Abstract
Unnatural cyclic amino acids (UNAA) are a new class of boron delivery agents that are in a pre-clinical stage of evaluation. In the present study, the biodistribution of racemic forms of the cis-and trans- isomers of the boronated UNAA 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC) and 1-amino-3-boronocycloheptanecarboxylic acid (ABCHC) was studied in B16 melanoma bearing mice and this was compared to L-p-boronophenylalanine (BPA). Boron concentrations were determined by inductively coupled plasma-optical emission spectroscopy (ICPOES) at 2.5 hrs following intraperitoneal (i.p.) injection of the test agents at a concentration equivalent to 24 mg B/kg. While all compounds attained comparable tumor boron concentrations, the tumor/blood (T/Bl) boron concentration ratios were far superior for both cis-ABCPC and cis-ABCHC compared to BPA (T/Bl = 16.4, and 15.1 vs. 5.4). Secondary ion mass spectrometry (SIMS) imaging revealed that the cis-ABCPC delivered boron to the nuclei, as well as the cytoplasm of B16 cells. Next, a biodistribution study of cis-ABCPC and BPA was carried out in F98 glioma bearing rats following i.p. administration. Both compounds attained comparable tumor boron concentrations but the tumor/brain (T/Br) boron ratio was superior for cis-ABCPC compared to BPA (6 vs. 3.3). Since UNAAs are water soluble and cannot be metabolized by tumor cells, they potentially could be more effective boron delivery agents than BPA. Our data suggest that further studies are warranted to evaluate these compounds prior to the initiation of clinical studies.
Keywords: Unnatural cyclic amino acids, melanomas, gliomas
1. Introduction
Boronated analogues of unnatural cyclic amino acids (UNAA’s) belong to a new class of compounds that are gaining increasing attention as potentially more effective boron delivery agents for boron neutron capture therapy (BNCT) (Barth et al., 2012; Kabalka et al., 2001, 2011, 2004, 2003, 2006). The impetus for the design and synthesis of these compounds originated from the remarkable positron emission tomography (PET) observations in patients with the glioblastoma multiforme (GBM) and metastatic melanoma using fluorine-18 labeled boronophenylalanine (BPA) and carbon-11 labeled 1-aminocyclobutanecarboxylic acid. These studies revealed that the cyclic amino acids localized in these tumors at higher concentrations than BPA (Hubner et al., 1998; Nichols et al., 2002). The fact that they are water soluble and cross the blood-brain barrier (BBB) (Aoyagi et al., 1988) provided the stimulus to further evaluate them as delivery agents for BNCT. This was especially true because of their ability to target infiltrating tumor cells within normal brain (Chandra et al., 2013; Kabalka et al., 2001, 2004, 2006, 2009). In the present report we describe biodistribution studies that have been carried out with the racemic forms of the cis and trans isomers of ABCPC and ABCHC and compared them to BPA using the B16 murine melanoma and F98 rat glioma models (Barth et al., 2009).
2. Materials and Methods
2.1 Synthesis and purification of cis and trans 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC) and of 1-amino-3-borono-cucloheptanecarboxylic acid (ABCHC)
The chemical structures of the cis and trans molecules are shown in Fig. 1. Only one enantiomer of each is shown but the molecules were isolated as racemic mixtures and the structures were confirmed by X-ray analysis. It should be noted that the labeling of these compounds was transposed in an earlier report relating to ABCPC (Kabalka et al., 2009). The synthesis of both compounds involved the preparation of hydantoin precursors (nitrogen heterocycles) which could be separated by crystallization. The cis isomer precipitated out whereas the trans remained in solution and it was subsequently isolated by evaporation of the solvent. The hydantoins then were hydrolyzed to yield the desired amino acids. However, unexpectedly the process generated ammonium chloride as a byproduct that complexed with the desired products. To remove NH4Cl from them, the boronic acid functionality was converted to the corresponding insoluble trifluoro-borate, which did not form a complex due to its ionic character. The trifluoroborate derivatives were isolated by filtration and hydrolyzed back to the boronic acids by mixing them with alumina, microwaving the mixture, and then washing off the alumina. Final purification was achieved by column chromatography using an alumina column with water as the eluent. The chemical structures of the cis and trans ABCHC are also shown in Fig. 1. Again, only one enantiomer of each is shown but the molecules were isolated as racemic mixtures. These were prepared from the corresponding hydantoins in a manner completely analogous to that used to synthesize the ABCPC compounds.
Fig.1.
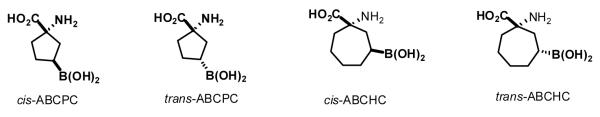
Structures of the UNAA used in the study. Although the molecules were used as racemates, only one enantiomer is drawn.
2.2 Biodistribution of ABCPC, ABCHC and BPA in B16 melanoma bearing mice
All of the animal studies described in this report were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and our protocol was approved by the Institutional Animal Care and Use Committee of The Ohio State University (Permit #: A-3261-01 and IACUC protocol number 2011A0000003). B16 melanoma cells were implanted subcutaneously (s.c.) into the right dorsum of syngeneic female C57BL/6 mice. Approximately 10 -12 d later, when tumors had attained a diameter of ~1 cm, biodistribution studies were initiated. The cis and trans compounds of ABCPC and ABCHC were used as a mixture of their enantiomers, which were dissolved in phosphate buffered saline (PBS) for the biodistribution studies. BPA (Katchem Ltd, Prague) was converted to a fructose complex as previously described (Yang et al., 1996). Based on our previous studies with BPA (Barth et al., 1997; Yang et al., 1997) a dose equivalent to 24 mg boron/kg body weight (b.w.) of each compound was administered by i.p. injection to groups of four mice. They were euthanized 2.5 hr. post injection, which based on previous studies with BPA (Barth et al., 1997), yielded the maximum T/Br and T/Bl boron concentration ratios. Blood and various organs and tissues were removed and processed for boron determinations by means of inductively coupled plasma-optical emission spectroscopy (ICP-OES).
2.3 SIMS imaging studies of cis-ABCPC in B16 melanoma bearing mice
Small portions of the B16 melanoma were frozen for correlative SIMS studies from mice that had received a mixture of L- and D- enantiomers of cis-ABCPC. As described in the previous section, this was carried out in order to determine the cellular and subcellular localization of the compounds. For SIMS analysis, 4 μm thick cryosections were attached to silicon wafers (~1 cm2), freeze-dried and sputter coated with a 10 Å layer of Au/Pd for enhancing their electrical conductivity. A CAMECA IMS-3f ion microscope was used for SIMS analyses (Chandra et al., 2013, 2000). Pixel-by-pixel image quantification of boron signals (11B+) was achieved by the secondary ion 12C+ carbon normalization approach and using the relative-sensitivity-factors (RSF) of boron isotopes to the 12C+ tissue matrix signals (Ausserer et al., 1989; Chandra et al., 2000; Smith et al., 1996). The absolute boron concentrations determined by this approach were converted into estimated wet weight concentrations by assuming 85% cellular water content.
2.4 Biodistribution of cis-ABCPC and BPA in F98 glioma bearing rats
The F98 rat glioma (#CRL-2397, American Type Culture Collection, Manasus, VA) has been used in a wide variety of studies in experimental neuro-oncology (Barth et al., 2009). F98 glioma cells (104) were implanted stereotactically into the right caudate nucleus of syngeneic Fischer rats (Yang et al., 1996) and ~12 days later biodistribution studies were carried out. The racemic cis-ABCPC compound, as a mixture of L- and D- enantiomers, was dissolved in PBS for administration and BPA was converted to a fructose complex. Both compounds were administered i.p. at a dose of 250 mg/kg b.w., corresponding to 12 mg/kg for BPA and 16 mg/B/kg for cis-ABCPC. The rats were euthanized 2.5 hrs later and the organs were removed and processed for boron determinations by ICP-OES.
3. Results
3.1 Boron biodistribution of cis-ABCPC, trans-ABCPC, and BPA in B16 melanoma bearing mice
Boron uptake values, as determined by ICP-OES, for tumor, brain and blood for racemates of the cis and trans-ABCPC compounds and BPA in B16 melanoma bearing mice are summarized in Table 1. Although the tumor boron values for cis and trans-ABCPC were equivalent, the T/Bl ratio of the former was 16.4 vs. 3.9 for the latter suggesting that the pharmacodynamics of the two compounds were different. Although the tumor uptake of BPA was higher than that of cis-ABCPC (28.6 vs. 16.4 μg/g), the blood value of cis-ABCPC was very low (1.0 vs. 5.3 μg/ml). Consequently, the T/Bl ratio of cis-ABCPC was 16.4 vs. 5.4 for BPA suggesting that the former is more rapidly cleared from the blood. These observations indicate that the racemic cis-ABCPC had desirable characteristics as a boron-delivery agent and these might be improved by further separating them into their L- and D- enantiomers.
Table 1.
Boron biodistribution of cis-ABCPC, trans-ABCPC and BPA in B16 melanoma bearing mice. Boron concentrations are expressed in ppm wet weight (mean ± SD).
| Compounda | Boron concentration (μg/g)b |
Ratios |
|||
|---|---|---|---|---|---|
| Tumor | Brain | Blood | T/Br | T/Bl | |
| cis-ABCPC | 16.4 ± 9.0 | 4.1 ± 3.5 | 1.0 ± 0.9 | 4.0 | 16.4 |
| trans- ABCPC | 13.0 ± 2.4 | 3.3 ± 1.1 | 3.3 ± 1.1 | 3.9 | 3.9 |
| BPA | 28.6 ± 4.6 | n.d. | 5.3 ± 0.8 | n.d. | 5.4 |
All compounds at a dose of 24 mg B equivalent/kg b.w. were administered i.p. and animals were euthanized at 2.5 hrs post-dosing.
Boron concentrations were determined by ICP-OES for groups of 4 mice. “n.d. is not determined.
3.2 Boron biodistribution of cis- and trans-ABCHC and BPA in B16 melanoma bearing mice
Boron uptake values for tumor, blood, liver, kidney, spleen and brain and for the racemic cis and trans-ABCHC compounds and BPA in B16 melanoma bearing mice are summarized in Table 2. This detailed biodistribution study revealed that the pharmacodynamics of the cis and trans-ABCHC are quite different. Cis-ABCHC was superior to the trans-compound in delivering boron to the tumor (31.7 vs. 13.9 μg/g). In a direct comparison to BPA, cis-ABCHC was comparable to BPA in the amount of boron delivered to the tumor, but it had a far greater T/Bl ratio (15.1 for cis-ABCHC vs.5.4 for BPA, Table 2). Furthermore, the boron concentrations of cis-ABCHC in blood, kidneys and spleen were lower compared to BPA indicating faster clearance while maintaining higher tumor boron concentrations, which were comparable to BPA. The more rapid blood clearance of cis-ABCHC also was consistent with observations relating to cis-ABCPC, as discussed above, and this would be a desirable property of these compounds as clinical boron delivery agents (Barth et al., 2012).
Table 2.
Boron biodistribution of cis-ABCHC, trans-ABCHC, and BPA in B16 melanoma bearing mice.
| Mean Boron Concentration (μg/g tissue) ± SDb |
Ratios |
|||||||
|---|---|---|---|---|---|---|---|---|
| Compounda | Tumor | Blood | Liver | Kidney | Spleen | Brain | T/Bl | T/Br |
| cis-ABCHC | 31.7 ± 11.2 | 2.1 ± 0.9 | 3.6 ± 2.1 | 5.8 ± 1.6 | 4.1 ± 1.5 | 2.6 ± 0.6 | 15.1 | 12.2 |
| trans-ABCHC | 13.9 ± 2.7* | 3.2 ± 0.6 | 4.7 ± 1.0 | 8.3 ± 3.2 | 5.3 ± 1.4 | n.d. | 4.3 | - |
| BPA | 28.6 ± 4.6 | 5.3 ± 0.8* | 5.3 ± 1.0 | 18.9 ± 1.3* | 14.1 ± 5.3* | n.d. | 5.4 | - |
All compounds at a dose of 24 mg B equivalent/kg b.w. were administered i.p. and animals were euthanized at 2.5 hrs post-dosing.
Boron concentrations were determined by means of ICP-OES. Means and standard deviations were calculated for each group of 4 mice. “n.d.” designates “not determined.” Ratios of boron concentrations in tumor (T) to blood (Bl) and tumor to brain (Br) were calculated.
Boron concentrations for the different compounds in each group of tissue and blood samples were compared using analysis of variance (ANOVA) and Tukey’s post hoc test for multiple comparisons. A p value of less than 0.05 was considered significant. The tumor uptake value of trans-ABCHC was significantly different (p<0.05) from cis-ACCHC and BPA.
3.3 Single cell boron imaging SIMS studies of cis-ABCPC in B16 melanoma bearing mice
In contrast to ICP-OES determinations of boron concentrations of the test compounds, SIMS imaging for boron provides data on their subcellular localization. As shown in the frozen section photomicrograph (Fig. 2A) of a hematoxylin and eosin (H & E) stained section, the B16 melanoma was composed of a monomorphic population of cells with hyperchromatic nuclei and cytoplasmic melanin. SIMS analyses were made in adjacent cryosections and revealed the boron distribution from cis-ABCPC in the B16 melanoma cells. In positive secondary ion SIMS images of 39K and 11B, individual tumor cells were discernible (Fig. 2B). Boron was localized in nuclei and cytoplasm of B16 cells with discernible heterogeneity (Fig. 2C). The regions of interest (ROI) in each SIMS image represented approximately 10-15 individual cells, taken together for quantitation of boron by SIMS images. As determined by SIMS from 47 ROIs in 7 SIMS imaging fields, the boron concentration in tumor cells at 2.5 hrs was 14 ± 5 μg/g (mean + SD). Both ICP-OES measurements (Table 1) and SIMS analyses revealed close agreement in boron concentrations in tumor and individual tumor cells at 2.5 hrs following administration. A plausible explanation for this close agreement can be attributed to the fact that most of the cis-ABCPC had cleared from the blood and extracellular fluids by 2.5 hrs and the intracellular cis-ABCPC was bound to matrix components.
Fig.2.
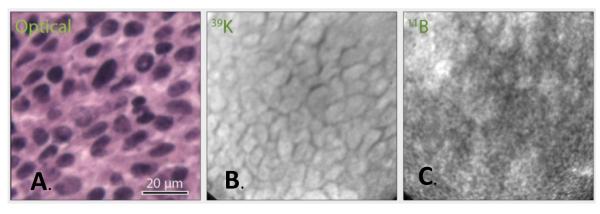
SIMS imaging analysis of B16 tumor from cis-ABCPC treated B16 melanoma bearing mice. The optical image was recorded from H&E stained 4 μm thick tumor cryosection. SIMS images were recorded from adjacent 4 μm thick cryo-sections. The SIMS 39K+ image (B.) was integrated on the CCD camera for 0.2 sec and 11B+ image (C.) for 2 min.
3.4 Boron biodistribution studies of cis-ABCPC and BPA in F98 rat glioma model
F98 glioma cells (Yang et al., 1996) were implanted stereotactically into the right cerebral hemisphere of syngeneic Fischer rats and 12-14 d later they received either cis-ABCPC or BPA (24 B/kg b.w.) by i.p. injection. The rats were euthanized 2.5 hrs later and boron biodistribution was quantified by ICP-OES (Table 3). Both cis-ABCPC and BPA delivered comparable amounts of boron to the tumor, blood, kidneys and tumor bearing (ipsilateral) cerebral hemisphere. However, the contralateral, non-tumor bearing cerebral hemisphere had less boron (4.4 μg/g boron for cis-ABCPC vs. 6.5 μg/g for BPA, Table 3). This suggests that the uptake of cis-ABCPC in normal brain might be less compared to that of BPA, which would be a highly desirable property. A possible explanation as to why analysis of ipsilateral brain tissue, adjacent to the main tumor mass, did not reveal any significant differences between the compounds could have been due to the presence of infiltrative tumor cells in normal brain. Since ICP-OES boron measurements cannot differentiate between infiltrating tumor cells and normal brain tissue, they are a less accurate measure of the true boron content of normal brain.
Table 3.
Boron biodistribution of cis-ABCPC and BPA in F98 Glioma bearing Rats.
| Boron Concentrations (μg/g wt) ± SDc | Ratios | ||||||
|---|---|---|---|---|---|---|---|
| Test Agentb | Tumor | Ipsilat. Brain |
Contralat. Braind |
Blood | Kidney | T/Bl | T/Br |
|
|
|
||||||
| cis-ABCPC | 26.2 ± 9.7 | 7.9 ± 3.2 | 4.4 ± 0.5d | 6.7 ± 1.5 | 33.6 ± 7.7 | 3.9 | 6.0 |
|
|
|
||||||
| BPA | 21.5 ± 8.4 | 8.8 ± 2.1 | 6.5 ± 0.5 | 5.1 ± 1.4 | 30.1 ± 5.5 | 4.2 | 3.3 |
F98 glioma cells (104) were implanted into the right caudate nucleaus of syngeneic Fischer rats.
The test agents were administered i.p. at a dose of 250 mg/kg b.w. and rats were euthanized 2.5 hrs post-dosing.
Boron concentrations were determined by ICP-OES and mean and standard deviations were calculated from groups of 4 rats.
Contralateral brain tissue contained significantly less (p <0.05) boron in rats that received cis-ABCPC compared to BPA.
4. Discussion and Conclusions
In the present study has demonstrated that racemic cis-ABCPC and cis-ABCHC compounds showed comparable boron uptake by B16 melanoma but a far superior T/Bl boron concentration ratio was attained compared to that of BPA. Further separation of these compounds into their purified enantiomers might yield even better uptake data. Comparison of cis-ABCPC and BPA up-take data in the F98 rat glioma model provided evidence that the compound, even as a racemic mixture, was as effective as BPA in targeting the main tumor mass. The observation that the up-take of cis-ABCPC may be taken up in smaller amounts than BPA in normal brain deserves further investigation by SIMS imaging in order to better define the targeting of infiltrative tumor cells. This has been described in detail in a recently published report (Chandra et al., 2013). These types of unnatural amino acids have been shown to cross an intact BBB due to the presence of L-amino acid transporter (Aoyagi et al., 1988), which also is upregulated in high grade gliomas (Detta et al., 2009). Both of these characteristics may enhance the uptake of cis-ABCPC and cis-ABCHC by infiltrating tumor cells in the normal brain. Furthermore, both of these compounds are water soluble and cannot be metabolized, which may further enhance their retention in tumor cells compared to BPA, which is metabolized (Svantesson et al., 2002). Our observations suggest that further pre-clinical studies on enantiomerically pure cis-ABCPC and cis-ABCHC are warranted to optimize their delivery and dosing and to evaluate their toxicity prior to clinical evaluation.
Research Highlights.
Unnatural cyclic amino acids (UNAA) are a new class of boron delivery agents for neutron capture therapy
ABCPC and ABCHC attained higher tumor/blood ratios vs. BPA in B16 melanoma bearing mice
The tumor/brain ratio of cis-ABCPC was superior to BPA (6 vs. 3.3) suggesting that further studies are warranted
Acknowledgments
This study was funded by a NIH grant R01 CA129326 to RFB, SC, and GWK. The Kevin J. Mullin Memorial Fund for Brain Tumor Research provided partial support to RFB. SC thanks the Cornell Microscopy and Imaging Facility (MIF), Department of Biomedical Engineering for culturing the B16 melanoma cells used for the SIMS studies. The Cornell SIMS Laboratory, under the direction of S. Chandra, is affiliated with New York State Foundation for Science, Technology, and Innovation (NYSTAR). Finally, we thank Mrs. Heidi Bosworth for expert secretarial assistance in the preparation of this manuscript.
Footnotes
Present address: University of Florida College of Medicine Department of Cardiology Rm M420 1600 SW Archer Road Gainesville, FL 32608, U.S.A.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyagi M, Agranoff BW, Washburn LC, Smith QR. Blood-brain barrier transport of 1-aminocyclohexanecarboxylic acid, a nonmetabolizable amino acid for in vivo studies of brain transport. J Neurochem. 1988;50:1220–1226. doi: 10.1111/j.1471-4159.1988.tb10596.x. [DOI] [PubMed] [Google Scholar]
- Ausserer WA, Ling YC, Chandra S, Morrison GH. Quantitative imaging of boron, calcium, magnesium, potassium, and sodium distributions in cultured cells with ion microscopy. Anal Chem. 1989;61:2690–2695. doi: 10.1021/ac00199a002. [DOI] [PubMed] [Google Scholar]
- Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94:299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RF, Vicente MG, Harling OK, Kiger WS, 3rd, Riley KJ, Binns PJ, Wagner FM, Suzuki M, Aihara T, Kato I, Kawabata S. Current status of boron neutron cap ture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RF, Yang W, Rotaru JH, Moeschberger ML, Joel DD, Nawrocky MM, Good man JH, Soloway AH. Boron neutron capture therapy of brain tumors: enhanced sur vival following intracarotid injection of either sodium borocaptate or boronophenylalanine with or without blood-brain barrier disruption. Cancer Res. 1997;57:1129–1136. [PubMed] [Google Scholar]
- Chandra S, Barth RF, Haider SA, Yang W, Huo T, Shaikh AL, Kabalka GW. Biodistribution and subcellular localization of an unnatural boron-containing amino acid (Cis-ABCPC) by imaging secondary ion mass spectrometry for neutron capture therapy of melanomas and gliomas. PLoS One. 2013;8:e75377. doi: 10.1371/journal.pone.0075377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Smith DR, Morrison GH. Subcellular imaging by dynamic SIMS ion mi croscopy. Anal Chem. 2000;72:104A–114A. doi: 10.1021/ac002716i. [DOI] [PubMed] [Google Scholar]
- Detta A, Cruickshank GS. L-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res. 2009;69:2126–2132. doi: 10.1158/0008-5472.CAN-08-2345. [DOI] [PubMed] [Google Scholar]
- Hubner KF, Thie JA, Smith GT, Kabalka GW, Keller IB, Kliefoth AB, Campbell SK, Buonocore E. Positron emission tomography (PET) with 1-aminocyclobutane-1- [(11)C]carboxylic acid (1-[(11)C]-ACBC) for detecting recurrent brain tumors. Clin Positron Imaging. 1998;1:165–173. doi: 10.1016/s1095-0397(98)00010-7. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Das BC, Das S. Synthesis of novel boron containing unnatural cyclic amino acids as potential therapeutic agents. Tetrahedron Letters. 2001;42:7145–7146. [Google Scholar]
- Kabalka GW, Shaikh AL, Barth RF, Huo T, Yang W, Gordnier PM, Chandra S. Boronated unnatural cyclic amino acids as potential delivery agents for neutron capture therapy. Appl Radiat Isot. 2011;69:1778–1781. doi: 10.1016/j.apradiso.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabalka GW, Wu ZZ, Yao ML, Natarajan N. The syntheses and in vivo biodistribu tion of novel boronated unnatural amino acids. Appl Radiat Isot. 2004;61:1111–1115. doi: 10.1016/j.apradiso.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Yao ML. Synthesis of a novel boronated 1-amino-cyclobutane carboxyl ic acid as a potential boron neutron capture therapy agent. Appl Organomet Chem. 2003;17:398–402. [Google Scholar]
- Kabalka GW, Yao ML. The synthesis and use of boronated amino acids for boron neu tron capture therapy. Anticancer Agents Med Chem. 2006;6:111–125. doi: 10.2174/187152006776119144. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Yao ML, Marepally SR, Chandra S. Biological evaluation of boro nated unnatural amino acids as new boron carriers. Appl Radiat Isot. 2009;67:S374–379. doi: 10.1016/j.apradiso.2009.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TL, Kabalka GW, Miller LF, Khan MK, Smith GT. Improved treatment planning for boron neutron capture therapy for glioblastoma multiforme using fluorine-18 la beled boronophenylalanine and positron emission tomography. Med Phys. 2002;29:2351–2358. doi: 10.1118/1.1507780. [DOI] [PubMed] [Google Scholar]
- Smith DR, Chandra S, Coderre JA, Morrison GH. Ion microscopy imaging of 10B from p-boronophenylalanine in a brain tumor model for boron neutron capture therapy. Cancer Res. 1996;56:4302–4306. [PubMed] [Google Scholar]
- Svantesson E, Capala J, Markides KE, Pettersson J. Determination of boron-containing compounds in urine and blood plasma from boron neutron capture therapy patients. The importance of using coupled techniques. Anal Chem. 2002;74:5358–5363. doi: 10.1021/ac025798e. [DOI] [PubMed] [Google Scholar]
- Yang W, Barth RF, Carpenter DE, Moeschberger ML, Goodman JH. Enhanced delivery of boronophenylalanine for neutron capture therapy by means of intracarotid injection and blood-brain barrier disruption. Neurosurgery. 1996;38:985–992. doi: 10.1097/00006123-199605000-00027. [DOI] [PubMed] [Google Scholar]
- Yang W, Barth RF, Rotaru JH, Moeschberger ML, Joel DD, Nawrocky MM, Good man JH. Enhanced survival of glioma bearing rats following boron neutron capture ther apy with blood-brain barrier disruption and intracarotid injection of boronophenylalanine. J Neu rooncol. 1997;33:59–70. doi: 10.1023/a:1005769214899. [DOI] [PubMed] [Google Scholar]


