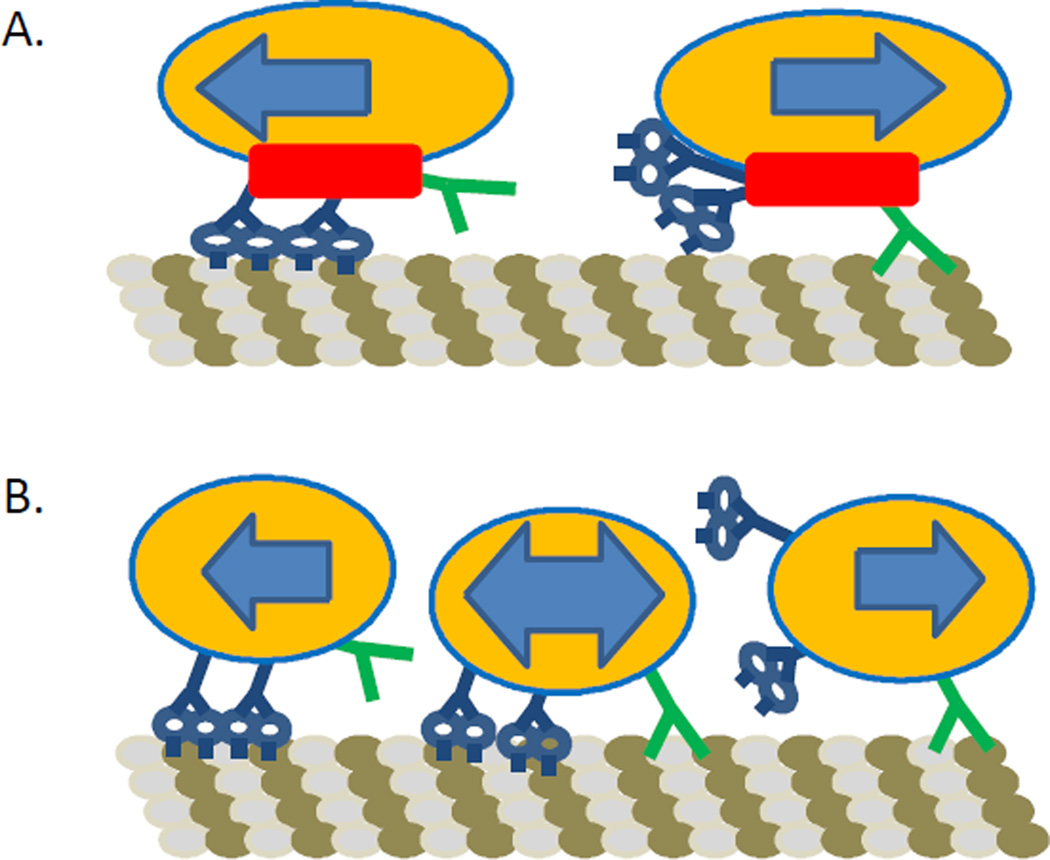
There is currently a wide array of models describing the interaction between kinesin and dynein. In this paper, the term interaction will mean any interplay between kinesin and dynein dynamics, such as through a cargo; not necessarily a direct, physical interaction. These models typically fall into two main categories: coordinated motion, which involves a secondary protein or complex that controls the states of kinesin and dynein, regulating their activity and determining the cargo’s directionality on the microtubule; and tug-of-war, which postulates that kinesin and dynein interact directly through force transductions via the cargo that determine directionality (Fig. 2). Historically the definitions of coordinated vs. tug-of war have varied somewhat.1a Today, however, there is general agreement. Coordinated motion typically involves only one particular type of motor being active at any time (kinesin or dynein). Tug-of-war models have several possible states, e.g. both motor(s) are pulling and the one that is pulling with more force wins out. Another tug-of-war scenario can have the “losing” motor come off the microtubule, or stay bound but walk or diffuse backward. It is possible that which set of motors “wins” depends on the particular number of the motors pulling, and that number may be regulated. In this paper, determination of cargo directionality by strain sensitivity is the definition of tug-of-war we will use. A tug-of-war can lead to stalling (e.g. yeast dynein and mammalian kinesin, as we will see), inefficient motility, or highly efficient motility (mammalian kinesin and mammalian dynein) depending on motor properties.10 Coordinated motion would be any other type of regulatory mechanism of cargo directionality that prevents motors from being simultaneously active (the existence of some external “coordinator” outside the motors and cargo). Higher order mechanisms could exist that modulate both of these models.
Figure 2.
Models of kinesin and dynein interaction. A. Coordination complex model. In this model there exists a complex that regulates kinesin and dynein’s activity such that they never interfere with each other. The complex turns on kinesin while keeping dynein off or vice versa. The complex is visualized here as a secondary protein with both motors attached, but could be a signaling molecule or other factor that activates and deactivates the motors. Under this model kinesin and dynein should never interfere with each other or be simultaneously active. B. The tug-of-war model. This model postulates that there is no external control of the motors. They regulate themselves and each other by force transduction through the cargo. Both motors can be active simultaneously and interfere with each other. Directionality is determined by the different motors interacting by force transduction through the cargo.
For many years, a coordinated model was popular because a tug-of-war model seemed unable to explain organelle motility. This was due to the fact that if both dynein and kinesin were active simultaneously, one would expect the organelle to stall (not move) quite often. Whenever both motors became active, the organelle would stop, and only restart when only either kinesin or dynein remained active. Such a scenario assumed that simultaneously active kinesin and dynein would act as anchors against one another. This sort of behavior can be seen in experimental systems with yeast dynein and mammalian kinesin.11 However, this particular case is an artificial system: yeast and mammalian motors are not designed to work together.
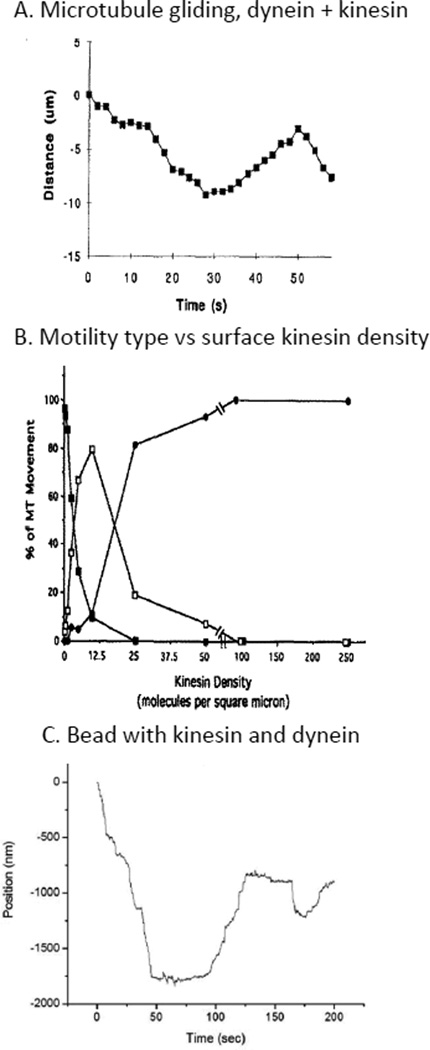
Recent theoretical arguments,10b,c and several experiments7d,12 have shown that a tug-of-war can lead to motility without constant stalling and with efficient directional switching. Vale et al., in 1992, for example, showed that attaching kinesin and dynein to a surface and laying down microtubules on them caused bidirectional gliding of the microtubules with reversals in direction occurring routinely (Fig. 3A-B).12c In a somewhat different arrangement, Blehm et al. and Deberg et al. attached kinesin and dynein to a polystyrene bead and watched it walk on a microtubule. They showed similar reversals and bidirectional motion, with saltations and directional switching (Fig. 3C).12a,13 The fact that directional switching can be seen in both these systems without any external coordinating complex is strong support for a local tug-of-war, although it is difficult to say how similar other transport properties are between in vitro and in vivo systems.
Figure 3.
In vitro bidirectional motion. A. Trace of a microtubule’s position while gliding on a surface coated with mammalian kinesin and mammalian dynein. Adapted with permission from ref.12c. Copyright 1992 The Rockefeller University Press. B. Polarity of microtubule gliding is dependent on kinesin surface density in microtubule gliding assays. The black circles indicate kinesin direction; black squares indicate dynein direction; clear squares indicate bidirectional motion. Microtubules observed either more than a minute, or greater than 30 microns were scored. Only within a specific range of kinesin densities can bidirectional motion be seen. Adapted with permission from ref.12c. Copyright 1992 The Rockefeller University Press. C. 500 nm fluorescent beads coated with kinesin and dynein, displayed bidirectional, saltatory motion in vitro, if the correct ratio of dynein and kinesin was used.13 The plus-end of the microtubule was determined by observing a GFP-labeled kinesin walk towards the positive end. Also, mammalian dynein must be used, as yeast dynein and kinesin do not typically display bidirectional motion.11 The ability of a simplified system, containing only a bead with kinesin and dynein attached, to display bidirectional, saltatory motion, indicates that kinesin and dynein can interact solely through force transduction through the cargo, a basic tug-of-war interaction. Data used in Fig 6B of De Berg et al.13
These experimental results are supported by several theoretical papers showing that by varying motor properties, such as stall force, on/off rate, and velocity, amongst others, different directionalities and types of motility can be engineered.10b,c,14 Interestingly, the theoretical results showed that by having a small detachment force (the force needed to pull a motor off the microtubule) compared to stall force (the force needed to prevent the cargo from proceeding), tug-of-war interactions could occur that result in minimal stalling. Tug-of-war events would happen quickly, with one set of motors quickly detaching, while the other would take control and transport the cargo. However, a large detachment force relative to the stall force would lead to a situation with both motors attached to the cargo and microtubule, and no motility occurring.10b
Bidirectional switching of purified organelles without any cytoplasmic signaling factors (instead of isolated motors, as discussed previously) has also been observed, further adding to the tug-of-war hypothesis.7d,15 Organelles were purified from living cells, with a small complement of motors still attached. When placed on microtubules, they exhibited bidirectional, saltatory motion similar to that predicted by tug-of-war models and similar to that seen in the cell. This bidirectional motility of cargoes in vitro shows that cytoplasmic factors are not necessary for directional switching,7d, 15b although a large array of accessory proteins could still be attached to the transport complex, making a firm conclusion impossible to reach.
Evidence for a tug-of-war also comes from work on vesicle fission, in Dictyostelium and rat liver cells, both in vitro and in vivo.7e,12b In the case of fission, it is clear that a tug-of-war is happening because pulling by both motors is causing the endosome to stretch.7e,12b In addition, in vivo trapping in both Dictyostelium and human epithelium cells has suggested a model where dynein remains attached to the microtubule during plus- and minus-end directed transport, while kinesin is only active during plus-end directed motion.12a This “dynein-drags tug-of-war model”7d,12a (Fig. 4B), posits that during kinesin-driven motion, dynein is dragged backwards along the microtubule, effectively reducing kinesin’s stall force. However, during dynein-driven motion, kinesin is detached from the microtubule.
Figure 4.
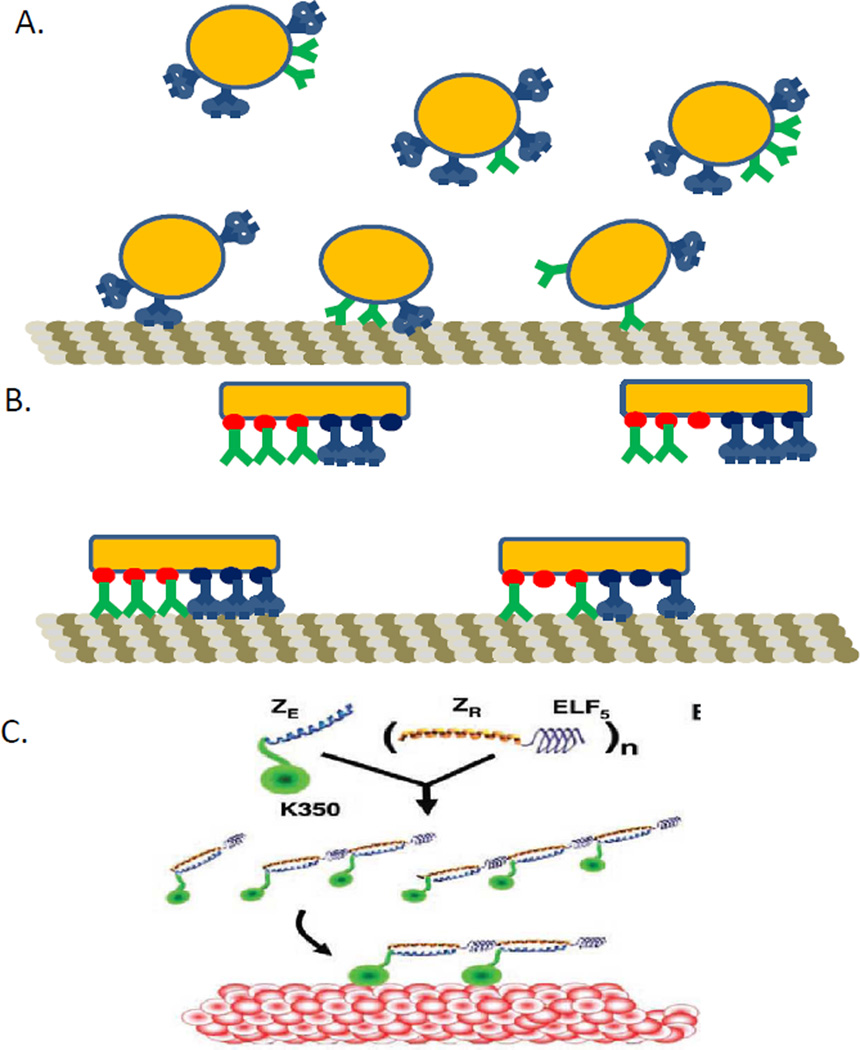
Counting active motors. One of the major issues with studying kinesin-dynein interaction during transport is that it is very difficult to directly measure the number of motors on the cargo and the number of active motors (motors attached to the microtubule generating force). This is a significant problem as a 1:1 ratio of kinesin and dynein can lead to beads with differing ratios of motors attached as can be seen in A. When taking force or other measurements on this sample, even though the concentration of motors is 1:1, any particular measurement could be on a cargo with a wildly different motor ratio. This makes it difficult to study the interaction of specific numbers of motors. Recent methods to establish fixed numbers of binding sites on cargos have improved the situation, but some still suffer from lack of complete occupancy at the binding sites, leading to fewer motors present than would be expected, as seen in B. This will also lead to different than expected numbers and ratios of active motors. C. Schematic representation of the synthesis of an engineered multi-motor assembly. The kinesin construct (K350) with part of the zipper attached (ZE) will bind to the other half of the zipper (ZR), which has the elastic spring attached to it. Schematic and caption adapted with permission from ref.45. Copyright 2006 American Association for the Advancement of Science.
Coordinated motion also has a large amount of support. A huge array of accessory proteins affect intracellular transport;16 there are known regulatory factors that bias directionality;17 and there is a lack of competition between opposite-directed motors.7c,18 The first two methods of coordination, accessory proteins and regulatory factors, do not necessarily exclude a tug-of-war--they could potentially modify the way the local tug-of-war works--but a lack of competition between motors seems to directly contradict any tug-of-war model. If the motors are not pulling against one another, how could a tug-of-war be occurring? For example, in many situations, eliminating a motor reduces motility in all directions,7c implying that there is a coordination factor that requires both motors be present to initiate motility. If a tug-of-war were occurring, eliminating one motor would naively be expected to increase the motility of the opposite motor (eliminating kinesin e.g. would increase dynein-driven motion). When dynein or dynactin function was disrupted in Drosophila embryos, plus-end directed motility was adversely affected: decreasing dynein-driven motion negatively affected kinesin-driven motion, opposite what would be expected in a tug-of-war.7c However, it is possible that impairing a motor in one direction could impair motion in all directions due to the presence of obstacles in vivo.1b.
Other experiments in Ustilago maydis (yeast-like fungus), and Xenopus melanophores have shown that down-regulating dynein or kinesin-driven motion showed no effect on the opposite motor’s motility.18b This clearly indicates that the motors are not interfering with one another.18a In vivo optical trapping also provides support for coordinated motion models. In Drosophila embryos, organelles that are detached from microtubules using an optical trap tend to go the same direction they had been going when they reactivate.19 This could indicate that only one set of motors is active at a time during transport, which clashes with the idea of both motors being active simultaneously, i.e. a tug-of-war.
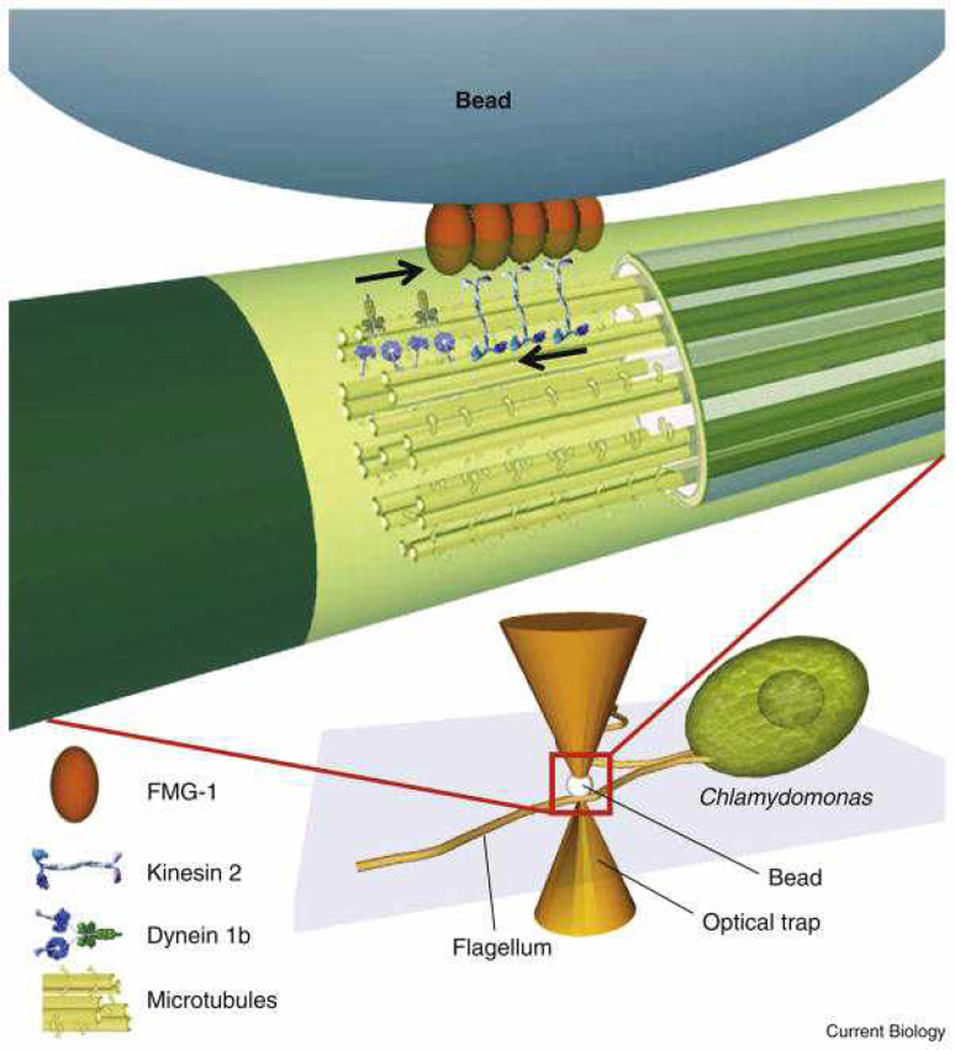
It is also clear that cells must have a way to regulate cargo directionality in the cell, and that higher order mechanisms other than motor copy number and tug-of-war between motors might regulate transport.7f,20 Comparing the predictions of unregulated tug-of-war models (transport models where only tug-of-war regulates directionality), to in vivo transport behavior, revealed discrepancies, indicating that additional levels of regulation are required on top of tug-of-war.20 For example, changes in motor-copy number in Drosophila embryos had minimal effect on transport behavior, indicating a mechanism other than tug-of-war regulates transport.7f In vivo trapping work with Chlamydomonas also indicated that coordinated motion occurs during intra-flagellar transport.21 In this case, large groups of motors of one type appeared to work together--50 pN stall forces were generated!--with no tug-of-war occurring. The generation of these large forces, with rare directional changes and saltations (commonly seen in other systems), suggests coordinated motion, with minimal competition between motor types. Also in this study, the knock-out of one motor had no effect on transport by the other motor, again opposing tug-of-war.21
Fu et al. looked at a another mammalian system—mouse neurons— and found evidence for coordinated motion.22 They showed that phosphorylation of an adaptor protein, the JNK interacting protein 1 (JIP1), acted as a molecular switch to control the direction of axonal amyloid precursor protein (APP) cargo transport, involved in Alzheimer’s disease. When JIP1 was unphosphorylated, dynein was bound to the microtubule and kinesin was not; after JIP1 phosphorylation, the opposite was true; a clear example of coordinated motion, as kinesin and dynein were never simultaneously active.
Characteristics of the tug-of-war and coordinated motion models are now being merged into more sophisticated models where transport is regulated at the level of motor properties such as stall force, release force, microtubule binding and unbinding rates, and the relationship between load and motor velocity.20 These models assume a local tug-of-war in which motors engage stochastically with the microtubule, with random binding and force events determining directionality, but motors which rarely engage in a prolonged tug-of-war. Instead their properties are such that, when one set of motors has an upper hand, the other motors stop interfering with transport, either by unbinding, or by simply getting pulled along behind the ‘winners’.7d,10b,12a These more complex models postulate an interplay between local tug-of-war interactions on the cargos, and larger regulatory events, such as change in motor number, motor properties (through phosphorylation or accessory protein binding), or even microtubule modification.20 12a
Conclusion
Evidence for local tug-of-wars occurring in most transport systems is very strong.7d,12,15b,20,23 Similarly, regulation of motility and directionality at higher levels than a local tug-of-war has been demonstrated in several systems.7f,20–21 Some of the current debate between which model is correct is based on different groups focusing on different behaviors.1a,9b,20 In vitro systems with just kinesin and dynein clearly display tug-of-war behavior,12a,12c,23b but this simple behavior cannot explain all transport behavior in vivo.20 Most systems might have a tug-of-war method for regulating directionality, with a higher level of regulation and control set on top, which controls motor number, type of motor, phosphorylation of motor(s), etc. In addition, the diversity of transport systems also seems to indicate that both coordinated motion systems and tug-of-war systems exist, with some model systems (Chlamydomonas, Ustilago maydis, APP transport) showing mostly coordination,18b,21a,22 while others (A549 cells, Dictyostelium phagosomes, and others) show clear evidence of tug-of-war behavior.7d,12a,b
2 Controlling the transport complex from the bottom up
This section will describe techniques that have been used to study kinesin-dynein interactions in vitro, how these techniques work and what results have been obtained using them. We have organized them from the simplest, two-or-three component systems, to the most complex systems, consisting of large constructs or entire purified organelles.
Various motors and accessory proteins associated with the transport complex have been purified,24 and are being added piecemeal to in vitro systems to study their effects in isolation. By altering the motor(s), accessory proteins, and cargo types, specific interactions between components of the transport complex can be observed. Tug-of-war interactions of groups of kinesin,24b,25 dynein,26 and kinesin and dynein7d,12a have been observed using this type of assay.
These assays have strongly demonstrated that pairs of motors (kinesin and mammalian dynein12a,12c,13 or kinesin and yeast dynein)11 undergo a tug-of-war in vitro without external signals or cofactors. In addition, when cofactors are examined in vitro with a motor (e.g. dynactin-dynein), these cofactors modulate motor properties, which then could influence the tug-of-war between motors.27 Finally, different teams of a single-motor type (teams of kinesin-only, dynein-only, or NCD-only) have been shown to have different cooperative behaviors.26,28 Kinesin-only teams appear to be poor cooperators, particularly on fixed-surfaces (that exist on polystyrene beads, for example). In contrast, dynein and NCD appear to be particularly good at sharing the load equally between motors to generate forces greater than that of a single motor.26,28 This impacts dual-motor transport in that teams of dynein would apparently be able to overwhelm kinesin in a tug-of-war, another method of modulating transport behavior.
2.1 Mixing multiple motors in vitro: tug-of-war, cofactors and teams
Single motor behavior for kinesin and dynein has been individually well characterized using various fluorescence and force spectroscopy techniques. Stepping behavior,29 force-velocity curves,30 and the interactions of various structural elements in the motors have been observed.31 More remains to be done, but observing the interplay between multiple motors in simplified in vitro environments has also started to reveal interesting information about the motors.
The most basic dual-motor experiments began with gliding assays, where kinesin and dynein were attached to a surface, and the gliding of microtubules over the surface was observed.12c In 1992, Vale et al. showed that coupling kinesin and dynein through a microtubule did not lead to stalled motion, but instead led to bidirectional motility of the microtubule, with stochastic directional switching (Fig. 3A). The motor-motor force interactions through the microtubule affected motor binding and unbinding events, leading to the hypothesis that the motors’ mechanical properties, as opposed to outside activating factors, played a key role in determining directionality. In addition, motor density on the surface tightly regulated the directionality of the microtubules. That is, more kinesin led to more plus-end directed motion (Fig. 3B) and the velocity of microtubule gliding was decreased when opposite polarity motors were present. This is clear evidence that a tug-of-war was occurring.
Gliding assays have also shown how two different kinesin motors (OSM-II and kinesin-2 from C. elegens) interact to drive plus-end directed IntraFlagellar Transport (IFT).32 This supports previous in vivo data indicating that OSM-II and kinesin-2 are simultaneously active during plus-end directed IFT.33 By varying the ratios of the two motors attached to the surface, they were able to continuously vary the velocity of the gliding microtubules between the velocities of each individual motor. This strongly indicated that during IFT, the motors were undergoing a mechanical competition to drive plus-end directed motion. Therefore a coordination mechanism outside of mechanical coupling through the cargo is unnecessary for two different kinesin motors to drive plus-end directed motion, i.e. a tug-of-war can also drive interactions between motors going in the same direction.32
A somewhat more physiological situation, with a bead labeled with kinesin and dynein walking on microtubules attached to a coverslip surface, was also tested.12a,13,23b In experiments by Muresan et al., using latex beads coated with kinesin and dynein, the beads always walked in the kinesin direction, i.e. bidirectional motility was not observed.23b However, when kinesin was inhibited (by an anti-kinesin antibody), dynein would take over, indicating that directionality was regulated by the presence of kinesin. Similarly, DeBerg et al. observed the motility of polystyrene beads coated with kinesin and dynein (without dynactin).13 They, however, found that the cargos underwent bidirectional, saltatory motion (motion with directional reversals and repeated pauses, Fig. 3C). The direction of motion and stall force behavior could be biased by altering the ratio of dynein and kinesin, but only within a fairly tight range.12a,12c,13 This indicates that one way to regulate directionality in a tug-of-war is by altering the motor ratio, and that the ratio of kinesin to dynein must be tightly controlled if bidirectional motion is to occur.
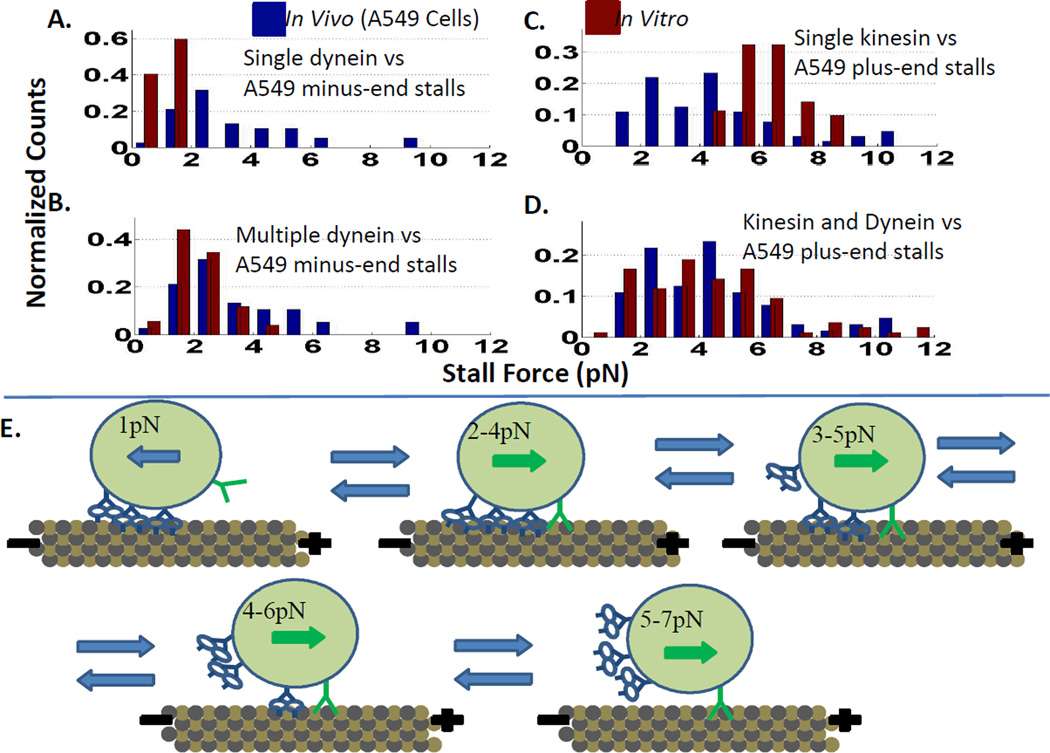
In vitro stall force measurements have also been made on beads with kinesin and dynein attached by Blehm et al.12a They used an optical trap to measure stall forces in both the plus and minus directions. A single-kinesin and also a single-dynein on a bead showed the “normal” stall forces of individual kinesin and dynein (6 pN and 1 pN respectively, Fig. 8A, C). A bead pulled by kinesin and dynein, however, showed a reduced stall force in the plus direction (that is with kinesin “winning’), and a stall force equal to or greater than that of a single dynein in the minus, dynein-driven direction, see Fig. 8D (presumably because a multiple dyneins were working together).12a This indicates that when moving toward the microtubule plus-end, kinesin has to drag dynein behind it, with dynein presumably still bound to the microtubule even though dynein is forced to move backwards. When the cargo is moving toward the microtubule minus-end, dynein operates freely as a team, presumably with kinesin detached from the microtubule--i.e. the kinesin(s) adds no drag. This provides support for a dynein-drags tug-of-war model (see Fig 8E).
Figure 8.
Dynein drags. A. In vitro (red) beads with a single dynein have a lower stall force than minus-end directed lipid droplets in vivo (blue). B. Beads coated with multiple dynein in vitro have stall forces similar to minus-end directed lipid droplets in vivo. C. A single kinesin on a bead in vitro has a narrower and higher stall force distribution than plus-end directed lipid droplets in vivo. D. In vitro, beads coated with kinesin and dynein stall at similar forces while walking towards the microtubule plus-end as lipid droplets and phagosomes in vivo (less than the stall force of a single-kinesin)12a. E. Dynein being dragged by kinesin during plus-end directed motion appears to be the simplest explanation for why the beads with kinesin and dynein in vitro, and organelles in vivo, have reduced plus-end directed stall forces. Adapted with permssion from ref. 12a. Copyright 2013 National Academy of Sciences.
Cofactors
Several elaborations on in vitro motor-cargo systems have also been explored. For example, cofactors, such as the dynactin complex, and Lis1, a regulator of dynein,24c,27,34 have been added to in vitro systems. These cofactors have been shown to have significant effects on the behavior of dynein alone, and have also been suggested to link kinesin and dynein during intracellular transport.
Lis1 appears to act as a “clutch” causing dynein to remain attached to the microtubule for extended periods of time, particularly increasing its binding time (time attached to the microtubule) under load.34–35 This would theoretically help teams of dynein motors apply large forces for the movement of large objects such as nuclei.
Dynactin was shown to increase dynein’s processivity and enhance microtubule binding in vitro.27. This was shown for dynein from yeast and from chick embryo brains. In addition, Ross et al. have shown that dynein with dynactin in vitro can undergo bidirectional motion. This surprising result was suggested to be a method of modifying dynein’s properties so as to allow obstacles on microtubules to be bypassed.24c However, experiments by Ross et al. also showed that groups of dynein-dynactin complexes only displayed unidirectional motion.24c This indicates that multiple dynein working together (thought to be typical)7d,12a,b,26 would not display bidirectional behavior without kinesin present. In addition, it is possible that the plus-end directed motion of the dynein-dynactin complex was diffusive, although its velocity was ATP dependent.24c If plus-end directed motion was diffusive, dynactin-dynein complexes could not drive net plus-end directed motion, indicating dynactin merely acts as a tether to keep dynein attached to the microtubule. Dynactin’s effects on dynein and bidirectional motility in general are still unclear, although they are definitely significant.3
Another cofactor is JIP1, which interferes with kinesin autoinhibition in cultured mouse neurons, thereby activating kinesin when bound to it in vitro. In addition, when p150Glued (a dynactin subunit) binds to JIP1, it counteracts JIP1’s effects on kinesin, inhibiting it again. So it appears that JIP1 and p150Glued can act as a switch to regulate transport.22 This is a clear case of a cofactor directing coordinated motion. This cofactor can turn kinesin on and off, thereby preventing it from being simultaneously active with dynein.
These experiments show that cofactor modulation of intracellular transport by altering motor properties appears to be very significant method of transport regulation. It is particularly important for dynein, as there is only one type of cytoplasmic dynein, while there are many types of kinesins.36 Different kinesins could therefore be used in different situations in the cell, but a single type of cytoplasmic dynein must play several roles, and cofactors could help modify its behavior. Cofactor modulation is an example a higher order method of regulating motion, with a tug-of-war often occurring between the local motors on the organelle, while cofactors modulate the individual motors’ behavior so as to bias the outcome of the tug-of-war, or even, as in the case of JIP1, prevent a tug-of-war from occurring altogether.
Multiple Motors
The interaction between several motors of the same type is also of interest, as it appears that many cargoes carry several kinesins and dyneins. Whether dyneins cooperate with other dyneins, and kinesins with kinesins, will have a large effect on transport. These same-motor interactions could potentially enhance or impair cargo transport by a single motor type. This, in turn, could be an important method of influencing the outcome of a tug-of-war. For example, it is well known that increasing the number of dyneins or kinesins can increase processivity under conditions of no load.37 However, when the motors have to share load and actively exert force, how well they cooperate is not clear. Several papers have shown that multiple kinesins do not cooperate well,24b,26,28,38 while multiple dyneins work extremely well as a team.26 In addition, a minus-end directed kinesin, NCD, also behaves cooperatively as a group.28 The cooperativity within groups of a single type of motor now appears to be a major component of the tug-of-war model, with the significant differences between kinesin and mammalian dynein playing a large part in the intracellular tug-of-war.26,28
Why dynein is more cooperative as a group than kinesin is driven by the fact that a single dynein may function as a gear in response to load, taking smaller steps as the load increases.39 To show this, Mallik et al. applied force to dynein attached to a bead (using an optical trap in vitro). As the force increased, the step size of dynein decreased (from 32 to 8 nm). This allowed motors with reduced load to catch up and increase their load, so as to share the load more equitably. In contrast, kinesin reduces its velocity under load (while its step size remains constant), but not in the same fashion as dynein. Consequently, kinesins do not cooperate well with other kinesins to generate large forces.26,28 However, some studies have shown that kinesin can cooperate more effectively at lower velocities, achieved in this case by lowering the ATP concentration.40 The fact that dynein is more cooperative as a team than kinesin is another high-order method for regulating the tug-of-war interaction between the two motor types. A single kinesin is likely to win out over a few dynein motors, but as the number of dynein motors increases, their ability to cooperate will overwhelm kinesin, driving motion toward the minus-end of microtubules.
Conclusion
Simple in vitro systems consisting of just kinesin, dynein, and cargo, with no external coordinating factors, have shown bidirectional motility is possible without a coordinating complex, and that tug-of-war is how motors interact in vitro.12a,12c,23b Additional studies on groups of either dyneins or kinesins have revealed how they cooperate, and the potential impact this will have on directional tug-of-war. Kinesin typically wins tug-of-wars with a dynein, but since dyneins are better at working as a team, enough dynein can overwhelm a kinesin.24b,26,28 Finally, the effect of cofactors and how they modulate motor behavior has also been studied, such as the effect of dynactin, Lis1, and JIP1 on dynein, and indicates that accessory proteins can significantly modulate motor behavior in vitro.27,34 These in vitro experiments have clearly shown that while a tug-of-war occurs with just kinesin and dynein present, cofactors and motor copy number are methods of modifying the outcome of a tug-of-war, sometimes completely eliminating it (JIP1), and therefore are a higher-order method of regulating cargo directionality.
2.2 Beyond Beads: Synthetic Cargos
It is possible that some of the in vitro systems used to study motors create artifacts due to their artificial nature. In particular, beads restrain the bound motors to remain in one spot along the bead surface, while on lipid vesicles, and presumably organelles in the cell, the free flow of motors allows multiple motors to be recruited to one spot on a vesicle. In addition, the number of motors bound to beads is very difficult to ascertain, as even at a specific concentration of motors, different numbers will bind to different beads and only some of the motors will be bound to microtubules at one time. To overcome these issues, synthetic cargos have been developed to more closely mimic in vivo cargos, while others have been created to control the number and type of motors present.
Motor diffusion on cargo surfaces
The idea that the properties of the cargo influence kinesin cooperativity was demonstrated recently on giant unilamellar vesicles coated with kinesin.41 Studies where kinesins were firmly tethered in place (attached to a solid surface, like a polystyrene bead), showed poor kinesin- 1 cooperativity in vitro.24b,28,42 Here again we mean the ability of motors of a single type to work together (as opposed to kinesin and dynein cooperating with each other). However, on giant unilamellar vesicles, kinesins were recruited to the microtubule-binding site, as they freely diffused around the vesicle until they attached to the microtubule.41 This increased the number of available motors at the microtubule, and enhanced their cooperativity. Other evidence that motor cooperativity can be altered by the cargo’s properties is shown in the cooperative pulling of nanotubes,25,43 microtubule gliding,44 and intra-flagellar transport.21a These studies all showed that groups of kinesin would generate forces greater than a single kinesin could against different types of load (membrane elasticity, magnetic traps, and optical traps respectively).
Building a scaffold to control motor number
A second major concern about bead experiments (and motor experiments in general) is that there is currently no good way to determine the number of motors on the bead or the number of motors that are active. Attempts to determine this have been made by using stall force assays, with the assumption that stall force increases more or less linearly with motor number (for a single motor type).7f,12a,17c,24a However, it can be difficult to take stall force measurements in every instance, as the number of active motors can vary in a single sample with a single concentration of motors, along with a large number of other issues that can occur (see figure 4A). In addition, some recent studies have shown that single-motor stall forces are not as additive as one might expect,17c,28,42 and multimotor assays display a much more complex picture due to interactions between kinesin and dynein affecting the stall force.12a,15b For example, if a cargo had 2 kinesins (2×7 pN = 14 pN stall force) that were dragging 7 dyneins (7×1 pN = 7 pN), a net stall force of 7 pN might be measured. This cannot be differentiated from a single kinesin (7 pN) using stall force measurements. Without knowing the number of active motors present, understanding motor cooperativity and interaction is very difficult.
The first step in elucidating the number of active motors is to control the number of motors present on the cargo. Various scaffolds have been tried to replace beads as artificial cargo (Figure 4B). An early scaffold (Fig. 4C), built of protein linkers and “springs” was built to study the effects of elastic coupling and linker distance on transport by multiple monomeric kinesin motors.45 In this paper, the elastic coupling and linker distance did not play a large role, only slightly increasing the velocity and processivity of the kinesin monomers. Another group used DNA to tether two kinesin-monomers together to determine if the structure of the kinesin outside the motor domain was necessary for processivity. They determined that the neck-linker was important (removal abolishes processivity), and that inter-motor strain allows processive motion. The native kinesin coiled-coil structure was not necessary for processivity, but was the most efficient of all alternatives tested.46
Kinesin and NCD on a DNA scaffold
While previous studies focused on kinesin monomers, a study by Furata et al. in 2013 focused on dimeric motors-- kinesin-1 and NCD, a minus-end directed kinesin. They used DNA as a scaffold and provided evidence for a “dragging” tug-of-war model, with NCD being dragged rather than dynein.28 Short pieces of double-stranded DNA with binding site tags and fluorophores were used to stitch together groups of motors in a line (Fig 5A). Each motor was connected to the ones around it with a short, stiff DNA linker and the various motor assemblies’ behavior was observed. The linkers were flexible at the motor attachment site, so this allowed some freedom of movement for the motors. As the number of motors increased, the processivity of both kinesin and NCD increased, but only NCD’s velocity increased (and then mostly at the 1 to 2 motor transition, with minimal increase after). Force production was similar, as NCD’s stall-force was positively correlated with the number of motors, while kinesin’s showed almost no correlation. Kinesins sometimes cooperated to generate stall forces larger than a single kinesin, but were much less likely to do so than NCD. This experiment indicated that NCD cooperates well in groups, while kinesin gains little advantage from increasing motor number.
Figure 5.
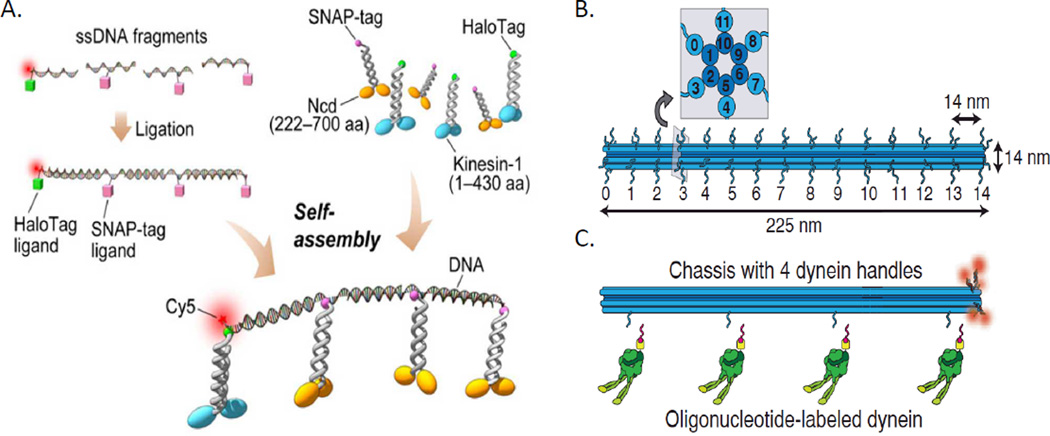
Synthetic DNA cargos. A. Schematic representation of DNA-motor construction (not drawn to scale). The typical spacing between motors is 22.7 nm, and the lengths of kinesin, SNAP-tag, and HaloTag are ~17, 4.3, and 4.8 nm respectively. Note that the DNA was fully ligated and annealed before the motors were attached enzymatically with a Halo or SNAP tag. By altering the number of each tag, the motor ratio could be controlled. Caption and figure adapted with permission from ref 28. Copyright 2006 American Association for the Advancement of Science. B. Schematic of the 12-helix-bundle chassis structure with 6 inner and 6 outer helices. Each outer helix contains up to 15 optical handles, yielding 90 uniquely addressable sites. Each handle consists of an unpaired 21-bp (~7nm) oligonucleotide sequence for hybridization to complementary anti-handle sequences covalently attached to motors or fluorophores. The inset shows an orthogonal cross section. The chassis is substantially larger and more complex than in Furuta et al., although it is more customizable. C. Schematic of a chassis labeled with five fluorophores (red) at handle position 14 on each of five outer helices and dynein at handles at positions 1, 5, 9, 13 on a single outer helix. Oligonucleotide-labeled dynein is also shown. Note that the motors are attached to a piece of single-stranded DNA through a SNAP tag and then the DNA is attached to the chassis. Figure and caption adapted with permission from ref 11. Copyright 2012 American Association for the Advancement of Science.
A final experiment by Furuta et al. tethered kinesin and NCD together with the DNA scaffold previously used to couple together only-kinesin or only-NCD (Fig 5A). The ratios of the motors (kinesin/NCD) were changed and the behavior of coupled plus and minus-end directed motors was observed.28 This experiment showed that the velocity of plus-end directed motion decreased with increasing numbers of NCD motors. This indicated that some sort of tug-of-war was going on, reducing kinesin’s velocity. It is interesting that NCD, as a weak, cooperative, minus-end directed motor, behaved so similarly to mammalian dynein when coupled to kinesin.12a,26 This is evidence that motors with certain properties function together better in tug-of-war situations, as NCD and mammalian dynein behave similarly, and both display bidirectional motility when coupled with kinesin.
Kinesin and yeast dynein on a DNA origami scaffold
A final, more complex, but also more versatile, synthetic cargo also uses DNA, in the form of DNA “origami”.47 This allowed the construction of more or less arbitrary structures that can be used to determine specific numbers, types, and placement of binding sites on a single cargo, up to 90 unique sites on the DNA construct demonstrated in this paper.11 In this technique, a large, cylindrical scaffold consisting of 12 helical pieces of DNA was the synthetic cargo, and 21-base-pair DNA handles were left at specific sites of the scaffold (Fig 5B). Then appropriate anti-handles could be attached to the motors of interest (in this case kinesin-1 and yeast dynein). The effects of increasing numbers of kinesin and yeast dynein were observed, and run length increased with motor number, while velocity did not change (kinesin) or decreased (yeast dynein) with increasing number. In this experiment, mixed motor ensembles of kinesin and yeast dynein were routinely immobile; when motile, they moved more slowly than single motor ensembles, and yeast dynein in general “won” over (mammalian) kinesin in terms of the direction of motility. This is very different from (mammalian) kinesin’s interaction with mammalian dynein.12a,b,13 Finally, the authors incorporated a photocleavable handle that allowed severance of specific motors. They used this to detach one motor type or the other, and showed that the immobile cargos became mobile again after removing one of the motor types, directly proving that a tug-of-war was going on between the yeast dynein and the mammalian kinesin.11
Issues with DNA scaffolds and origami
There is a significant issue with the DNA origami technique, in that currently, the binding sites available for each motor type are not completely filled. The authors saw about 80% occupancy of each site, leaving uncertainty in the actual number of motors present on the complex.11 This occupancy issue could be due to the fact the authors attached DNA linkers to the motors (with a SNAP-tag), which were then annealed to the chassis. Therefore the final step was the binding of an oligonucleotide already attached to the motor. Furuta et al. when using kinesin and NCD held together by a DNA scaffold,28 however, annealed the whole scaffold together and then attached the motors with SNAP tags, which showed essentially 100% occupancy. Furuta et al. did have difficulty obtaining full occupancy with Halo tags however. In addition, although the DNA for both these techniques is commercially available, it still requires design and assembly, which appears to be quite complicated, in particular for the larger origami structures.
Dynein’s stall force
Another important note about the experiments by Derr et al.,11 and other experiments, is that in vitro, single yeast dynein and single mammalian dynein have been shown to have very different behaviors. A single yeast dynein has been shown to be a slow, strong (7 pN stall force) motor in vitro,48 while, also in vitro, a single mammalian dynein has been measured to be weak (1–2 pN) and of a similar speed to mammalian kinesin.12a,b,15b,26,39 Mammalian dynein’s stall force and behavior is disputed: some contend it has a stall force of 5–7 pN.49 A substantial comparison of mammalian and yeast dynein will not be undertaken here as it is outside the purview of this review. However, we note that in the experiment with yeast dynein and mammalian kinesin, it was routine for cargos to be stationary as the motors were engaged in a balanced tug-of-war, preventing motility.11 This might indicate that these motors’ properties are not well balanced for each other. This is in contrast to mammalian kinesin and mammalian dynein, which rarely stall due to a tug-of-war in vitro, and appear to have complementary properties: dynein is dragged by kinesin, but is weaker, and can cooperate in groups as opposed yeast dynein.12a,b,26,39
Conclusion
Synthetic cargos have shown that groups of only-kinesin motors can cooperate if they are attached to fluid membranes that allow free-motor diffusion.41 In addition, synthetic DNA cargos can be constructed that control the number of motors attached (along with allowing detachment with specific signals) and have clearly shown that a tug-of-war occurs between kinesin and dynein.11,28 Interestingly, dyneins with different properties (yeast vs. mammalian) display extremely different behaviors, indicating that motor properties (stall force, velocity, detachment force) play a significant role in regulating intracellular transport.
2.3 Cellular Organelles in Vitro: Examining Parts of the Transport Complex
Being able to purify intact organelles with the entire transport complex present and active, and examine their behavior, is another way to observe transport behavior in a controlled environment. However it is difficult to ensure that all factors involved in transport are present and functional. Nonetheless, even organelles with part of the transport complex can reveal useful information about intracellular transport. Purified organelles have shown tug-of-war behavior and that the viscoelasticity of the cellular cytoplasm has minimal effect on organelle transport.7d,12a,b,50
The power of examining intact organelles can be seen in that kinesin and cytoplasmic dynein were both discovered as the motors that powered directional transport using this technique.51 Organelles have long been purified and studied to see which proteins will co-purify with them, in an attempt to determine what proteins are part of the transport complex. One of the first non-motor parts of the transport complex identified was dynactin, a separate protein from dynein, but one that evidently plays an important role in the transport complex. This was examined with in vitro motility assays.52 A more recent example is in vitro studies of organelle fission properties. For example, studies on the transition from early-to late-endocytic vesicles have compared the motile properties of the two populations to characterize the difference in proteins present in the transport complex.15a Similarly, Huntingtin protein has been shown to be necessary for dynein-mediated transport in vitro.53 Herpes Simplex virus transport in vitro was shown to be predominantly mediated by kinesin and associated with the trans- Golgi network marker TGN46.54
Purified organelles can also display very similar motile behavior to that seen in the cell, with bidirectional, saltatory motion.7d Some of the first evidence for a tug-of-war was shown using purified organelles.23b. This study showed that the presence of kinesin determined directionality. Only plus-end directed purified vesicles had kinesin present, while both plus-end and minus-end directed vesicles had dynein. Furthermore, when kinesin was inhibited, plus-end directed vesicles became uniformly minus-end directed. This study saw no bidirectional movement, but clearly some sort of local tug-of-war or interaction between kinesin and dynein was occurring, as similar behavior occurred when the motors were bound to beads.23b
Optical trapping of purified organelles has recently been used to measure the behavior of the transport complex without the interference of the highly viscoelastic and complex cellular environment. Organelles purified from a wide array of cells have been trapped, including Dictyostelium,55 A549,12a and mouse macrophages, J774A.115b. Different methods of purification led to different parts of the motor complex being present; specifically, some of the harsher purification techniques stripped away dynein,12a,b while others did not.12b Trapping of these organelles has shown that kinesin’s stall force (with dynein stripped away) is not affected by the remaining transport complex, but kinesin and dynein together on a cargo can interact in surprising ways, leading at times to stretching of the organelle and effectively reducing kinesin’s stall force.12a,b The stretching reveals that kinesin and dynein do engage in a tug-of-war: only kinesin and dynein pulling simultaneously would be able to cause the stretching of the organelle.
Effect of viscoelasticity in a cell
In addition, in two independent studies it was found that that there is little difference in the forces exerted by purified organelles that display bidirectional motion in vitro and their behavior in vivo.12a,b,15b Given that viscoelastic behavior is extremely different in vitro and in vivo, this is a surprising result. (Viscoelastic moduli of a cell range from 102 to 105 dyne/cm,12a,15b,56 leading to viscoelasticities several orders of magnitude higher than that of water, which has a viscosity of 1 centipoise and essentially no elasticity50,57). Apparently the high viscoelasticity of the cell has minimal effect on transport.12a,15b,50
Conclusion
The interplay of the components of the transport complex appears to be the major factor determining transport behavior (See also section 3.3). Organelles purified with only one motor behave similarly to single motor experiments. In contrast, having both motors present on purified cargos can lead to stretching of the cargo, and a reduction in kinesin-driven stall forces. Since the simple addition of dynein causes stretching of the cargo and reduces kinesin’s stall force, the motors must be engaging one another in a tug-of-war. When directional motion occurs with purified organelles, the lack of difference between their in vivo and in vitro force behavior also indicates that external factors to regulate motility are not necessary for short-range bidirectional motility. The standardization of purification techniques,58 and their increasing use is creating a middle ground between in vivo and in vitro studies. Purified organelles analyzed via in vitro techniques may be more suited to exploring the exact components of the transport complex, and thus lead to clearer conclusions than can be extracted from in vivo studies.
Overall, the preponderance of in vitro data indicates that a tug-of-war occurs between motors present on in vitro cargos.11–12,23b,28 Motors’ properties--in particular dynein’s properties--are regulated by the presence of regulatory cofactors, and this regulation could potentially influence the outcome of a tug-of-war. 22,24c,27b,34,36 In addition, the outcome of the tug-of-war can be determined by the number of each motor type present, and how well these motors can cooperate amongst themselves.11–12,13,23b,26,28 Assays with motor-coated beads,12a,13 DNA scaffolds,11,28 and purified organelles7d,12a,b all support these conclusions.
3 The Transport Complex in Vivo: Examining Organelles in Living Cells
Observing organelle motility in the living cell is a huge area with decades of research behind it. A great variety of techniques exist for observing organelle behavior, from basic light microscopy, phase contrast and DIC (Differential Interference Contrast), to modern fluorescence (TIRF, Total Internal Reflection Microscopy,59 and FRAP, Fluorescence Recovery After Photobleaching)60 and super-resolution techniques (STED or STimulated Emission Depletion,61 and SIM or Structured Illumination Microscopy).62 In vivo optical trapping studies have shown that a tug-of-war is likely occurring,7d,12a,b but have also revealed that higher order mechanisms must exist to regulate and coordinate motor behavior in the cell.7f,20
3.1 Tracking Organelles in Living Cells
Recent discoveries in the area of kinesin-dynein interaction in living cells have revealed a wide variety of behaviors across organelle and cell types. How cells regulate directionality is one of the major questions about bidirectional intracellular transport, and the experiments discussed in this section cover the wide variety of mechanisms observed in the cell: motor concentration, opposite polarity motors, myosin, regulatory factors, and external signaling. Many of these mechanisms may be unique to a specific cellular system, while others may be more broadly applied.
Motor concentration regulates tug-of-war
During endosome transport in the fungus Ustilago maydis, kinesin-1, kinesin-3, and dynein, have been shown to have a complex interaction that alternates between cooperation and competition.18b Dynein dominates transport near the cell tip, but kinesin-3 takes over outside of the cell tip for long-range transport, necessitating a cooperative “hand-off” between the motors for transport across the cell.18b However, near the plus-ends of microtubules, motion driven by kinesin-3 towards the plus-end appears to be stopped by a high dynein concentration. The high dynein concentration eventually overwhelms kinesin-3 and prevents it from running endosomes off the plus-end. As only minus-end directed endosomes had dynein present, while both plus- and minus-end directed endosomes had kinesin-3, the presence of dynein apparently regulates directionality in this system.63 This high concentration of dynein is maintained by dynein transport to the plus-end of the microtubule by kinesin-1.64 This supports a model in which the cell regulates the dynein concentration, thereby regulating overall motion. Locally, at the cargo, the decision of which direction to travel is driven by a tug-of-war where net force or motor number is intermittently tested to determine directionality.
Opposite polarity motors are both required for in vivo transport
In Drosophila S2 cells, it has been shown that opposite polarity motors must both be present on certain organelles for motility to occur.65 However, it apparently does not matter what the specific motor is.65b While tracking fluorescently labeled peroxisomes (GFP was targeted to the organelles), it was observed that knocking out kinesin-1 or cytoplasmic dynein caused all motility to stop. Knocking out kinesin-1 and replacing it with kinesin-3 (specifically unc104 from Caenorhabditis elegans, in this case), restored motility.65b Similarly, replacing dynein with NCD, a minus-end directed kinesin, would restore motility. (In this case, motility was heavily plus-end biased as NCD has different properties than dynein.) If kinesin or dynein was replaced by a non-motile mutant, motility would not resume. This indicates that active plus- and minus-end directed motors are simultaneously required during intracellular transport.65b What this means is unclear. It could indicate that there is a coordinating complex that requires the presence of both motors to function.
Kinesin and dynein interact to bypass obstacles
Another potential reason for the simultaneous presence of kinesin and dynein on many cargos is that they might aid in bypassing obstacles on microtubules, such as tau protein patches and microtubule intersections.1b,9a,66 Research on nuclear migration in C. elegans has indicated that while kinesin is the driver for nuclear movement, the deletion of dynein seriously slows and impairs transport, likely due to the inability to back the nucleus up to bypass obstacles.67 Also, kinesin and dynein are differentially regulated by a microtubule associated protein, tau. Kinesin generally detaches in the presence of tau-decorated microtubules, while dynein reverses at patches of tau. This indicates that dynein and kinesin interact to allow more efficient intracellular transport, and could explain why knocking out one motor stops transport in all directions.
Myosin can modulate microtubule-based transport
Myosin is often also present on organelles with kinesin and dynein, and its interaction with microtubule-based transport is an open question. Mitochondrial motility on microtubules in Drosophila neurons appears to be inhibited by myosin V in both directions, and myosin VI during retrograde transport, while myosin II has no effect on microtubule-based transport. This appears to indicate that myosin inhibits long-range microtubule-based motility and may aid organelle docking and pausing.68 Myosin’s effect on transport is still unclear, but it could have an override effect on kinesin-dynein tug of wars, with myosin perhaps superseding kinesin and dynein to allow switching to actin-based transport.
Regulatory factors modulate transport
A level of complexity typically only present in vivo is external regulatory factors, such phosphatases and kinases. For example, in the Xenopus laevis melanophore system, melanophores (pigment granules) can be dispersed or aggregated by external signals.69 Recently, it was shown that this control of directionality is mediated by phosphorylation of the dynein intermediate chain, where phosphorylating dynein apparently stimulates its activity.70 This type of regulation has also been seen in Huntington’s disease, as one of the downstream effects of pathogenic huntingtin protein is kinesin phosphorylation, which appears to inhibit kinesin.71 In addition, phosphorylation of the scaffolding protein JIP-1 modulates the directionality of Amyloid Precursor Protein (APP) motility in mouse axons. JIP-1 phosphorylation may fully coordinate APP motility, as experiments indicated that kinesin and dynein were not simultaneously active in this system.22,72
Controlling in vivo motility with external signals
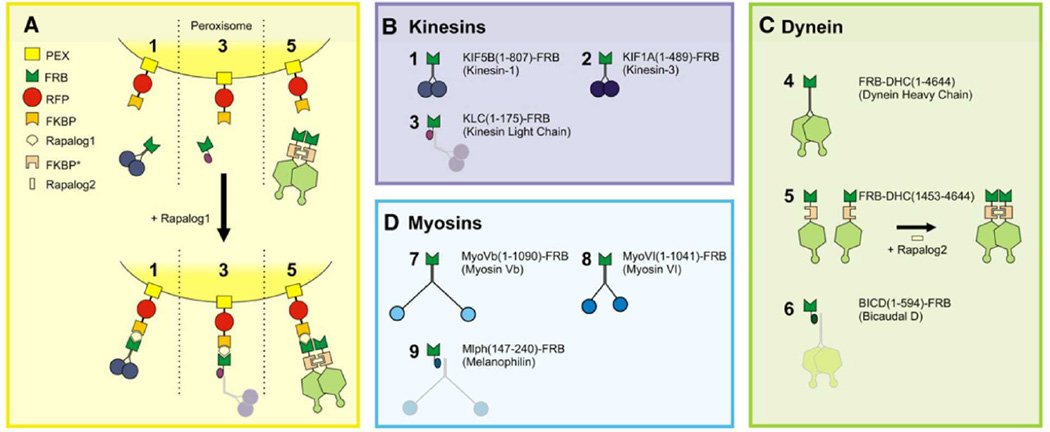
An exciting new method to study intracellular transport is the creation of an in vivo transport system, which can be induced to move upon addition of an external ligand. This allows much greater control of in vivo transport, and control over the motors attached to the tracked organelle. This creates an artificial system where the scientist has control over an external regulatory factor, the motors to be regulated, and the timing of transport. One such system is peroxisomes, which can be targeted by labeling motors with a 93 aa FRB domain (see Fig 6). By adding an FRB domain to endogenous or exogenous kinesins and dyneins, motility could be stimulated by addition of rapalog, which crosslinks the motor to the peroxisome. As was expected, when FRB-labeled kinesin was present, rapalog led to the rapid in vivo dispersal of peroxisomes, while aggregation at the center occurred for FRB-labeled dynein.73 Currently, the effects of rapalog addition are irreversible, and there is only one binding motif present, so only one binding event can be controlled (although it was possible to induce bidirectional motility by stimulating kinesin and dynein simultaneously). In addition, the paper showed that peroxisomes with only a single motor type attached (kinesin or dynein) underwent motion (the native motors caused only minimal motility). They also showed that kinesin- or dynein-only peroxisomes underwent pauses and changed directions at microtubule intersections, indicating that saltatory motion is not necessarily indicative of a tug-of-war. The ability to control transport with exo-or endogenous motor proteins is quite promising, as the construction of arbitrary in vivo transport systems could reveal much about intracellular transport.
Figure 6.
Inducible intracellular motility assays. A. Assay: a fusion construct of PEX, RFP, and FKBP targets peroxisomes. This PEX-RFP-FKBP construct consists of PEX, a peroxisome membrane targeting signal, RFP, a red-fluorescent protein, and FKBP, a domain that can be cross-linked to an FRB domain using rapamycin analogs. This PEX construct is recruited to peroxisomes, and consequently fusions of FRB with motor constructs (B-D) (1, 2, 4, 5, 7, and 8) or adaptor protein fragments (3, 6, and 9) are recruited to FKBP and the peroxisomes upon addition of rapalog. This ability to target specific motors to receptors on the peroxisome allows the control of intracellular motility. Caption and figure adapted with permission from ref. 73. Copyright 2010 Elsevier.
A similar system was also used to probe transport behavior in dendrites.74 Microtubules are of mixed orientations in dendrites (as compared to axons, where they all run in parallel), and it was unclear how transport to the dendrite was targeted. Recruiting motors to specific cargoes showed that dynein preferentially drives cargos to the dendrites, but also moves bidirectionally in dendrites, presumably by switching between oppositely oriented microtubules. Due to the small size of the dendrite, this random, bidirectional motion appeared to be capable of maintaining a stable density of cargos in the dendrite. Recruiting kinesin (Kif5 and Kif17, conventional kinesin and another kinesin family member) appeared to increase axonal transport, but had minimal effect on dendritic transport.74 In this system, the recruitment of a specific motor type appeared to regulate cargo placement.75 This is a clear system where both motor types do not appear to be required for transport, and could possibly be an example of an exclusionary coordination mechanism. Directionality is controlled by the fact that only one motor type is present at a time.
Conclusion
These in vivo studies have shown how the cell biases motion in one direction or the other. In a couple of cases, the cell appears to completely coordinate motion.22,74 However, in general, the cell appears to modulate a tug-of-war by modifying the number of motors,63 the properties of the motors (through phosphorylation),71 or even the types of motors (dynein, myosin or kinesin).68,74 In addition, both kinesin and dynein appear to be necessary for intracellular transport in certain systems,65b although evidence to the contrary has been found in artificial in vivo systems.73 This could be due to the regulation of motor behavior by microtubule-associated proteins such as tau.66 These sorts of mechanisms can determine overall directional bias, while a local tug-of-war between different motor types determines directionality of a specific cargo.
3.2 New Techniques for Tracking Organelles in Living Cells
In addition to standard brightfield and fluorescence techniques, new methods and super-resolution techniques are being applied to tracking organelle motility in living cells. These techniques all have their advantages and disadvantages, with many techniques increasing spatial resolution at the expense of temporal resolution, or increasing the lifetime or brightness of an imaged molecule at the expense of increasing the difficulty of labeling or interfering with the labeled molecule’s behavior.
The first new technique that is just beginning to be applied to in vivo transport is STED microscopy. This technique uses a focused beam to excite fluorescent molecules, and a second donut-shaped beam to stimulate emission around the excited spot at a slightly longer wavelength than normal fluorescence would occur. By ignoring this stimulated emission (cutting out the longer wavelength) the size of the fluorescent spot is reduced, yielding sub-diffraction spatial information.76 This technique has been used to image several organelle types in cellular areas smaller than the diffraction limit. In particular it has imaged synaptic vesicle motility in synaptic boutons, areas so small that typical widefield techniques have trouble accurately tracking organelles.77 Synaptic vesicles were mostly diffusive, but there was a strong flux of vesicles through the axon before reaching the synaptic bouton. The small area being imaged allowed this scanning technique to image vesicle dynamics with a reasonable time resolution (18Hz). The resolution, however, is still somewhat limited due to the photostability of dyes, leading to a 50–100 nm resolution.77
Another sub-diffraction imaging technique is Structured Illumination Microscopy (SIM), with a resolution about 100 nm.78 A non-linear version of the technique, known as saturated SIM, has theoretically unlimited resolution, although only 50 nm resolution has been achieved.79 SIM uses patterned illumination light to transfer information from high spatial frequencies past the diffraction limit to lower spatial frequencies within the limit. This technique requires computational processing after image acquisition, and requires multiple images of a widefield area under different illumination patterns. While it only doubles the lateral resolution, it is more amenable to vesicle tracking than STED as it requires less time to take a complete image. It has successfully tracked vesicles and kinesin-driven transport in Retinal Pigment Epithelium cells and Drosophila S2 cells.80 Saturated SIM, while getting higher-resolution, requires more images under alternating illumination patterns, thereby requiring more time per complete image.79,81 It also requires saturation of the fluorophores being imaged, which quickly photobleaches most fluorophores.
Other new imaging techniques use novel labels, although these labels tend to be large, generally 20–40 nm in diameter. Quantum dots or gold/silver nanoparticles are two ways to increase brightness and reduce photobleaching. Gold nanoparticles have been used in A549 cells to track endosomes at extremely high spatial and temporal resolution (25 µsec and 1.5 nm).82 This was achieved using dark field imaging and a quadrant photodiode to record the nanoparticles’ images. This technique was able to resolve both dynein and kinesin steps, and does not suffer from photobleaching.82a Similar work was done earlier on quantum dots with a fast CCD camera instead of a Quadrant Photo Diode.82b Upconverting nanoparticles (UCNPs) also have been shown to display excellent properties for cellular tracking, as they do not photobleach, they require minimal excitation power, and are minimally cytotoxic. In addition, since UCNPs up-convert two long-wavelength photons, the excitation causes minimal cellular auto-fluorescence.83 Another technique, bFIONA (bright-Field Imaging with One- Nanometer Accuracy), uses brightfield imaging of highly absorbing particles. It was demonstrated on melanophores, which contain dark melanin-containing vesicles.17d By saturating the CCD everywhere except the melanophore, it leaves an organelle-sized spot that can be fit with a 2D Gaussian, creating the opposite situation to typical FIONA (Fluorescence Imaging with One-Nanometer Accuracy).29 This allows highly accurate fits to organelles and will not photobleach, although it is limited to highly light-absorbent organelles.
Conclusion
These new techniques have allowed imaging of in vivo intracellular transport at higher resolution and for longer periods of time.17d,82 In addition, they have imaged in vivo motor stepping, revealing that motors step similarly in vivo as they do in vitro.82 All these techniques however, involve serious trade offs. Both STED and SIM trade temporal for spatial resolution. STED, being a scanning technique, allows high speed (full frame rate of 28 Hz) scanning of small areas at high spatial resolutions (50 nm), however it is fairly complex to setup, and loses time resolution as one scans larger areas, similar to confocal microscopy.76–77 SIM, on the other hand, can take large wide-field images at the same rate as smaller image areas, but is limited to a doubling of resolution and requires significant post-processing.78 New labels can increase photostability and brightness, but come with their own costs. Most are significantly larger than fluorophores (qDots, gold nanoparticles, UCNPs) and present significant difficulties when labeling.82 bFIONA is inherently limited to absorbent particles (or organelles).17d
3.3 Optical Trapping in Vivo
Although one of the first uses of an optical trap was in a living cell, where Ashkin et al. attempted to measure the forces exerted by mitochondria,84 over the next 20 years, trapping was rarely used in the cell. This was because making quantitative force measurements in the cell was extremely difficult due to the complex nature of the cellular environment; this initial paper made many assumptions that may or may not have been appropriate when taking in vivo measurements. In the cell, the viscous and elastic properties of the cytoplasm are unknown and can vary both temporally and spatially, making experimental results difficult to interpret. In contrast, in vitro, the spring constant of the trap (the stiffness) can be easily measured, largely because water’s viscous properties are constant and well-known, yielding nanometer and sub-nanometer results with a millisecond or greater time resolution.85
Optical trap calibration
In the last few years, in vivo optical trapping has been rejuvenated due to new calibration techniques.7f,12a,b,15b,26,86 As a result, new questions about motor transport in the cell can now be answered.87 The spring constant or stiffness (k) of an optical trap in a purely viscous medium—i.e. an in vitro optical trap—can be calibrated using a wide variety of methods. All these methods measure essentially the same thing: the strength of the trap when compared to a known force acting on it. This can be done by measuring the trap strength against viscous drag (drag method), or by measuring the trap strength against Brownian motion—either the equipartition method or the power spectrum method.88 For a more thorough review of these techniques see Neuman et al.85a
The most common technique used to calibrate traps in living cells is to estimate the trap stiffness by measuring the index of refraction of the cellular cytoplasm, the index of refraction and size of the trapped organelle, and then to create a viscous, in vitro environment that replicates these indices of refraction, and measure the trap stiffness in it.7f,19,26,86 In this index-of-refraction matched environment, standard in vitro calibration techniques are used. Then one creates an organelle size vs. stiffness calibration in this in vitro environment. After that, the stiffness of the trap is assumed to be directly related to the size of the organelle being trapped, i.e. trap stiffness is linearly related to r, the radius of the trapped object (assumed to be spherical). This technique is more straightforward than the actual in vivo calibrations described later, but suffers from the fact that it has many more assumptions. This type of technique and the in vivo calibration technique described below appear to measure very similar trap stiffnesses, indicating that this technique may be sufficient for most measurements. Actual results (force measurements) are quite variable between differing calibrations and systems though, so it is difficult to determine how well various techniques work relative to each other.
For fully in vivo optical trap calibration, the assumptions going into a calibration must be minimized. There are two different in vivo calibration techniques that were developed and tested in vitro and in vivo,15b,89 and are based on the Fluctuation Dissipation Theorem (FDT). Compared to in vitro techniques they make minimal assumptions about the environment. The minimal assumptions are: the trapped particle’s environment must be locally homogeneous, and the response of the system to a small-applied force must be the same as its response to a similar spontaneous thermal force. These techniques require more complex setups and analysis, but in return allow trap calibration in situ, calibrating the trap on each organelle being observed. In addition, these techniques actively measure the local viscoelasticity.
The fundamental issue in vivo is that the trap is in an environment with 3 variables: the environment’s elasticity, the environment’s viscosity, and the trap’s stiffness (effectively, a second form of elasticity). Both techniques take 3 measurements, one from passive observation of the trapped object, and two from actively applying force to the trapped object: the trapped object’s oscillation, in-phase and out-of-phase relative to the trap’s oscillation. The passive measures the combination of the trap’s damping and environment’s damping of the organelle’s thermal vibration. The active measures the trap’s ability to apply force against the local environment. The ratio of the in-phase and out-of-phase measurements corresponds to the relative strengths of the environment’s elasticity and viscosity vs. the trap’s stiffness. Since there are 3 measurements and 3 variables, all 3 variables can be determined. Therefore, the trap stiffness can be extracted.
The method developed by Hendricks et al15b is very similar to the FDT method,89a with the main differences being in some slightly different mathematical terminology and transformations, and the fact that Hendricks et al. performs global fits to the active (or forced) and passive (or thermal) spectra. Fisher et al. applies their equations individually at each frequency. The actual calibration is very similar, with a measurement of the response to an applied force by moving the trapping laser or stage, and a measurement of the passive response of the system to thermal fluctuations. These in vivo calibration techniques differ from in vitro methods mainly in adding one extra step for the in vivo case--the active calibration--where the response of the trapped object to the trap’s applied force is tested. This extra measurement allows the measurement of the local viscoelasticity of the trapped object. This then allows the removal of the effect of the local environment on the trap, and the calibration of the trap’s stiffness.
In vivo optical trapping results
The wide variety of calibration methods, and wide-variety of in vivo systems, has led to divergent results. This should not be unexpected though, as different types of transport (nuclear, intraflagellar, axonal …) would be expected to have varying properties and purposes.
One of the big questions in the motor field is how many and what types of motors are simultaneously present and active on cargo. Studies on Drosophila embryos suggested that there are 1–2 kinesin and 1–2 dynein motors present and active on most cargos, and that the number of motors present on the droplets changes as the embryo develops.7f,90 Their data also suggests that both motors have a stall force of about 2.6 pN in the cell.7f They argue for a coordinated motion model: motor number had a minimal effect on motility-- knocking down kinesin by half, as determined by western blot and stall force assays, had no effect on motility. In addition, droplets going in one direction were more likely to go in that direction again after being pulled off a microtubule by the trap. They infer from this that although motors of both polarities are present on the droplet, only one polarity is active at a time, in opposition to the tug-of-war model where both polarities are simultaneously active.19 These papers all used in vitro calibration techniques dependent on calibrating outside the cell.
Another study on lipid droplets, this time in a human lung cancer cell line, A549, revealed that stall forces in both directions of transport were similar.86 This paper, by Sims and Xie, also used an in vitro calibration technique. Outward-directed motion, presumably driven by kinesin, had steps around 8 nm in size, while inward directed motion had a variable step size as has been reported by Mallik et al., for dynein in vitro.39 In addition, most transport appeared to be driven by one motor, and the fact that the stall forces in both directions were peaked around 7 pN was taken to indicate that dynein and kinesin both have a stall force of around 7 pN 86.
However, Rai and Mallik showed that there were typically 6–10 weak dynein (1 pN stall force) and 1 kinesin on a typical phagosome in mouse macrophages.26 This is in agreement with this same group’s previous data in Dictyostelium.12b They also showed that multiple kinesins do not cooperate well during force generation because there were few force events greater than 7 pN in the plus-direction in vitro and in vivo. Dynein however appears to be excellent at collectively generating force, and appears to form a catch-bond (stronger bond as the load increases) to the microtubule under high opposing forces, making it unlikely to detach.35 Catch-bond behavior was also seen in Drosophila embryos.19
Dynein’s catch-bond behavior was shown by pulling back on groups of dynein attached to organelles in the cell. The larger the stall force of the cargo (i.e. the more dyneins attached), the longer the dynein would hold onto the microtubule before release under a super-stall force (a force pulling back greater than that required to stall dynein-driven motion). They also observed dynein’s variable step-size in the cell, and kinesin’s 8 nm step size.26 This group calibrated outside the cell. While catch-bond behavior might look like it would prevent dynein from being dragged backward by kinesin, (dynein-drags model, Figure 8E) it is important to note that the behavior demonstrated here is not what would be expected for an organelle undergoing a tug-of-war. In the experiments where catch-bond behavior was observed, a vesicle was moving in the dynein-driven direction when a sudden, large force was applied to it; the vesicle was suddenly jerked backwards against the dynein-driven motion. However, in a tug-of-war situation, kinesin would reattach during dynein-driven motion, and the amount of force applied against the dynein would slowly grow as kinesin stepped away. This should not initiate catch-bond behavior. Catch-bond behavior would prevent dynein detachment in the case of sudden, large forces, and would aid in preventing organelle detachment, but would not be expected to be significant during typical tug-of-war behavior.
Two groups have also demonstrated optical trap calibration in living cells.12a,15b In these studies all calibration was done in the cell, accounting for any effects of a viscoelastic environment. Hendricks et al. looked at phagocytosed latex beads in a mouse macrophage line, J774A.1.15b They discovered that inward and outward directed forces were nearly balanced, and found that at high loads (> 10 pN), 8 nm stepping occurred, indicating that teams of motors (dyneins, kinesins, or dyneins and kinesins) had correlated stepping (Fig 7).
Figure 7.
Asynchronous vs. synchronous stepping: do kinesins and dyneins coordinate their stepping? A. If multiple motors coordinate stepping such that they all step simultaneously (or nearly so), the cargo center of mass will move the same size as a motor step (8 nm for kinesin). B. If motors do not coordinate stepping, small movements of the cargo will occur that are fractions of a motor step (< 8 nm for kinesin) because if there are multiple kinesin, 1 stepping forward will drag the cargo forward, while the remaining kinesin will pull back, preventing the cargo from moving the full 8 nm forward.
The other work, by Blehm et al. compared inward and outward forces exerted by lipid droplets in a human lung cancer cell line (A549), phagocytosed polystyrene beads in Dictyostelium, and dynein and kinesin coated beads in vitro.12a This paper indicated that there were typically several dyneins and generally 1 kinesin on most cargos. This was concluded because the stall force in the minus-direction was typically greater than 1 pN: since a single dynein was shown to have a stall force ~ 1 pN in vitro, several dyneins were necessary to generate this large force (Fig. 8A-B). In the positive direction the stall force was rarely greater than 6 pN, and hence only one kinesin was pulling at a time (Fig. 8C).
Blehm et al. also found that the plus-end directed stalls in the cell (i.e. outward or presumably kinesin-driven) looked very similar to the in vitro plus-end directed stalls for kinesin- and dynein-coated beads (Fig. 8D). In particular, a range of stall forces from 1–7 pN was found, equal to or more-often less than the 7 pN in vitro stall force of a kinesin-alone (Fig. 8C). This is important as it indicates that kinesin’s stall force was reduced by the presence of dynein, not due to some other effect of the cell. This suggested that dynein reduces kinesin’s stall force, perhaps by remaining attached to the microtubule during plus-end directed motion, and acting as a drag (Fig. 8E). This behavior could potentially help bypass obstacles, allowing for quicker directional switching or stronger attachment to the microtubule if kinesin detaches. It could also simply be the most efficient way these two motors interact to implement a tug-of-war. Data from both the Blehm and Hendricks papers support a weak, 1 pN dynein, and a strong 7 pN kinesin in vivo.12a,15b They also strongly support a tug-of-war model, with cooperative dynein working in a group against a single (perhaps 2) kinesin during intracellular transport.
In contrast, Laib et al. measured extremely high in vivo stall forces in an Intra- Flagellar Transport (IFT) system, indicating large, coordinated groups of kinesin or dynein. They bound a microsphere to the outside of Chlamydomonas’ flagella by attaching the sphere to a transmembrane protein undergoing IFT (Fig. 9).21a They could then measure the force the molecular motors inside the cell were exerting, even though the bead was outside the cell. This allowed typical in vitro stiffness calibration techniques to work.21a This system appears to be quite different than most others studied, as the forces measured were extremely high, around 60 pN in both directions. This indicates upwards of 10 motors cooperating during transport assuming a stall force similar to kinesin’s, 5–7 pN. Shih et al., using the same technique and system, have also seen similar behavior, with a somewhat lower peak stall force of 20–30 pN.21b The reason for the difference in peak forces between these papers was unclear, but could potentially be due to the microsphere being non-specifically bound in the Laib et al. paper, while Shih et al. specifically bound the bead with an antibody. In addition, temperature-sensitive kinesin-2 and dynein-1b were used to measure the effect of each motor on transport. When kinesin-2 was knocked-out, dynein-driven motion continued unaffected, with the typical pauses that accompanied two-motor transport still occurring.21 Similarly with dynein-1b.21b This was taken as evidence for reciprocal coordination as opposed to a tug-of-war model; in a tug-of-war model, knocking out kinesin should have improved dynein-driven transport and removed most saltations, and vice versa.21a
Figure 9.
Schematic of the assay developed by Laib et al.21a for the study of the transport of FMG-1-bound beads on the surface of a flagellum. The flagellum of a single Chlamydomonas cell is immobilized on a coverslip, and a polystyrene bead held in an optical trap is lowered onto the flagellum. A cluster of FMG-1 proteins on the flagellar surface is believed to bind to the bead (red box; blown-up region). Back-and-forth surface motion of the bead thus represents FMG-1 motion driven by opposing microtubule motors (cytoplasmic dynein 1b and kinesin 2; arrows indicate direction of motion) within the flagellum. The optical trap exerts a controlled force (‘load’) on the bead (and connected motors), directed towards the trap center and proportional to the bead’s displacement. Thus, the displacement is used to determine the force produced by the motors. Caption and figure reprinted with permission from ref. 23a. Copyright 2009 Elsevier.
The Shih et al. and the Laib et al. papers seem to show that there are perhaps many ways in which kinesin and dynein can interact. That kinesin (and dynein) could yield up to 60 pN of force, indicates excellent cooperativity in their system, in addition to coordination between the two motors to prevent them from interfering with each other. Other systems seem to indicate poor kinesin cooperativity, and rarely if ever saw motor cooperation on this scale.
Conclusion
From the wide variety of in vivo optical trapping results, the different kinesin-dynein transport systems present quite a bit of complexity. In addition, all the different techniques used to observe and measure force in these in vivo systems may or may not be entirely compatible. In addition to differing calibration techniques, some groups looked at maximum force,15b,86 some looked at stalls,7f,12a,39 and some looked at escape forces (the minimum force required to escape the trap).12b Each of these methods has its merits, as does each cellular system, but it will be difficult to compare and contrast these systems until more data is collected, so it can be understood how and why these systems act so differently. It might be expected that transport systems with different functions and purposes, i.e. intraflagellar transport21a as opposed to neuronal transport, would have different characteristics, which could explain much of the variability encountered in different systems.
4 Conclusion
Recent interest in how kinesin and dynein interact during bidirectional motion has led to many new systems and techniques with which to explore this interaction. New data and theoretical work has also led to much more nuanced models of kinesin-dynein interaction, with evidence for local directional regulation (individual organelles) being mainly through tug-of-war between motors, while intermediate and large scale regulation (regional and whole cell) is dominated by other factors (motor number, phosphorylation state, microtubule modifications …).
Motors also seem to be somewhat specialized in working with each other, in that mammalian kinesin and dynein appear to have characteristics allowing them to work together when simultaneously present on a cargo--or at least not interfere with each other to the point of preventing transport.7d,12b,15b Dynein apparently is dragged behind kinesin during some plus-ended transport, whereas kinesin routinely releases to allow unhindered minus-end transport.7d,12a This behavior has also been shown with mammalian NCD and kinesin.28 This is in contrast to yeast dynein and human kinesin,11 which seem to actively work against each other, leading to stalled cargoes. Compatible motors seem to be able to coordinate with each other naturally through a local tug-of-war,7d with weak, cooperative dyneins acting as a foil to strong, weakly cooperative kinesin. Non-compatible motors (yeast dynein and mammalian kinesin) act similarly to early tug-of-war predictions in that both motors appear to be active, and neither can win out, leading to stalling and failed transport.11
While evidence in a majority of systems looked at so far seems to point to a local tug-of-war to determine directionality and motility behavior for individual cargos,7d,10b,c,11–12,15b and general transport bias being regulated by some higher signaling mechanism,7c,7f,17a,b,18,23a,37a only a few systems have been examined. In particular there are systems that seem to show strong coordination (no tug-of-war occurring at all). Intraflagellar transport21 and amyloid precursor protein transport22 both displayed heavily coordinated motion: kinesin or dynein were active, but rarely, if ever, simultaneously.
More complex systems, such as systems with more motor types present,68 or other specialized types of transport promise to add more complex regulatory mechanisms. Intraflagellar transport systems have already shown this to be the case, displaying very different behavior than that seen so far in other in vivo systems.18b,63–64
New techniques are being developed to count motor-number in vitro and observe organelle behavior in the living cell and in various purified in vitro systems. As these techniques improve, and more systems are explored, a more complete understanding of how motor-motor interactions (the local tug-of-war) interplay with cellular regulation (motor number, phosphorylation, signaling) to control bidirectional transport will be uncovered.
Acknowledgments
Funding: Funding for this work was provided by: NIH GM068625 and NSF 0822613
Biographies

Benjamin H. Blehm is currently a post-doctoral fellow at the National Institutes of Health, and previously received his PhD at the University of Illinois, Urbana-Champaign. Current research interests include the role of force and tension in mediating cell-microenvironment interactions and developing new optical trapping and imaging techniques.

Paul R. Selvin: is a Professor of Physics, Biophysics and Cell and Developmental Biology at the University of Illinois, Urbana-Champaign. He has developed many single-molecule fluorescence techniques, as well as optical trap techniques, with the goal of understanding molecular motors, and more recently, neuronal synapses.
References
- 1.(a) Gross SP. Phys. Biol. 2004;1:R1. doi: 10.1088/1478-3967/1/2/R01. [DOI] [PubMed] [Google Scholar]; (b) Welte MA. Curr. Biol. 2004;14:R525. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Mallik R, Gross SP. Curr. Biol. 2004;14:R971. doi: 10.1016/j.cub.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Schroer TA. Annu. Rev. Cell Dev. Biol. 2004;20:759. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hirokawa N, Noda Y, Tanaka Y, Niwa S. Nat. Rev. Mol. Cell Biol. 2009;10:682. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]; (b) Walker RA, Salmon ED, Endow SA. Nature. 1990;347:780. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- 5.Barlan K, Rossow MJ, Gelfand VI. Curr. Opin. Cell Biol. 2013;25:483. doi: 10.1016/j.ceb.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamal A, Goldstein LS. Curr. Opin. Cell Biol. 2002;14:63. doi: 10.1016/s0955-0674(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 7.(a) Jolly AL, Gelfand VI. Biochem. Soc. Trans. 2011;39:1126. doi: 10.1042/BST0391126. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Deacon SW, Nascimento A, Serpinskaya AS, Gelfand VI. Curr. Biol. 2005;15:459. doi: 10.1016/j.cub.2004.12.074. [DOI] [PubMed] [Google Scholar]; (c) Gross SP, Welte MA, Block SM, Wieschaus EF. J. Cell Biol. 2002;156:715. doi: 10.1083/jcb.200109047. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hendricks AG, Perlson E, Ross JL, Schroeder HW, Tokito M, Holzbaur ELF. Curr. Biol. 2010;20:697. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Murray JW, Sarkar S, Wolkoff AW. Traffic. 2008;9:833. doi: 10.1111/j.1600-0854.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP. Cell. 2008;135:1098. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebhun LI. J. Gen. Physiol. 1967;50:223. doi: 10.1085/jgp.50.6.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Ross JL, Ali MY, Warshaw DM. Curr. Opin. Cell Biol. 2008;20:41. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lipowsky R, Klumpp S. Physica A. 2005;352:53. [Google Scholar]
- 10.(a) Berger F, Keller C, Klumpp S, Lipowsky R. Phys. Rev. Lett. 2012;108:208101. doi: 10.1103/PhysRevLett.108.208101. [DOI] [PubMed] [Google Scholar]; (b) Muller MJI, Klumpp S, Lipowsky R. Proc. Natl. Acad. Sci. 2008;105:4609. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Muller MJI, Klumpp S, Lipowsky R. J. Stat. Phys. 2008;133:1059. [Google Scholar]
- 11.Derr N, Goodman B, Jungmann R, Leschziner A, Shih W, Reck-Peterson S. Science. 2012;338:662. doi: 10.1126/science.1226734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Blehm BH, Schroer TA, Trybus KM, Chemla YR, Selvin PR. Proc. Natl. Acad. Sci. 2013;110:3381. doi: 10.1073/pnas.1219961110. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R. Proc. Natl. Acad. Sci. 2009;106:19381. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vale RD, Malik F, Brown D. J. Cell Biol. 1992;119:1589. doi: 10.1083/jcb.119.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deberg HA, Blehm BH, Sheung J, Thompson AR, Bookwalter CS, Torabi SF, Schroer TA, Berger CL, Lu Y, Trybus KM, Selvin PR. J. Biol. Chem. 2013;288:32612. doi: 10.1074/jbc.M113.515510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y. Phys. Rev. E: Stat. Phys. Plasmas. Fluids. 2009;79:061918. doi: 10.1103/PhysRevE.79.061918. [DOI] [PubMed] [Google Scholar]
- 15.(a) Bananis E, Nath S, Gordon K, Satir P, Stockert RJ, Murray JW, Wolkoff AW. Mol. Biol. Cell. 2004;15:3688. doi: 10.1091/mbc.E04-04-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hendricks AG, Holzbaur ELF, Goldman YE. Proc. Natl. Acad. Sci. 2012;109:18447. doi: 10.1073/pnas.1215462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Deacon SW, Serpinskaya AS, Vaughan PS, Fanarraga ML, Vernos I, Vaughan KT, Gelfand VI. J. Cell Biol. 2003;160:297. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Haghnia M, Cavalli V, Shah SB, Schimmelpfeng K, Brusch R, Yang G, Herrera C, Pilling A, Goldstein LSB. Mol. Biol. Cell. 2007;18:2081. doi: 10.1091/mbc.E06-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Rozdzial MM, Haimo LT. J. Cell Biol. 1986;103:2755. doi: 10.1083/jcb.103.6.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sammak P, Adams S, Harootunian A, Schliwa M, Tsien R. J. Cell Biol. 1992;117:57. doi: 10.1083/jcb.117.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Proc. Natl. Acad. Sci. 2007;104:87. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kural C, Serpinskaya AS, Chou YH, Goldman RD, Gelfand VI, Selvin PR. Proc. Natl. Acad. Sci. 2007;104:5378. doi: 10.1073/pnas.0700145104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nascimento AA, Roland JT, Gelfand VI. Annu. Rev. Cell Dev. Biol. 2003;19:469. doi: 10.1146/annurev.cellbio.19.111401.092937. [DOI] [PubMed] [Google Scholar]
- 18.(a) Levi V, Serpinskaya AS, Gratton E, Gelfand V. Biophys. J. 2006;90:318. doi: 10.1529/biophysj.105.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schuster M, Kilaru S, Fink G, Collemare J, Roger Y, Steinberg G. Mol. Biol. Cell. 2011;22:3645. doi: 10.1091/mbc.E11-03-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leidel C, Longoria Rafael A, Gutierrez FM, Shubeita George T. Biophys. J. 2012;103:492. doi: 10.1016/j.bpj.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunwar A, Tripathy SK, Xu J, Mattson MK, Anand P, Sigua R, Vershinin M, McKenney RJ, Yu CC, Mogilner A, Gross SP. Proc. Natl. Acad. Sci. 2011;108:18960. doi: 10.1073/pnas.1107841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Laib JA, Marin JA, Bloodgood RA, Guilford WH. Proc. Natl. Acad. Sci. 2009;106:3190. doi: 10.1073/pnas.0809849106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shih SM, Engel BD, Kocabas F, Bilyard T, Gennerich A, Marshall WF, Yildiz A. Elife. 2013;2:e00744. doi: 10.7554/eLife.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu MM, Holzbaur ELF. J. Cell Biol. 2013;202:495. doi: 10.1083/jcb.201302078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Mallik R, Gross SP. Curr. Biol. 2009;19:R416. doi: 10.1016/j.cub.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Muresan V, Godek CP, Reese TS, Schnapp BJ. J. Cell Biol. 1996;135:383. doi: 10.1083/jcb.135.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Curr. Biol. 2005;15:2075. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]; (b) Jamison DK, Driver JW, Diehl MR. J. Biol. Chem. 2012;287:3357. doi: 10.1074/jbc.M111.296582. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur ELF. Nat. Cell Biol. 2006;8:562. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- 25.Campas O, Leduc C, Bassereau P, Casademunt J, Joanny JF, Prost J. Biophys. J. 2008;94:5009. doi: 10.1529/biophysj.107.118554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rai A, Rai A, Ramaiya A, Jha R, Mallik R. Cell. 2012;152:172. doi: 10.1016/j.cell.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 27.(a) Kardon JR, Reck-Peterson SL, Vale RD. Proc. Natl. Acad. Sci. 2009;106:5669. doi: 10.1073/pnas.0900976106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) King SJ, Schroer TA. Nat. Cell Biol. 2000;2:20. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 28.Furuta K, Furuta A, Toyoshima YY, Amino M, Oiwa K, Kojima H. Proc. Natl. Acad. Sci. 2013;110:501. doi: 10.1073/pnas.1201390110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildiz A, Tomishige M, Vale RD, Selvin PR. Science. 2004;303:676. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 30.Svoboda K, Block SM. Cell. 1994;77:773. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 31.Vale RD, Milligan RA. Science. 2000;288:88. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 32.Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. J. Cell Biol. 2006;174:1035. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Nat. Cell Biol. 2004;6:1109. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Roberts AJ, Leschziner AE, Reck-Peterson SL. Cell. 2012;150:975. doi: 10.1016/j.cell.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. Cell. 2010;141:304. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kardon JR, Vale RD. Nat. Rev. Mol. Cell Biol. 2009;10:854. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(a) Gross SP, Welte MA, Block SM, Wieschaus EF. J. Cell Biol. 2000;148:945. doi: 10.1083/jcb.148.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Block SM, Goldstein LS, Schnapp BJ. Nature. 1990;348:348. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 38.Driver JW, Jamison DK, Uppulury K, Rogers AR, Kolomeisky AB, Diehl MR. Biophys. J. 2011;101:386. doi: 10.1016/j.bpj.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Nature. 2004;427:649. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Shu Z, King SJ, Gross SP. Traffic. 2012:9999. doi: 10.1111/j.1600-0854.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herold C, Leduc C, Stock R, Diez S, Schwille P. ChemPhysChem. 2012;13:1001. doi: 10.1002/cphc.201100669. [DOI] [PubMed] [Google Scholar]
- 42.Jamison DK, Driver JW, Rogers AR, Constantinou PE, Diehl MR. Biophys. J. 2010;99:2967. doi: 10.1016/j.bpj.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leduc C, Campas O, Zeldovich KB, Roux A, Jolimaitre P, Bourel-Bonnet L, Goud B, Joanny JF, Bassereau P, Prost J. Proc. Natl. Acad. Sci. 2004;101:17096. doi: 10.1073/pnas.0406598101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallesen T, Macosko J, Holzwarth G. Eur. Biophys. J. 2011;40:1071. doi: 10.1007/s00249-011-0724-1. [DOI] [PubMed] [Google Scholar]
- 45.Diehl MR, Zhang K, Lee HJ, Tirrell DA. Science. 2006;311:1468. doi: 10.1126/science.1122125. [DOI] [PubMed] [Google Scholar]
- 46.Miyazono Y, Hayashi M, Karagiannis P, Harada Y, Tadakuma H. EMBO J. 2009;29:93. doi: 10.1038/emboj.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.(a) Douglas SM, Dietz H, Liedl T, Högberg B, Graf F, Shih WM. Nature. 2009;459:414. doi: 10.1038/nature08016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rothemund PWK. Nature. 2006;440:297. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 48.(a) Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Cell. 2007;131:952. doi: 10.1016/j.cell.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Cell. 2006;126:335. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.(a) Walter WJ, Brenner B, Steffen W. J. Struct. Biol. 2010;170:266. doi: 10.1016/j.jsb.2009.11.011. [DOI] [PubMed] [Google Scholar]; (b) Walter WJ, Koonce MP, Brenner B, Steffen W. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5289. doi: 10.1073/pnas.1116315109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Proc. Natl. Acad. Sci. 2006;103:5741. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holzwarth G, Bonin K, Hill DB. Biophys. J. 2002;82:1784. doi: 10.1016/S0006-3495(02)75529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.(a) Schroer TA, Schnapp BJ, Reese TS, Sheetz MP. J. Cell Biol. 1988;107:1785. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schroer TA, Steuer ER, Sheetz MP. Cell. 1989;56:937. doi: 10.1016/0092-8674(89)90627-2. [DOI] [PubMed] [Google Scholar]; (c) Vale RD, Reese TS, Sheetz MP. Cell. 1985;42:39. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Vale RD, Schnapp BJ, Mitchison T, Steuer E, Reese TS, Sheetz MP. Cell. 1985;43:623. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]; (e) Dabora SL, Sheetz MP. Cell Motil. Cytoskeleton. 1988;10:482. doi: 10.1002/cm.970100405. [DOI] [PubMed] [Google Scholar]
- 52.Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. J. Cell Biol. 1991;115:1639. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caviston JP, Ross JL, Antony SM, Tokito M, Holzbaur ELF. Proc. Natl. Acad. Sci. 2007;104:10045. doi: 10.1073/pnas.0610628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee GE, Murray JW, Wolkoff AW, Wilson DW. J. Virol. 2006;80:4264. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartsch TF, Longoria R, Florin EL, Shubeita GT. Biophys. J. 2013;104:475A. doi: 10.1016/j.bpj.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nam W, Epureanu BI. J. Phys.: Condens. 2012:24. doi: 10.1088/0953-8984/24/37/375103. [DOI] [PubMed] [Google Scholar]
- 57.Yamada S, Wirtz D, Kuo SC. Biophys. J. 2000;78:1736. doi: 10.1016/S0006-3495(00)76725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dermietzel R. The cytoskeleton : imaging, isolation, and interaction. Humana Press/Springer: New York; 2013. [Google Scholar]
- 59.Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Nagai S, Nakamichi Y, Nagamatsu S. Biochem. J. 2004;381:13. doi: 10.1042/BJ20040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith AJ, Pfeiffer JR, Zhang J, Martinez AM, Griffiths GM, Wilson BS. Traffic. 2003;4:302. doi: 10.1034/j.1600-0854.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 61.Hein B, Willig KI, Hell SW. Proc. Natl. Acad. Sci. 2008;105:14271. doi: 10.1073/pnas.0807705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shao L, Kner P, Rego EH, Gustafsson MG. Nat. Methods. 2011;8:1044. doi: 10.1038/nmeth.1734. [DOI] [PubMed] [Google Scholar]
- 63.Schuster M, Lipowsky R, Assmann MA, Lenz P, Steinberg G. Proc. Natl. Acad. Sci. 2011;108:3618. doi: 10.1073/pnas.1015839108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinberg G. Curr. Opin. Microbiol. 2011;14:660. doi: 10.1016/j.mib.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 65.(a) Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Proc. Natl. Acad. Sci. 2004;101:17428. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. J. Cell Biol. 2009;187:1071. doi: 10.1083/jcb.200908075. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kim H, Ling SC, Rogers GC, Kural C, Selvin PR, Rogers SL, Gelfand VI. J. Cell Biol. 2007;176:641. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Science. 2008;319:1086. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fridolfsson HN, Starr DA. J. Cell Biol. 2010;191:115. doi: 10.1083/jcb.201004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pathak D, Sepp KJ, Hollenbeck PJ. J. Neurosci. 2010;30:8984. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers SL, Tint IS, Fanapour PC, Gelfand VI. Proc. Natl. Acad. Sci. 1997;94:3720. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ikeda K, Zhapparova O, Brodsky I, Semenova I, Tirnauer JS, Zaliapin I, Rodionov V. Mol. Biol. Cell. 2011;22:1321. doi: 10.1091/mbc.E10-09-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, Huang CF, Banker G, Pigino G, Brady ST. Nat. Neurosci. 2009;12:864. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reis GF, Yang G, Szpankowski L, Weaver C, Shah SB, Robinson JT, Hays TS, Danuser G, Goldstein LS. Mol. Biol. Cell. 2012;23:1700. doi: 10.1091/mbc.E11-11-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kapitein LC, Schlager MA, van der Zwan WA, Wulf PS, Keijzer N, Hoogenraad CC. Biophys. J. 2010;99:2143. doi: 10.1016/j.bpj.2010.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Curr. Biol. 2010;20:290. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 75.Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N. J. Cell Biol. 2011;194:245. doi: 10.1083/jcb.201104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klar TA, Hell SW. Opt. Lett. 1999;24:954. doi: 10.1364/ol.24.000954. [DOI] [PubMed] [Google Scholar]
- 77.Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW. Science. 2008;320:246. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 78.Gustafsson MGL. J. Microsc. 2000;198:82. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 79.Gustafsson MG. Proc. Natl. Acad. Sci. 2005;102:13081. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.(a) Ach T, Best G, Rossberger S, Heintzmann R, Cremer C, Dithmar S. Br. J. Ophthalmol. 2012;96:1141. doi: 10.1136/bjophthalmol-2012-301547. [DOI] [PubMed] [Google Scholar]; (b) Kner P, Chhun BB, Griffis ER, Winoto L, Gustafsson MG. Nat. Methods. 2009;6:339. doi: 10.1038/nmeth.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rego EH, Shao L, Macklin JJ, Winoto L, Johansson GA, Kamps-Hughes N, Davidson MW, Gustafsson MG. Proc. Natl. Acad. Sci. 2011;109:E135. doi: 10.1073/pnas.1107547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.(a) Nan X, Sims PA, Xie XS. Chemphyschem. 2008;9:707. doi: 10.1002/cphc.200700839. [DOI] [PubMed] [Google Scholar]; (b) Nan X, Sims PA, Chen P, Xie XS. J. Phys. Chem. B. 2005;109:24220. doi: 10.1021/jp056360w. [DOI] [PubMed] [Google Scholar]
- 83.Nam SH, Bae YM, Park YI, Kim JH, Kim HM, Choi JS, Lee KT, Hyeon T, Suh YD. Angew. Chem. Int. Ed. Engl. 2011;50:6093. doi: 10.1002/anie.201007979. [DOI] [PubMed] [Google Scholar]
- 84.Ashkin A, Schutze K, Dziedzic JM, Euteneuer U, Schliwa M. Nature. 1990;348:346. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- 85.(a) Neuman KC, Block SM. Rev. Sci. Instrum. 2004;75:2787. doi: 10.1063/1.1785844. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Abbondanzieri EA, Greenleaf WJ, Shaevitz JW, Landick R, Block SM. Nature. 2005;438:460. doi: 10.1038/nature04268. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Moffitt JR, Chemla YR, Izhaky D, Bustamante C. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9006. doi: 10.1073/pnas.0603342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sims PA, Xie XS. Chemphyschem. 2009;10:1511. doi: 10.1002/cphc.200900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oddershede LB. Nat. Chem. Biol. 2012;8:879. doi: 10.1038/nchembio.1082. [DOI] [PubMed] [Google Scholar]
- 88.Tolić-Norrelykke SF, Schäffer E, Howard J, Pavone FS, Jülicher F, Flyvbjerg H. Rev. Sci. Instrum. 2006:77. [Google Scholar]
- 89.(a) Fischer M, Berg-Sorensen K. J. Optic. Pure Appl. Optic. 2007;9:S239. [Google Scholar]; (b) Fischer M, Richardson AC, Reihani SN, Oddershede LB, Berg-Sorensen K. Rev. Sci. Instrum. 2010;81:015103. doi: 10.1063/1.3280222. [DOI] [PubMed] [Google Scholar]; (c) Mas J, Richardson AC, Reihani SN, Oddershede LB, Berg-Sorensen K. Phys Biol. 2013;10:046006. doi: 10.1088/1478-3975/10/4/046006. [DOI] [PubMed] [Google Scholar]
- 90.Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Cell. 1998;92:547. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]