Abstract
The objective of this study was to examine the influence of sensory experience on the synaptic circuitry of the cortex. For this purpose, the quantitative distribution of the overall and of the gamma-aminobutyric acid (GABA) population of synaptic contacts was investigated in each layer of the somatosensory barrel field cortex of rats which were sensory deprived from birth by continuously removing rows of whiskers. Whereas there were no statistically significant changes in the quantitative distribution of the overall synaptic population, the number and proportion of GABA-immunopositive synaptic contacts were profoundly altered in layer IV of the somatosensory cortex of sensory-deprived animals. These changes were attributable to a specific loss of as many as two-thirds of the GABA contacts targeting dendritic spines. Thus, synaptic contacts made by GABA terminals in cortical layer IV and, in particular, those targeting dendritic spines represent a structural substrate of experience-dependent plasticity. Furthermore, since in this model of cortical plasticity the neuronal receptive-field properties are known to be affected, we propose that the inhibitory control of dendritic spines is essential for the elaboration of these functional properties.
Full text
PDF
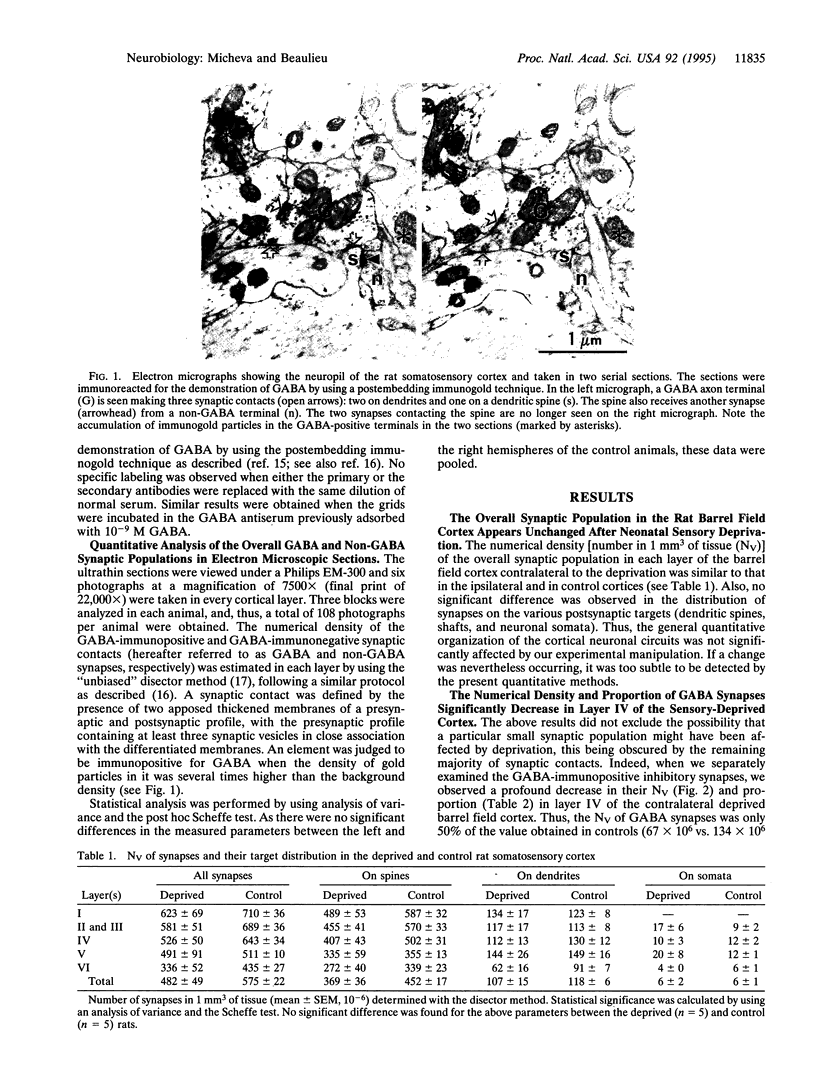
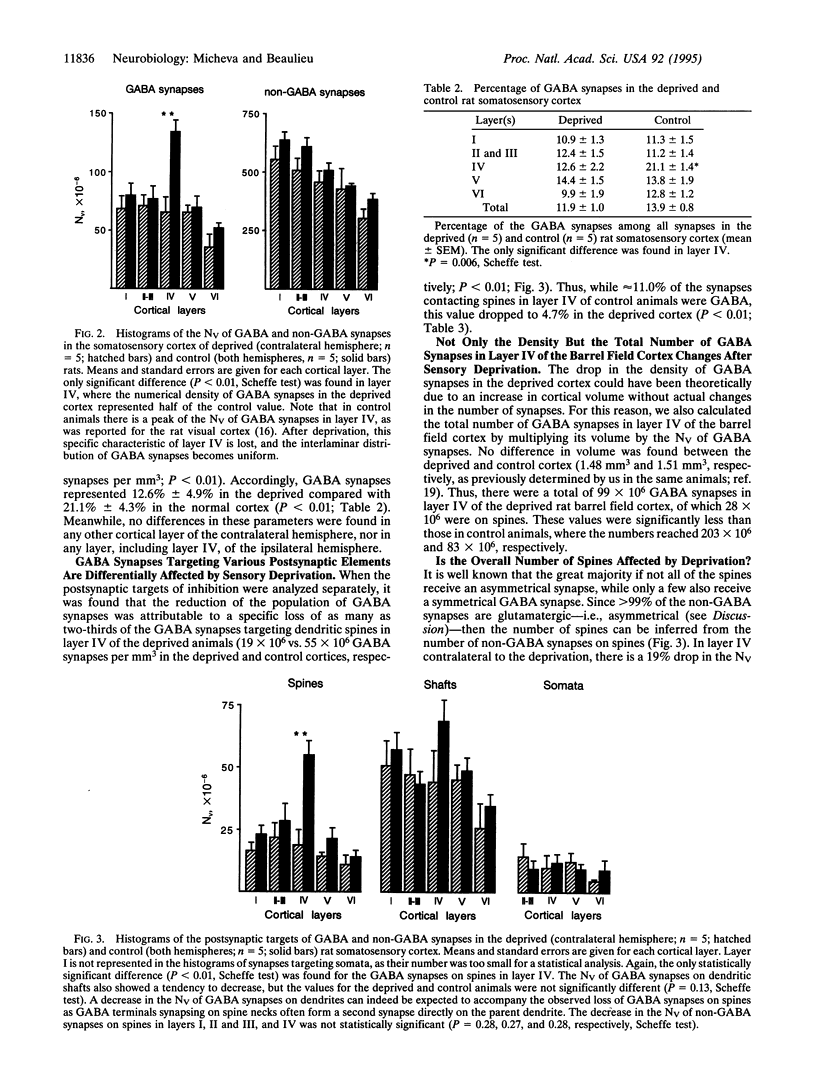
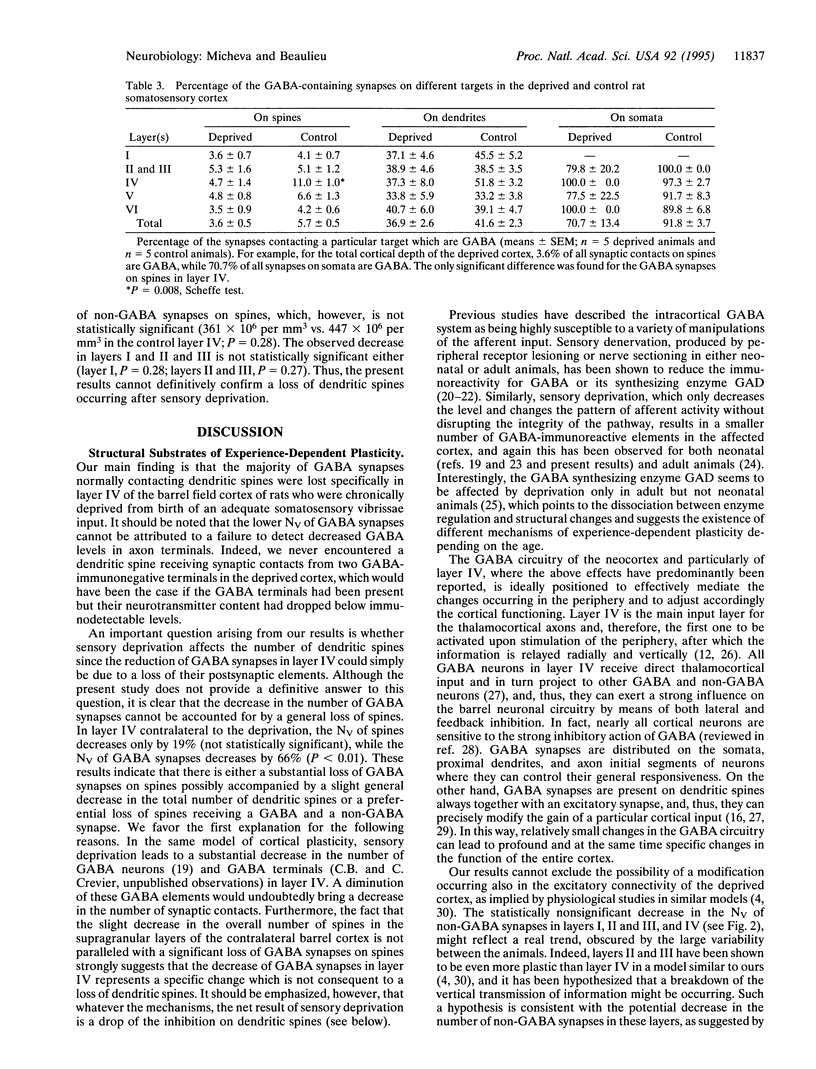

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar N. D., Land P. W. Activity-dependent regulation of glutamic acid decarboxylase in the rat barrel cortex: effects of neonatal versus adult sensory deprivation. J Comp Neurol. 1991 May 8;307(2):200–213. doi: 10.1002/cne.903070204. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K., Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992 Oct;68(4):1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Audet M. A., Descarries L., Doucet G. Quantified regional and laminar distribution of the serotonin innervation in the anterior half of adult rat cerebral cortex. J Chem Neuroanat. 1989 Jan-Feb;2(1):29–44. [PubMed] [Google Scholar]
- Audet M. A., Doucet G., Oleskevich S., Descarries L. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. J Comp Neurol. 1988 Aug 15;274(3):307–318. doi: 10.1002/cne.902740302. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Campistron G., Crevier C. Quantitative aspects of the GABA circuitry in the primary visual cortex of the adult rat. J Comp Neurol. 1994 Jan 22;339(4):559–572. doi: 10.1002/cne.903390407. [DOI] [PubMed] [Google Scholar]
- Benevento L. A., Bakkum B. W., Port J. D., Cohen R. S. The effects of dark-rearing on the electrophysiology of the rat visual cortex. Brain Res. 1992 Feb 14;572(1-2):198–207. doi: 10.1016/0006-8993(92)90470-t. [DOI] [PubMed] [Google Scholar]
- Dehay C., Douglas R. J., Martin K. A., Nelson C. Excitation by geniculocortical synapses is not 'vetoed' at the level of dendritic spines in cat visual cortex. J Physiol. 1991;440:723–734. doi: 10.1113/jphysiol.1991.sp018732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L., Lemay B., Doucet G., Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987 Jun;21(3):807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992 May;12(5):1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J Neurosci. 1994 Dec;14(12):7665–7679. doi: 10.1523/JNEUROSCI.14-12-07665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty P. E., LaChica E. A., Kaas J. H. Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosens Mot Res. 1991;8(4):347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986 Apr 24;320(6064):750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Hicks T. P., Dykes R. W. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983 Sep 5;274(1):160–164. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. Morphological variations in the dendritic spines of the neocortex. J Cell Sci. 1969 Sep;5(2):509–529. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Merzenich M. M., Killackey H. P. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Keller A., White E. L. Synaptic organization of GABAergic neurons in the mouse SmI cortex. J Comp Neurol. 1987 Aug 1;262(1):1–12. doi: 10.1002/cne.902620102. [DOI] [PubMed] [Google Scholar]
- Kossut M., Stewart M. G., Siucinska E., Bourne R. C., Gabbott P. L. Loss of gamma-aminobutyric acid (GABA) immunoreactivity from mouse first somatosensory (SI) cortex following neonatal, but not adult, denervation. Brain Res. 1991 Jan 4;538(1):165–170. doi: 10.1016/0006-8993(91)90393-a. [DOI] [PubMed] [Google Scholar]
- Land P. W., Simons D. J. Cytochrome oxidase staining in the rat SmI barrel cortex. J Comp Neurol. 1985 Aug 8;238(2):225–235. doi: 10.1002/cne.902380209. [DOI] [PubMed] [Google Scholar]
- Qian N., Sejnowski T. J. When is an inhibitory synapse effective? Proc Natl Acad Sci U S A. 1990 Oct;87(20):8145–8149. doi: 10.1073/pnas.87.20.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Silverman M. S., Tootell R. B. Modified technique for cytochrome oxidase histochemistry: increased staining intensity and compatibility with 2-deoxyglucose autoradiography. J Neurosci Methods. 1987 Jan;19(1):1–10. doi: 10.1016/0165-0270(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Simons D. J., Land P. W. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987 Apr 16;326(6114):694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., Chubb I. W., Penke B., Erdei A. Antisera to gamma-aminobutyric acid. II. Immunocytochemical application to the central nervous system. J Histochem Cytochem. 1985 Mar;33(3):240–248. doi: 10.1177/33.3.2579123. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Warren R., Tremblay N., Dykes R. W. Quantitative study of glutamic acid decarboxylase-immunoreactive neurons and cytochrome oxidase activity in normal and partially deafferented rat hindlimb somatosensory cortex. J Comp Neurol. 1989 Oct 22;288(4):583–592. doi: 10.1002/cne.902880405. [DOI] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Welker C., Woolsey T. A. Structure of layer IV in the somatosensory neocortex of the rat: description and comparison with the mouse. J Comp Neurol. 1974 Dec 15;158(4):437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- Woolsey T. A. Somatosensory, auditory and visual cortical areas of the mouse. Johns Hopkins Med J. 1967 Aug;121(2):91–112. [PubMed] [Google Scholar]




