Abstract
Systemic inflammation might modulate the microenvironment in the lungs and promotes metastasis. E-selectin, an inflammation inducible endothelial cell adhesion molecule, has been reported to play an important role in homing metastatic cancer cells. To study the effects of E-selectin expression induced by systemic inflammation on breast cancer metastasis, we first treated BALB/c mice with lipopolysaccharide (LPS) to induce systemic inflammation. Pulmonary tissues were analyzed by wet/dry ratio, hematoxylin and eosin (H&E) staining and immunohistochemistry. Then 4T1 cells were injected via tail vein. Lung surface metastasis was counted and detected by histological analysis. LPS-induced E-selectin expression and tumor cells adhesion were assessed by western blotting and immunofluorescence. The circulating levels of proinflammatory cytokines in sera were evaluated by ELISA. Our results showed that a significant increase in breast cancer metastasis to lungs was observed in LPS-treated mice vs. the PBS-treated mice, accompanying with an increased E-selectin expression in pulmonary tissue of LPS-treated mice. In vitro studies showed a significant elevation of E-selectin production in MPVECs which enhanced the adhesion activity of 4T1 cells. Treatment with anti-E-selectin antibody significantly reduced the development of metastasis in vivo, and significantly reduced the adhesion of 4T1 cells to MPVECs in vitro. Our results suggest that systemic inflammation may increase the expression of E-selectin which mediated the lung metastasis of breast cancer in mouse model.
Keywords: breast cancer, E-selectin, metastasis, microenvironment, inflammation, LPS, lung
Introduction
Breast cancer is the leading cause of cancer-related death in women.1 While advances have been made in breast cancer therapies, breast cancer metastasis remains an incurable disease, thus the prevention of metastasis must be a priority.2 Breast cancer metastasis is regulated not only by intrinsic genetic changes in malignant cells, but also by the tumor microenvironment. Several studies have demonstrated that systemic inflammation may influence the adhesion of circulation tumor cells in the microvasculature of distant organs.3,4 The adhesion of tumor cells to the vascular endothelium is a key step in the metastasis cascades, and interactions between endothelial selectins and tumor cell-expressed selectin ligands have been implicated in the metastatic process.5-7
The selectin family is represented by three receptors which are classified by their site of expression into E-selectin (activated endothelium), P-selectin (platelets and endothelial cells), and L-selectin (lymphocytes).8 E-selectin, also known as CD62E, is an important transmembrane glycoprotein mediating the initial cell–endothelial adhesion, which is expressed specifically on activated endothelial cells.9,10 Endothelial expression of E-selectin is induced by exposure to various inflammatory cytokines stimuli such as VEGF, TNF- α, IL-1, and LPS. Direct attachment of monocytes and change of hemodynamic conditions can also induce the production of E-selectin.11-13 E-selectin has been found to recognize particular oligosaccharide ligand sialylated Lewis x (sLex) expressed on leukocytes and tumor cells. The interaction between E-selectin and its ligands play an important role in metastatic tumor cell tethering and extravasation.14-16
A previous study has shown that the incidence of breast cancer associated lung metastasis is significantly higher in mice with arthritis.17 Moreover, celecoxib, an anti-inflammation drug, significantly reduces metastasis to lungs.18,19 These findings indicate the correlation between inflammatory microenviroment and the homing of circulating tumor cells. However, the mechanism of systemic inflammation in the metastatic spread of breast cancer has not been adequately studied.
In the present study, we used a murine breast cancer model of lung metastasis to determine if systemic inflammation induced by LPS contributes to increased breast cancer lung metastasis. In addition, we analyzed the effect of inflammation reaction induced by LPS to E-selectin expression both in vivo and in vitro. The role of E-selectin in 4T1 cells lung metastasis was studied in the mouse model. Our results show a significant increase in lung metastasis in LPS-treated mice vs. PBS-treated mice. E-selectin is upregulated in LPS-treated mice. Blocking the E-selectin significantly reduced lung metastasis in LPS-treated mice. This study demonstrates systemic inflammation promotes 4T1 cell lung metastasis, and this effect is mediated by E-selectin upregulation.
Results
LPS induced pulmonary inflammation and promoted metastasis
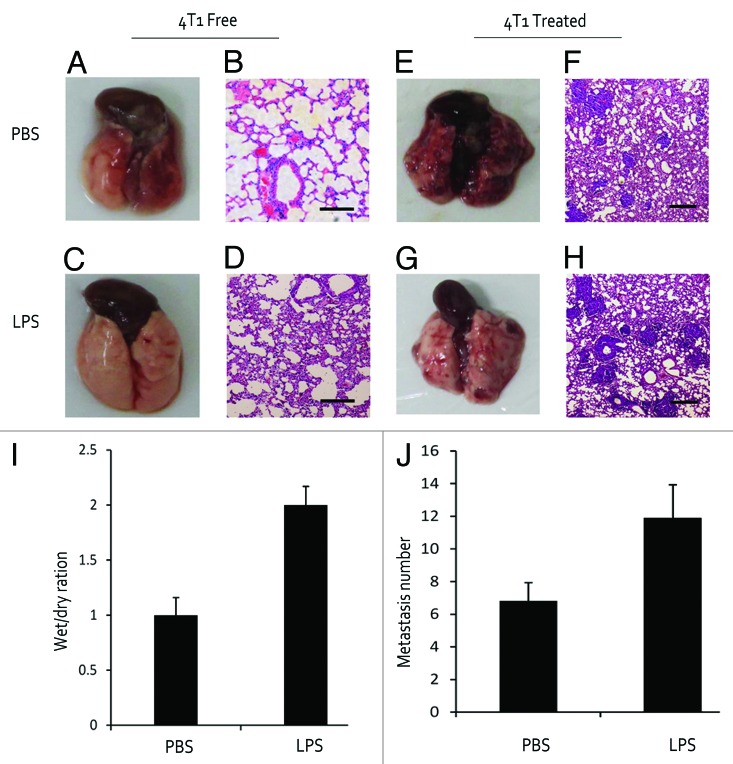
To demonstrate the inflammatory response in the lungs of LPS-treated mice, we examined the lung morphology in various mice. There was a significant increase in edema and lung wet/dry ratio in LPS-treated mice compared with PBS-treated mice (Fig. 1A, C, and I). From H&E staining, all lungs in LPS-treated mice were packed with inflammatory cellular infiltrates characterized by prominent neutrophilic and granulocytic cells. In comparison, PBS-treated lungs rarely showed inflammation reaction (Fig. 1B and D). The number and size of the lung metastasis were found to be significantly higher in LPS-treated mice than those in PBS-treated mice (Fig. 1E–H and J). The inflammatory response in the lung is correlated with the incidence of lung metastasis, suggesting the critical role of inflammatory response in promoting metastasis.
Figure 1. LPS induced pulmonary inflammation and promoted metastasis. (A and B) PBS-treated mouse was disposed as a control. (C and D) Mouse was injected with LPS for 3 d to confirm an inflammation model. (E–H) Mouse received 4T1 cell inoculation and lung metastasis number was counted after 3 wk. (I and J) Wet/dry weight ratio of lungs and number of metastatic foci per mice. Bar is 200 μm.
E-selectin expression in LPS-treated mice and metastatic foci
In the study of pulmonary E-selectin expression, we evaluated the presence of E-selectin in lung tissues. Strong expression of E-selectin was observed in lungs of LPS-treated mice, while in the lungs of PBS-treated mice E-selectin expression was scarcely detected (Fig. 2A and B). In addition, serum samples were assessed for E-selectin concentration by ELISA. The results showed that the concentration of serum E-selectin (sE-selectin) increased in LPS-treated mice in comparison with PBS-treated group (Fig. 2E) (P < 0.01). Slight E-selectin expression was detected on metastatic foci in lungs of PBS-treated mouse. However, in lungs of LPS-treated mouse, a stronger expression of E-selectin was observed accompanied with obvious metastastic foci (Fig. 2C, D, and F).
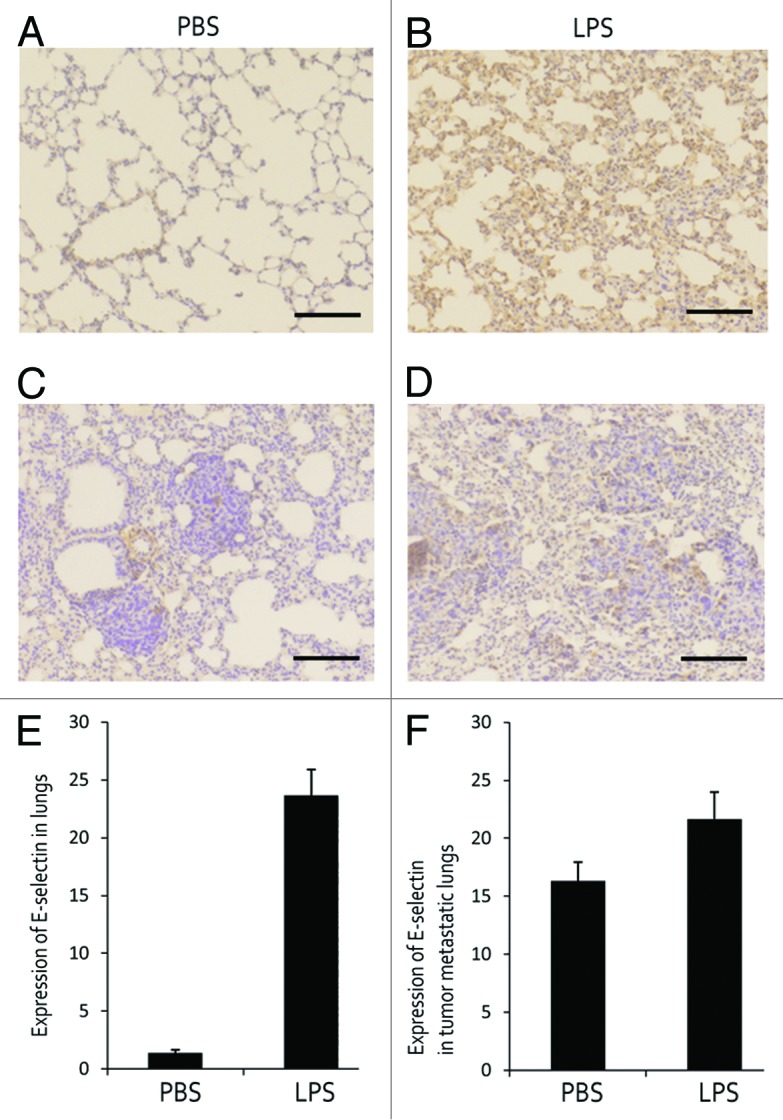
Figure 2. E-selectin expression in LPS-treated mice and metastatic foci. (A, B, and E) E-selectin expression in lungs of PBS- and LPS-treated mouse. (C, D, and F) E-selectin expression in lungs of tumor inoculated mouse.
Anti-E-selectin treatment reduced lung metastasis
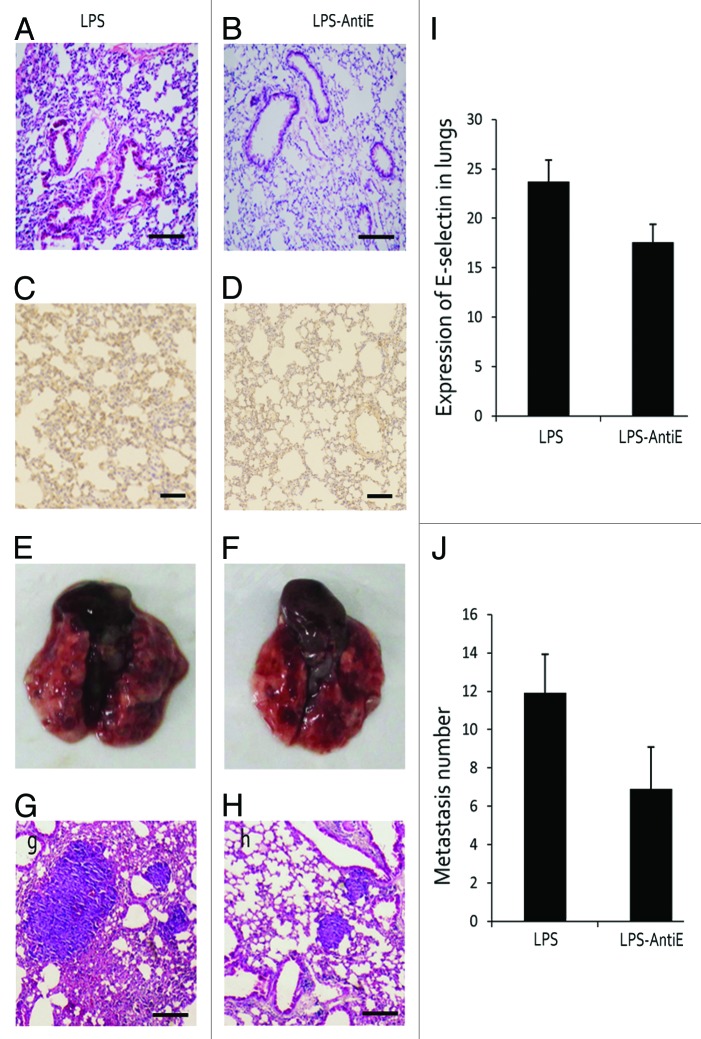
In order to study the association between E-selectin expression and metastasis, an anti-E-selectin antibody (AntiE) was used to block the E-selectin. As shown in Figure 3A and B, no difference of inflammatory response was observed between the two groups. E-selectin expression in the two groups exhibited appreciable difference under the anti-E-selectin treatment (Fig. 3C, D, and I), and the number of metastasis showed a significant decrease when treated with anti-E-selectin antibody (Fig. 3E–H and J).
Figure 3. Anti-E-selectin treatment reduced lung metastasis. (A and B) Mice were treated with LPS alone or with LPS and anti-E-selectin antigen together (LPS-Anti-E), there lung structures were showed by H&E staining. (C, D, and I) E-selectin expression was detected and contrasted in LPS and LPS-Anti-E group. (E–H) H&E staining showed the metastasis foci in lungs of LPS and LPS-Anti-E group. (J) The metastasis number was also analyzed.
LPS directly induced E-selectin expression in MPVECs
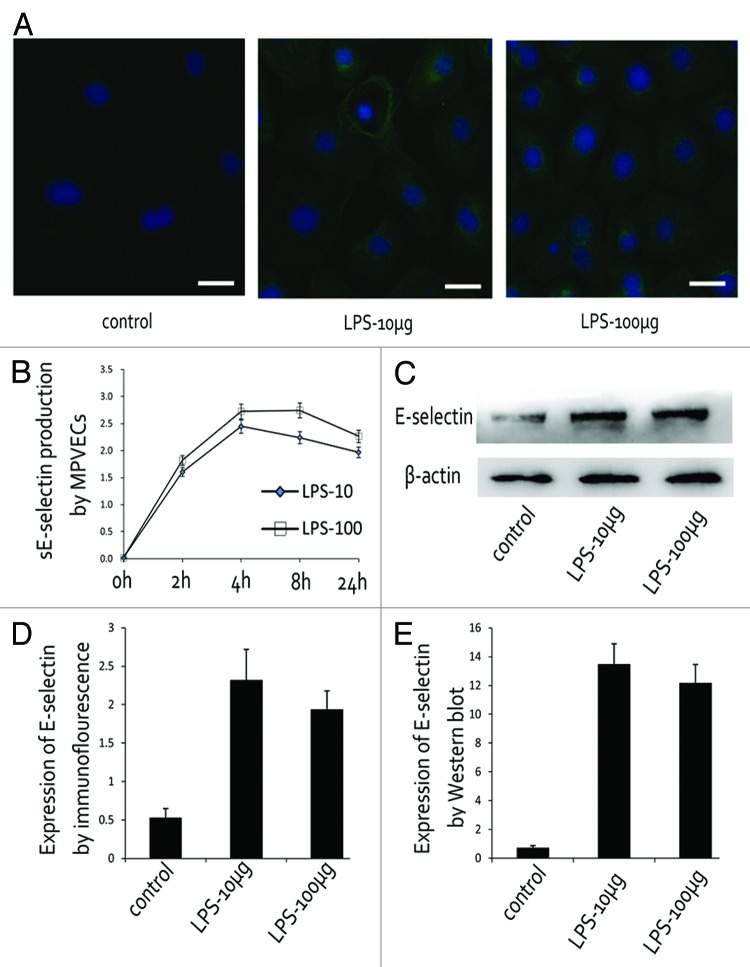
Soluble E-selectin derived from culture MPVECs was analyzed by ELISA. In the supernatant of PBS-treated MPVECs, no E-selectin expression was detected. On the contrary, both the lower dose (10 μg/mL) and the higher dose of LPS (100 μg/mL) caused a rapid induction of E-selectin (Fig. 4A). In the lower dose group, E-selectin level began to rise within 2 h and peaked at 4 h. In comparison, the higher dose group exhibited a rapid increase in E-selectin expression within 2 h and reached maximal level at 8 h after LPS stimulation. Consistent with the strong stimulation activity detected in the conditioned media from LPS-treated MPVECs, E-selectin expression level also significantly increased in LPS-treated MPVECs, as evaluated by immunofluorescence and western blot.
Figure 4. LPS induced E-selectin expression in MPVECs. (A and D) E-selectin expression in LPS-treated MPVECs detected by immunofluorescence. (B) Soluble E-selectin produced by MPVECs at different time point. (C and E) E-selectin expression in LPS-treated MPVECs detected by western blot.
4T1 cells homing to pulmonary microenvironment depended on E-selectin
BCECF-AM-labeled 4T1 cells were incubated and intravenously injected via tail vein. Tumor cells homing to lungs after LPS stimulation were significantly reduced by treatment with anti-E-selectin antibody (Fig. 5A and C). Next, we evaluated the role of E-selectin induction by LPS in 4T1cells–endothelial cell adhesion in an in vitro cancer cell adhesion assay. BCECF-AM-labeled 4T1 cells were incubated with monolayer cultured MPVECs for 30 min and then washed. LPS stimulation increased the number of BCECF-AM-labeled cancer cells attaching to lung endothelial cells. Addition of neutralizing anti-mouse E-selectin antibodies abolished the tumor cell adhesion to the endothelial cells. (Fig. 5B and D)
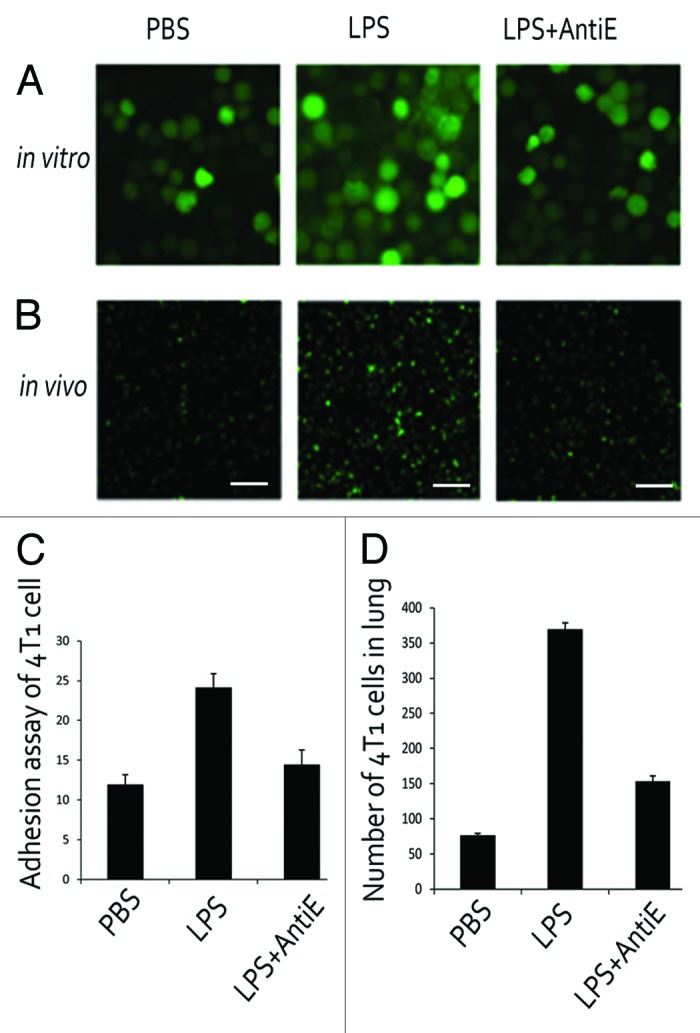
Figure 5. 4T1 cells homing to pulmonary microenvironment depends on E-selectin. (A) Tumor cells-MPVECs adhesion assays under three conditions: PBS, LPS (10 μg/mL), and LPS with anti-E-selectin antibody. (B) Tumor cell homing to lungs after LPS stimulation was significantly reduced by treatment with anti-E-selectin antibody in mice. Bar is 100 μm, and the pictures below were under 10 times magnification. (C) The number of adhering tumor cells was counted. (D) The number of tumor cells homing in lungs was counted.
LPS treatment had no effect on 4T1 proliferation
4T1 cells cultured in 96-well-plate were treated with different concentration of LPS, and no significantly increased proliferation was observed in LPS-treated groups compared with control group (Fig. 6).
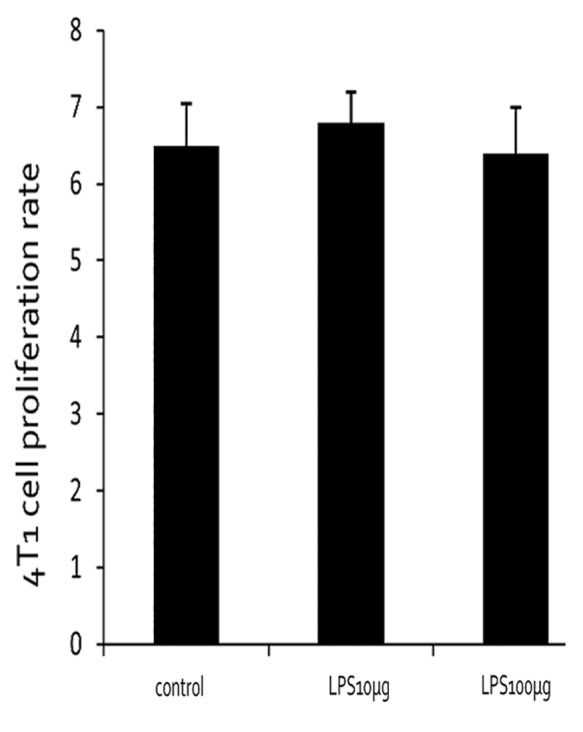
Figure 6. 4T1 proliferation rate under different LPS-treated conditions.
Discussion
Our results showed a significant increase in breast cancer lung metastasis was observed in the LPS-treated vs. PBS-treated mice. Compared with the control mice, the lungs of the LPS-treated mice expressed moderate levels of inflammatory response before breast cancer cells challenge. These findings suggest that an inflammatory milieu may be responsible for homing of the breast cancer metastatic cells to the lungs.
Several studies suggest that inflammation play a regulatory role in cancer progression and metastasis.20-22 Systemic inflammation facilitates cancer metastasis by promoting neovascularization and providing growth factors to the metastatic cells growth.23-25 Host inflammatory cells can participate in regulating tumor cells dissemination through the release of proinflammatory cytokines and chemokines, proangiogenic factors, and extracellular matrix-degrading proteinases.26-28 Consistent with these reports, our results showed that the inflammatory response in the lung is correlated with the incidence metastasis, suggesting the critical role of inflammatory response in promoting metastasis.
In our study, strong expression of E-selectin was observed in lungs of LPS-treated mice, while in the lungs of PBS-treated mice, E-selectin expression was scarcely detected. Treatment with anti-E-selectin antibody significantly reduced the development of metastasis. E-selectin is normally expressed at low levels on the sinusoidal endothelium, but can be upregulated by factors such as proinflammatory cytokines and bacterial products such as LPS.29,30 E-selectin has been shown to contribute to the metastatic spread of multiple cancers, including lung and colon carcinoma and melanoma.31-33 Functional inhibition of E-selectin has been shown to prevent the development of metastases in vivo. In human malignant disease, soluble E-selectin is significantly higher in the gastrointestinal, ovarian, and breast cancers.34,35 Further clinical research reported that sE-selectin were significantly elevated in patients with stage 4 breast carcinoma compared with controls.36,37 Furthermore, the serum level of sE-selectin was significantly higher in women with estrogen receptor-negative tumors.38 These clinical researches indicate that E-selectin is associated with the progress of human breast cancer.
In further examination of the mechanism, we found that tumor cells homing to lungs was significantly reduced by treatment with anti-E-selectin antibody in mice, suggesting that E-selectin could mediate tumor endothelial cell adhesion. These results were in agreement with results of our in vitro adhesion assays that anti-E-selectin antibodies markedly reduced numbers of 4T1 cells adhesion. E-selectin was upregulated in the metastatic microenvironment several hours after the microvascular arrest of tumor cells.39 The detected E-selectin expression was concomitant to the upregulation of VCAM-1, indicating an inflammatory activation of the metastatic microenvironment.40,41 This notion was further supported by the observed recruitment of inflammatory cells to metastatic tumor cells.42 A similar inflammatory activation was also observed in the sinusoidal endothelial to the upregulation of E-selectin.43 Furthermore, inhibition of E-selectin expression by antisense oligonucleotides or by function-blocking anti-E-selectin antibody attenuated experimental liver metastasis of tumor cells injected in the spleen of a mouse.44 Tumor cell extravasation is considered to be an important step during lung colonization. In the absence of E-selectin, we observed no reduction of metastasis, indicating that E-selectin expression is unlikely to facilitate tumor cell extravasation during lung colonization. Our results indicate that E-selectin expression directly facilitate tumor cell homing in lungs and thereby metastasis.
We have also shown that LPS does not directly promote 4T1 cells proliferation. The specific mechanism of LPS induced E-selectin is still under exploration. It has been demonstrated that LPS is recognized by TLR4 together with myeloid differentiation factor 2 on the cell surface.45 The complex then activates downstream signaling involving NFκB pathway. NFκB inhibitors can inhibit the expressions of E-selectin on LPS activated endothelial cells.46 However, the exact mechanism is still under hypothesis and needs further research.
Our results provide direct evidence that systemic inflammation can upregulate E-selectin in lung microenvironment, promote breast cancer cells adhesion and ultimately increase lung metastasis.
Materials and Methods
Tumor cell culture
Murine breast cancer 4T1 cells were purchased from the China Cell Culture Center. Tumor cells were cultured in DMEM supplemented with 10% FBS (GIBCO) and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin). Cells were routinely cultured in humidified air with 5% CO2 at 37 °C.
Animals
Female BALB/c mice (6–8 wk old) were purchased from Laboratory Animal Center of Shandong University and maintained under laminar-airflow condition. Laboratory chow and water were freely available. All experiments were approved by the Shandong University Experimental Animal Committee.
Lung metastasis colonization assay
In this experiment, mice were separated into two groups, each with 12 mice. One group was pretreated with LPS (5 mg/kg) for 3 d before tumor cell inoculation, and another group was treated with isometric phosphate-buffered saline (PBS). Then a total of 1 × 105 of 4T1 cells (>90% viability) were suspended in 0.1 mL of serum-free DMEM and injected into coupled abdominal mammary glands of each BALB/c mice. Isometric PBS was injected in the same way as a control. After 3 wk, mice were sacrificed and metastasis tumor nodules in lungs were counted under a dissecting microscope.
H&E staining and immunohistochemistry of lung tissues
Lungs from different groups of mice were dissected, fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, cut into slices with a thickness of 3–4 μm and stained with H&E. For immunohistochemistry, tissue sections were blocked with 3% bovine serum albumin for 1 h and then incubated with polyclonal antibodies against mouse E-selectin (Abcam) at a dilution of 1:100 overnight at 4 °C. After being washed with PBS, the sections were incubated with secondary antibody and streptavindin–peroxindase complex each for 1 h and visualized with 3,3-diaminobenzidine (DAB). The slices were observed under a light microscope.
Isolation and culture of mouse pulmonary vascular endothelial cells (MPVECs)
The method of MPVECs isolation has been described previously.47 Briefly, a three-week old BALB/c mouse was anesthetized by i.p. administration of 0.5% pentobarbital (10 mg/kg) and accepted a laparotomy to expose the lungs. The blood remaining in the pulmonary vascular was washed out by sufficient perfusion with Hank’s solution. Lungs were isolated and the lateral 1/3 part of each lob was cut into small pieces at size of 1 mm3, which were carefully transferred to a cell flask coated by 0.1% gelatin and cultured in DMEM supplemented with 20% FBS. The tissue pieces were removed after 72 h and cells were expanded with 0.25% trypsin for further incubation in 6- or 24-well plates. The primary cultured MPVEs were characterized by expression of CD31. These assays ensured that over 90% of cells in the primary cultures were endothelial cells.
Immunofluorescence of lung tissue and MPVECs
MPVECs were cultured in a 24-well plate with various concentrations of LPS for 6 h and then fixed by 4% paraformaldehyde. After being washed with PBS, cells were incubated with 3% bovine serum albumin at room temperature for 30 min, followed by incubation of E-selectin antibodies at a dilution of 1:100 overnight at 4 °C. For the immunofluorescence of lung tissue, the procedures on the first day had been described above. Similarly, a FITC-conjugated fluorescent secondary antibody was added next day, and cells (or tissue sections) were detected under an inverted fluorescent microscope.
Tumor cell–MPVECs adhesion assay
MPVECs were cultured in 24-well plates with various concentrations of LPS for 6 h. For blocking of 4T1 cell adhesion to MPVECs, the antibody to E-selectin was used. 4T1 cells were suspended at a concentration of 1 × 106 cells/mL in DMEM and labeled using 2’,7’-bis-5-carboxyfluorescetin-acetoxymethyl ester (BCECF-AM) at 37 °C for 15 min before being added to 24-well plates. The plate was incubated at 37 °C for 30 min, and then washed 3 times with PBS to remove unattached cells. The number of adhered BCECF-AM-labeled 4T1 cells was counted using an inverted fluorescent microscope.
Detection of E-selectin by ELISA
MPVECs were cultured in 6-well plates at 105 cells/well concentration for 1 d, then treated with different doses of LPS. Supernatants were collected for cytokine ELISA. Previous studies have shown that maximum expression of E-selectin was at 6 h, so we detected the expression of E-selectin at these time points. The procedures were operated following the instructions from the manufacturer.
Western blot analysis
Cell samples were collected in lysis buffer at ice for 30 min and cell lysates were cleared by centrifugation at 1.5 × 104 g for 5 min at 4 °C. Total proteins (40 μg) from cell lysates were separated on 10% sodium dodecyl sulfate-PAGE (SDS-PAGE) and transferred onto PVDF membranes (Millipore) using a Mini-Genie blotting system (Bio-Rad). The membrane was blocked with TBST containing 5% BSA for 1 h and then incubated with a polyclonal antibody against murine E-selectin (1:1000) at 4 °C overnight. Then membranes were washed with TBST 3 times and incubated with goat-anti-rabbit secondary antibodies (1:5000) for 1 h at room temperature. The protein bands on the membranes were visualized with an enhanced chemiluminescence system (ECL).
Statistical analysis
Differences between the results of two treatments were evaluated using the Student t test. Statistical significance was defined as P < 0.05.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by General programs of Natural Science Foundation (81272351), General programs of Natural Science Foundation of Shandong Province (ZR2012HM020), and Key Development Program for Basic Research of Shandong Province (2012G0021826).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–18. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 3.Taranova AG, Maldonado D, 3rd, Vachon CM, Jacobsen EA, Abdala-Valencia H, McGarry MP, Ochkur SI, Protheroe CA, Doyle A, Grant CS, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 2008;68:8582–9. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabodi S, Taverna D. Interfering with inflammation: a new strategy to block breast cancer self-renewal and progression? Breast Cancer Res. 2010;12:305. doi: 10.1186/bcr2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St Hill CA, Krieser K, Farooqui M. Neutrophil interactions with sialyl Lewis X on human nonsmall cell lung carcinoma cells regulate invasive behavior. Cancer. 2011;117:4493–505. doi: 10.1002/cncr.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–35. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, Teodoru M, Tanase CP. Cytokine patterns in brain tumour progression. Mediators Inflamm. 2013;2013:979748. doi: 10.1155/2013/979748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jubeli E, Moine L, Vergnaud-Gauduchon J, Barratt G. E-selectin as a target for drug delivery and molecular imaging. J Control Release. 2012;158:194–206. doi: 10.1016/j.jconrel.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 9.Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008;27:19–30. doi: 10.1007/s10555-007-9101-z. [DOI] [PubMed] [Google Scholar]
- 10.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- 12.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 13.Stannard AK, Khurana R, Evans IM, Sofra V, Holmes DI, Zachary I. Vascular endothelial growth factor synergistically enhances induction of E-selectin by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2007;27:494–502. doi: 10.1161/01.ATV.0000255309.38699.6c. [DOI] [PubMed] [Google Scholar]
- 14.Gout S, Tremblay PL, Huot J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin Exp Metastasis. 2008;25:335–44. doi: 10.1007/s10585-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–79. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das Roy L, Pathangey LB, Tinder TL, Schettini JL, Gruber HE, Mukherjee P. Breast-cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Res. 2009;11:R56. doi: 10.1186/bcr2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han M, Xu J, Bi Y, Jiang M, Xu X, Liu Q, Jia J. Primary tumor regulates the pulmonary microenvironment in melanoma carcinoma model and facilitates lung metastasis. J Cancer Res Clin Oncol. 2013;139:57–65. doi: 10.1007/s00432-012-1299-7. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478–83. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 23.Grote K, Schütt H, Schieffer B. Toll-like receptors in angiogenesis. ScientificWorldJournal. 2011;11:981–91. doi: 10.1100/tsw.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31:4499–508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 27.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–9. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Liu Q, Sun Q, Zhang C, Chen T, Cao X. TLR4 signaling in cancer cells promotes chemoattraction of immature dendritic cells via autocrine CCL20. Biochem Biophys Res Commun. 2008;366:852–6. doi: 10.1016/j.bbrc.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr., Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 30.Wong D, Dorovini-Zis K. Regualtion by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 1996;55:225–35. doi: 10.1097/00005072-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Fukami A, Iijima K, Hayashi M, Komiyama K, Omura S. Macrosphelide B suppressed metastasis through inhibition of adhesion of sLe(x)/E-selectin molecules. Biochem Biophys Res Commun. 2002;291:1065–70. doi: 10.1006/bbrc.2002.6572. [DOI] [PubMed] [Google Scholar]
- 32.Khatib AM, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M, Meterissian S, Brodt P. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol. 2005;167:749–59. doi: 10.1016/S0002-9440(10)62048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci (Landmark Ed) 2011;16:3233–51. doi: 10.2741/3909. [DOI] [PubMed] [Google Scholar]
- 34.Banks RE, Gearing AJ, Hemingway IK, Norfolk DR, Perren TJ, Selby PJ. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993;68:122–4. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velikova G, Banks RE, Gearing A, Hemingway I, Forbes MA, Preston SR, Hall NR, Jones M, Wyatt J, Miller K, et al. Serum concentrations of soluble adhesion molecules in patients with colorectal cancer. Br J Cancer. 1998;77:1857–63. doi: 10.1038/bjc.1998.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38:2252–7. doi: 10.1016/S0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 37.Gassmann P, Hemping-Bovenkerk A, Mees ST, Haier J. Metastatic tumor cell arrest in the liver-lumen occlusion and specific adhesion are not exclusive. Int J Colorectal Dis. 2009;24:851–8. doi: 10.1007/s00384-009-0694-2. [DOI] [PubMed] [Google Scholar]
- 38.Sheen-Chen SM, Eng HL, Huang CC, Chen WJ. Serum levels of soluble E-selectin in women with breast cancer. Br J Surg. 2004;91:1578–81. doi: 10.1002/bjs.4513. [DOI] [PubMed] [Google Scholar]
- 39.Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356–61. [PubMed] [Google Scholar]
- 40.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, Jain RK. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011;108:3725–30. doi: 10.1073/pnas.1100446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, Siddiqui J, Pienta KJ, Furie B, Dimitroff CJ. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci U S A. 2009;106:19491–6. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay PL, Huot J, Auger FA. Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008;68:5167–76. doi: 10.1158/0008-5472.CAN-08-1229. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression. Cancer Res. 2000;60:3978–84. [PubMed] [Google Scholar]
- 45.Wang X, Grace PM, Pham MN, Cheng K, Strand KA, Smith C, Li J, Watkins LR, Yin H. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 2013;27:2713–22. doi: 10.1096/fj.12-222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JE, Lee S, Han KS, Kim HK. Aurintricarboxylic acid inhibits the nuclear factor-κB-dependent expression of intercellular cell adhesion molecule-1 and endothelial cell selectin on activated human endothelial cells. Blood Coagul Fibrinolysis. 2011;22:132–9. doi: 10.1097/MBC.0b013e32834356b6. [DOI] [PubMed] [Google Scholar]
- 47.Chen SF, Fei X, Li SH. A new simple method for isolation of microvascular endothelial cells avoiding both chemical and mechanical injuries. Microvasc Res. 1995;50:119–28. doi: 10.1006/mvre.1995.1044. [DOI] [PubMed] [Google Scholar]