Abstract
Purpose
High-risk prostate cancer (PC) has poor outcomes due to therapeutic resistance to conventional treatments, which include prostatectomy, radiation, and hormone therapy. Previous studies suggest that anticoagulant (AC) use may improve treatment outcomes in PC patients. We hypothesized that AC therapy confers a freedom from biochemical failure (FFBF) and overall survival (OS) benefit when administered with radiotherapy in patients with high-risk PC.
Materials and Methods
Analysis was performed on 74 high-risk PC patients who were treated with radiotherapy from 2005 to 2008 at UT Southwestern. Of these patients, 43 were on AC including aspirin (95.6%), clopidogrel (17.8%), warfarin (20%), and multiple ACs (31.1%). Associations between AC use and FFBF, OS, distant metastasis, and toxicity were analyzed.
Results
Median follow-up was 56.6 mo for all patients. For patients taking any AC compared with no AC, there was improved FFBF at 5 years of 80% vs. 62% (P = 0.003), and for aspirin the FFBF was 84% vs. 65% (P = 0.008). Aspirin use was also associated with reduced rates of distant metastases at 5 years (12.2% vs. 26.7%, P = 0.039). On subset analysis of patients with Gleason score (GS) 9–10 histology, aspirin resulted in improved 5-year OS (88% vs. 37%, P = 0.032), which remained significant on multivariable analysis (P < 0.05).
Conclusions
AC use was associated with a FFBF benefit in high-risk PC which translated into an OS benefit in the highest risk PC patients with GS 9–10, who are most likely to experience mortality from PC. This hypothesis-generating result suggests AC use may represent an opportunity to augment current therapy.
Keywords: aspirin, anticoagulant, prostate cancer, radiotherapy
Background
Prostate cancer (PC) is the most common male malignancy in the United States with 238 000 cases and 29 720 deaths expected in 2013,1 of which 20–30% have high-risk features.2 For high-risk PC defined by the National Comprehensive Cancer Network (NCCN) as Gleason score (GS) ≥ 8, stage ≥ cT3a, prostate-specific antigen (PSA) ≥ 20, standard treatment guidelines include definitive radiation therapy with neo-adjuvant, concurrent, and long-term androgen deprivation therapy in the United States.3 In spite of the multimodality therapy, long-term outcomes are suboptimal with 5-y biochemical progression free survival of 60–70% and 5-y mortality rate of 15–25%.4-7 These poor outcomes have led to efforts to investigate novel strategies for high-risk PC.2
There is substantial pre-clinical and clinical evidence that anticoagulants (ACs) including aspirin, clopidogrel, warfarin, and enoxaparin have anti-neoplastic properties in several types of malignancies including PC.8 A potential association between the use of ACs and improved biochemical control for PC patients treated with radiotherapy was first reported by Choe et al.9 In that study, the 4-y FFBF was 91.2% among AC users, compared with 78% in patient not taking any ACs. Within the high-risk group, the difference was more robust (82% vs. 58%, P = 0.0007). The overall survival (OS) rates, however, were not different. The association between AC use and improved PC outcomes was further supported by a large registry study of 5995 patients from the Cancer of the Prostate Strategic Urologic Research Endeavor database.10 PC specific mortality was reduced from 19% to 4% in high-risk PC at 10 y in patients treated with definitive radiation therapy or radical prostatectomy. These lines of evidence suggest that AC use has the potential to improve therapeutic outcomes in patients with PC. The purpose of this study is to explore our hypothesis that AC used in conjunction with radiation therapy may lead to improved OS in patients with high-risk PC.
Results
Patient characteristics
The clinical and pathologic characteristics of these high-risk PC patients are described in Table 1. The median age for the control group was 66 y and 70 y for the AC group. Median follow-up was 56.6 mo (1.9–88.3 mo). A total of 45 patients reported use of ACs during radiotherapy, and 29 patients did not take ACs (control group). Within the AC group, 43 patients were using aspirin (95.6%), 8 patients were using clopidogrel (17.8%), 9 patients took warfarin (20%), and 14 patients (31.1%) reported taking multiple ACs (generally aspirin and another AC). All patients with GS 9 or 10 tumors had a primary GS pattern of 5. Age was the only patient characteristic found to be significantly different between the two groups, with the control group slightly younger (median age of 66 y vs. 70 y in the AC group, P = 0.04). Otherwise, no statistically significant differences were noted with respect to other major baseline prognostic factors.
Table 1. Patient characteristics compared with respect to major prognostic variables.
| Characteristic | Control | AC | P value |
|---|---|---|---|
| n = | 29 | 45 | N/A |
| Age (years) | |||
| Median | 66 | 70 | 0.04 |
| Range | 49–84 | 53–86 | |
| Gleason score | |||
| 6 | 3 (10.3%) | 2 (4.4%) | 0.52 |
| 7 | 8 (27.6%) | 9 (20%) | |
| 8 | 8 (27.6%) | 17 (38.8%) | |
| 9 | 8 (27.6%) | 16 (35.6%) | |
| 10 | 2 (6.9%) | 1 (2.2%) | |
| Initial PSA | |||
| Mean | 40 | 49 | 0.47 |
| Median | 30.9 | 21.6 | |
| Standard deviation | 33 | 69.6 | |
| Range | 4.7–147 | 1.8–332.7 | |
| Tumor clinical stage | |||
| T1c-T2b | 14 (48.3%) | 23 (51.1%) | 0.81 |
| T2c-T3b | 14 (48.3%) | 20 (44.4%) | |
| Unknown | 1 (3.4%) | 2 (4.4%) | |
| Follow-up (mo) | |||
| Mean | 47 | 53.5 | 0.27 |
| Median | 51.3 | 61.6 | |
| Standard deviation | 26 | 22.2 | |
| Range | 3.2–88.3 | 1.9–81.9 | |
| Pelvic lymphatic irradiation | |||
| Yes | 25 (86.2%) | 40 (89.9%) | 0.73 |
| No | 4 (13.8%) | 5 (11.1%) | |
| Hormone therapy | |||
| Yes | 24 (82.8%) | 40 (95.6%) | 0.36 |
| No | 3 (10.3%) | 2 (4.4%) | |
| Duration of hormone therapy | |||
| None | 3 (10.3%) | 2 (4.4%) | 0.25 |
| <6 mo | 4 (13.8%) | 4 (8.9%) | |
| 6–23 mo | 10 (34.4%) | 12 (26.7%) | |
| ≥24 mo | 10 (34.4%) | 27 (60%) | |
| Unknown | 2 (6.9%) | 0 (0%) |
PSA, prostate specific antigen; AC, anticoagulant; P, P value.
Effect of AC use on 5-y FFBF and OS
To investigate the potential the relation between AC use and PC outcomes, univariate analysis was performed with known prognostic factors and AC use (Table 2). For 5-y FFBF, there were no significant associations with T-stage, pelvic irradiation, or duration of hormone deprivation. However, there was a statistically significant improvement in the 5-y FFBF in the AC group compared with the control group (84% vs. 62%, P = 0.003). Among the specific ACs studied, only aspirin use was associated with a significant improvement in biochemical failure (BF) (16% vs. 35%, P = 0.008). Kaplan–Meier FFBF curves were generated showing significant improvement in FFBF (Fig. 1A), with median FFBF of 4.5 y in the non-aspirin group and median FFBF not yet reached in the aspirin group (P = 0.008).
Table 2. Univariate analysis of major prognostic factors and AC use with respect to 5-y FFBF, and OS.
| Variable | 5-y BF % | HR | 95% CI | P value | 5-y OS% | HR | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|
| T-stage | ||||||||
| T1c-T2b vs. T2c-T3b | 19% vs. 32% | 1.956 | 0.761–5.076 | 0.155 | 84% vs. 75% | 1.624 | 0.562–4.688 | 0.365 |
| Pelvic irradiation | ||||||||
| Yes vs. no | 25% vs. 22% | 1.047 | 0.240–4.558 | 0.952 | 81% vs. 78% | 1.262 | 0.282–5.654 | 0.761 |
| Hormone deprivation | ||||||||
| ≤6 mo vs. >6 mo | 8% vs. 27% | 0.339 | 0.045–2.556 | 0.271 | 67% vs. 84% | 2.714 | 0.833–8.842 | 0.084 |
| Any AC use | ||||||||
| Yes vs. no | 20% vs. 38% | 0.262 | 0.100–0.685 | 0.003 | 87% vs. 70% | 0.410 | 0.142–1.186 | 0.089 |
| Aspirin | ||||||||
| Yes vs. no | 16% vs. 35% | 0.294 | 0.113–0.765 | 0.008 | 86% vs. 72% | 0.444 | 0.154–1.282 | 0.123 |
| Warfarin | ||||||||
| Yes vs. no | 22% vs. 25% | 0.874 | 0.201–3.804 | 0.857 | 78% vs. 81% | 1.227 | 0.273–5.513 | 0.789 |
| Clopidogrel | ||||||||
| Yes vs. no | 12.5% vs. 26% | 0.493 | 0.066–3.707 | 0.483 | 75% vs. 81% | 1.345 | 0.142–1.186 | 0.698 |
| Multiple AC use | ||||||||
| Yes vs. no | 21% vs. 25% | 0.843 | 0.244–2.913 | 0.787 | 71% vs. 83% | 1.626 | 0.511–5.237 | 0.402 |
HR, hazard ratio; CI, confidence interval; P, P value.
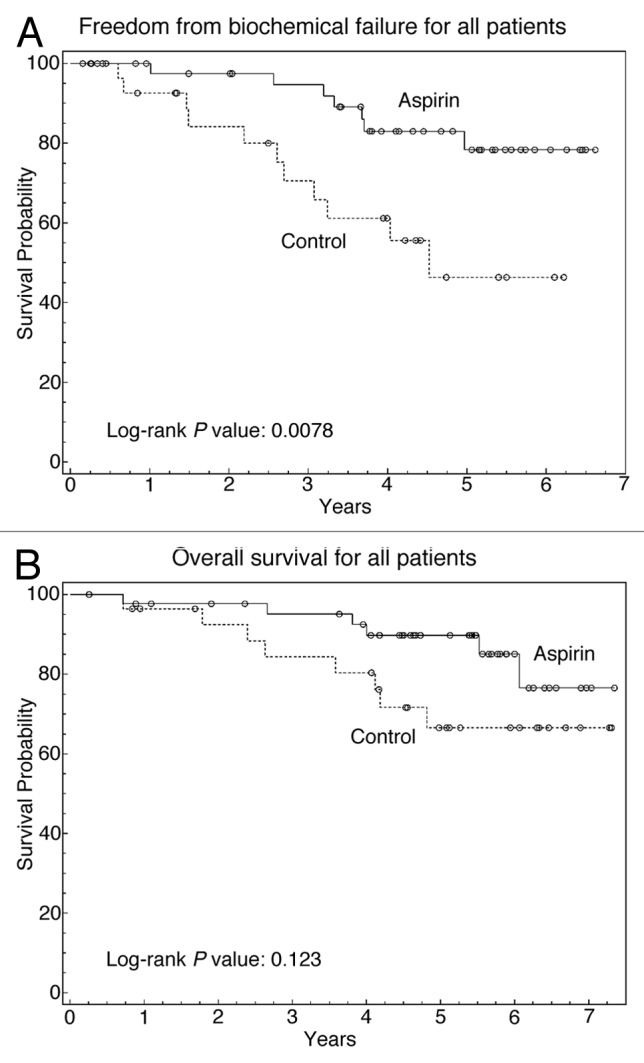
Figure 1. The effect of aspirin use on 5-y FFBF and OS. (A) Kaplan–Meier curve comparing FFBF in patients who took aspirin compared with those who did not. (B) Kaplan–Meier curve comparing OS in patients who took aspirin compared with those who did not. Solid line, patients who took aspirin; dashed line, patients who did not take aspirin.
On univariate analysis, there was a trend for improved OS in patients who took any AC (Table 2). It is important to note that although the two groups had a statistically significant difference among patient ages, the trend of improved survival occurred in the AC group which had the more elderly patients. In the AC group, 5-y OS was 87% compared with 70% in those who did not take ACs (P = 0.089). AC use did not significantly impact OS, although the aspirin group also had a numerical trend of improved 5-y OS of 86% compared with 72% in those who did not take aspirin (Fig. 1B). There were no significant differences in 5-y OS with respect to other prognostic variables. The trend of improved survival in patients who took aspirin led to further analysis in order to identify subgroups in which aspirin might confer an OS advantage.
Effect of AC use on distant metastasis
In order to find a potential explanation for improved FFBF and trend toward improved OS in the AC group, univariate analysis was performed with respect to AC use and the rate of distant metastases at 5 y (Table 3). A significant reduction in the rate of distant metastases was seen in the AC group (11.6% vs. 28.6%, P = 0.023). The only type of AC associated with a significant improvement in distant metastasis rate was aspirin (P = 0.039). For those taking aspirin, there was a substantial reduction in the distant metastasis rate of 12.2% compared with 26.7% in patients who did not report aspirin use.
Table 3. The effect of AC use on 5-y rates of distant metastasis.
| Variable | Distant metastasis % | HR | 95% CI | P value |
|---|---|---|---|---|
| Any AC | 11.6% vs. 28.6% | 0.293 | 0.095–0.901 | 0.023 |
| Aspirin | 12.2% vs. 26.7% | 0.325 | 0.106–0.998 | 0.039 |
| Clopidogrel | 0% vs. 20.6% | N/A | N/A | 0.183 |
| Warfarin | 12.5% vs. 19% | 0.694 | 0.089–5.387 | 0.726 |
| Multiple agents | 7.7% vs. 20.7% | 0.351 | 0.045–2.712 | 0.294 |
Impact of AC use on OS in GS 9–10 subgroup
We next investigated whether aspirin use might be associated with an OS benefit in certain subgroups such as those with GS 9–10 tumors who may be at highest risk for distant metastases. We therefore generated Kaplan–Meier survival curves to compare the OS of patients between the AC and no-AC groups, stratified by their Gleason grade. In patients with GS ≤ 8 (Fig. 2A), there was no significant association between aspirin use and OS (P = 0.943), as was the case for all patients (Fig. 1B). However, in patients with GS 9–10 histology (Fig. 2B), there was a significant improvement in OS (P = 0.012).
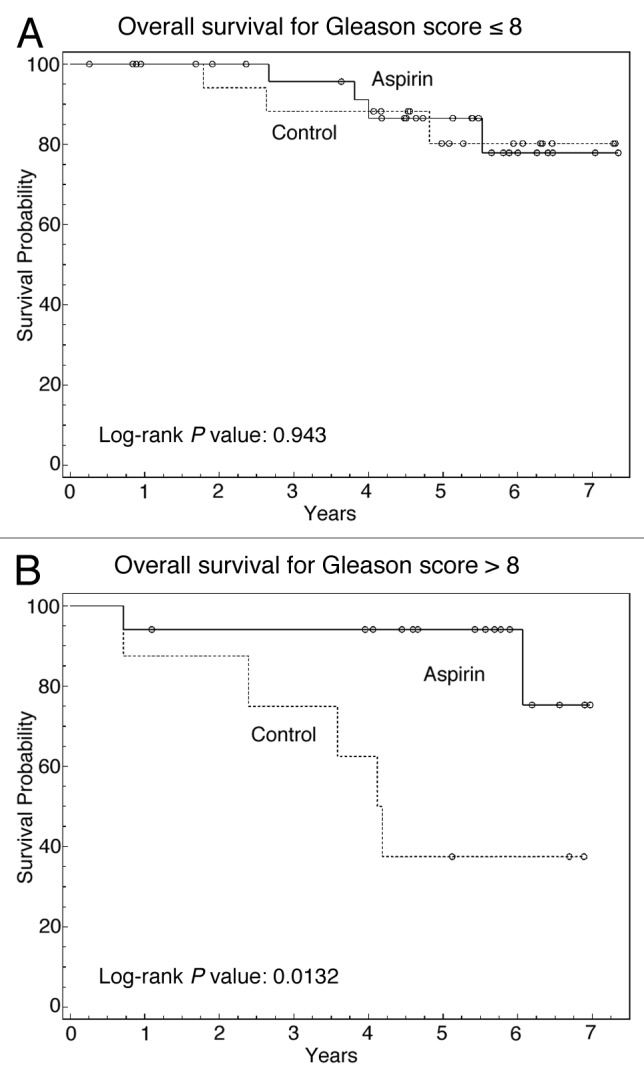
Figure 2. Subgroup analysis comparing effect of aspirin on 5-y OS based upon GS. (A) Kaplan–Meier curve comparing OS with respect to aspirin use in GS ≤ 8 tumors. (B) Kaplan–Meier curve comparing OS with respect to aspirin use in GS 9–10 tumors. Solid line, patients who took aspirin; dashed line, patients who did not take aspirin.
In order to confirm the independent significance of aspirin use on OS in this group of patients, additional statistical analyses were performed on these 27 patients who had GS 9–10 disease. First, univariate analysis was used to examine the effect of key prognostic factors and aspirin use on 5-y OS in patients with GS 9–10 tumors (Table 4A). Only age and aspirin use were associated with a statistically significant impact on OS (P < 0.05), and there was a trend toward improved OS in patients who received hormone deprivation ≥ 24 mo (P = 0.063). For patients who took aspirin with GS 9–10 tumors, the 5-y OS was 88% compared with 37% in those who did not take aspirin, representing a 2.4-fold improvement in OS associated with aspirin use. When looking specifically at prostate cancer deaths in these patients with GS 9–10 tumors, only 5.9% (1/17) of patients in the aspirin group died from prostate cancer, where as 30% (3/10) of patients in the non-aspirin group died from prostate cancer.
Table 4A. Subset analysis of the effect of aspirin use in patients with GS 9–10 histologies.
| Variable | 5-y OS% | HR | 95% CI | P value |
|---|---|---|---|---|
| Age per year | N/A | 1.162 | 1.102–1.327 | 0.027 |
| Gleason score per unit | N/A | 0 | 0.00–0.00 | 0.996 |
| T-stage per unit | N/A | 1.43 | 0.857–2.386 | 0.171 |
| % cores positive | N/A | 11.16 | 0.105–1184.064 | 0.311 |
| Pelvic irradiation | ||||
| Yes vs. no | 70% vs. 100% | 0 | 0.00–0.00 | 0.996 |
| Hormone deprivation | ||||
| ≥24 mo vs. <24 mo | 92% vs. 54% | 0.133 | 0.016–1.112 | 0.063 |
| N-stage | ||||
| N0 vs. N1 | 75% vs. 67% | 1.782 | 0.199–15.991 | 0.606 |
| Aspirin | ||||
| Yes vs. no | 88% vs. 37% | 0.165 | 0.032–0.853 | 0.0315 |
Univariate analysis on the effect of major prognostic variables and aspirin on 5-y OS
Multivariable analysis was performed on the variables with most significant impact on OS. In GS 9–10 patients, when variables with P ≤ 0.1 were included (Table 4B), only aspirin use remained a significant factor associated with improved survival (P = 0.036). Moreover, when variables with P ≤ 0.05 were considered (Table 4C), aspirin again remained significantly associated with improved OS in GS 9–10 tumors (P = 0.017) suggesting that aspirin use is independently associated with survival benefit in this subgroup of patients.
Table 4B. Subset analysis of the effect of aspirin use in patients with GS 9–10 histologies .
| Variable | 5-y OS% | HR | 95% CI | P value |
|---|---|---|---|---|
| Age per year | N/A | 1.162 | 1.102–1.327 | 0.027 |
| Gleason score per unit | N/A | 0 | 0.00–0.00 | 0.996 |
| T-stage per unit | N/A | 1.43 | 0.857–2.386 | 0.171 |
| % cores positive | N/A | 11.16 | 0.105–1184.064 | 0.311 |
| Pelvic irradiation | ||||
| Yes vs. no | 70% vs. 100% | 0 | 0.00–0.00 | 0.996 |
| Hormone deprivation | ||||
| ≥24 mo vs. <24 mo | 92% vs. 54% | 0.133 | 0.016–1.112 | 0.063 |
| N-stage | ||||
| N0 vs. N1 | 75% vs. 67% | 1.782 | 0.199–15.991 | 0.606 |
| Aspirin | ||||
| Yes vs. no | 88% vs. 37% | 0.165 | 0.032–0.853 | 0.0315 |
Multivariate analysis of variables with P ≤ 0.1
Table 4C. Subset analysis of the effect of aspirin use in patients with GS 9–10 histologies .
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.151 | 1.025–1.293 | 0.018 |
| Aspirin use | 0.113 | 0.019–0.676 | 0.017 |
Multivariate analysis of variables with P ≤ 0.05
Toxicity
Radiation therapy and AC use was tolerated well with low rates of acute and long-term gastrointestinal (GI) toxicity. All patients with bowel toxicity had grade 1–2 toxicity, and no patient experienced acute or late toxicity ≥ grade 3. In patients who took any AC, the rate of acute GI toxicity was 15% compared with 20% in control group (P = 0.49), and the rate of late GI toxicity was 14.2% compared with 14.3% in control group (P = 1.00). In patients taking multiple ACs, the rate of acute GI toxicity was 8% compared with 19.6% in those who did not take multiple ACs (P = 0.35), and 23.1% vs. 12.25% respectively for late GI toxicity (P = 0.32). Thus, no significant increases in GI toxicity could be attributed to AC use in this study.
Discussion
In our experience of patients with high-risk PC treated with definitive radiotherapy, we observed a 5-y FFBF benefit associated with AC use, as well as a 5-y OS benefit in patients with GS 9–10 tumors. Of the ACs evaluated, aspirin had a significant association with improved FFBF as well as OS. These findings are consistent with previous analyses that have reported improved biochemical control and PC specific survival in high-risk PC.10 The finding of improved OS in patients with GS 9–10 histology taking aspirin is notable given that even with a relatively short follow-up and with many of the patients being on hormone therapy, this study suggests that particularly in the patients with the highest risk of disease recurrence and distant metastases, aspirin may confer a survival advantage.
One concern regarding AC use and radiation therapy for PC is the development of worse acute and long-term radiation toxicity. AC use is potentially associated with exacerbation of radiation-induced GI bleeding and ulceration. There are reports of increased GI toxicity associated with AC use during radiotherapy for PC,11 but no significant impact on acute or late gastrointestinal toxicity was observed in our study. Additionally, there were no toxicities greater than grade 2 observed in any patient. The observation of a non-significant difference in mild GI toxicity may be attributed to the small number of patients observed in our study. Of note, all of these patients were treated using IMRT techniques and with strict dose constraints on the rectum. Moreover, the majority of patients treated in our study received a dose of 75.6 Gy, prior to data showing a benefit for further dose escalation. It is also likely that AC use was stopped if GI bleeding occurred, thereby preventing a serious further progression of the GI complication. While AC use was well tolerated in our study, it was shown in other studies to increase rectal bleeding.12 Therefore, we still advise caution, strict rectal dose volume histogram constraints, and close follow-up when dose escalated radiotherapy is used in PC patients taking ACs.
Although there has been an abundance of research investigating the molecular basis for the beneficial effect of aspirin and ACs in prostate and other malignancies, the precise mechanisms for this effect remains elusive. Multiple mechanisms including inhibition of platelet functions, cyclooxygenase (COX)-2 inhibition,13 and reduction in pro-neoplastic inflammatory cytokines have been hypothesized,14 but this phenomenon remains a topic of active interest and research. An interesting finding in our study that may support the platelet dysfunction hypothesis is that 0% of the patients who received clopidogrel were observed to develop distant metastases whereas 20.6% of patients in the control group did. It is also possible that ACs increase blood flow to potentially hypoxic cancer cells thereby enhancing radiation-induced reactive oxygen species and improving cell kill, which may account for the improved outcomes in patients receiving radiation therapy and ACs. However, the benefit of AC use is not limited to concurrent treatment with radiotherapy and thus, radiation sensitization is unlikely to be the primary mode of action.10 Since aspirin represents the overwhelming majority of AC use in our cohort studied, it brings into question whether there may be other mechanisms of actions related to aspirin that may be mediating the improvement in radiation outcomes. For example, there is significant literature suggesting COX-2’s importance in prostate cancer,15-18 and its inhibition as a potential mechanism for improving radiation outcomes in cancer.19 However, COX-2 inhibition requires much higher doses than the aspirin doses typically used by patients, and which showed antineoplastic effects in these studies.20
When analyzed as a group, there were no statistically significant differences between the AC group and control with respect to duration of hormone deprivation therapy (Table 1). However, a potential issue which we needed to clarify for this study was the observation that a slightly higher percentage of patients in the GS 9–10 group with aspirin use had hormone duration >24 mo compared with those who did not use aspirin (53% of patients on aspiringroup compared with 40% for those not on aspirin). However, we felt that this small difference was unlikely to account for the 50.7% difference in survival benefit seen at 5 y in the GS 9–10 patients who were taking aspirin. In support of this, we performed multivariable analysis (Table 4B), which demonstrated that differences in OS associated with aspirin remained independently significant when adjusting for duration of hormone deprivation. Furthermore, when we consider experiences from a large randomized study, long-term androgen deprivation therapy compared with short-term androgen deprivation therapy was associated with only a 3.8% improvement in mortality in the EORTC trial, suggesting that the magnitude of OS benefit observed in our study with aspirin use is unlikely to be solely due to differences due to variances in the duration of hormone therapy.5
Another potential confounder in our study is that the age in the control group was significantly younger than patients who took AC therapy. There has been some retrospective data suggesting that older patients may have decreased risk of developing metastatic prostate cancer after initiation of androgen deprivation therapy (ADT).21 However, there are conflicting studies which do not clearly show age to be a prognostic factor for prostate cancer specific survival, particularly in patients with high Gleason grade.21-23 In our study, in multivariate analysis of patients with GS 9–10 tumors, while the effect of age (P = 0.068) only trended toward significance, aspirin remained a highly statistically significant predictor of overall survival (P = 0.036). Thus, while our sample size precludes definite conclusions, it is unlikely that age was responsible for the highly statistically significant survival advantage in patients with GS 9–10 who took aspirin.
While our study suggests that the subgroup of patients with GS 9–10 disease may benefit from aspirin use, there are caveats. In our study, all patients with GS 9–10 disease had a primary GS of 5. Therefore, we may need to caution against generalizing this observation to patients with GS 9 (4 + 5) disease. Furthermore, only 3 patients had GS 10 disease in our study. We anticipate that any benefit conferred by aspirin on GS 9 (5 + 4) patients should also be seen in the GS 10 patients. However, this will need to be verified in a larger study of high-risk patients. Finally, a major limitation of this study is the small number of patients (n = 27) included in the subset analysis of those with GS 9–10 in which the survival benefit was noted. Therefore, these findings though intriguing, at best, are hypothesis generating, and not conclusive for this finding.
Taken together, these hypothesis generating results suggest that AC and particularly aspirin use may have the potential to improve outcome in high-risk PC. Along with previous studies examining the association of AC use with radiation therapy,9,10 these data would support a larger prospective trial examining AC use in PC patients treated with definitive radiotherapy to clarify the associations observed in these retrospective analyses. Such data along with ongoing trials examining aspirin in the metastatic setting will clarify whether routine use of ACs should be considered in PC.
Materials and Methods
The Institutional Review Board (IRB) approved registry protocol (STU 052012-019) at the University of Texas Southwestern Medical Center permitted this study. A retrospective review of all patients with high-risk PC treated at the University of Texas Southwestern Medical Center with definitive radiation therapy from 2005 to 2008 was performed.
Associations between AC (warfarin, clopidogrel, and/or aspirin) use and FFBF, distant metastasis, and OS in high-risk PC patients treated with definitive radiation therapy were examined in a cohort of 74 patients who met NCCN criteria for high-risk. BF was censored from FFBF analysis at the time of recurrence, and deaths were censored from OS analysis at time of occurrence. Patients were included in the AC group if warfarin, aspirin, and/or clopidogrel were included on their medication list at the time of initial consultation or subsequent visits.
All patients were treated using intensity-modulated radiation therapy (IMRT) to a target area which included the prostate, seminal vesicles, and the pelvic lymph nodes. The median dose to the prostate gland was 75.6 Gy (range 75.6–79.2 Gy) and the median pelvic lymphatic irradiation dose was 45 Gy (range 45–54 Gy), all delivered in 1.8 Gy per fraction daily. The planning target volume (PTV) included the prostate and seminal vesicles as well as the internal and external iliac vessels with 6–10 mm expansions modified at the discretion of treating physician. BF was defined as a PSA increase of >2 ng/mL from the post-treatment nadir per Radiation Therapy and Oncology Group (RTOG)/American College of Radiology (ACR) Phoenix consensus definition.24 FFBF was defined as patients who did not meet Phoenix criteria for BF at time of follow-up. Patients were followed once every 3 mo for the first year, every 6 mo for the second year, and yearly thereafter upon completion of radiation therapy. Toxicity analysis was documented as defined by the Common Toxicity Criteria version 4.0. Median follow-up time was 4.7 y, and ranged from 56 d to 7.3 y.
Comparisons between the AC group and non-AC control group were made using the Fisher Exact test for categorical values and paired t test or Wilcoxon 2-sample test for continuous variables. Univariate analyses were performed to determine the association between AC use and FFBF, distant metastasis rate, and 5-y OS. Multivariable analysis was performed using Cox proportional-hazards model, which included covariates that were significant or approached significance in the univariate analysis. Kaplan–Meier curves for 5-y FFBF and OS were compared by log-rank test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We express our gratitude to Ms Jean Wu and Clinical Research Staff in the Department of Radiation Oncology at the University of Texas at Southwestern Medical Center for their assistance in data collection and coordination of this study.
Glossary
Abbreviations:
- AC
anticoagulant
- ACR
American College of Radiology
- BF
biochemical failure
- FFBF
freedom from biochemical failure
- GI
gastrointestinal
- IMRT
intensity-modulated radiation therapy
- IRB
Institutional Review Board
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- PC
prostate cancer
- PSA
prostate-specific antigen
- RTOG
Radiation Therapy and Oncology Group
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Punnen S, Cooperberg MR. The epidemiology of high-risk prostate cancer. Curr Opin Urol. 2013;23:331–6. doi: 10.1097/MOU.0b013e328361d48e. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA, Sandler HM, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, Gez E, Kil P, Akdas A, Soete G, et al. EORTC Radiation Oncology Group and Genito-Urinary Tract Cancer Group Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 7.Shilkrut M, McLaughlin PW, Merrick GS, Vainshtein JM, Feng FY, Hamstra DA. Interval to biochemical failure predicts clinical outcomes in patients with high-risk prostate cancer treated by combined-modality radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:721–8. doi: 10.1016/j.ijrobp.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Choe KS, Liauw SL. Effects of aspirin on cancer initiation and progression. Expert Rev Anticancer Ther. 2013;13:115–7. doi: 10.1586/era.12.166. [DOI] [PubMed] [Google Scholar]
- 9.Choe KS, Correa D, Jani AB, Liauw SL. The use of anticoagulants improves biochemical control of localized prostate cancer treated with radiotherapy. Cancer. 2010;116:1820–6. doi: 10.1002/cncr.24890. [DOI] [PubMed] [Google Scholar]
- 10.Choe KS, Cowan JE, Chan JM, Carroll PR, D’Amico AV, Liauw SL. Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy. J Clin Oncol. 2012;30:3540–4. doi: 10.1200/JCO.2011.41.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamstra DA, Conlon AS, Daignault S, Dunn RL, Sandler HM, Hembroff AL, Zietman AL, Kaplan I, Ciezki J, Kuban DA, et al. PROSTQA Consortium Study Group Multi-institutional prospective evaluation of bowel quality of life after prostate external beam radiation therapy identifies patient and treatment factors associated with patient-reported outcomes: the PROSTQA experience. Int J Radiat Oncol Biol Phys. 2013;86:546–53. doi: 10.1016/j.ijrobp.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Choe KS, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer patients on anticoagulation therapy: how significant is the bleeding toxicity? Int J Radiat Oncol Biol Phys. 2010;76:755–60. doi: 10.1016/j.ijrobp.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Moon CM, Kwon JH, Kim JS, et al. Nonsteroidal anti-inflammatory drugs suppress cancer stem cells via inhibiting PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in colorectal cancer. Int J Cancer. 2014;134:519, 29. doi: 10.1002/ijc.2838. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C, Jin Y, Shi S. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells. 2013;31:1383–95. doi: 10.1002/stem.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khor L-Y, Bae K, Pollack A, Hammond ME, Grignon DJ, Venkatesan VM, Rosenthal SA, Ritter MA, Sandler HM, Hanks GE, et al. COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92-02 trial. Lancet Oncol. 2007;8:912–20. doi: 10.1016/S1470-2045(07)70280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, Wallen E. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006;12:2172–7. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24:2723–8. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 18.Pruthi RS, Derksen JE, Moore D. A pilot study of use of the cyclooxygenase-2 inhibitor celecoxib in recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. BJU Int. 2004;93:275–8. doi: 10.1111/j.1464-410X.2004.04601.x. [DOI] [PubMed] [Google Scholar]
- 19.Saha D, Pyo H, Choy H. COX-2 inhibitor as a radiation enhancer: new strategies for the treatment of lung cancer. Am J Clin Oncol. 2003;26:S70–4. doi: 10.1097/01.COC.0000074161.92815.93. [DOI] [PubMed] [Google Scholar]
- 20.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 21.Abouassaly R, Paciorek A, Ryan CJ, Carroll PR, Klein EA. Predictors of clinical metastasis in prostate cancer patients receiving androgen deprivation therapy: results from CaPSURE. Cancer. 2009;115:4470–6. doi: 10.1002/cncr.24526. [DOI] [PubMed] [Google Scholar]
- 22.Tward JD, Lee CM, Pappas LM, Szabo A, Gaffney DK, Shrieve DC. Survival of men with clinically localized prostate cancer treated with prostatectomy, brachytherapy, or no definitive treatment: impact of age at diagnosis. Cancer. 2006;107:2392–400. doi: 10.1002/cncr.22261. [DOI] [PubMed] [Google Scholar]
- 23.Russo AL, Chen MH, Aizer AA, Hattangadi JA, D’Amico AV. Advancing age within established Gleason score categories and the risk of prostate cancer-specific mortality (PCSM) BJU Int. 2012;110:973–9. doi: 10.1111/j.1464-410X.2012.11470.x. [DOI] [PubMed] [Google Scholar]
- 24.Roach M, 3rd, Hanks G, Thames H, Jr., Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]


