Abstract
The objective of this research was to establish a chloroplast transformation technique for Platymonas (Tetraselmis) subcordiformis. Employing the gfp gene as a reporter and the bar gene as a selectable marker, transformation vectors of P. subcordiformis chloroplast were constructed with endogenous fragments rrn16S–trnI (left) and trnA–rrn23S (right) as a recombination site of the chloroplast genome. The plasmids were transferred into P. subcordiformis via particle bombardment. Confocal laser scanning microscopy indicated that the green fluorescence protein was localized in the chloroplast of P. subcordiformis, confirming the activity of the Chlamydomonas reinhardtii promoter. Cells transformed with the bar gene were selected using the herbicide Basta. Resistant colonies were analyzed by PCR and Southern blotting, and the results indicated that the bar gene was successfully integrated into the chloroplast genome via homologous recombination. The technique will improve genetic engineering of this alga.
Introduction
Platymonas (Tetraselmis) subcordiformis is a marine unicellular green alga that is a widely used feed in aquaculture for its high nutrient levels. Recently, P. subcordiformis was observed to evolve H2 in a two-stage bio-photosynthesis, making it a potential resource for H2 [1]. In microalgae, photosynthetic hydrogen is catalyzed by hydrogenase with electric flow from electron transport chain of PSII in the chloroplast, while the activity of hydrogenase was inhibited by molecular oxygen evaluated from PSI.
Chloroplast transformation has many advantages over nuclear transformation, such as the high expression level of foreign genes [2], the homologous integration of foreign genes [3], the lack of gene silencing and position effects [4], and co-expression of multi-genes [5]. For P. subcordiformis, chloroplast transformation could be more suitable than nuclear transformation to raise its nutrient value. Meanwhile, a strategy to increase hydrogen production is to regulate PS I and PS II to maintain cells anaerobic by manipulating genes encoding PS proteins via chloroplast transformation. However, the current lack of chloroplast transformation protocols is obstructing this promising and fundamental research.
The first chloroplast transformation was reported in the green microalga Chlamydomonas reinhardtii [6]–[7]. The technology was rapidly applied to tobacco [8] and subsequently to many higher plant species [9]–[11]. However, algal plastid transformation research focused on C. reinhardtii, and only two other species [12]–[13] have stable chloroplast transformation systems.
An efficient selectable marker gene is critical for successful transformation. For chloroplast transformation, the commonly used primary markers are the aadA gene [14]–[15] and the neo gene [16], which confer resistance to spectinomycin–streptomycin and kanamycin, respectively; these antibiotics inhibit protein synthesis by the plastid ribosome [17]. We previously found [18] that P. subcordiformis was not sensitive to spectinomycin, streptomycin, or kanamycin but was very sensitive to the herbicide Basta. Basta contains a tripeptide of two Ala residues and an analog of Glu called phosphinothricin (PPT). After the two Ala are removed by an intracellular peptidase, the released PPT inhibits glutamine synthetase (GS) activity, leading to rapid buildup of intracellular ammonia, disruption of chloroplast structure, and cell death [19]. The bar gene encoding phosphinothricin acetyltransferase (PAT) confers tolerance to PPT. The interaction of PPT, GS, and PAT can be used to select positive colonies in nuclear transformations of higher plants and algae [18], [20]–[23]. In tobacco chloroplast transformation, the foreign bar gene could confer resistance to PPT in transformed plants but failed in direct selection for positive colonies [24]. This result may be related to the subcellular localizations of the expressed foreign PAT and GS isoforms.
In eukaryotic autotrophic cells, there are basically two isoforms of GS: GS1 localized in the cytoplasm and GS2 in chloroplasts and mitochondria [25]–[26]. In higher plants, the GS2 protein is usually encoded by a single nuclear gene and is expressed specifically in leaves [27]. The major role of GS2 is to re-assimilate the ammonium released by photorespiration in chloroplast and mitochondria.
In nuclear transformation, PAT localized in the cytoplasm could stop the PPT inhibition of GS1, which is critical to cell ammonia assimilation. The amount of GS1 is higher than that of GS2 in leaf cells. As a result, the recovered GS1 could then assimilate ammonia from photorespiration in chloroplasts before ammonia builds up to a level that disrupts chloroplast structure.
Unlike the single nuclear genome, plant cells contain multiple chloroplasts, each with multiple genomic copies. After transformation, there is usually one chloroplast with the foreign gene inserted into one copy of the genome. In the beginning of selection, the expressed level of PAT is much lower to detoxify PPT. The relatively low level of GS2 in one chloroplast compared with GS1 could not detoxify the inhibition of PPT to GS1 in cytoplasm in the time in order to the high level of ammonia, leading to cell death.
The subcellular localization of PAT, the amount of GS isoforms, and the lethal effect of PPT are the main factors for the failure of PPT in direct selection of tobacco chloroplast transformation. As a unicellular alga, transformed P. subcordiformis does not need the process of tissue culture without mature chloroplasts. Also, this alga has a single huge chloroplast, which may reduce the impact of PAT subcellular localization. Here, to understand the interaction between PAT and GS isoforms in algal chloroplasts, the bar gene was adopted as a selectable marker in P. subcordiformis chloroplast transformation.
Materials and Methods
Strain, Growth Condition, and Medium
Platymonas subcordiformis was obtained from Dalian Institute of Chemical and Physics, Chinese Academy of sciences, China. It was cultured in f/2 liquid medium or on agar plates at 23°C under a light intensity of 80–90 µmol photons m−2 s−1, with a photoperiod of 12 h/12 h (light/darkness).
Construction of Plasmids
The reported sequences of P. subcordiformis chloroplast genome were limited. Therefore, the chloroplast genomes of ten other green algae (Table 1) were used to design degenerate primers (Table 2) to isolate the fragment rrn16 (16S rRNA)–rrn23 (23S rRNA) in P. subcordiformis. Then, based on the sequence of rrn16–rrn23, the target fragments rrn16–trnI and trnA–rrn23 were amplified by specific primers as described in table 3.
Table 1. Chloroplast genome of 10 green algae for degenerate primers.
| Algae | GenBank accession number of chloroplast genome |
| Chlamydomonas reinhardtii | FJ423446 |
| Chlorella vulgaris | NC_001865 |
| Dunaliella salina | GQ250046 |
| Mesostigma viride | NC_002186 |
| Nephroselmis olivacea | NC_000927 |
| Oltmannsiellopsis viridis | NC_008099 |
| Oocystis solitaria | FJ968739 |
| Parachlorella kessleri | FJ968741 |
| Pycnococcus provasolii | NC_012097 |
| Scenedesmus obliquus | DQ396875 |
Table 2. Degenerate primers for homologous region.
| Primers | Sequences |
| 16S/trnA-for | CACTGGGACTGAGACACG |
| 16S/trnA-rev | CCSBYGRCVYCYGCMWTGC |
| trnI/23S-for | CAGYTGGTAGAGCRYYGCMYTT |
| trnI/23S-rev | CTTCGGCAGRYYDYTTAG |
| 23S-for | CCCTTBAAAGAGTGCGTAA |
| 23S-rev | AKTTTGCCGAGTTCCTTA |
Table 3. Primers and restriction sites of the fragments for the chloroplast vectors.
| fragments | Primers | Sequences | Enzymic sites | Length |
| rrn16–trnI | 16-I for | cggggtaccTCCTACGGGAGGCAGCAGT | KpnI | 1288 bp |
| 16-I rev | gcgtcgacTTGAGGCAAATGGGCTATGC | SalI | ||
| trnA–rrn23 | A-23 for | ttgcggccgcACAACGGAGTTTCGGAATA | NotI | 1405 bp |
| A-23 rev | cgagctcCGTTACTCAAACCGACATTC | SacI | ||
| atpA 5′ UTR | 5′atpA for | gcgtcgacAAGCTTATCGATGACTTTATTAG | SalI | 668 bp |
| 5′atpA rev | ccgatatcGGACATTTTCACTTCTGGAGT | EcoRV | ||
| rbcL 3′ UTR | 3′rbcL for | cgggatccGTACTCAAGCTCGTAACGAAG | BamHI | 448 bp |
| 3′rbcL rev | gctctagaGGATCGCACTCTACCGATT | XbaI | ||
| the bar gene | bar for | ccgatatcATGAGCCCAGAACGACGCC | EcoRV | 567 bp |
| bar rev | cgggattcTCATCAAATCTCGGTGACGG | BamHI | ||
| the gfp gene | GFP for | ccgatatcGTACTCAAGCTCGTAACGAAG | EcoRV | 732 bp |
| GFP rev | cgggattcGGATCGCACTCTACCGATT | BamHI |
Because of a lack of information on gene expression regulation in P. subcordiformis chloroplasts, the foreign gene cassette was made by amplifying a 5′ untranslated region (UTR) of atpA and a 3′ UTR of rbcL from a Chlamydomonas reinhardtii chloroplast transformation plasmid (p64D, provided by Biotechnology Research Institute, CAAS). The two fragments showed high efficiency in C. reinhardtii chloroplast transformation [28]. Meanwhile the gfp (green fluorescent protein) gene and the bar gene, were cloned respectively from pEGFP-N1 (Clontech, Palo Alto, CA, USA) and pSVB [23]. A unique restriction site was added to each fragment. Primers for these fragments are listed in Table 3. All the PCR products were sequenced by Sangon Biotech (Shanghai, China).
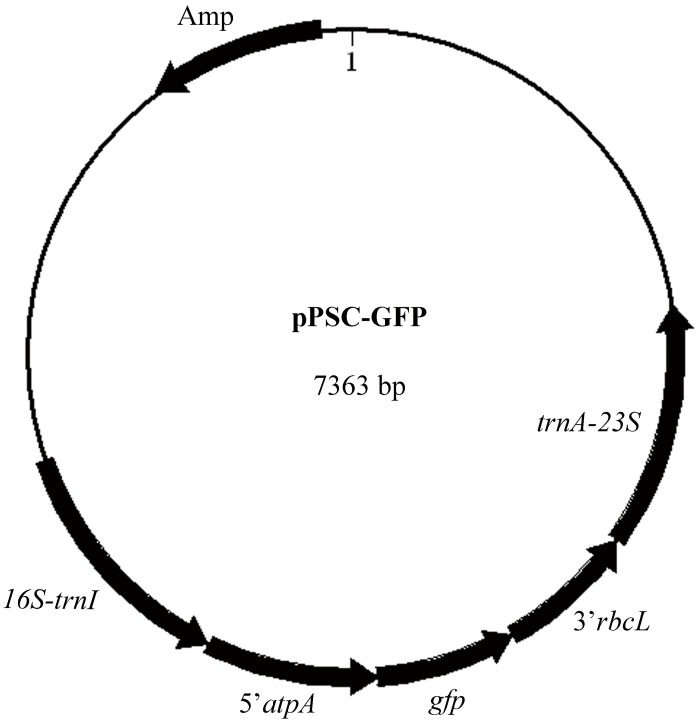
The resulting fragments were then cloned into the pMD18-T vector individually with a TA cloning kit (Tiangen, China). After restriction enzyme digestion, the fragments were inserted into the plasmid pBluescript KS (+) (Promega, USA) in the fit sites one by one. The plasmid containing the gfp gene was named as pPSC-GFP, while that containing the bar gene was pPSCB.
Microparticle Bombardment
The pPSC–GFP and pPSCB plasmids were used for P. subcordiformis chloroplast transformation. Alga preparation and bombardment parameters were as previously described [18]. Gold particles 0.8–1.5 µm in diameter were used as plasmid carriers, and 2–3 µg of plasmid DNA was used for each bombardment following protocols described by Bio-Rad (Hercules, CA, USA). The rupture pressure was 900 psi and the bombardment distance 6 cm. The cells bombarded with uncoated gold particles were set as negative controls. All experiments were performed in triplicated.
Microscopy Analysis
Cells transformed with pPSC–GFP were first examined with a Nikon Eclipse 50i microscope (Nikon, Tokyo, Japan) with blue-light excitation 48 h after bombardment. Transformed cells were selected by comparing their fluorescence intensity with that of negative controls using a fluorescence-assisted cell sorting (FACS) (Vantage SE, Becton Dickinson, Franklin Lakes, NJ, USA) with 488 nm excitation and standard filter set-up. Then, the localization of GFP in the harvested cells was examined by confocal laser scanning microscope (CLSM) (FluoView FV1000, Olympus, Tokyo, Japan) with excitation at 488 nm and 635 nm for GFP and chlorophyll fluorescence, respectively.
Herbicide Selection
After 2 days of recovery, the cells transformed with pPSCB were transferred to selective f/2 medium with 15 µg ml−1 of Basta for a month. Then, surviving cells were spread on solid medium with 10 µg ml−1 of Basta. Colonies appearing after 2 weeks were selected and streaked on agar plates of f/2 medium with 5 µg ml−1 Basta. After another 2 weeks of cultivation, 40 colonies were selected and cultured in liquid f/2 medium without the herbicide.
Detection of the Integration Events
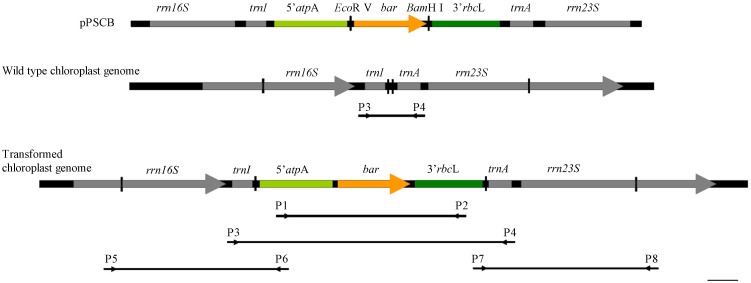
After 3 weeks of cultivation, cells originating from resistant colonies were harvested and genomic DNA was extracted using a plant genomic DNA kit (Tiangen). According to the map of pPSCB and the construct of the P. subcordiformis chloroplast genome (Fig. 1), four pairs of primers (Table 4) were designed to examine the bar gene in these resistant colonies. A fragment of 5′atpA–bar–3′rbcL was amplified from genomic DNA using primers p1 and p2 to confirm the existence of the bar gene. Colonies with positive amplification were further analyzed by PCR with primers p5 and p6, p7 and p8 to test homologous integration, and with primers p3 and p4 to test for homoplasmic integration. All PCR products were sequenced.
Figure 1. Construct map of homologous recombinant in P. subcordiformis chloroplast.
Bar = 100 bp.
Table 4. Primers for testing homologous recombination in P. subcordiformis chloroplast.
| Primers | Sequences |
| P1 | GCTTATCGATGACTTTATTAG |
| P2 | GGATCGCACTCTACCGATT |
| P3 | ACAAGCAACGGGCTATTA |
| P4 | TTTAGGCTGTTCCCATTT |
| P5 | TATGCTGAGGAGTAAAACGGTA |
| P6 | ACAGGCGTCGTAAGCAACTA |
| P7 | AAGCGGATGTAACTCAAT |
| P8 | TCCTGACTAACCCTCCAT |
Finally, samples with positive amplification in all PCR steps were analyzed by Southern blotting. A fragment of pSVB amplified with primers a (5′-GCACCATCGTCAACCACTA-3′) and b (5′-CAGAAACCCACGTCATGC-3′) (Tan et al. 2005) was used as the probe. Total DNA (≥4 µg) of each sample was first digested with EcoR V and BamH I. Southern blotting was conducted with a DIG DNA labeling and detection kit (Roche, Penzberg, Germany).
Results
Plasmids
The plastid fragment cloned from P. subcordiformis was 3729 bp in length (GenBank no. JN561782) with the organization rrn16–trnI–trnA–rrn23, which is similar to that of higher plants. Fragments of rrn16–trnI (1288 bp) and trnA–rrn23 (1405 bp) were separated as the homologues regions for plastid transformation vectors.
Two vectors, pPSCB (KJ668650) and pPSC–GFP (KJ668651), for P. subcordiformis chloroplast transformation were constructed. Maps of the two plasmids were similar, as described in Fig. 2. In the plasmid pPSCB, the bar gene was used as the selectable marker to confer algal resistance to the herbicide Basta, while the plasmid pPSC–GFP used the gfp gene as the reporter. The bar and gfp genes were each driven by the C. reinhardtii chloroplast regulators 5′ UTR of atpA and 3′ UTR of rbcL. The expression cassettes of the bar and gfp genes in the respective plasmids were both between the plastid border regions rrn16–trnI and trnA–rrn23, so homologous recombination of the foreign genes was allowed.
Figure 2. Physical map of the vectors pPSC-GFP.
GFP Expression
After bombardment of pPSC–GFP, some cells showed green–yellow fluorescence with blue-light excitation under fluorescence microscope, while the negative controls showed red chlorophyll fluorescence (Fig. 3). This result indicated that GFP was expressed in some cells.
Figure 3. GFP fluorescence of Transformed P. subcordiformis by Microscope.
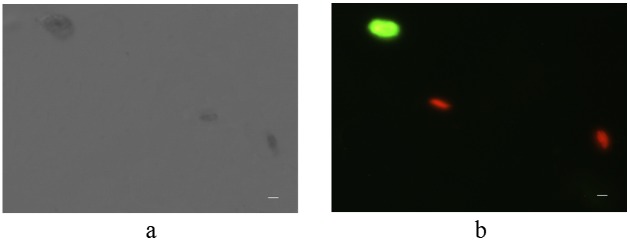
Fig. 3a, View of cells under white light; Fig. 3b, View of cells under fluorescence excitation. Bar = 5 µm.
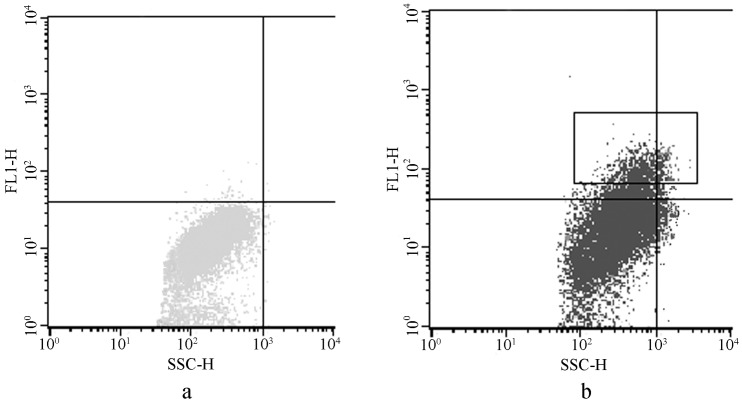
Chlorophyll fluorescences with an excitation wavelength of 488 nm, but the cells transformed with GFP showed more fluorescence with 488 nm excitation than did the negative controls. Therefore, we quantitatively compared these fluorescence differences by FACS, and the cells with more intense fluorescence (box in Fig. 4b) were harvested for further CLSM analysis. The positive fraction was 4.95% in the transformed cells.
Figure 4. Quantitative comparison fluorescence variant between cells of the negative controls and the transformed lines.
The axis FL1-H means relative intensity of GFP florescence, and the axis SSC-H means side scatter of each alga cell. Fig. 4a, Fluorescence intensity of the negative cells. Fig. 4b, Fluorescence intensity of the transformed cells. The cells with higher fluoresce intension in the rectangle in Fig. 4b, were harvested for further LSCM scanning for GFP positive cells. These cells may contain wild type cells, cells with plasmids inserted into chromosomes, and cells with plasmids inserted in chloroplast genome.
By combining images of GFP and chlorophyll fluorescence in some harvested cells by CLSM scanning (Fig. 5), GFP was found to be co-localized with chlorophyll in the cup-shaped chloroplasts of P. subcordiformis. The GFP fluorescence in P. subcordiformis chloroplasts indicated the activity of the regulating factors from C. reinhardtii in P. subcordiformis. The positive rate of pPSC-GFP transformed to P. subcordiformis chloroplast is 500–1000 cell per 1 µg DNA according to fifteen independent experiments.
Figure 5. Imagines of GFP and chlorophyll fluorescence in P. subcordiformis by LSCM.

Fig. 5a, GFP fluorescence. Fig. 5b, Chlorophyll fluorescence. Fig. 5c, Imagine with white light. Fig. 5d, Combination of the imagines a, b and c. Bar = 5 µm.
Detection of the Bar Gene Integration
Herbicide resistance was tested first by cultivating the bombarded alga in f/2 medium containing Basta. After selection, 40 resistant colonies were chosen for further PCR detection. Two colonies (named PSB1 and PSB2) gave positive results with all four pairs of primers (Fig. 6). The bands of about 1700 bp with the correct sequence (Fig. 6a) indicated the presence of the bar gene expression cassette in P. subcordiformis. The bands of about 1300 bp (Fig. 6b) indicated the homologous insertion in the left border, while the bands of 1500 bp (Fig. 6c) indicated the homologous insertion in the right border. Primers p3 and p4 could yield three types results: a single band of about 500 bp, which represents the wild type colony; a single band of about 2000 bp, which represents the homoplasmic colony, or two bands of 500 bp and 2000 bp, representing a non-homoplasmic transformed colony. Both colonies produced two bands with these primers (Fig. 6d), indicating that the bar gene has been inserted into some chloroplast genomes in each. Homoplasmic strains can be obtained by repeated selection. The green alga C. reinhardtii [29] and the red alga Porphyridium [13] yielded homoplasmic mutants after more than 6 month selection. Ultimately, both colonies showed positive bands in the Southern blotting analysis (Fig. 7), demonstrating that the bar gene was stably integrated into the P. subcordiformis chloroplast genome.
Figure 6. PCR detection of resistant P. subcordiformis colonies.
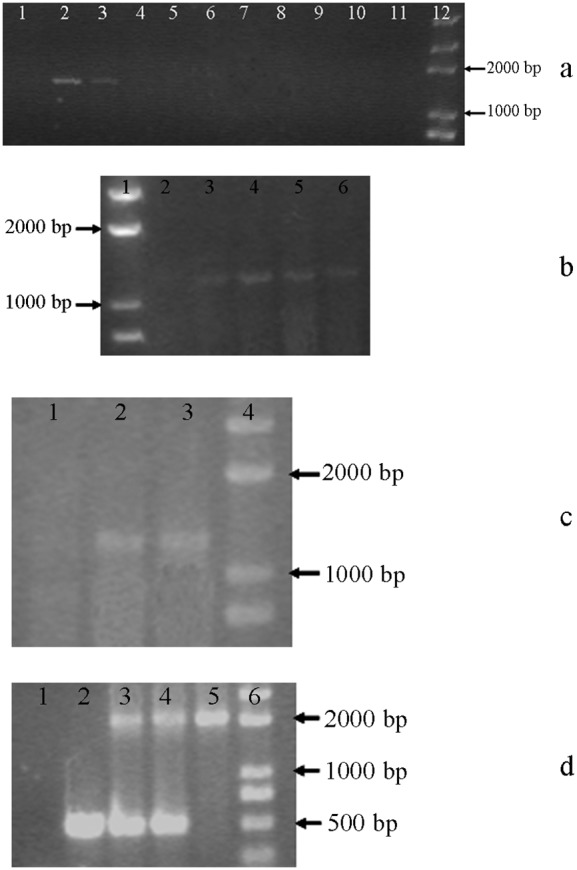
Fig. 6a, PCR product with primers p1 and p2. Lane 1, wild type of P. subcordiformis; lane 2, PSB1; lane3, PSB2; lanes 4–11, other resistant colonies; lane 12, DNA Marker Trans 2K. Fig. 6b, PCR product with primers p5 and p6. Lane 1, DNA Marker Trans 2K; lane 2, wild type of P. subcordiformis; lanes 3–6, resistant colonies. Fig. 6c, PCR product with primers p7 and p8. Lane 1, wild type of P. subcordiformis; lanes 2 and 3, PSB1 and PSB2; lane 4, DNA Marker Trans 2K. Fig. 6d is PCR product with primers p3 and p4. Lane 1, negative control; lane 2, wild type of P. subcordiformis; lanes 3 and 4, PSB1 and PSB2; lane 5, plasmid pPSCB; lane 6, DNA Marker Trans 2K.
Figure 7. Southern blotting analysis of the PCR-positive P. subcordiformis colonies.
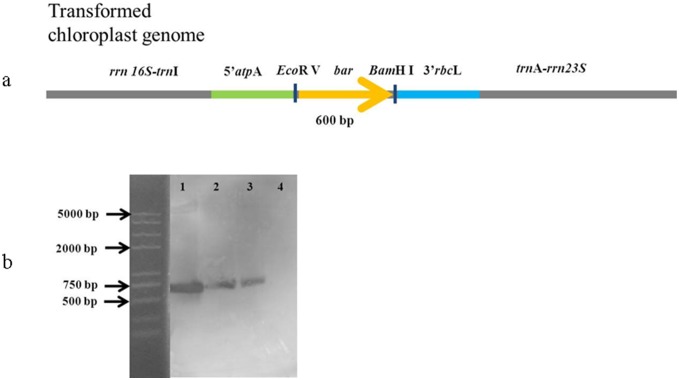
Total DNA (≥4 µg) of each sample was firstly digested with EcoR V and BamH I. The probe was hybridized with the bar gene. The size of hybridized fragment should be 600 bp. Lane 1, pPSCB; lane 2, PSCB1; Lane 3, PSCB2; lane 4, wild-type of P. subcordiformis.
Discussion
Here, a stable transformation of P. subcordiformis chloroplast was achieved using the gfp gene as a reporter and the bar gene as a selectable marker. This technique will facilitate the genetic manipulation of this organism to express foreign genes or modify the expression of endogenous ones. This successful algal chloroplast transformation system will promote research on other algal chloroplast systems.
An efficient chloroplast transformation system requires four parts, including an endogenous plastome fragment as a homologous insertion site, a promoter originating from endogenous or heterogeneous chloroplasts, a suitable selectable marker or a reporter gene, and a practical method to transfer the foreign plasmid into host cells. Particle bombardment is the most widely used method for plasmid delivery into chloroplasts [6], [8] and the transforming frequency is quite high. In the present research, particle bombardment was successfully used to transfer the foreign plasmid into the P. subcordiformis chloroplast. However, this method was less efficient for nuclear transformation of P. subcordiformis than glass-bead agitation [18]. This difference may be related to the fact that P. subcordiformis has a single large cup-shaped chloroplast surrounding the nucleolus, which may facilitate particles entering the former while blocking them from the latter.
The gfp gene is a widely-used reporter gene for chloroplast transformation [30]–[32]. In photosynthetic cells, chlorophyll and heterogonous GFP can fluoresce with excitation. Based on the difference in fluorescence between chlorophyll and GFP, positively-transformed cells were harvested by FACS [33]–[34]. The difference was also used to obtain separate fluorescence images of both chlorophyll and GFP by CLSM [30], [35], allowing GFP to be localized in the chloroplast.
Platymonas subcordiformis is compressed and obovate in outline with dimensions of 11–16 µm in length, 7–9 µm in breadth and 3.5–5 µm in depth [36]. Under CLSM, the cells always lay flat, with depth as the Z-axis, which resulted in just five to seven images during Z-series scanning. These images were not sufficient for a very clear three-dimensional image. Further research is needed to find a method to orient P. subcordiformis cells so that the length is the Z-axis, and technical improvements are also needed for a thinner optical section to obtain more images within a Z-axis to create a clear three-dimensional image.
The bar gene conferring resistance to Basta is an excellent selective marker in nuclear transformation of plants [20]–[21] and algae [18], [22]–[23], but it failed in the direct selection of tobacco chloroplast transformation [24]. The reasons for this failure are not presently clear and predicted from the lethal selection of Basta in the initial selection of the tissue culture before a sufficient proportion of the homologous recombinant plastomes established, while the selection of antibiotics was non-lethal [37].
In the present research, we tested the bar gene as the direct selectable marker and found that bar is suitable for P. subcordiformis chloroplast transformation. The results may be explained in two ways. First, as a unicellular alga with rapid growth rate, the transformed P. subcordiformis could recover within about 8 h of dark culture, unlike the long tissue culture period in tobacco leaf transformation. Also, because the alga has a single chloroplast, the plastome inserted with foreign gene could be easier to reach a high portion in the chloroplast. As a result, the bar gene could be expressed quickly and at high levels in P. subcordiformis. This is the first research to employ the bar gene as a selectable marker in algal plastid transformation. Further research is needed on expression pattern of the bar gene in the P. subcordiformis chloroplast.
Conclusion
This paper reports the successful chloroplast transformation of P. subcordiformis. Much further work is needed to develop a sophisticated system. The regulation of gene expression in P. subcordiformis chloroplasts is in urgent need for the endogenous promoters. Furthermore, to express several genes at the same time, the construct of a polycistron in this alga is needed.
Acknowledgments
The authors thank Prof. Song Xue of Dalian Institute of Chemical and Physics, Chinese Academy of sciences, China, for her help during the research.
Funding Statement
This work was supported by Ocean Public Welfare Scientific Research Project, State Oceanic Administration of China (201205027), National Natural Science Foundation of China (41176144). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Guo Z, Chen Z, Zhang W, Yu X, Jin M (2008) Improved hydrogen photoproduction regulated by carbonylcyanide m-chlorophenylhrazone from marine green alga Platymonas subcordiformis grown in CO2-supplemented air bubble column bioreactor. Biotechnol Lett 30: 877–883. [DOI] [PubMed] [Google Scholar]
- 2. DeCosa B, Moar W, Lee SB, Miller M, Daniell H (2001) Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol 19: 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daniell H, Khan MS, Allison L (2002) Milestones in chloroplast genetic engineering: an environmentally friendly era in biotechnology. Trends Plant Sci 7: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SB, Kwon HB, Kwon SJ, Park SC, Jeong MJ, et al. (2003) Accumulation of trehalose within transgenic chloroplasts confers drought tolerance. Mol Breeding 11: 1–13. [Google Scholar]
- 5. Quesada-Vargas T, Ruiz ON, Daniell H (2005) Characterization of heterologous multigene operons in transgenic chloroplasts. Transcription, processing, and translation. Plant Physiol 138: 1746–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, et al. (1988) Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240: 1534–1538. [DOI] [PubMed] [Google Scholar]
- 7. Blowers AD, Bogorad L, Shark KB, Sanford JC (1989) Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell 1: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Nat Acad Sci 87: 8526–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidorov VA, Kasten D, Pang SZ, Hajdukiewicz PTJ, Staub JM, et al. (1999) Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J 19: 209–216. [DOI] [PubMed] [Google Scholar]
- 10. Hou BK, Zhou YH, Wan LH, Zhang ZL, Shen GF, et al. (2003) Chloroplast transformation in oilseed rape. Transgenic Res 12: 111–114. [DOI] [PubMed] [Google Scholar]
- 11. Lee SM, Kang K, Chung H, Yoo SH, Xu XM, et al. (2006) Plastid transformation in the monocotyledonous cereal crop, rice (Oryza sativa) and transmission of transgenes to their progeny. Mol cells 21: 401–410. [PMC free article] [PubMed] [Google Scholar]
- 12. Doetsch NA, Favreau MR, Kuscuoglu N, Thompson MD, Hallick RB (2001) Chloroplast transformation in Euglena gracilis: splicing of a group III twintron transcribed from a transgenic psbK operon. Curr Genet 39: 49–60. [DOI] [PubMed] [Google Scholar]
- 13. Lapidot M, Raveh D, Sivan A, Arad SM, Shapira M (2002) Stable chloroplast transformation of the unicellular red alga Porphyridium species. Plant Physiol 129: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker for site-directed transformation of Chlamydomonas . Nucleic Acids Res 19: 4083–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Nat Acad Sci 90: 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carrer H, Hockenberry TN, Svab Z, Maliga P (1993) Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet 241: 49–56. [DOI] [PubMed] [Google Scholar]
- 17. Maliga P (2002) Engineering the plastid genome of higher plants. Curr Opin Plant Biol 5: 164–172. [DOI] [PubMed] [Google Scholar]
- 18. Cui YL, Jiang P, Wang JF, Li FC, Chen YJ, et al. (2012) Genetic transformation of Platymonas (Tetraselmis) subcordiformis (Prasinophyceae, Chlorophyta) using particle bombardment and glass-bead agitation. Chin J Oceanol Limnol 30: 471–475. [Google Scholar]
- 19. Ye GN, Colburn SN, Xu CW, Hajdukiewicz PTJ, Staub JM (2003) Persistence of unselected transgenic DNA during a plastid transformation and segregation approach to herbicide resistance. Plant Physiol 133: 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White J, Chang S, Bibb MJ (1990) A cassette containing the bar gene of Streptomyces hygroscopicus: a selectable marker for plant transformation. Nucleic Acids Res 18: 1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang C, Han P, Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40: 711–717. [DOI] [PubMed] [Google Scholar]
- 22. Tan C, Qin S, Zhang Q, Jiang P, Zhao F (2005) Establishment of a micro-particle bombardment transformation system. J Microbiol 43: 361–365. [PubMed] [Google Scholar]
- 23. Zhang YC, Jiang P, Gao JT, Liao JM, Sun SJ, et al. (2008) Recombinant expression of rt-PA gene (encoding reteplase) in gametophytes of the seaweed Laminaria japonica (Laminariales, Phaeophyta). Sci China Ser C Life Sci 51: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 24. Lutz KA, Knapp JE, Maliga P (2001) Expression of bar in the plastid genome confers herbicide resistance. Plant Physiol 125: 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirel B, Perrot-Rechenmann C, Maudimas B, Gadal P (1982) Glutamine synthetase in alder (Alnus glutinosa) root nodules. Purification, properties and cytoimmunochemical localization. Physiol Plantarum 55(2): 197–203. [Google Scholar]
- 26. Ghoshroy S, Binder M, Tartar A, Robertson DL (2010) Meseoarlceh acrtuicllear evolution of glutamine synthetase II: Phylogenetic evidence of a non-endosymbiotic gene transfer event early in plant evolution. BMC Evol Biol 10: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McNally SF, Hirel B, Gadal P, Mann AF, Stewart GR (1983) Glutamine synthetases of higher plants. Plant Physiol. 72: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Su ZL, Qian KX, Tan CP, Meng CX, Qin S (2005) Recombination and heterologous expression of allophycocyanin gene in the chloroplast of Chlamydomonas reinhardtii . Acta Biochim Biophys Sin 37(10): 709–712. [DOI] [PubMed] [Google Scholar]
- 29. Rasala BA, Muto M, Lee PA, Jager M, Caedoso RMF, et al. (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J 8: 719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hibberd JM, Linley PJ, Khan MS, Gray JC (1998) Transient expression of green fluorescent protein in various plastid types following microprojectile bombardment. Plant J 16: 627–632. [Google Scholar]
- 31. Lelivelt CLC, McCabe MS, Newell CA, Desnoo CB, Dun KMP, et al. (2005) Stable plastid transformation in lettuce (Lactuca sativa L.). Plant mol Biol 58: 763–774. [DOI] [PubMed] [Google Scholar]
- 32. Limaye A, Koya V, Samsam M, Daniell H (2006) Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J 20: 959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang TT, Sinai P, Green G, Kitts PA, Chen YT, et al. (1998) Improved fluorescence and dual color detection with enhanced blue and green variants of the green fluorescent protein. J Biol Chem 273: 8212–8216. [DOI] [PubMed] [Google Scholar]
- 34. Rieseberg M, Kasper C, Reardon KF, Scheper T (2001) Flow cytometry in biotechnology. Appl Microbiol Biotechnol 56: 350–360. [DOI] [PubMed] [Google Scholar]
- 35. Kohler RH, Cao J, Zipfel WR, Webb WW, Hanson MR (1997) Exchange of protein molecules through connections between higher plant plastids. Science 276: 2039–2042. [DOI] [PubMed] [Google Scholar]
- 36. Salisbury J, Swanson J, Floyd G, Hall R, Maihle NJ (1981) Ultrastructure of the flagellar apparatus of the green alga Tetraselmis subcordiformis . Protoplasma 107: 1–11. [Google Scholar]
- 37. Maliga P (2004) Plastid transformation in higher plants. Annu Rev Plant Biol 55: 289–313. [DOI] [PubMed] [Google Scholar]