Abstract
Bt crops are able to produce Cry proteins, which were originally present in Bacillus thuringiensis bacteria. Although Bt maize is very efficient against corn borers, Spanish crops are also attacked by the earworm H. armigera, which is less susceptible to Bt maize. Many mechanisms could be involved in this low susceptibility to the toxin, including the insect's metabolic resistance to toxins due to cytochrome P450 monooxygenases. This paper examines the response of last instar H. armigera larvae to feeding on a diet with Bt and non-Bt maize leaves in larval development and in the gene expression of three P450 cytochromes: CYP6AE14, CYP6B2 and CYP9A12. Larvae fed on sublethal amounts of the Bt toxin showed reduced food ingestion and reduced growth and weight, preventing most of them from achieving the critical weight and pupating; additionally, after feeding for one day on the Bt diet the larvae showed a slight increase in juvenile hormone II in the hemolymp. Larvae fed on the non-Bt diet showed the highest CYP6AE14, CYP6B2 and CYP9A12 expression one day after feeding on the non-Bt diet, and just two days later the expression decreased abruptly, a finding probably related to the developmental programme of the last instar. Moreover, although the response of P450 genes to plant allelochemicals and xenobiotics has been related in general to overexpression in the resistant insect, or induction of the genes when feeding takes place, the expression of the three genes studied was suppressed in the larvae feeding on the Bt toxin. The unexpected inhibitory effect of the Cry1Ab toxin in the P450 genes of H. armigera larvae should be thoroughly studied to determine whether this response is somehow related to the low susceptibility of the species to the Bt toxin.
Introduction
Bt crops are able to produce Cry proteins, which were originally present in Bacillus thuringiensis bacteria. Spain is the European country with the highest number of hectares sown with Bt maize, 25.3% of the total of 97 346 hectares devoted to the maize crop, and the area has been increasing since Bt maize was introduced in 1998 [1]. The only Bt event sown is MON 810, which expresses the Cry1Ab type protein which is highly effective against the corn borers Ostrinia nubilalis [2] and Sesamia nonagrioides [3]. Maize crops in Spain are attacked by these two corn borers and by other Lepidoptera larvae that are less susceptible to Bt toxin: the leaf feeder Mythimna unipuncta and the maize earworm Helicoverpa armigera [4]. Pérez-Hedo et al [4] sugested that multiple mechanisms may be involved in the low susceptibility of these two Lepidoptera, including a high rate of toxin elimination inside the peritrophic membrane. The toxin elimination inside the peritrophic membrane could be due to rapid excretion, as occurs in M. unipuncta larvae [5] or to a high rate of degradation inside this space. Recently Gonzalez-Cabrera et al [6] indicated that the low levels of proteolytic enzymes involved in Cry toxin activation could be another reason for the low susceptibility of M. unipuncta to the Bt toxin.
Insects possess three enzyme systems providing metabolic resistance to toxins: esterases, cytochrome P450 monooxygenases and glutathione-S-transferases [7]. P450s are a multigenic superfamily of enzymes that are found in the biosynthetic pathways of ecdysteroids and juvenile hormones [8] and may be the greatest detoxification mechanism available to insects when they are exposed to a foreign agent [9]. Several authors have published excellent reviews of the roles played by these enzymes in insects [9], [10], [8], including growth, development, feeding, resistance to pesticides and tolerance to plant toxins [11]–[12]. When the resistance to insecticides is mediated by monooxygenases, it is usually due to increased detoxification or decreased activation of the xenobiotics [11] through overexpression or induction of the P450 genes [13]–[16]. The response of insects to plant allelochemicals and other xenobiotics is also mediated by monooxygenases [17]–[20].
In phytophagous caterpillars high P450 gene expression is also related to the periods of active feeding [10], [8], [21]. However, caterpillars do not feed continuously during each larval instar but show different phases that could be related to the rise and fall of the P450 expression during the instar reported by Agosin [9]. Moreover, last instar larvae undergo a phagoperiod that lasts a few days, followed by a phase of cessation of feeding and a massive purge of material from the gut [22]. The cessation of feeding due to the achievement of the critical weight [23] coincides with a change of developmental programme characterized by the turning off of the corpora allata and the secretion of juvenile hormone (JH), which leads to the release of the prothoracicotropic hormone and the activation of the prothoracic glands, followed by the pupation of the insect.
Recent studies have demonstrated that H. armigera has developed field resistance to the Cry1Ac toxin in China [24] and to fenvalerate in cotton in Australia [25]. Also, Zhou et al [26] reported the overexpression of several P450 genes of H. armigera in response to xenobiotics.
Therefore, considering the possibility of H. armigera developing resistance to the Cry1Ab toxin of maize in field conditions, we decided to analyse the response of larvae to the ingestion of sublethal amounts of the Bt toxin with respect to feeding behaviour, level of JH and expression of the several P450 genes identified as monooxygenases responding to xenobiotics.
Materials and Methods
Insects
H. armigera larvae were originally collected with permission of the owner (Josep Piqué) from a commercial non-Bt maize field in Lleida Spain (GPS coordinates 41°46’55.48’’N, 0°31’39.3’’E) and renewed every season. Larvae were reared on a semi-artificial diet [27]. The adults were supplied with a sugar solution (10%) and maintained at 21°C and high humidity (>60%) under a 16:8 h light: dark photoperiod.
Effects of Bt toxin on larval development
Newly moulted caterpillars of 6th instars (L60) were selected and provided with semi-artificial diets containing 9% lyophilized maize non-Bt (non-Bt diet) or Bt (Bt diet) maize leaves and 3% maize flour (all percentages refer to wet prepared diet) [28]. Every day until pupae or death, larvae (24 larvae in each type of diet) were changed to a clean box, and the larvae, ingested food and frass produced were weighed. The assimilation of ingested food (digestibility) and the ability to convert ingested and digested food into growth were evaluated by analysis of covariance [29]. The assimilation of food was examined by adjusting the amount of frass produced with food intake as covariate. The ability to convert ingested food into growth was examined by subtracting the weight of frass from the weight of ingested food and using the result as a covariate for larval weight gain [30]. Moreover, the food intake of the larvae fed on the Bt or non-Bt diet for all the instars was compared with the food intake of larva fed on a Bt diet for three days and then with the non-Bt diet for the rest of the instar.
The duration of the instars and the weight of the pupae were also recorded. A one-way ANOVA was used to analyse the effects of the Bt and non-Bt diet on pupal weight with the JMP statistical package [31]. In cases of significant differences the least significant difference Student t-test was used. Mortality and pupation were analysed using the normal approximation of the binomial test.
Hemolymph collection
Hemolymph was extracted from eight live larvae fed on the Bt diet and eight fed on the non-Bt diet on the first and third day of the sixth instar (L6d1, L6d3) by cutting a proleg with microscissors and collecting 40 µL from each larvae in a graduated glass micropipette.
JH II quantification
Hemolymph was collected in a vial with methanol/isooctane (1:1, vol:vol) and methoprene as an internal standard (5 ng). The hemolymph-solvent solution was vortexed for 20s and allowed to stand at room temperature for 30 min. Then, the whole sample was centrifuged at 8500 x g for 15 min, and the isooctane phase was transferred to a new glass vial. The remaining methanol phase was vortexed again, centrifuged at 10000 x g for 30 min, and combined with the isooctane phase in the same vial. The extracts were dried under nitrogen flow down and stored a −80°C. The extracts were diluted with 100 µL of methanol/water (80:20, v/v) for immediate analysis [32]. JH II, the predominant hormone in H. armigera [33], was measured. Five-point calibration curves, as standard, were obtained with methanol and by spiking sample blank extract free from JHII to cover a range in both cases of 1 to 100 ng/mL, with 18 ng/mL methoprene as the internal standard. To obtain blank extracts free from JHII, L6d1 larvae were decapitated and the hemolymph was extracted five days after decapitation [3]. The instrumental parameters used were an Acquity UPLC coupled to a QqQ-MS TQD (Waters, Milford, MA), that is, a triple quadrupole mass spectrometer using the ESI, APCI, and APPI interfaces, and the system was operated under Masslynx 4.1 software. The chromatographic separation was carried out at 28°C in the isocratic mode using methanol (Waters) (80+20, vol : vol) as the mobile phase. The injection volume was 15 µl in partial loop with needle overfill. The column used was a 100 mm×2.1 mm i.d., 1.7 µm, Acquity UPLC BEH C18 (Waters) at a flow rate of 400 µL/min. A total separation of 7 min was needed.
Tissue extraction
Midguts, the tissues with the highest expression of P450 [8], were dissected from L6 larvae fed one (L6d1) and three (L6d3) days in Bt or non-Bt diet and from larvae fed for three days on the Bt diet and one (L6d4) or three (L6d6) days on the non-Bt diet, immediately frozen in liquid N2 and stored at −80°C.
RNA isolation and cDNA synthesis
Total RNA was isolated from five midguts pooled together using TRIzol (INvitrogen, CA, USA) following the manufacturer's instructions. The RNA was quantified and its quality was assessed by agarose gel electrophoresis and absorbance measurements at λ260/λ280 nm with the Nanodrop ND-1000 spectrophotometer. Total RNA was treated with the Turbo DNA-free DNase kit (Applied Biosystems) according to the manufacturer's protocol in order to eliminate any traces of genomic DNA.
The first-strand cDNA was synthesized from 2 µg of total RNA with random hexamer primers (50 ng/µL) and dNTPs at 65°C for 5 min, then by reverse transcription in 20-µL reactions with the SuperScript III First-Strand Synthesis System kit (Invitrogen, Carlsbad, CA, USA) following the recommended protocol. Three independent RNA preparations representing three biological replicates for each treatment were used for cDNA synthesis.
The primer sets used in this study are listed in Table 1. Three primers of two different families, with different response to allelochemicals [26] were chosen. Two of the primers tested were identical to described by Zhou et al [26], the other primer was re-designed using Genomics expression software. Specificity of the polymerase chain reaction (PCR) amplification was checked by a melt curve analysis and by sequencing the PCR products.
Table 1. Primers used for amplification and quantification of Helicoverpa armigera cDNA.
| Gene | Primers | Sequence (from 5′ to 3′) | Length | T°m | Amplicon size |
| EF-1α | EF-1α-F EF-1α-R | GACAAACGTACCATCGAGAAG GATACCAGCCTCGAACTCAC | 21 20 | 62 | 279 |
| CYP6AE14 | CYP6AE14-F CYP6AE14-R | TGTGCATTTGGCGTTGAA TCCGAGATGTGGGCGTAT | 18 18 | 54 | 241 |
| CYP6B2 | CYP6B2-F CYP6B2-R | CCTGAAAGATTCTTCGCGGAGGAATTGGACAGCAGCTTCGTGATGC | 24 22 | 70 | 140 |
| CYP9A12 | CYP9A12-F CYP9A12-R | ATCACCTCATAGAAGATATCC CATGTCTTTCCATTCTTGACC | 21 21 | 59 | 233 |
cDNA was used in subsequent PCR reactions carried out in the Eppendorf Mastercycler DNA Engine Thermal Cycler PCR (Eppendorf AG, Hamburg, Germany). The reaction mixtures of 25 µL contained 2.5 µL 10x buffer, 1 µL 200 µM dNTP mix, 1 µL 10 µM of each primer (Table 1), 1U of Taq polymerase (BIOTOOLS, Madrid, Spain) and 2 µL of the cDNA solution. The initial denaturing step of 1 min at 94°C was followed by 20 cycles of 20 s at 60°C with a −0.5°C change per cycle, and 1 min at 72°C; then 30 cycles of 1 min at 94°C, 1 min at 50°C and 1 min at 72°C; the reaction was concluded with 5 min at 72°C. PCR products were separated by electrophoresis in 2% agarose gel.
Sequencing
PCR products were cleaned and extracted with the QIAquick PCR purification kit (QIAGEN, Düsseldorf, Germany) and then sequenced using the BigDye Terminator Sequencing kit v3.1 (Applied Biosystems, Foster City, CA, USA) and the ABI-3130 capillary electrophoresis system. Sequence homologies were confirmed by a nucleotide BLAST search.
Quantitative analysis of cytochrome p450 gene expression
The expression level of P450 cytochromes in midgut tissues was analysed by quantitative real-time PCR (q-PCR), following to the MIQE guidelines [34].
q-PCR was done using a CFX 96 system and IQ SYBR Green Supermix (Bio-Rad Laboratories, 2000 Alfred Nobel Drive, Hercules, CA 94547 USA). EF-1α was used as a reference gene to normalize the target gene expression levels among samples. q-PCR of each cDNA sample and a template-free control was performed at least in triplicate. The primer sets used in this study are listed in Table 1.
Specificity of the PCR amplification was checked by a melt curve analysis (Bio-Rad CFX Manager 3.0 software) and by sequencing the PCR products. q-PCR was run in a 25 µL reaction containing 22 µL RealMasterMix/SYBR solution, 0.5 µL each of forward and reverse primer (10 µM) and a 2 µL cDNA template using the following cycling parameters: 95°C for 5 min, followed by 45 cycles of 95°C for 30 s, 55–60°C for 30 s and 68°C for 40 s. The melting curves of amplicons were measured by taking continuous fluorescence reading while increasing temperature from 58 to 95°C with 0.5°C increments for 10 s. For each gene, a serial dilution from 10- to 1000-fold of each cDNA template was performed in order to assess the efficiency of the PCR. The relative expression levels of target genes were calculated using Bio-Rad CFX Manager Software. Values represent the mean of the different replicates ± standard error. Amplification efficiencies were compared by plotting the ΔCt values of different primer combinations of serial dilutions against the log of starting template concentrations using the CFX96 software. The Ct values were adjusted to standard curves and were normalized against the levels of EF-1α.
The transformed data (sqrt (x+0.5)) were analysed by two-way ANOVA. Results were expressed as mean expression ratio (±SE) of 1-day, 3-day, 4-day and 6-day L6 larvae fed with the Bt and non-Bt diet.
Results
Effects of Bt toxin on feeding behaviour and larval development
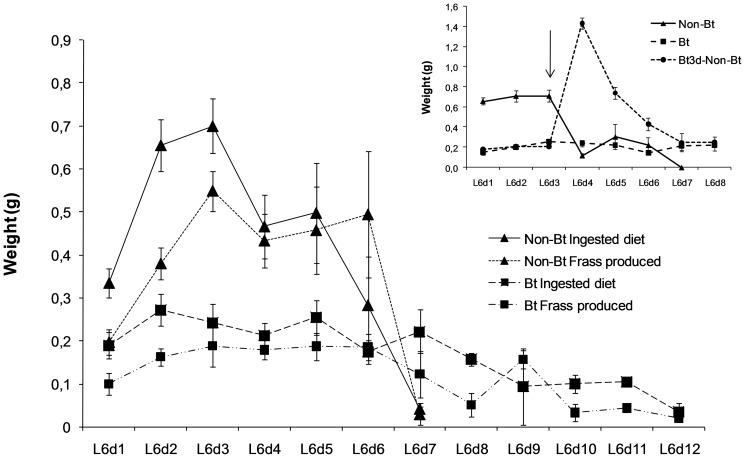
The feeding behaviour of the larvae fed on the non-Bt diet shown in Figure 1 had three different periods. In the first period, from L6d1 to L6d3, the larvae fed actively, ingesting a greater amount of diet than the amount of frass produced. In the second period, from L6d4 to L6d5, the amount of ingested food and the frass produced were quite similar. In the third period the amount of frass produced was much greater than the amount of food ingested, signalling the purging period, which ended with the pupation of the larvae.
Figure 1. Feeding behaviour, daily mean (±SE) of ingested diet (g) and frass produced (g) of the L6 larvae of of Helicoverpa armigera fed on the Bt or non-Bt diet.
Inset compares the daily ingested diet of the larvae fed for the whole last instar on the Bt or non-Bt diet with the ingested food of the larvae fed on the Bt diet for three days and then on the non-Bt diet for the rest of the instar. Arrow in the inset indicate the day the lavae were changed from the Bt to non-Bt diet.
Feeding behaviour of the larvae fed on the Bt diet was quite different (Figure 1). The food intake was higher than the amount of frass produced until the twelfth day of the instar, except for the ninth day, and no abrupt changes in behaviour were observed. The larvae ate less than the larvae fed on the non-Bt diet but fed for a longer period. The first pupae appeared one day later than the larvae fed on the non-Bt diet (Table 2) and the majority of the larvae died without pupating. Larvae fed for three days on the Bt diet and then on the non-Bt diet for the rest of the instar displayed a sharp increase in the food intake immediately after the change of diet followed by a decrease in the feeding activity similarly to, but three days later than the larvae fed on the non-Bt diet (inset in Figure 1).
Table 2. Accumulated pupation (%) and mortality (%) of Helicoverpa armigera larvae fed on the non-Bt and Bt diet during the whole last larval instar or for three days with the Bt diet and then with the non-Bt diet during the rest of the instar (Bt3d-nonBt).
| % pupation | % mortality | |||||
| Non-Bt | Bt | Bt3d- non-Bt | Non-Bt | Bt | Bt3d- non-Bt | |
| L6d5 | 0.0 | 0.0 | 0 | 0.0 | 12.5 | 0 |
| L6d6 | 9.1 | 0.0 | 0 | 4.5 | 16.7 | 0 |
| L6d7 | 45.5 | 4.2 | 4 | 4.5 | 16.7 | 0 |
| L6d8 | 72.7 | 8.3 | 16 | 4.5 | 20.8 | 0 |
| L6d9 | 90.9 | 12.5 | 64 | 4.5 | 20.8 | 4 |
| L6d10 | 95.5 | 16.7 | 92 | 4.5 | 37.5 | 4 |
| L6d11 | 16.7 | 92 | 50.0 | 4 | ||
| L6d12 | 16.7 | 92 | 66.7 | 4 | ||
| L6d13 | 16.7 | 92 | 83.3 | 4 | ||
| L6d14 | 16.7 | 94 | 83.3 | 4 | ||
Pupation and mortality were significantly different for the larvae fed on the Bt diet and the non-Bt (Z = −2.85, P = 0.002) and for three days on the Bt diet and then on the non-Bt diet (Z = 2.31, P = 0.001). Larvae fed on the non-Bt diet started pupation on the sixth day and finished by the tenth day of L6 instar (Table 2). The pupation occurred in 7.7 days on average, while in larvae fed on the Bt diet the pupation occurred in 8.8 days on average. The weight of pupae from larvae fed on the Bt diet was different (0.17 g on average), of the pupae from larvae fed on the non-Bt diet (0.32 g) and of the pupae from larvae fed 3 days on the Bt diet and then on the non-Bt diet (0.29 g) (F = 107.32; df = 2,66; P = 0.001).
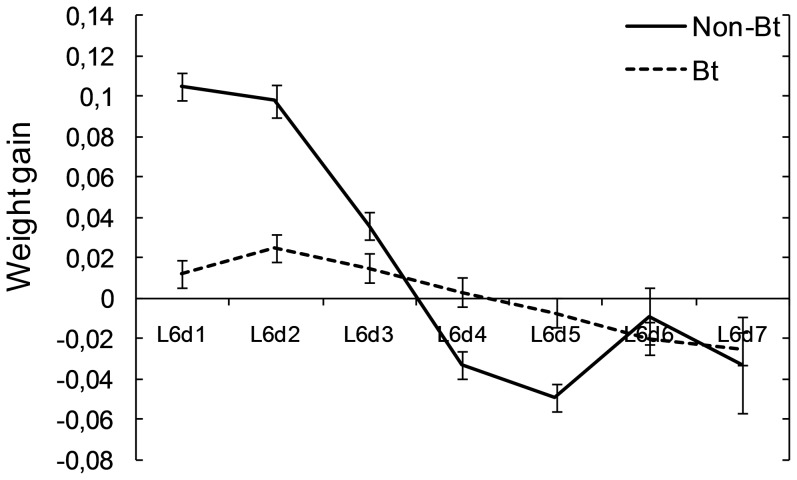
Assimilation of food examined by adjusting the amount of frass produced with food intake as covariate was influenced by the type of diet (F = 5.21; df = 1,253; P = 0.002). Two-way (diet and day) interaction was not significant (F = 0.76; df = 6, 253; P = 0.60). The influence of the diet on the weight gain significantly interacted with the day of the last instar larvae (F = 25.5; df = 6, 253; P>0.001) (Figure 2) when the subtraction food intake minus faeces was used as covariate (F = 10.07; df = 1, 253; P>0.001). The efficiency of conversion of digested food during the first two days of the last instar was significantly higher for the larvae fed on the non-Bt diet than for the larvae fed on the Bt diet. The efficiency decreased on the third day, being similar for the larvae fed on the Bt and non-Bt diet. On the fourth and fifth days of the last instar it was higher for the larvae fed on the Bt diet and on the remaining days of the instar it was similar for the larvae fed on both diets. The abrupt change in the ability of conversion of digested food of the larvae fed on the non-Bt diet on days 4 and 5 of the last instar indicated that the purge process leading to pupation had already started.
Figure 2. Ability to convert digested food into growth: relation between larval weight gain and the subtraction of weight of frass produced from weight of the food ingested during the last instar of Helicoverpa armigera larvae fed on a diet with the Bt and non-Bt leaves.
Bars show least significant differences (P<0.05) for each mean in the ANCOVA of weight gain when ‘food intake-faeces’ is the covariate.
These results indicate that there was another factor that influenced the growth rate of the last instar larvae apart from the food intake or frass produced. The differences between the growth rate of the larvae fed on the Bt diet and the non-Bt diet in the first two days of the instar are probably due to the lower food intake together with the toxic effect of the Bt toxin.
Effects of Bt toxin ingestion on the level of JH II in the hemolymph
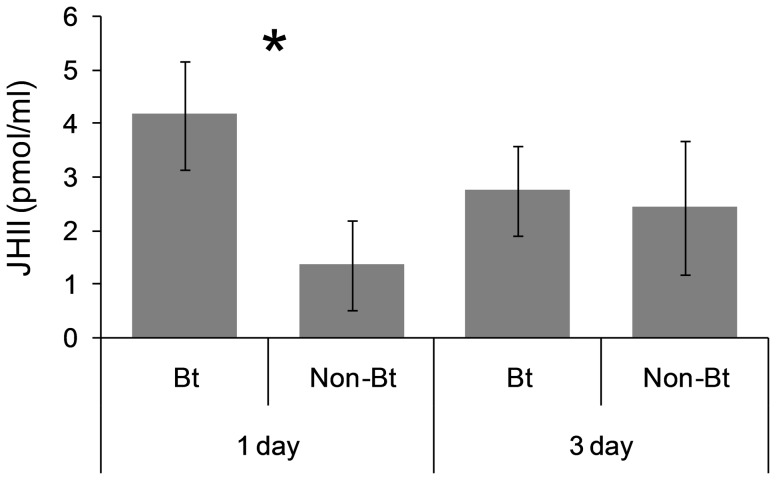
Figure 3 shows the concentration of JHII in the hemolymph of the first and third day of the sixth instar (L6d1 and L6d3, respectively) of larvae fed on the Bt and non-Bt diet. Concentration of JH II in the hemolymph was higher in the larvae fed on the Bt diet for one day than in the larvae fed on the non-Bt diet (F = 6.62; df = 1,14; P = 0.02) but two days later there were not differences in the JHII concentration according to the type of diet ingested (F = 0.06; df = 1,15; P = 0.81).
Figure 3. Effect of Bt toxin in the diet on the concentration of JHII in the larval hemolymph of Helicoverpa armigera.
Larvae were fed on the non-Bt or Bt leaves for one (L6d1) or three (L6d3) days. An asterisk above the columns indicates differences between treatments, and bars indicate SE.
Quantitative analysis of cytochrome p450 gene expression
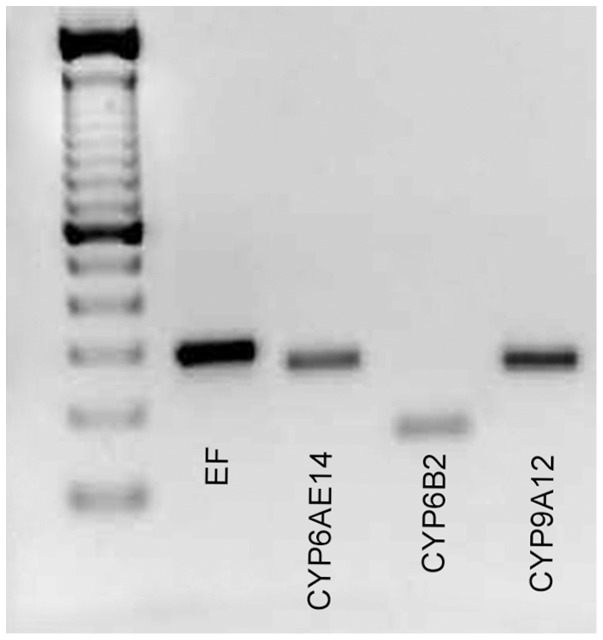
cDNA was amplified by PCR with specific primers and separated by electrophoresis. Expected size was detected for each cytochrome (Figure 4) and confirmed by sequencing.
Figure 4. cDNA fragments produced from midgut tissues of Helicoverpa armigera larvae.
Specific products were obtained for the elongation factor EF-1α (279bp), CYP6AE14 (241bp), CYP6B2 (140bp), and CYP9A12 (233bp).
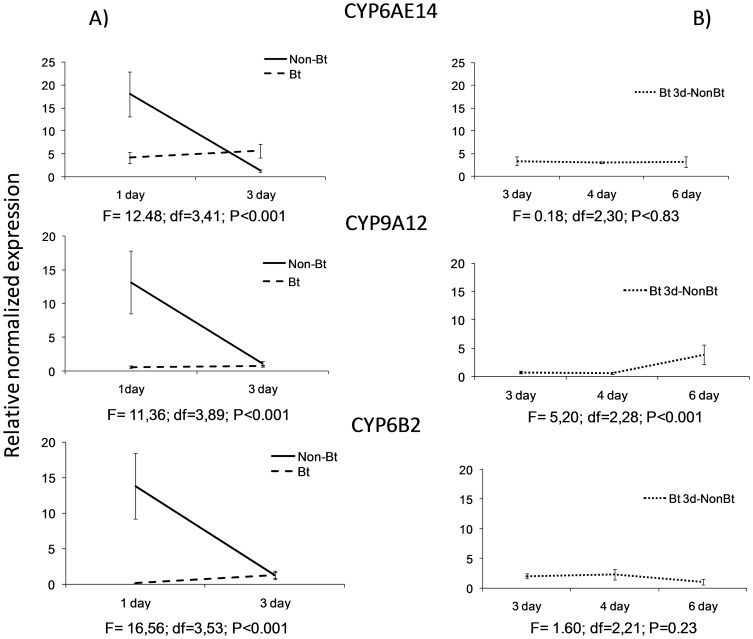
The results of the quantitative q-PCR signalled that the expression of the cytochromes CYP6AE14, CYP6B2 and CYP9A12 was differentially affected by diet and feeding days (Figure 5).
Figure 5. Relative normalized expression of the CYP6AE14, CYP9A12 and CYP6B2 genes in L6 larvae of Helicoverpa armigera fed A) for one (1 day) or three (3day) days on a the non-Bt or Bt diet and B) for three days on the Bt diet (day 3) and then one (4 day) or three (6 day) days on the non-Bt diet (Bt 3d-non Bt).
Values represent the mean of at least three replicates ± SE.
Although the three genes compared belonged to two different families, CYP 6 and CYP 9, the response to the non-Bt diet (Figure 5A) was similar for all three. The highest expression occurred one day after feeding and the expression decreased sharply on day 3, suggesting a higher feeding activity during the first few days of the instar.
The response to the Bt toxin was quite different. Those feeding on the Bt diet almost completely suppressed the gene expression, and the expression of the CYP6AE14, CYP6B2 and CYP9A12 genes of the larvae fed on the Bt diet were 4.4, 125, and 25 times lower than those of the larvae fed for one day on the non-Bt diet. The expression of the three genes in the larvae fed on the Bt diet rose two days later but no significantly, and was higher than that of the larvae fed on the non-Bt diet only for the gene CYP6AE14 (Figure 5A). When the larvae changed the type of diet three days after feeding on the Bt diet (Figure 5B), the expressions of the genes CYP6AE14 and CYP6B2 remained low has happened in the larvae fed on the Bt diet. Only the response of the CYP9A12 gene increased after the change to the non-Bt diet in one of the repetition performed, showing a double (sample x repetition) interaction (F = 21.43; df = 2,17; P<0.001).
Discussion
The development of the last instar of H. armigera followed the pattern described by Nijhout and Williams [22]. Larvae increased their weight to achieve a critical weight three days after the moult to the instar. Then, they reduced the weight drastically due to the purging, in the process leading to pupation. The achievement of the critical weight coincides with a change of developmental programme characterized by the turning off of the corpora allata and the secretion of JH, which leads to the pupation of the insect [23].
However, larvae of H. armigera fed on sublethal amounts of the Bt toxin showed reduced food ingestion and reduced growth and weight, which did not allow the majority of them to achieve the critical weight and pupate. This reduction of food intake, which occurs in other caterpillars in response to sublethal amounts of pesticides [35], followed by the increase of food intake that took place as a response of the change from the Bt to the non-Bt diet, coincides with the behaviour described by Slansky and Scriber [36]. These authors indicated that this behaviour may be an adaptive response to a feeding deterrent in which the reduced intake allows the larvae not to overload their detoxification and excretory system, thus avoiding death by toxicity or starvation until the change of food. This feeding behaviour may also be the consequence of the metabolic interference of the toxin with the larva's growth. Both of these phenomena may occur in the response of H. armigera larvae to Bt toxin ingestion.
The three P450 genes studied in the local population of H. armigera larvae belonged to the CYP 6 and 9 families. Some of them, such as CYP 6B2, are larval-specific [11]. The CYP 6 family is one of the most studied insect monooxygenase families in relation to insecticide resistance and plant allelochemical responses [37], [19]. CYP 9 monooxygenases have been less studied but also in relation to the response to plant allelochemicals and sometimes the response to xenobiotics [18].
Larvae fed on the non-Bt diet showed the highest CYP6AE14, CYP6B2 and CYP9A12 expression one day after feeding on the non-Bt diet, and just two days later the expression decreased abruptly. This decrease is probably related to the developmental programme of the last instar, which involves a sharp reduction in feeding after the critical weight is achieved. This change, related to JH suppression and prothoracicotropic hormone activation, may have been a consequence of P450 gene suppression or activation, because it is known that some P450 genes are involved in hormone synthesis and metabolization [10], [38]. Interestingly, the ingestion of Bt toxin led to the suppression of the three P450 genes tested, indicating that the response was not gene- or family-specific, and produced a slight increase in the JH titer in the hemolymph as was detected in the S. nonagrioides larvae fed on sublethal amounts of Bt toxin [3] or Chilo suppressalis fed on imidacloprid [39]. When larvae were changed from the Bt diet to the non-Bt diet, they recovered the feeding activity but the expression of the genes did not improve. Therefore, the suppression of the gene expression may be due not only to the reduction of the feeding activity [10], [8], [21] but also to the Bt toxin effect.
The response of P450 genes to plant allelochemicals and xenobiotics has been related in general to overexpression of the genes in the insect's resistance to insecticides, or induction of the genes when the feeding takes place [14], [17], [19], [40]. The inhibition of gene expression, although occurring in some cases [26], has been little studied. In relation to the insect's resistance to insecticides, the response of some P450 inhibitors has been studied from the point of view of the synergists of the insecticides [41], [14].
The results of the present study support the hypothesis that feeding on a Bt diet causes an suppression in the P450 expression, then reduces the feeding activity, and then the expression increases slightly and so does the feeding activity, so growth is more limited and slower. Mao et al [42] demonstrated that the larvae of H. armigera fed on transgenic cotton plants expressing dsCYP6AE14 showed a reduced expression level of CYP6AE14 and drastically retarded growth, so the effect achieved with the gene suppression by the dsRNA plants was somewhat similar to the effect produced by the gene suppression by the Bt toxin. It must be pointed out that the response of the P450 genes of insects to Bt ingestion has been studied very little [43].
H. armigera larvae have developed resistance to many insecticides [25] and to the Cry1Ac toxin in a Bt cotton in field in China [24], and have been reported to be tolerant to Bt maize in Europe [4]. The unexpected suppressive effect of the Cry1Ab toxin in the P450 genes of the CYP6 and CYP9 families of H. armigera larvae deserves to be further studied in order to determine whether the response to other Cry toxins is similar, whether the suppressive effect of the toxin can act as a synergist for other xenobiotics or other Cry toxins, how the strains of H. armigera resistant to insecticides respond to Bt toxins, and whether this response is related in some way to the low tolerance of the species to the Bt toxin.
Acknowledgments
The authors thank Joan Safont, Aurora Ribes, Dr Gemma Farré, Dr Ariadna Peremarti, Dr Gina Sanahuja, Dra Romi Pena, David Almuzara, Eva Puig, and Isabel Sánchez for their technical assistance.
Funding Statement
This work was supported by a grant from the Spanish R&D Agency (Comisión Interministerial de Ciencia y Tecnología) through project AGL2011-23996. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.MARM (2011) Ministerio de Medio Ambiente Medio Rural y Marino. Available at: www.marm.es/estadistica (accessed 13 November 2011).
- 2. Barry BD, Darrah LL, Huckla DL, Antonio AQ, Smith GS, et al. (2000) Performance of transgenic corn hybrids in Missouri for insect control and yield. J Econ Entomol 93: 993–999. [DOI] [PubMed] [Google Scholar]
- 3. Pérez-Hedo M, Albajes R, Eizaguirre M (2011a) Modification of hormonal balance in larvae of the corn borer Sesamia nonagrioides due to Bacillus thuringiensis protein ingestion. J Econ Entomol 104: 853–861. [DOI] [PubMed] [Google Scholar]
- 4. Pérez-Hedo M, López C, Albajes R, Eizaguirre M (2012) Low susceptibility of non-target Lepidopteran maize pests to the Bt protein Cry1Ab. Bull Entomol Research 102: 737–743. [DOI] [PubMed] [Google Scholar]
- 5. Pérez-Hedo M, Reiter D, Lopez C, Eizaguirre M (2013) Processing of the maize Bt toxin in the gut of Mythimna unipuncta caterpillars. Entomol Exp Appl 148: 56–64. [Google Scholar]
- 6. González-Cabrera J, García M, Hernández-Crespo P, Farinós GP, Ortego F, et al. (2013) Resistance to Bt maize in Mythimna unipuncta (Lepidoptera: Noctuidae) is mediated by alteration in Cry1Ab protein activation. Insect Biochem Mol Biol 43: 635–643. [DOI] [PubMed] [Google Scholar]
- 7.Dauterman WC (1981) In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 12 . New York: Pergamon Press. pp. 713–730. [Google Scholar]
- 8. Feyereisen R (1999) Insect P450 enzymes. Annu Rev Entomol 44: 507–33. [DOI] [PubMed] [Google Scholar]
- 9.Agosin M (1985) Role of microsomal oxidation in insecticide degradation. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 12. New York: Pergamon Press. pp. 647–712. [Google Scholar]
- 10.Hodgson E (1985) Microsomal Mono-Oxigenasas pp. 225-321: In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 4. Pergamon Press; 1985. pp. 225–319. [Google Scholar]
- 11. Scott JG, Liu Na, Wen Z (1998) Insect cytochromes P450: diversity, insecticide resistance and tolerance to plant toxins. Comp Biochem Physiol C 121: 147–155. [DOI] [PubMed] [Google Scholar]
- 12. Scott JG (1999) Cytochromes P450 and insecticide resistance. Insect Biochem Mol Biol 29: 757–777. [DOI] [PubMed] [Google Scholar]
- 13. Bautista MAM, Tanaka T, Miyata T (2007) Identification of permethrin-inducible cytochrome P450s from the diamondback moth, Plutella xylostella (L.) and the possibility of involvement in permethrin resistance. Pestic Biochem Physiol 87: 85–93. [Google Scholar]
- 14. Brun-Barale A, Héma O, Martin T, Suraporn S, Audant P, et al. (2010) Multiple P450 genes overexpressed in deltamethrin-resistant strains of Helicoverpa armigera . Pest Manag Sci 66: 900–909. [DOI] [PubMed] [Google Scholar]
- 15. Jones CM, Daniels M, Andrews M, Slater R, Lind RJ, et al. (2011) Age-specific expression of a P450 monooxygenase (CYP6CM1) correlates with neonicotinoid resistance in Bemisia tabaci . Pestic Biochem Physiol 101: 53–58. [Google Scholar]
- 16. Karunker I, Benting J, Lueke B, Ponge T, Nauen R, et al. (2008) Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) Insect Biochem Mol Biol. 38: 634–644. [DOI] [PubMed] [Google Scholar]
- 17. Snyder MJ, Stevens JL, Andersen JF, Feyereisen R (1995) Expression of Cytochrome P450 genes of the CYP4 family in midgut and fat body of the tobacco Hornworm Manduca sexta . Arch Biochem Biophys 321: 13–20. [DOI] [PubMed] [Google Scholar]
- 18. Stevens JL, Snyder MJ, Koener JF, Feyereisen R (2000) Inducible P450s of the CYP9 family from larval Manduca sexta midgut. Insect Biochem Mol Biol 30: 559–568. [DOI] [PubMed] [Google Scholar]
- 19. Schuler MA (2011) P450s in plant-insect interactions. Biochim et Biophys Acta 1814: 36–45. [DOI] [PubMed] [Google Scholar]
- 20. Rupasinghe SG, Wen Z, Chiu T-L, Schuler MA (2007) Helicoverpa zea CYP6B8 and CYP321A1: different molecular solutions to the problem of metabolizing plant toxins and insecticides. Protein Eng Des Sel 20: 615–624 doi:10.1093/protein/gzm063 [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Yuan D, Ding L, Li P, Li F, et al. (2013) Expression of cytochrome P450 CYP6B6 in the different developmental stages of the insect Helicoverpa armigera (Lepidoptera: Noctuidae). Eur J Entomol 110: 39–45. [Google Scholar]
- 22. Nijhout HF, Williams MC (1974) Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): growth of the last-instar larva and the decision to pupate J Exp Biol. 61: 493–501. [DOI] [PubMed] [Google Scholar]
- 23. Nijhout HF, Williams MC (1974) Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol 61: 493–501. [DOI] [PubMed] [Google Scholar]
- 24. Zhang H, Yin W, Zhao J, Jin L, Yang Y, et al. (2011) Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS One 6(8): e22874 doi:–10.1371/journal.pone.0022874 20: 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jouβen N, Agnolet S, Lorenz S, Schöne SE, Ellinger R, et al. (2012) Resistance of Australian Helicoverpa armigera to fenvalerate is due to the chimeric P450 enzyme CYP337B3. Proc Natl Acad Sci U S A 109: 15206–15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X, Sheng C, Li M, Wana H, Liu D, et al. (2010) Expression responses of nine cytochrome P450 genes to xenobiotics in the cotton bollworm Helicoverpa armigera . Pestic Biochem Physiol 97: 209–213. [Google Scholar]
- 27. Eizaguirre M, Albajes R (1992) Diapause induction in the stem corn-borer, Sesamia nonagrioides (Lepidoptera, Noctuidae). Entomol Gen 17: 277–283. [Google Scholar]
- 28. Pérez-Hedo M, Marques T, Lopez C, Eizaguirre M (2011b) Determination of the Cry1Ab toxin in Helicoverpa armigera larvae fed on diet containing lyophilized Bt leaves. IOBC/ WPRS Bull 73: 75–81. [Google Scholar]
- 29. Martinez SS, Emden HFV (1999) Sublethal concentrations of azadirachtin affect food intake, conversion efficiency and feeding behaviour of Spodoptera littoralis (Lepidoptera: Noctuidae). Bull Entomol Res 89: 65–71. [Google Scholar]
- 30.Statgraphics (1997) Statgraphics plus version 3.0 Manugistics, Rockwille MD.
- 31.JMP 8.0 (2008) SAS Institute Inc., Cary, NC, USA.
- 32. Vilaró F, Pérez-Hedo M, Eras J, Canela R, Eizaguirre M (2012) UHPLC−MS Analysis of Juvenile Hormone II in mediterranean corn borer (Sesamia nonagrioides) Hemolymph using various ionization techniques J Agric Food Chem. 60: 3020–3025. [DOI] [PubMed] [Google Scholar]
- 33. Fan Y, Rafaeli A, Gileadi C, Applebaum SW (1999) Juvenile hormone induction of pheromone gland PBAN-responsiveness in Helicoverpa armigera females. Insect Biochem Molec Biol 29: 635–641. [Google Scholar]
- 34. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE guidelines: Minimum information for publication of quantitative real time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 35. Bernad L, Lagadic L (1993) Sublethal effects of dietary cyfluthrin on nutritional performance and gut hydrolase activity in larvae of the Egyptian cotton leafworm, Spodoptera littoralis . Pestic Biochem Physiol 46: 171–180. [Google Scholar]
- 36.Slansky FJ, Scriber JM (1985) Food consumption and utilization. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 4. New York: Pergamon Press. pp. 87–163. [Google Scholar]
- 37. Baek JH, Clark JM, Lee SH (2010) Cross-strain comparison of cypermethrin-induced cytochrome P450 transcription under different induction conditions in diamondback moth. Pestic Biochem Physiol 96: 43–50. [Google Scholar]
- 38. Scott JG, Wen Z (2001) Cytochromes P450 of insects: the tip of the iceberg. Pest Manag Sci 57: 958–987. [DOI] [PubMed] [Google Scholar]
- 39. Yu YS, Xue S, Wu JC, Wang F (2007) Changes in levels of juvenile hormone and moulting hormone in larvae and adult females of Chilo suppresalis (Lepidoptera: Pyralidae) after imidacloprid application to rice. J Econ Entomol 100: 1088–1193. [DOI] [PubMed] [Google Scholar]
- 40. Zhao G, Zhao S, Gao R, Wang R, Zhang T, et al. (2011) Transcription profiling of eight cytochrome P450s potentially involved in xenobiotic metabolism in the silkworm, Bombyx mori . Pestic Biochem Physiol 100: 25–255. [Google Scholar]
- 41. Berge J-B, Feyereisen R, Amichot M (1998) Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos Trans R Soc Lond B Biol Sci 353: 1701–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao YB, Tao XY, Xue XY, Wang LJ, Chen XY (2011) Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res 20: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munster M, Préfontaine G, Meunier L, Elias M, Mazza A, et al. (2007) Altered gene expression in Choristoneura fumiferana and Manduca sexta in response to sublethal intoxication by Bacillus thuringiensis Cry1Ab toxin. Insect Mol Biol 16: 25–35. [DOI] [PubMed] [Google Scholar]