Abstract
Androgen-deprivation therapy (ADT) has been reported to be active in androgen receptor (AR)-expressing, relapsed/metastatic (RM), salivary gland cancers (SGCs). Abiraterone, an inhibitor of androgen synthesis, has recently been approved as a second-line treatment in hormone-resistant (HR) prostate cancer (PCa) patients. Two patients with AR-positive HR-RM adenocarcinoma, NOS of the salivary glands have been treated with abiraterone. This is the first time that this agent has been reported to be active in tumors other than HRPCa. Immunohistochemical analysis showed overexpression of EGFR, HER2, and HER3 in both untreated primary tumors. Sequencing analysis revealed a TP53 non-functional mutation in one case and a PIK3CA-activating mutation in the other. In conclusion, second line activity of ADT in AR-expressing, adenocarcinoma, NOS of salivary glands further strengthens the pathogenic and therapeutic role of AR signaling in AR-positive SGCs.
Keywords: androgen receptor, androgen deprivation therapy, HER3, PIK3CA, TP53, abiraterone, salivary gland cancers
Introduction
About half of the cases of adenocarcinoma, NOS and salivary duct cancer (SDC) express androgen receptor (AR).1 The biological mechanisms that explain the presence of AR only in selected SGCs histotypes, as well as its pathogenetic role, are currently unknown. AR is a promising molecular target for tailored therapy in SGCs, in fact response to androgen deprivation therapy (ADT) has already been reported in AR-expressing, relapsed/metastatic (RM) SGCs, irrespective of gender.2,3 Abiraterone, an inhibitor of androgen synthesis, was approved in 2011 for metastatic hormone-resistant prostate cancer (HRPCa) patients following docetaxel and, more recently, before chemotherapy.4,5 Two ADT-resistant patients with AR-expressing RM-SGCs were treated with abiraterone and the results are reported herein. Moreover, immunohistochemical, cytogenetic, and molecular analyses of AR and other biological markers involved in the AR signaling and pathogenesis of SGCs were performed.
Clinical Cases
Case 1
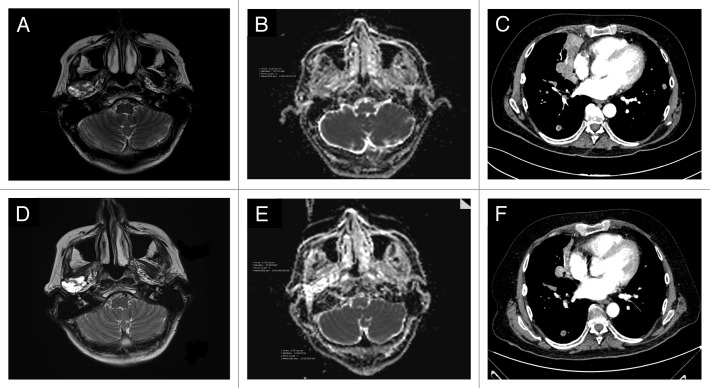
In August 2007, a 59-y-old man had surgery for an adenocarcinoma, NOS of the submandibular gland (pT2N0). He had two local recurrences (October 2008 and March 2009) treated by surgery plus radiotherapy (the second time combined with platinum-based chemotherapy). In April 2011, an MRI documented an unresectable local recurrence, involving the jaw, soft tissues, and pterigoid muscles. The IHC analysis of AR expression in the primary tumor revealed the presence of AR in up to 90% of the nuclei of tumor cells (Table 1; Fig. 1A). In May 2011 the patient started a complete androgen blockade (CAB) (bicalutamide 50 mg daily plus triptorelin 3.75 mg q28 d) with partial regression of the disease lasting until May 2012 (progression free-survival [PFS] 12 mo) when CAB was stopped due to local progression. Abiraterone at a daily dose of 1 g plus prednisone was started one month later. A clinical response to abiraterone was observed two months later lasted until January 2013 (PFS 8 mo). The polylobulated mass present in the MRI of May 2012 (Fig. 2A), evolved into a liquid mass, without a clear solid component (Fig. 2D). The two diffusion weighted (DW)-MRIs performed in May and July 2012 showed a different apparent diffusion coefficient (ADC) maps (Fig. 2B and E, respectively), suggestive of drug activity.6,7
Table 1. Immunohistochemical, cytogenetic, and molecular analyses by FFPE samples.
| Immunohistochemistry | FISH | Sequencing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR | ER | PgR | EGFR | HER2 | HER3 | AR | HER2 | AR | TP53 | PIK3CA | PTEN | |
| Case 1 | ||||||||||||
| Primary tumor | Pos | Neg | Neg | Low | 3+ | Pos | Not amplified | Amplified | wt | R175H (not functional) | wt | wt |
| Case 2 | ||||||||||||
| Primary tumor | Pos | Neg | Neg | Intermediate | 2+ | Pos | Not amplified | Not amplified | wt | wt | H1047R | wt |
| HR− bone metastasis | Pos | Neg | Neg | ND* | ND* | ND* | Not amplified | ND* | wt | wt | H1047R | wt |
FISH, fluorescent in situ hybridization; pos, positive; neg, negative; wt, wild type; ND*, not done for FFPE material exhausted.
Figure 1. Case 1: MRI performed before starting abiraterone (A); MRI done two months later, showed the evolution of the solid tumor lesion into a liquid mass (D). The DW-MRI images (B and E) demonstrated a different ADC maps, confirming the activity of the drug: the region of interest (ROI) corresponding to the tumor had a mean/standard deviation ADC value of 1200/178 in May 2012 (B) while in (E) (July 2012), the mean ADC/standard deviation value was 2410/166. Case 2: CT scan performed on March 2012 (baseline) (C) and on May 2012 (F); this latter showed a regression of the right paracardiac mass and the disappearance of a solid lesion in the lower left lobe.
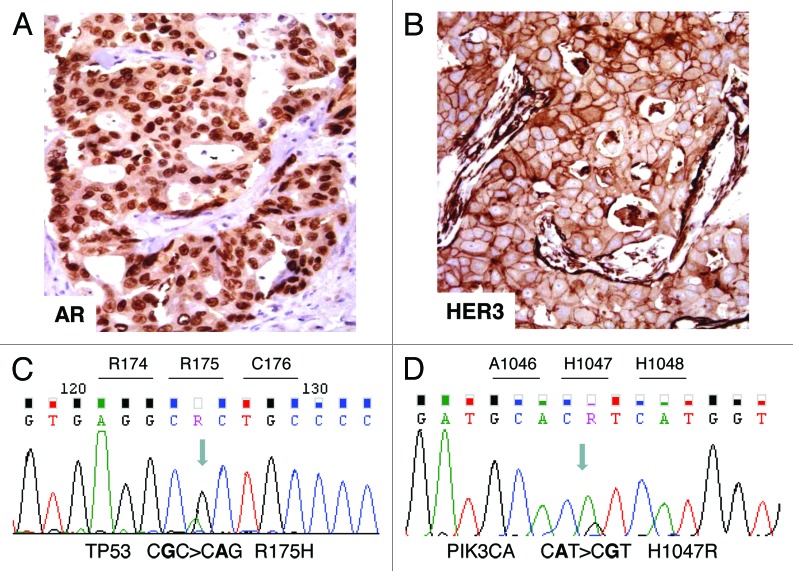
Figure 2. Immunohistochemical and molecular analyses. (A) AR-positive immunostaining in tumor nuclei. (B) HER3-positive membrane and cytoplasm immunostaining. (C) Case 1: electropherogram of TP53 mutation analysis showing the missense mutation R175H in exon 5. (D) Case 2: electropherogram of PIK3CA mutation analysis showing the missense mutation H1047R in the kinase domain (exon 20).
Case 2
In April 2004, a 44-y-old man underwent a total parotidectomy and radiotherapy (60 Gy) due to an adenocarcinoma, NOS (pT2N0). For a local relapse plus lung metastases, in October 2006 he received chemotherapy for 3 cycles (carboplatin plus paclitaxel) with disease progression (right coroide and spleen). In December 2006, the immunohistochemistry (IHC) analysis performed on both the primary tumor and lung metastasis demonstrated a strong AR-expression in almost all the nuclei of the tumor cells (Table 1). CAB was started and after 4 mo we observed a partial response with the disappearance of the spleen lesion and a significant reduction of the local recurrence. CAB was administered from December 2006 to January 2008, and then stopped for mandibular progression treated by palliative RT (PFS 25 mo); CAB was restarted in November 2008 and continued until January 2012, at which time a laminectomy was performed for a new spinal lesion (PFS 38 mo). Histopathology was consistent with adenocarcinoma showing an immunophenotypic AR profile superimposable to the primary tumor. In March 2012, he started abiraterone. Two months later a CT scan showed a partial regression of lung lesions (Fig. 2C and F) along with disease stabilization at the locoregional level. Abiraterone was interrupted on October 2012 (PFS 7 mo) due to local progression.
Immunohistochemical, Cytogenetic, and Molecular Analyses
The results are summarized in Table 1. In both primary tumors aside from AR overexpression (Fig. 1A), IHC revealed a null ER/PgR immunophenotype as well as AR gene fluorescence in situ hybridization (FISH). Sequencing showed the absence of AR amplification and mutation, respectively. Moreover, we observed overexpression of EGFR, HER2, and HER3 (Fig. 1B).
In case 1, HER2 overexpression paralleled gene amplification, assessed by FISH. TP53 sequencing revealed in exon 5 the presence of the single substitution CGC > CAC leading to the missense mutation R175H (Fig. 1C). Following Kato classification,8 this mutation results to be “non-functional”.
In case 2, the sequencing of PIK3CA, encoding the catalytic subunit of PIK3, showed in exon 20 the presence of the single substitution CAT > CGT leading to the missense activating mutation H1047R (Fig. 1D). A part from the PIK3CA mutation already described in the primary tumor, no additional AR, PTEN, or TP53 deregulations were observed in the metastatic bone sample. In both the cases, no PTEN mutations were observed.
Discussion
To our knowledge, we report for the first time the activity of abiraterone in two ADT resistant AR-expressing, RM-SG adenocarcinomas, NOS. Both patients were responsive to CAB, then they developed a secondary resistance and abiraterone was administered with clinical benefit. The duration of abiraterone exposure and the number of cycles (mean 7.5) was comparable to that reported in HRPCa patients.4 Treatment was well tolerated in both patients without significant adverse events. The clinical activity of CAB and abiterone supports an AR addiction in this selected histotype, that persists after the development of a secondary resistance. Indeed, likewise to HRPCa data,5 the rechallenging to a second line of ADT yielded a clinical benefit, in line with AR overexpression still present in the bone metastasis of Case 2.
The biological mechanism underpinning AR overexpression in selected SGCs histotypes such as adenocarcinoma, NOS and SDC is poorly understood. In order to acquire pathogenetic insights we performed thorough immunohistochemical, cytogenetic, and molecular analyses in the formalin-fixed paraffin-embedded (FFPE) available sections. Analyses were backed up from the evidence that adenocarcinoma, NOS mimics breast cancer and PCa both in terms of morphology and immunophenotype. Our findings showed a null ER/PgR immunophenotype in both primary tumors, together with a relevant deregulation of all the three members of ErbB family, as well as a normal AR monosomic pattern along with a wild-type AR gene status, in spite of a strong AR immunostaining. Moreover, sequencing analysis of TP53, PI3KCA, and PTEN revealed the presence of R175H mutation of TP53 in case 1 and an activating mutation in the kinase domain (exon 20) of PI3KCA in case 2. No PTEN mutation was observed, in keeping with a recent study.9 Notably, R175 TP53 mutation leads to a “non-functional” p53 protein lacking transcriptional activity.8 Abrogation of p53 function could have a relevant implications in AR expression regulation: it has been demonstrated that p53 wild type inhibits AR transcription10 and the removal of the negative regulation of AR could lead to an AR overexpression. Furthermore, clinical data in ER/PgR-negative AR-positive breast cancer have demonstrated that PI3KCA kinase domain mutations (exon 20) significantly correlate with higher level of AR as compared to AR expression in presence of the mutation in helical domain (exon 9) or a wild-type PIK3CA gene.11
All these findings reinforce the notion that AR expression in selected SGCs, i.e., SDC and adenocarcinoma, NOS may be similarly regulated to more common closely related histotypes, such as PCa and breast cancer.
Our results also confirm that ErbB family deregulation is a trait which characterizes SGCs1,12 and in particular among ErbB members, we would underline the overexpression of HER3, found in both primary tumors. An increase of HER3 protein is reported to occur after ADT in HRPCa, rising the AR transcriptional activity which in turn leads to PI3K/AKT cascade.13 Since our preliminary data showed the presence of HER3 in untreated tumors, a combined block of AR and PI3K signaling could be beneficially applied as first line treatment. Silencing of PI3K/AKT pathway might also counteract signals from activating mutation of PIK3CA accounting for 5% of SGCs.9
In case 2, we also analyzed the bone metastasis developed during CAB, in order to identify biomarkers potentially related to resistance to ADT such as AR mutation/amplification and PI3K/PTEN mutation pathway,14 whereas due to the exiguity of the FFPE material we were not able to investigate PTEN loss, recently reported in AR-positive SDCs.15 Primary tumor and metastatic bone lesion presented the same immunohistochemical, cytogenetic, and molecular profile, suggesting that secondary hormone resistance mechanisms require further investigations in AR-positive SGCs.
In conclusion, even if the role of the AR and the biological reasons of its narrow histotype distribution among SGCs have yet to be clarified, the clinical activity of abiraterone in these two male patients provides further convincing data on the efficacy in inhibiting the AR-signaling in AR-expressing SGCs. Whether abiraterone is active in AR-positive SGCs irrespective of the gender as demonstrated in case of androgen-blockade1,2 remains an open question.
Materials and Methods
Formalin-fixed paraffin-embedded (FFPE) unstained slides of the primary untreated tumor were obtained in the 2 cases under study, both referred. A FFPE bone biopsy, obtained at the time of ADT resistance appearance, was also available in case 2.
Protein expression was investigated by IHC performed on an automated immunostainer (BenchMark Ultra, Ventana Medical Systems, Inc.) according to the manufacturer’s instructions. The following antibodies were used: Androgen Receptor AR441 (Dako); ER clone EP1 M3643 (Dako); PgR clone 636S M3569 (Dako); EGFR (kit Dako); polyclonal HER2 A0485 (Dako); c-erbB3 polyclonal rabbit antibody 20-786-255900 (GenWay). AR, ER, and PgR positivity was defined when an immunodecoration was observed in >70% of nuclei; EGFR was scored as previously described;16 HER2 staining was interpreted on the basis of the Dako HercepTest scoring guidelines; HER3 was assessed as positivity when an immunodecoration was present in >50% of tumors cells.
AR and HER2 gene copy number was assessed by FISH performed on 2 μm FFPE sections, as previously described.16 The following probes were used: AR gene Xq12 Spectrum Orange (Vysis); HER2/neu/CEP 17SG probe 30-171060 (Abbot Molecular). The AR and HER2 copy number in each nucleus was assessed in relation to the centromeric signals.
To perform gene sequencing, genomic DNA was extracted by using the Qiamp FFPE DNA kit (Qiagen) following manufacturer’s instructions. TP53 (exons 5 to 8), PIK3CA (exons 9 and 20), and PTEN (exons 5 to 9) mutational analysis was performed by means of PCR using specific primers previously described.17,18 Specific primers for AR (exons 5 to 8 corresponding to the ligand binding domain) were designed using Primer3 program: exon 5: forward 5′-CAGACTGACC ACTGCCTCTG-3′ reverse: 5′-CAGATCAGGG GCGAAGTAGA-3′; exon 6: forward 5′-AGGTACCGCA TGCACAAGTC-3′ reverse 5′- TCCCTGCACT TCTAGGCACT-3′; exon 7: forward 5′-TTCATCCCAC ATCAGTTCCA-3′ reverse 5′- GGAGCTTGGT GAGCTGGTAG-3′; exon 8: forward 5′-AGATTGCGAG AGAGCTGCAT-3′ reverse 5′-GCTTCACTGG GTGTGTGGAA AT-3′. The PCR products were subjected to direct sequencing using a 3500 DX Genetic Analyzer (Applied Biosystems) and then evaluated by means of the ChromasPro software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank our patients and our colleagues, Dr E Verzoni and Dr G Procopio, for their kind collaboration.
References
- 1.Locati LD, Perrone F, Losa M, Mela M, Casieri P, Orsenigo M, Cortelazzi B, Negri T, Tamborini E, Quattrone P, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs) Oral Oncol. 2009;45:986–90. doi: 10.1016/j.oraloncology.2009.05.635. [DOI] [PubMed] [Google Scholar]
- 2.Jaspers HC, Verbist BM, Schoffelen R, Mattijssen V, Slootweg PJ, van der Graaf WT, van Herpen CM. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29:e473–6. doi: 10.1200/JCO.2010.32.8351. [DOI] [PubMed] [Google Scholar]
- 3.Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14:1327–8. doi: 10.1093/annonc/mdg331. [DOI] [PubMed] [Google Scholar]
- 4.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F, et al. COU-AA-301 Investigators Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, et al. COU-AA-302 Investigators Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, Poptani H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–94. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32:2–16. doi: 10.1002/jmri.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, Ishioka C. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci U S A. 2003;100:8424–9. doi: 10.1073/pnas.1431692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cros J, Sbidian E, Hans S, Roussel H, Scotte F, Tartour E, Brasnu D, Laurent-Puig P, Bruneval P, Blons H, et al. Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumours. Ann Oncol. 2013;24:2624–9. doi: 10.1093/annonc/mdt338. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Alimirah F, Panchanathan R, Chen J, Zhang X, Ho SM, Choubey D. Expression of androgen receptor is negatively regulated by p53. Neoplasia. 2007;9:1152–9. doi: 10.1593/neo.07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, Traina TA, Hudis C, Hortobagyi GN, Gerald WL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–8. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 12.Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–74. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jathal MK, Chen L, Mudryj M, Ghosh PM. Targeting ErbB3: the New RTK(id) on the Prostate Cancer Block. Immunol Endocr Metab Agents Med Chem. 2011;11:131–49. doi: 10.2174/187152211795495643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–7. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith CC, Seethala RR, Luvison A, Miller M, Chiosea SI. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am J Surg Pathol. 2013;37:1201–7. doi: 10.1097/PAS.0b013e3182880d5a. [DOI] [PubMed] [Google Scholar]
- 16.Perrone F, Suardi S, Pastore E, Casieri P, Orsenigo M, Caramuta S, Dagrada G, Losa M, Licitra L, Bossi P, et al. Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2006;12:6643–51. doi: 10.1158/1078-0432.CCR-06-1759. [DOI] [PubMed] [Google Scholar]
- 17.Licitra L, Perrone F, Bossi P, et al. How High-Risk Human Papillomavirus affects prognosis of patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–6. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 18.Perrone F, Da Riva L, Orsenigo M, Losa M, Jocollè G, Millefanti C, Pastore E, Gronchi A, Pierotti MA, Pilotti S. PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro Oncol. 2009;11:725–36. doi: 10.1215/15228517-2009-003. [DOI] [PMC free article] [PubMed] [Google Scholar]