Abstract
Background and Objectives:
The fibrosing form of lung injury (occupational, environmental, infective or drug induced) is associated with significant morbidity and mortality. Amiodarone (AM), often prescribed for control of arrhythmias is considered a potential cause. No effective treatment was confirmed, except lung transplantation. Intravenous (IV) stem cell therapy may produce pulmonary emboli or infarctions. Despite being commonly used in clinical practice, the intraperitoneal (IP.) route has been rarely used for cell delivery. The present study aimed at investigating and comparing the possible effect of IP stem cell therapy (SCT) on pulmonary toxicity versus the intravenous route in a rat model of amiodarone induced lung damage.
Methods and Results:
36 adult male albino rats were divided into 4 groups. Rats of AM group were given 30 mg/kg daily orally for 4 weeks. Rats of IV SCT group were injected with stem cells in the tail vein. Rats of IP SCT group received IP cell therapy. Histological, histochemical, immunohistochemical and morphometric studies were performed. Obstructed bronchioles, overdistended alveoli, reduced type I pneumocytes, increased thickness of alveolar septa and vessels wall besides increased area% of collagen fibers regressed in response to IV and IP SCT. The improvement was more obvious in IV group. The area% of Prussion blue +ve and CD105 +ve cells was significantly higher in IV group.
Conclusions:
Cord blood MSC therapy proved definite amelioration of lung injury ending in fibrosis. The effect of IP SCT was slightly inferior to that of IV SCT, which may be overwhelmed by repeated IP injection.
Keywords: Mesenchymal stem cells, Amiodarone, Cord blood, Lung injury
Introduction
The fibrosing form of lung injury is associated with significant morbidity and mortality. Lung injury may be secondary to occupational or environmental exposures (1), in addition to infection (2). Drug induced pathogenesis has to be taken into consideration and amiodarone, often prescribed for the control of atrial and ventricular arrhythmias is considered a potential cause (3).
However, it was reported that no effective treatment was confirmed for pulmonary fibrosis except lung transplantation (4). This was recently approved by the absence of causal treatment option of pulmonary toxicity ending in fibrosis (5).
Mesenchymal stem cells (MSCs) are stromal cells that have the ability to self-renew and exhibit multilineage differentiation, which makes them an attractive choice for possible development of clinical applications (6). Recent studies suggest that cord blood transplant (CBT) is a safe and effective strategy for stem cell transplant patients (7). Immediate availability, absence of risk for donors and lower risk of acute graft-versus-host disease suggested CBT as a widely used source of mesenchymal cell support (8).
However, concentrated cells injected into tissues can form aggregates and intravenous (IV) injection can produce pulmonary emboli or infarctions (9). IV transplantation showed significant improvement of lung function, but 6∼17% treatment-related mortality (10). Pre-engraftment syndrome (PES) developed in 26.8% of patients receiving IV therapy (11). Despite being commonly used in clinical practice, the intraperitoneal (i.p.) route has been rarely used for cell delivery (12).
The present study aimed at investigating and comparing the possible effect of intraperitoneal human cord blood mesenchymal stem cell therapy on pulmonary toxicity versus the intravenous route. This was accomplished by using amiodarone as a model of induced lung damage in albino rat.
Materials and Methods
Drug
Amiodarone (Cordarone): Used as 200 mg tablets, crushed, the required dose for each rat weighed and dissolved in 0.5 ml tween 80 (Sanofi Corporation).
Animals
Thirty six adult male albino rats weighing 150–200g were divided into 4 groups. Each group was kept in a separate cage under good hygienic conditions, fed ad libitum and allowed for free water supply in Histology Department Animal House, Faculty of Medicine, Cairo University. The rats were treated in accordance with guidelines approved by the Animal Use Committee of Cairo University.
Control Group: 6 rats, 2 for each experimental group. Two rats were given 0.5 ml tween 80 (solvent of amiodarone) daily orally for 4 weeks. The latter being water insoluble at room temperature. Next 2 rats were given 0.5 ml tween 80 daily orally for the same period and 0.5 ml phosphate buffer saline (PBS) by intravenous injection (IVI) in tail vein for 2 days. Last 2 rats received tween 80 daily orally for the same period and 0.5 ml PBS by intraperitoneal injection (IPI) for 2 days.
Group A (Amiodarone group): 10 animals were used in this group. Each received 30 mg/kg of amiodarone daily orally (13) for 4 weeks,. then left for 4 weeks without therapy (14). A stock solution of the drug was prepared weekly and kept at 4°C. The required dose for each rat was introduced into the mouth using a syringe with a metal tube instead of the needle.
Group IV (IVI Stem cell therapy group): 10 rats were given amiodarone in the same dose, by the same route and for the same duration as in group A. Then the animals were injected each with 0.5 ml of cultured and labeled mesenchymal stem cells (MSCs) suspended in phosphate buffer saline (PBS) on two successive days in the tail vein (15).
Group IP (IPI Stem cell therapy group): 10 rats were given amiodarone in the same dose, by the same route and for the same duration as in group A. Then the animals were injected each with 0.5 ml of cultured and labeled MSCs suspended in phosphate buffer saline (PBS) on two successive days (16).
Stem cells were isolated from cord blood (17). Cord blood collection was performed at Gynaecology Department, Faculty of Medicine, Cairo University. Stem cell culture and labeling were performed at Hematology Unit, New Kasr El Aini Teaching Hospital, Cairo University. The animals were sacrificed 4 weeks following therapy (18).
Cord blood collection (19)
The storage and transport temperature was 15∼22°C, transport time was 8∼24 hours, sample volume was 65∼250 ml, and no sample had signs of coagulation or hemolysis.
Mononuclear cell fraction isolation and propagation (16)
The mononuclear cell fraction (MNCF) was isolated by loading 30 ml of whole blood onto 10 ml of Ficoll density media (Healthcare Bio-Sciences) in 50 ml polypropylene tubes, centrifuge for 30 minutes at room temperature. The interphase collected after aspirating and discarding the supernatant. The interphase was washed with 20 ml PBS and centrifuged at room temperature. The supernatant was aspirated and the cells were washed with PBS a second time. The cells were re-suspended in the isolation media and transferred to culture dishes. The isolation media was low-glucose DMEM (Dulbecco’s modified Eagles medium) (Cambrex Bio Science) supplemented with low dexamethazone (10−7 M) (Sigma-Aldrich), penicillin (100 IU/ml) (Invitrogen), streptomycin (0.1 mg/ml) (Invitrogen), and ultraglutamine (2 mM) (Cambrex Bio-Science). Incubation was at 38.5°C in humidified atmosphere containing 5% CO2.
Culture (19)
The isolation media were replaced after overnight incubation (12∼18 hours) in order to remove non-adherent cells. The media were replaced every 3 days until MSC colonies were noted. The cultures were inspected daily for formation of adherent spindle-shaped fibroblastoid cell colonies. Sub-culturing was done by chemical detachment using 0.04% trypsin. Later, when cell numbers allowed, expansion was done in 25 cm2 or 75 cm2 tissue culture flasks.
Labeling (20)
Mesenchymal stem cells were labeled by incubation with ferumoxides injectable solution (25 microgramFe/ml, Feridex, Berlex Laboratories) in culture medium for 24 hours with 375 nanogram/ml poly L lysine added 1 hour before cell incubation. Labeling was histologically assessed using Prussian blue. Feridex labeled HMSCs were washed in PBS, trypsinized, washed and resuspended in 0.01 Mol/L PBS at concentration of 1×1,000,000 cells/ml.
Cell viability analysis
Cell viability was done using trypan blue dye exclusion test. This method is based on the principle that viable cells do not take up certain dyes, whereas dead cells do.
Flow cytometry (21)
Flow cytometric analyses were performed on a Fluorescence Activated Cell Sorter (FACS) flow cytometer (Coulter Epics Elite, Miami, FL, USA). HMSC were trypsinized and washed twice with PBS. A total number of 1 ×105 HMSC were used for each run. To evaluate the HMSC marker profile, cells were incubated in 100 μL of PBS with 3 μL of CD105-FITC for 20 min at room temperature. Antibody concentration was 0.1 mg mL−1. Cells were washed twice with PBS and finally diluted in 200 μL of PBS. The expression of surface marker was assessed by the mean fluorescence. CD105 (mesenchymal stem cell marker), CD133 (early hematopoietic & endothelial progenitor stem cell marker) and CD45 (panleucocytic marker) were also used. The percentage of cells positive for CD 105 was determined by subtracting the percentage of cells stained non-specifically with isotype control antibodies.
Histological Study
The animals were sacrificed by decapitation. Lung specimens were removed and fixed in 10% formol saline for 24 hours. Paraffin blocks were prepared and 5 μm thick sections were subjected to hematoxylin and eosin (H&E) (22) and Masson’s trichrome stain (23).
Histochemical Study
Lung sections were stained with Prussian blue (PB) stain (24) for demonstration of iron oxide labeled therapeutic stem cells.
Immunohistochemical Study
CD105 immunostaining (25) the marker for mesenchymal stem cells. 0.1 ml prediluted primary antibody (CD105) rabbit polyclonal Ab (ab27422) and incubate at room temperature in moist chamber for 30∼60 minutes. Tonsil used as positive control specimens. Cellular localization is the cell membrane. On the other hand, one of the lung sections was used as a negative control by passing the step of applying the primary antibody.
Morphometric Study
Using Leica Qwin 500 LTD image analysis, assessment of the thickness of the alveolar septa and the thickness of pulmonary vessels (indicated by the distance parameter) was performed in H&E stained sections. In addition, the area of collagen fibers was done in Masson’s trichrome sections using interactive measurements menu. The measurments were done in 10 high power fields (HPF) in control and experimental groups. The area% of Pb +ve and that of CD105 +ve cells were assessed. The measurements were done in 10 HPF in control and experimental groups.
Statistical analysis
Quantitative data were summarized as means and standard deviations and compared using one-way analysis-of-variance (ANOVA). p-values <0.05 were considered statistically significant. Calculations were made on SPSS software (26).
Results
Haematoxylin and Eosin (H&E) Stained Sections
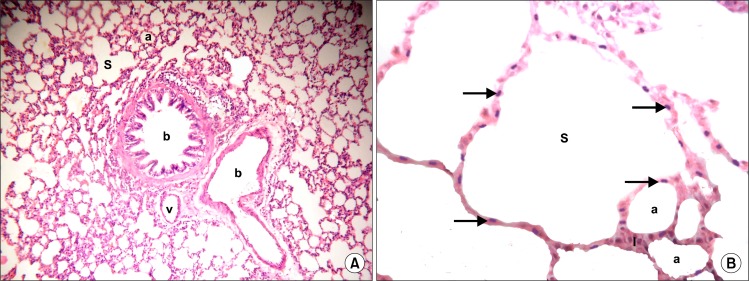
Sections in the lung of control rats showed normal structure of bronchioles, alveoli, alveolar sacs, vessels and alveolar septa (Fig. 1A). Close observation revealed alveoli and alveolar sacs lined by multiple cells exhibiting flat nuclei (Fig. 1B).
Fig. 1.
(A) Section in the lung of a control rat showing two bronchioles (b), alveoli (a), alveolar sacs (S) and a vessel (v) (H&E, ×100). (B) Section in the lung of a control rat showing alveoli (a) and alveolar sacs (S) lined by multiple cells exhibiting flat nuclei (arrows). Note alveolar septa (I) (H&E, ×400).
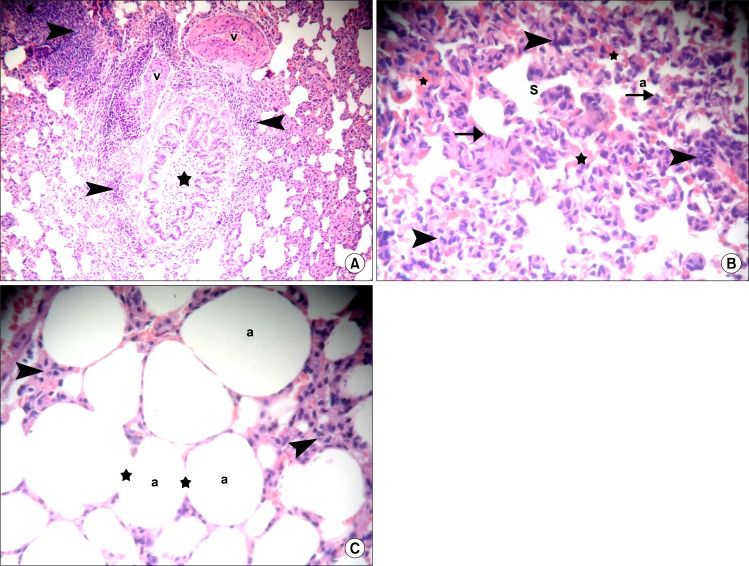
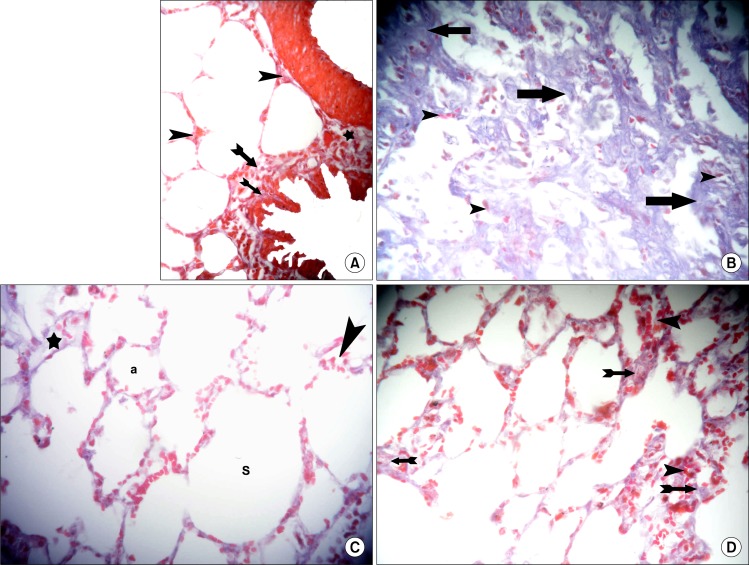
Sections in the lung of rats belonging to amiodarone group demonstrated small bronchioles with partial obliteration of the lumen by shed epithelial cells and cellular infiltration in the adventitia in multiple fields. Thickened wall of some vessels and bvious cellular aggregates were found in some alveolar septa (Fig. 2A). Close observation recruited thickening of the alveolar septa exhibiting dense cellular infiltration and extravasated RBCs in some fields. Irregular alveoli and alveolar spaces were noticed, which were lined by few cells exhibiting flat nuclei (Fig. 2B). On the other hand individual fields contained overdistended alveoli with disrupted alveolar walls and cellular infiltrates in the alveolar septa (Fig. 2C).
Fig. 2.
(A) Section in the lung of a rat in amiodarone group showing a small bronchiole with partial obliteration of the lumen by shed epithelial cells (*), thickened wall of two vessels (v), cellular aggregates in alveolar septa and cellular infiltration in the adventitia of bronhiole (arrowheads) (H&E, ×100). (B) Section in the lung of a rat in amiodarone group showing thickening of the alveolar septa exhibiting dense cellular infiltration (arrowheads), irregular alveoli (a) and alveolar spaces (S) lined by few cells exhibiting flat nuclei (arrows). Note multiple extravasated RBCs (*) (H&E, ×100). (C) Section in the lung of a rat in amiodarone group showing over-distended alveoli (a) with disrupted alveolar walls (*). Note cellular infiltrates in the alveolar septa (arrowheads) (H&E, ×400).
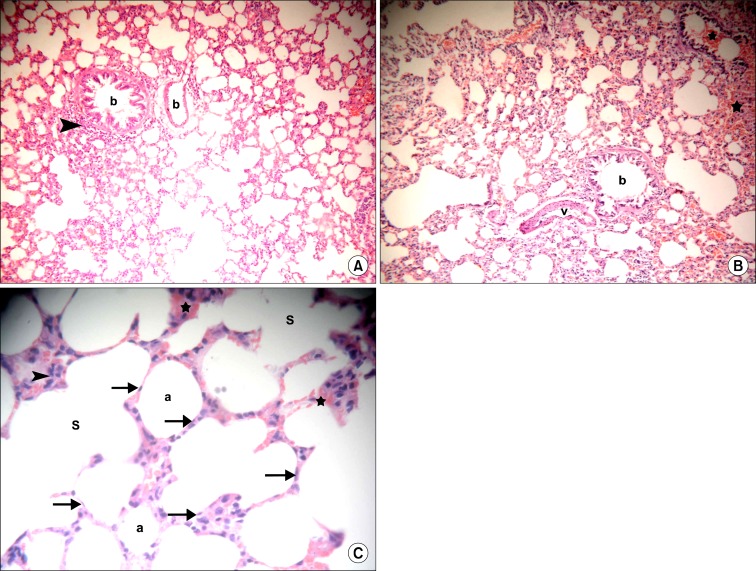
Sections in the lung of rats in IVI stem cell therapy group showed normal bronchioles, few of which demonstrated cellular infiltrate in the adventitia and few congested vessels in some fields (Fig. 3A). Other fields revealed occasional vessels with partially thickened wall. Multiple congested vessels and some extravasated RBCs were also recognized (Fig. 3B). Normal alveoli and alveolar sacs were lined by multiple cells exhibiting flat nuclei. Cellular infiltrates and extravasated RBCs were noted in few alveolar septa by close observation (Fig. 3C).
Fig. 3.
(A) Section in the lung of a rat in IVI stem cell therapy group showing two bronchioles (b), one of them demonstrates cellular infiltrate in the adventitia (arrowhead) and a congested vessel (c) (H&E, ×100). (B) Section in the lung of a rat in IVI stem cell therapy group showing a bronchiole (b), a vessel with partially thickened wall (v), congested vessels (c) and extravasated RBCs (*) (H&E, ×100). (C) Section in the lung of a rat in IVI stem cell therapy group showing normal alveoli (a) and alveolar sacs (S) lined by multiple cells exhibiting flat nuclei (arrows). Note cellular infiltrates (arrowheads) and extravasated RBCs in few alveolar septa (*) (H&E, ×400).
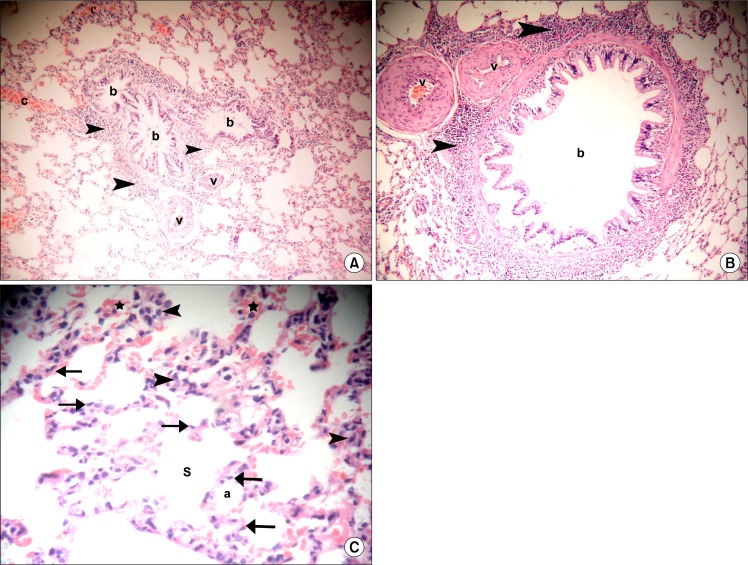
Sections in the lung of rats in IPI stem cell therapy group showed normal bronchioles surrounded by infiltrating cells, few vessels with partial thickening of their walls and some congested vessels (Fig. 4A). Occasional fields contained few vessels with marked thickening of their walls (Fig. 4B). By close observation normal alveoli and alveolar sacs were lined by multiple cells exhibiting flat nuclei. Cellular infiltrates and extravasated RBCs were found in multiple alveolar septa (Fig. 4C).
Fig. 4.
(A) Section in the lung of a rat in IPI stem cell therapy group showing three normal bronchioles (b) surrounded by infiltrating cells (arrowheads) and two vessels (v) with partial thickening of their walls. Note some congested vessels (c) (H&E, ×100). (B) Section in the lung of a rat in IPI stem cell therapy group showing a normal bronchiole (b) surrounded by infiltrating cells (arrowheads) and two vessels (v) with marked thickening of their walls (v) (H&E, ×100). (C) Section in the lung of a rat in IPI stem cell therapy group showing normal alveoli (a) and alveolar sacs (S) lined by multiple cells exhibiting flat nuclei (arrows). Note cellular infiltrates (arrowheads) and extravasated RBCs in multiple alveolar septa (*) (H&E, ×400).
Masson’s Trichrome Stained Sections
Sections in the lung of control rats showed fine collagen fibers in the alveolar septa and denser collagen fibers in the wall of bronchioles and the adventitia of blood vessels (Fig. 5A). In amiodarone group dense collagen fibers and infiltrating cells were evident in the alveolar septa (Fig. 5B). In IVI stem cell therapy group dense collagen fibers were seen in occasional septa and infiltrating cells in other occasional septa around normal alveoli and alveolar sacs (Fig. 5C). In IPI stem cell therapy group dense collagen fibers were observed in some septa and infiltrating cells in other septa (Fig. 5D).
Fig. 5.
(A) Section in the lung of a control rat showing fine collagen fibers (arrowheads) in the alveolar septa, denser collagen fibers in the wall of a bronchiole (arrows) and in the adventitia (*) of a blood vessel (Masson’s trichrome, ×400). (B) Section in the lung of a rat in amiodarone group showing dense collagen fibers (arrows) and infiltrating cells (arrowheads) in the alveolar septa (Masson’s trichrome, ×400). (C) Section in the lung of a rat in IVI stem cell therapy group showing dense collagen fibers (*) in a septum and infiltrating cells (arrowhead) in another septum around normal alveoli (a) and alveolar sacs (S) (Masson’s trichrome, ×400). (D) Section in the lung of a rat in IPI stem cell therapy group showing dense collagen fibers (arrows) in some septa and infiltrating cells (arrowheads) in other septa (Masson’s trichrome, ×400).
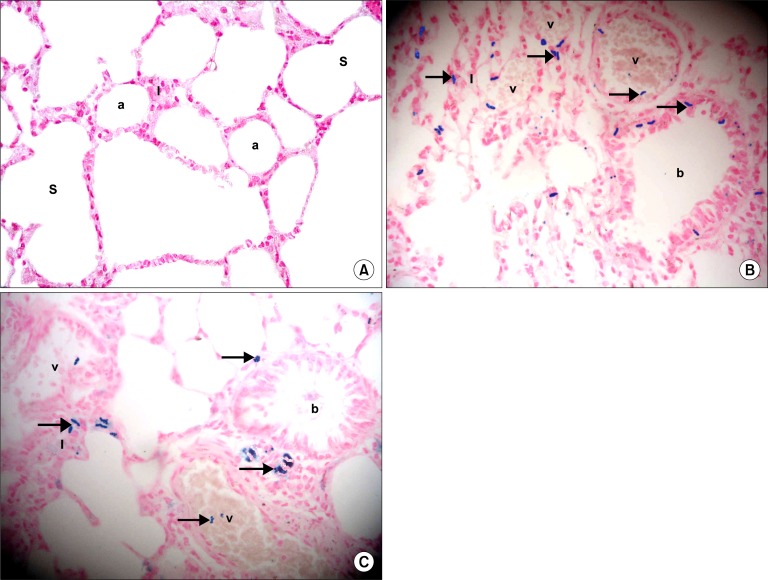
Prussian Blue Stained Sections
Sections in the lung of control rats showed negative staining with Prussian blue in the alveoli, alveolar sacs and alveolar septa (Fig. 6A). In IVI stem cell therapy group multiple Pb+ve were detected inside and around blood vessels. In addition, the +ve cells were noticed in some alveolar septa and among the lining epithelium of bronchioles (Fig. 6B). In IPI stem cell therapy group fewer Pb+ve were detected inside blood vessels. In addition, the +ve cells were noticed in some alveolar septa and in the adventitia of bronchioles (Fig. 6C).
Fig. 6.
(A) Section in the lung of a control rat showing negative staining in the alveoli (a), alveolar sacs (S) and alveolar septa (I) (Prussian blue, ×400). (B) Section in the lung of a rat in IVI stem cell therapy group showing multiple Prussian blue positive cells (arrows) inside and around blood vessels (v), in the alveolar septa (I) and among the epithelial lining of a bronchiole (b) (Prussian blue, ×400). (C) Section in the lung of a rat in IPI stem cell therapy group showing some Prussian blue positive cells (arrows) inside blood vessels (v), in the alveolar septa (I) and in the adventitia of a bronchiole (b) (Prussian blue, ×400).
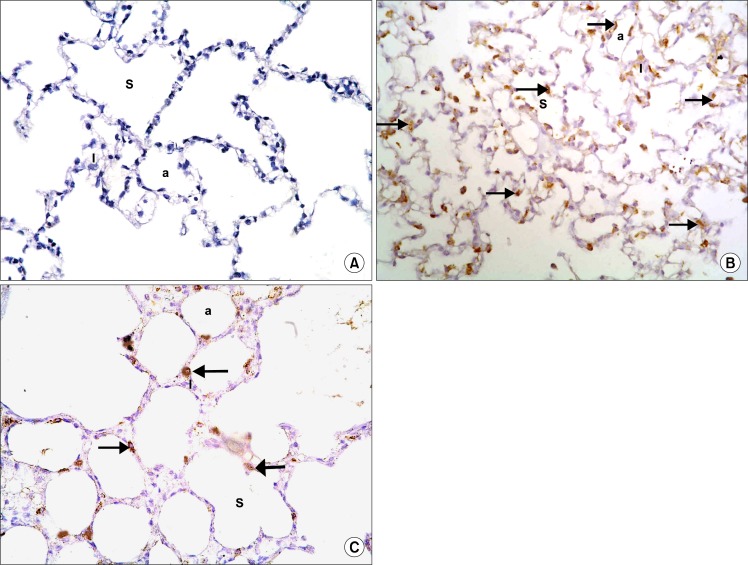
CD105 Immunostained sections
Sections in the lung of control rats showed negative immunostaining with CD105 in the alveoli, alveolar sacs and alveolar septa (Fig. 7A). In IVI stem cell therapy group multiple CD105 +ve cells were detected in the alveolar septa and among the lining the alveoli and alveolar sacs (Fig. 7B). In IPI stem cell therapy group fewer CD105 +ve cells were detected in the alveolar septa and among the lining the alveoli and alveolar sacs (Fig. 7C).
Fig. 7.
(A) Section in the lung of a control rat showing negative immunostaining in the alveoli (a), alveolar sacs (S) and alveolar septa (I) (CD105 immunostaining, ×400). (B) Section in the lung of a rat in IVI stem cell therapy group showing multiple CD105 positive cells (arrows) in the alveolar septa and among the lining epithelium of the alveoli and alveolar sacs (CD105 immunostaining, ×400). (C) Section in the lung of a rat in IPI stem cell therapy group showing some CD105 positive cells (arrows) in the alveolar septa and among the lining epithelium of the alveoli and alveolar sacs (CD105 immunostaining, ×400).
Morphometric Results
The mean thickness of the interalveolar septa, the mean thickness of the wall of pulmonary vessels and the mean area% of collagen fibers were significantly increased in amiodarone group compared to control, IVI and IPI stem cell therapy groups. Concerning the area% of Pb+ve and CD105 +ve cells a significant increase was recorded in IVI stem cell therapy group compared to IPI group (Table 1).
Table 1.
Mean±standard deviation (SD) of the thickness of alveolar septa, thickness of the wall of pulmonary vessels, area % of collagen fibers, area % of Pb+ve and CD105+ve cells in control and experimental groups
| Groups | Thickness of alveolar septa | Thickness of wall of vessels | Area % of collagen fibers | Area % of Pb+ve cells | Area % of CD105+ve cells |
|---|---|---|---|---|---|
| Control group | 25.72±5.65 | 5.97±1.26 | 1.59±0.29 | – | – |
| Amiodarone group | 165.31±14.44* | 48.03±12.76* | 24.13±3.46* | – | – |
| IVI stem cell therapy group | 28.75±3.53 | 10.59±0.97 | 2.32±0.39 | 9.56±0.87* | 12.51±1.81* |
| IPI stem cell therapy group | 35.11±3.53 | 12.86±1.73 | 3.82±0.71 | 3.29±0.39 | 5.63±1.43 |
Significant at p value < 0.05.
Discussion
The current study demonstrated modulating effect of cord blood stem cell therapy whether IV or IP on amiodarone induced lung injury ending in fibrosis in albino rat. This was evidenced by histological, histochemical, immunohistochemical and morphometric studies.
Sections in the lung of rats belonging to amiodarone group demonstrated small bronchioles with partial obliteration of the lumen by shed epithelial cells and cellular infiltration in the adventitia in multiple fields. In agreement, it was stated that thoracic imaging in amiodarone lung disease (ALD) showed diffuse infiltrates and histologic review revealed follicular bronchiolitis (27).
Thickened wall of some vessels was confirmed by a significant increase in the mean thickness of the wall of pulmonary vessels. In addition, extravasated RBCs were found in some alveolar septa. This can be referred to the development of pulmonary hypertension. It was stated that the latter condition is characterized by smooth muscle proliferation and consequent vessel wall thickening (28). Diffuse alveolar hemorrhage was proved to be an uncommon reaction in ALD (29).
Thickened alveolar septa exhibiting cellular aggregates and dense cellular infiltration were detected in AM group. This was proved by a significant increase in the mean thickness of septa. In accordance, it was proved that lung tissues recruited edema and infiltration by inflammatory cells in ALD (30).
Irregular alveoli and alveolar spaces were noticed, which were lined by few cells exhibiting flat nuclei. This can be related to pneumocyte typeI apoptosis. On the other hand individual fields contained overdistended alveoli with disrupted alveolar walls. Concomitantly, it was reported that cell death in the lungs in ALD was followed by inflammation and fibrosis (31).
In AM group dense collagen fibers were evident in the alveolar septa, this was confirmed by a significant increase in the mean area% of collagen fibers. It was mentioned that in idiopathic pulmonary fibrosis and drug-induced severe interstitial pneumonia ending in fibrosis, the patient rapidly developed respiratory failure and required mechanical ventilation. Despite corticosteroid pulse therapy, no clinical improvement was noted (32).
Intravenous stem cell therapy group showed normal bronchioles, few of which demonstrated cellular infiltrate in the adventitia and few congested vessels. Few areas revealed occasional vessels with partially thickened wall, multiple congested vessels and some extravasated RBCs. Normal alveoli and alveolar sacs were lined by multiple cells exhibiting flat nuclei. Cellular infiltrates and extravasated RBCs were noted in few alveolar septa. Reduction of the collagen content was definite. In agreement, it was confirmed that on IV administration, MSCs were largely localized in pulmonary capillaries (33). On the other hand, it was documented that single-dose MSC infusion ameliorated hyperglycemia but failed to restore normoglycemia in diabetic animals. So it was hypothesized that multiple IV MSC infusions may reverse hyperglycemia in type 2 diabetes (34).
In IPI stem cell therapy group, normal bronchioles surrounded by infiltrating cells, few vessels with partial thickening of their walls and some congested vessels were detected. Occasional fields contained few vessels with marked thickening of their walls. Normal alveoli and alveolar sacs were lined by multiple cells exhibiting flat nuclei. Cellular infiltrates and extravasated RBCs were found in multiple alveolar septa. Reduction of the collagen content was also proved. The previous findings indicated less prominent effect of IP therapy compared to IV therapy. In accordance, IP injection of human MSCs was tested in multiple sclerosis, a currently incurable inflammatory demyelinating syndrome and proved a therapeutic potential (35). Moreover it was documented that IP delivery of human MSCs reduced inflammatory damage to the cornea without engraftment and primarily by secretion of an anti-inflammatory protein in response to injury signals from the cornea (36). Recently, IP injection of cells resulted in reduced cell infiltration in brain more effectively as compared to the IV route (37).
It can be concluded that cord blood MSC therapy proved definite amelioration of lung injury ending in fibrosis. The effect of IP SCT was slightly inferior to that of IV SCT, which may be overwhelmed by repeated IP injection.
Footnotes
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Kinder BW, Shariat C, Collard HR, Koth LL, Wolters PJ, Golden JA, Panos RJ, King TE., Jr Undifferentiated connective tissue disease-associated interstitial lung disease: changes in lung function. Lung. 2010;188:143–149. doi: 10.1007/s00408-009-9226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanfleteren LE, Linssen CF. Role of microorganisms in interstitial lung disease. Curr Opin Pulm Med. 2010;16:489–495. doi: 10.1097/MCP.0b013e32833b1c54. [DOI] [PubMed] [Google Scholar]
- 3.Van Cott TE, Yehle KS, DeCrane SK, Thorlton JR. Amiodarone-induced pulmonary toxicity: case study with syndrome analysis. Heart Lung. 2013;42:262–266. doi: 10.1016/j.hrtlng.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien K, Troendle J, Gochuico BR, Markello TC, Salas J, Cardona H, Yao J, Bernardini I, Hess R, Gahl WA. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011;103:128–134. doi: 10.1016/j.ymgme.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Range FT, Hilker E, Breithardt G, Buerke B, Lebiedz P. Amiodarone-induced pulmonary toxicity--a fatal case report and literature review. Cardiovasc Drugs Ther. 2013;27:247–254. doi: 10.1007/s10557-013-6446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding DC, Shyu WC, Lin SZ. Mesenchymal Stem Cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 7.Bashir Q, Robinson SN, de Lima MJ, Parmar S, Shpall E. Umbilical cord blood transplantation. Clin Adv Hematol Oncol. 2010;8:786–801. [PubMed] [Google Scholar]
- 8.Maurer MH. Proteomic definitions of mesenchymal stem cells. Stem Cells Int. 2011;2011:704256–704264. doi: 10.4061/2011/704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Laar JM, Sullivan K. Stem cell transplantation in systemic sclerosis. Curr Opin Rheumatol. 2013;25:719–725. doi: 10.1097/01.bor.0000434669.32150.ac. [DOI] [PubMed] [Google Scholar]
- 11.Park M, Lee SH, Lee YH, Yoo KH, Sung KW, Koo HH, Kang HJ, Park KD, Shin HY, Ahn HS, Chung NG, Cho B, Kim HK, Koh KN, Im HJ, Seo JJ, Han DK, Baek HJ, Kook H, Hwang TJ, Lee EK, Hah JO, Lim YJ, Jung HJ, Park JE, Jang MJ, Chong SY, Oh D, Korean Cord Blood Transplantation Working Party Pre-engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:640–646. doi: 10.1016/j.bbmt.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Ghionzoli M, Cananzi M, Zani A, Rossi CA, Leon FF, Pierro A, Eaton S, De Coppi P. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatr Surg Int. 2010;26:79–84. doi: 10.1007/s00383-009-2504-x. [DOI] [PubMed] [Google Scholar]
- 13.Kolettis TM, Agelaki MG, Baltogiannis GG, Vlahos AP, Mourouzis I, Fotopoulos A, Pantos C. Comparative effects of acute vs. chronic oral amiodarone treatment during acute myocardial infarction in rats. Europace. 2007;9:1099–1104. doi: 10.1093/europace/eum196. [DOI] [PubMed] [Google Scholar]
- 14.Osman FRO, Aboul Fotouh GI, Zickri MB, El Deeb D. Histological and ultrastructural study on the effect of some antiarrhythmic drugs on the thyroid gland of albino rat. MD Thesis. 2004 [Google Scholar]
- 15.Lee YG, Hwang JW, Park SB, Shin IS, Kang SK, Seo KW, Lee YS, Kang KS. Reduction of liver fibrosis by xenogeneic human umbilical cord blood and adipose tissue-derived multi-potent stem cells without treatment of an immunosuppressant. TERM. 2008;5:613–621. [Google Scholar]
- 16.Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A, Jr, Souza SA, Gutfilen B, Fonseca LM, Elia C, Madi K, Schanaider A, Rossi MI, Souza HS. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocum DL, Zupanc GK. Stretching the limits: stem cells in regeneration science. Dev Dyn. 2008;237:3648–3671. doi: 10.1002/dvdy.21774. [DOI] [PubMed] [Google Scholar]
- 18.Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye (Lond) 2003;17:877–885. doi: 10.1038/sj.eye.6700573. [DOI] [PubMed] [Google Scholar]
- 19.Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:26. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 21.Haasters F, Prall WC, Anz D, Bourquin C, Pautke C, Endres S, Mutschler W, Docheva D, Schieker M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214:759–767. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. London, New York, New Delhi: Arnold publisher; 2001. pp. 111–162. [Google Scholar]
- 23.Bancroft JD, Gamble M. Connective tissue stains. In: Gamble M, Bancroft JD, editors. Theory and practice of histological techniques. 6th ed. Edinburgh, London, Oxford, New York, Philadelphia, St Louis, Sydney and Toronto: Elsevier Health Sciences, Churchill Livingstone; 2008. p. 150. [Google Scholar]
- 24.Ellis R. Perls Prussian blue Staining Protocol. South Australia: Pathology Division, Queen Elizabeth Hospital; 2007. [Google Scholar]
- 25.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–1864. doi: 10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 27.Larsen BT, Vaszar LT, Colby TV, Tazelaar HD. Lymphoid hyperplasia and eosinophilic pneumonia as histologic manifestations of amiodarone-induced lung toxicity. Am J Surg Pathol. 2012;36:509–516. doi: 10.1097/PAS.0b013e318243fd9a. [DOI] [PubMed] [Google Scholar]
- 28.Sood BG, Wykes S, Landa M, De Jesus L, Rabah R. Expression of eNOS in the lungs of neonates with pulmonary hypertension. Exp Mol Pathol. 2011;90:9–12. doi: 10.1016/j.yexmp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Borders CW, 3rd, Bennett S, Mount C, Claassen SL. A rare case of acute diffuse alveolar hemorrhage following initiation of amiodarone: a case report. Mil Med. 2012;177:118–120. doi: 10.7205/milmed-d-11-00208. [DOI] [PubMed] [Google Scholar]
- 30.Gumuser G, Vural K, Varol T, Parlak Y, Tuglu I, Topal G, Sayit E. Assessment of lung toxicity caused by bleomycin and amiodarone by Tc-99m HMPAO lung scintigraphy in rats. Ann Nucl Med. 2013;27:592–599. doi: 10.1007/s12149-013-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth FC, Mulder JE, Brien JF, Takahashi T, Massey TE. Cytotoxic interaction between amiodarone and desethylamiodarone in human peripheral lung epithelial cells. Chem Biol Interact. 2013;204:135–139. doi: 10.1016/j.cbi.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Sato N, Kojima K, Horio Y, Goto E, Masunaga A, Ichiyasu H, Kohrogi H. Successful treatment of severe amiodarone pulmonary toxicity with polymyxin B-immobilized fiber column direct hemoperfusion. Chest. 2013;143:1146–1150. doi: 10.1378/chest.12-0994. [DOI] [PubMed] [Google Scholar]
- 33.Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, Rehman S, Malhotra A, Bhargava K, Palestro CJ, Gupta S, Singhal PC. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013;94:466–473. doi: 10.1016/j.yexmp.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou Q, Tong C, Ti D, Dong L, Cheng Y, Mu Y, Liu J, Fu X, Han W. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats. Biochem Biophys Res Commun. 2013;436:418–423. doi: 10.1016/j.bbrc.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 35.Gordon D, Pavlovska G, Glover CP, Uney JB, Wraith D, Scolding NJ. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neurosci Lett. 2008;448:71–73. doi: 10.1016/j.neulet.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Jr, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 37.Yousefi F, Ebtekar M, Soleimani M, Soudi S, Hashemi SM. Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE) Int Immunopharmacol. 2013;17:608–616. doi: 10.1016/j.intimp.2013.07.016. [DOI] [PubMed] [Google Scholar]