Abstract
L-glutamate is the primary neurotransmitter at excitatory synapses in the vertebrate CNS and at arthropod neuromuscular junctions (NMJs). However, the molecular mechanisms that trigger the recruitment of glutamate receptors at the onset of synaptogenesis and promote their stabilization at postsynaptic densities remain poorly understood. We have reported the discovery of a novel, evolutionary conserved molecule, Neto, essential for clustering of ionotropic glutamate receptors (iGluRs) at Drosophila NMJ. Neto is the first auxiliary subunit described in Drosophila and is the only non-channel subunit absolutely required for functional iGluRs. Here we review the role of Drosophila Neto in synapse assembly, its similarities with other Neto proteins and a new perspective on how glutamatergic synapses are physically assembled and stabilized.
Keywords: glutamatergic synapses, synapse assembly, glutamate receptors, Drosophila, neuromuscular junction, auxiliary subunits
Introduction
Making a chemical synapse involves a complex series of events including neuronal fate determination, axon guidance, cell-cell adhesion and localized induction of presynaptic and postsynaptic differentiation. Synaptogenesis culminates with the recruitment of neurotransmitter receptors at postsynaptic specializations, which confers functionality to the nascent synapse. Neurotransmitter receptors are stabilized at the synaptic junctions by a myriad of proteins packed in electron-dense structures called postsynaptic densities (PSDs). Synaptic activity triggers further synthesis and aggregation of receptor complexes and synapse maturation. While the synaptic activity is a major force in sculpting synapses, it is not essential for the initial steps of synapse assembly.1-4 Synapses form normally in vertebrates and invertebrates even when neurotransmitter release is blocked using pharmacological or genetic methods.5-10 The molecular and cellular mechanisms utilized at the onset of synaptogenesis appear to be re-employed during developmental and activity-dependent changes of neural circuits and thus constitute a critical toolbox for controlling the composition and function of neurotransmitter receptors to finely tune neural activities
Postsynaptic ligand-gated ion channels, or ionotropic receptors, include gamma amino-butyric acid (GABA) receptors, nicotinic acetylcholine receptors (nAChRs) and a variety of glutamate receptor subtypes, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartic acid (NMDA) and kainate (KA) receptor subtypes. Understanding the mechanisms of synapse assembly can be reduced to the pursuit of two fundamental biological questions: (1) What are the molecular components of a particular synapse? (2) How do these molecules come together and function to fulfill the job that a particular synapse must perform? In this oversimplified view understanding how to make the neuromuscular junction (NMJ) in Drosophila has the potential to cover double duty. First, fly NMJ is glutamatergic similar in composition and function to the mammalian central AMPA/KA synapses.11 In vertebrates, glutamate receptors play critical roles in learning and memory, development of the brain and neurological and information storage disorders. Second, fly NMJ controls an entire muscle fiber, a demanding task that requires higher levels of currents than the ones used at vertebrate central glutamatergic synapses. Because of these higher demands and the essential function of NMJ for fly’s survival, the range of observable deficits in the assembly and function of glutamatergic synapses is broader at Drosophila NMJ, compared with the more subtle phenotypes of altered glutamatergic transmission at vertebrate CNS. In addition, fly NMJ is relatively simple and easily accessible to physiological measurements, light and electron microcopy, and live dynamics studies. The sum of these features makes Drosophila NMJ a very powerful system to understand the assembly and development of glutamatergic synapses. In spite of these advantages and the power of Drosophila genetics, the molecular mechanisms that trigger the initial clustering of receptors and promote their stabilization at PSDs remain a mystery.
Much of what we know about the recruitment and clustering of neurotransmitter receptors at the onset of synaptogenesis comes from studies on vertebrate NMJ. Vertebrate motor neurons organize postsynaptic differentiation by releasing a heparan sulfate proteoglycan called Agrin.12 In mouse muscle fibers, nAChRs form primitive aneural clusters prior to the arrival of the nerve terminal (reviewed in ref. 13). Innervation leads to the appearance of large nAChR aggregates in the synaptic region with longer residence time in the membrane. MuSK (muscle, skeletal receptor tyrosine-protein kinase) and Lrp4 (low-density lipoprotein receptor-related protein 4) are required for both aneural and neural nAChR cluster formation.14-17 Lrp4 binds to MuSK and stimulates MuSK kinase activity to induce aneural cluster formation. Binding of Agrin to Lrp4 further stimulates association between Lrp4 and MuSK and increases MuSK kinase activity.18 MuSK interacts with a plethora of proteins and initiates signaling necessary for postsynaptic differentiation (reviewed in ref. 19). The binding of Agrin to Lrp4 appears to be necessary and sufficient to enable Agrin signaling. Recent studies of the crystal structure of Agrin-Lrp4 complex suggest that Agrin and Lrp4 initially form a binary complex, which promotes the synergistic formation of tetrameric receptor complexes crucial for Agrin-induced nAChR clustering.20
Neto is an auxiliary subunit required for functional receptors at Drosophila NMJ
Clustering of vertebrate ionotropic glutamate receptors (iGluRs) remains less understood. In recent years, intense research in vertebrate systems has revealed a role for auxiliary subunits in the modulation of iGluR functions. Auxiliary subunits are transmembrane proteins that selectively bind to mature iGluRs and form stable complexes at the cell surface. They can modulate the functional characteristics of iGluRs and may also mediate surface trafficking and/or targeting to specific subcellular compartments.21 We have recently reported the discovery of a novel, evolutionary conserved molecule, Neto (Neuropillin and Tolloid-like), required for synaptic clustering of iGluRs.22 Neto is the first auxiliary subunit described in Drosophila and is essential for the function of the striated muscle.
Drosophila Neto belongs to a family of proteins conserved from worms to humans, which appear to share ancestral roles in the formation and modulation of glutamatergic synapses.23-25 Vertebrate Netos (Neto1 and -2) have emerged as important auxiliary subunits that modulate the gating properties of KA-type glutamate receptors; however their roles in receptor clustering have not been examined carefully.26-31 Neto1/Neto2 double knockout mice have defects in long-term potentiation, learning and memory, though they are viable.29 C. elegans Neto/SOL-2 has been implicated in the modulation of glutamatergic transmission.25 Except for the Drosophila Neto, none of the known Netos are absolutely required for iGluRs clustering, nor essential for viability. This difference could be due to variations in the properties of individual domains of Netos, or it could reflect the functional requirements among synapse types and the nature and composition of multiprotein complexes in which Netos function. Notably, fly Neto is also present at various glutamatergic synapses, including central synapses, but Neto function is essential at the NMJ.22
Like in vertebrates, clustering of receptors at the Drosophila NMJ follows the arrival of the motor neuron at the muscle target.2,32 Presynaptic and postsynaptic components populate the area of the future NMJs prior to the neuron arrival, a phenomenon known as pre-patterning. For example, Bruchpilot, an essential component of the T bars, accumulates as detectable puncta at presynaptic terminals, while components of postsynaptic densities, such as p21-activated kinase, accumulate on the postsynaptic side.33,34 Before innervation, the iGluR subunits are also present and assembled in nascent, small clusters away from the neuronal arbor (Fig. 1).32,35-38 Innervation induces recruitment of receptors at developing fields opposite to the active zones and promotes further expression and recruitment of postsynaptic components and formation of functional synapses.32,36,37,39 Drosophila iGluRs are heterotetrameric complexes composed of three shared subunits, GluRIIC, GluRIID and GluRIIE, and either GluRIIA or GluRIIB (reviewed in ref. 40). The shared subunits are essential for viability: without any of them the animals are completely paralyzed, lack any peristaltic and hatching movements and die as late embryos. The receptor subunits are dependent on each other for synaptic recruitment. None of the receptor subunits clusters at the neuronal arbor in the absence of GluRIIC, GluRIID or GluRIIE, or GluRIIA and GluRIIB together (Fig. 1).5,41-43 The shared subunits are limiting factors, while GluRIIA and GluRIIB are competing for the limiting subunits.42 We have discovered that the absence of neto also induces complete paralysis and embryonic lethality.22 In addition, none of the receptor subunits clusters at the NMJ in neto null mutants (Fig. 1). Neto itself clusters at the NMJ at the onset of synaptogenesis and its clustering is dependent on the iGluRs. Neto associates with iGluRs in vivo and functions as an essential non-channel subunit of the iGluR complexes. Interestingly, GluRIIA was detected at the surface of striated muscles at suboptimal Neto levels. One way to explain this observation is that Neto associates with tetrameric iGluRs then traffics and clusters together. Suboptimal levels of limiting GluR subunits likely impact the assembly of tetrameric iGluRs, thought to occur in ER, and consequently their distribution on the muscle surface.
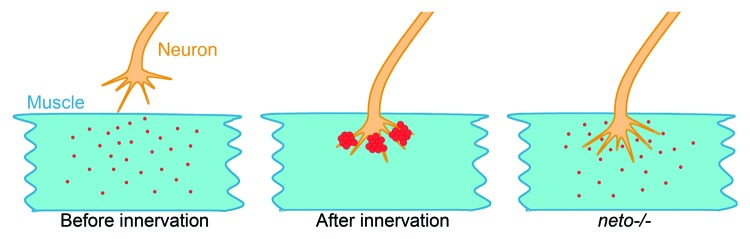
Figure 1. Innervation triggers recruitment of neurotransmitter receptors at the NMJ. Before innervation, receptor complexes (represented by red dots) are present in nascent, small clusters scattered on the muscle fiber. After innervation, the receptor complexes form large clusters/aggregates juxtaposing the active zones on the presynaptic termini. In the absence of Neto or any essential receptor subunit, iGluRs do not form synaptic clusters even after innervation.
Detailed analyses of neto hypomorphic allele revealed crucial roles for Neto in NMJ development. Suboptimal Neto levels induced dramatically reduced number of synaptic iGluR clusters and led to physiological and structural defects. Neto-deprived animals have reduced frequency and amplitude of miniature synaptic potentials, show no presynaptic compensation, and exhibit deficits in the maintenance of mature PSDs. Similar deficits were reported for NMJ synapses developing in the near absence of iGluRs.5,42 Neto deprivation does not affect net protein levels: all postsynaptic components tested showed normal levels. However, the distributions of iGluRs and other PSD components within the striated muscle have been altered toward extrajunctional location in neto hypomorphs. Live-imaging studies have shown that iGluRs stably integrate at growing PSDs from diffuse extrasynaptic pools, while other postsynaptic proteins remain highly mobile.33 A significant fraction of these extrasynaptic complexes must be at the cell surface, as fully functional iGluR complexes were detected on the muscle surface at extrajunctional locations.1 Neto is also distributed between junctional and extrajunctional locations on the muscle membrane.22 One possibility is that Neto and iGluR engage extrajunctionally then the complexes traffic together and are stabilized at the PSDs. In this scenario, only components engaged in the complexes could traffic and be stably incorporated at the synapse, consistent with the observed co-dependence of Neto and iGluRs for clustering at the synapse.
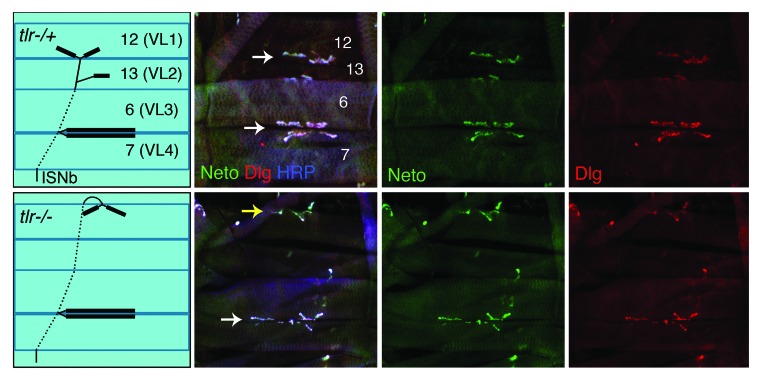
How does Neto mediate the clustering of iGluR complexes, and what does Neto tell us about mechanisms for recruitment and stable incorporation of iGluRs at the NMJ synapses? Neto does not seem to provide any instructive information that triggers receptor clustering. Synaptic Neto clusters appear to always accompany the iGluR complexes and follow the same developmental constraints. For example, Neto and iGluRs cluster together wherever the motor neuron makes contact with the target muscle, even when this contact is at inappropriate locations such as in axon guidance defective animals. In tolloid related (tlr) mutant larvae, the RP5 motor neurons fail to dissociate from the ISNb nerve bundle, and make ectopic synapses on the muscle 12.44,45 Neto forms clusters at the ectopic sites, instead of the normal location within the cleft of muscles 13/12 (Fig. 2).
Figure 2. Synaptic accumulation of Neto follows neuronal signaling. In control third instar larvae (upper panels) as well as in axon guidance mutants (tlrex[2–41]/tlrex[2–41, lower panels) Neto (in green) and the postsynaptic scaffold Discs large (Dlg, in red) accumulate at the site of neuronal innervation. HRP, in blue, marks the neuronal surface. ISNb innervates the ventrolateral muscles and includes axons of RP3, which innervates muscles 6 and 7, and RP5, which innervates muscle 12. RP3 makes appropriate synaptic contacts in both control and tlr animals (white arrow) but in tlr RP5 fails to defasciculate passes its muscle target, then returns to innervate muscle 12 from the dorsal side. White arrows mark the normal synaptic sites; yellow arrow marks the ectopic NMJ.
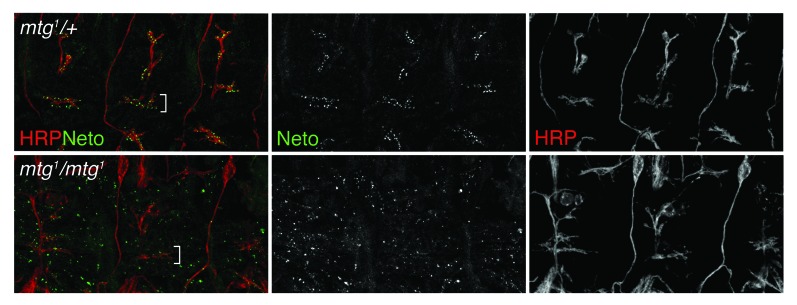
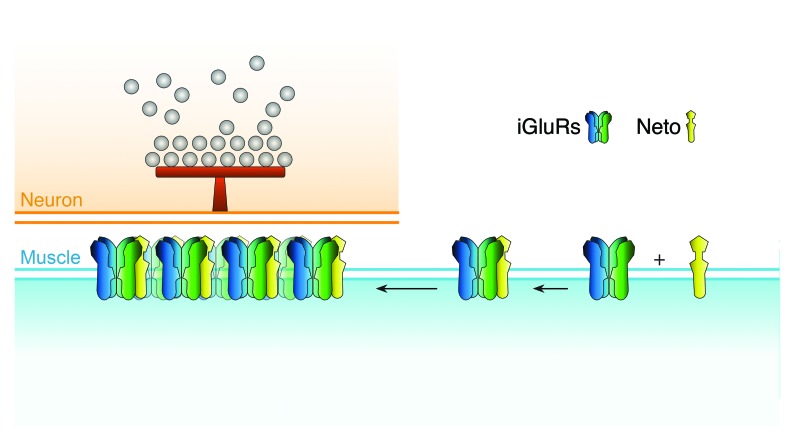
The only neuronally secreted protein known to influence the clustering of iGluRs at the Drosophila NMJ is the N-acetyl-glycosaminoglycan-binding glycoprotein called Mind the gap (Mtg).46,47 Mtg is thought to organize the extracellular matrix in synaptic cleft toward effective trans-synaptic signaling and proper clustering of iGluRs. An mtg null allele was identified in a screen for embryonically paralyzed mutants. In mtg null embryos, iGluRs remain clustered away from the neuronal arbor and fail to form functional synaptic clusters.46 We found that Neto also forms aggregates away from the neuronal arbor in the absence of Mtg (Fig. 3). In these aneural clusters, Neto immunoreactivities co-localize with the iGluR signals (not shown). The aneural Neto/iGluR aggregates present in mtg null late embryos are relatively large, comparable in size with the synaptic Neto/iGluR clusters found in wild-type embryos at this stage of development. In contrast, the aneural iGluR-positive puncta are very faint, barely distinguishable from the background in the absence of Neto.22 Thus, Neto appears to be required for both neural and aneural iGluRs clusters, similar to MuSK and Lrp4. While Mtg is required to organize the iGluR clusters in the proximity of the neuronal arbor, Neto appears to function at earlier steps in synapse assembly, synaptic targeting and clustering of the receptors. Neto does not contain any catalytic domains. Instead, it has a number of extracellular protein-protein interaction domains and an intracellular domain rich in putative phosphorylation sites and docking motifs. We favor a model in which Neto engages the iGluRs extrajunctionally and mediates their traffic to the synapses and/or their stable incorporation at the PSDs (Fig. 4).
Figure 3. Mtg affects Neto accumulation at the synapses. Neto recruitment and clustering at the synapses was analyzed in control (upper panels) and mtg mutant (lower panels) embryos 21 h after egg laying. HRP in red marks the neuronal surface. Neto (green) form clusters, which accumulate at the neuronal arbor (white bracket) in wt or mtg heterozygous animals but not in mtg mutants.
Figure 4. Model for Neto/iGluRs recruitment and clustering at the NMJ. Neto engages the iGluR complexes extrajunctionally and together they traffic and cluster at the synapses, opposite from the active zones marked by T-bars. Neto and the essential iGluR subunits are limiting for formation of functional iGluR complexes at the NMJ and for growth of synaptic structures.
How conserved are Neto activities across phylogenetic lineages?
Unlike other Netos, Drosophila Neto has essential roles in synapse formation and animal viability. However, several lines of evidences indicate that Netos constitute a family of highly conserved proteins that influence the function of glutamatergic synapses, which acquired species- and tissue-specific roles during evolution. First, all of the Neto proteins associate with iGluRs in vivo. In fact, vertebrate Neto2 was discovered in a screen for proteins that coimmunoprecipitated with GluK2/3 from rat cerebella.24 Neto1, originally identified because of its distribution in the brain PSD fraction, was shown to bind with NMDA receptors as well as hippocampal KA receptors, GluK2 and GluK5.23,29 Notably, Neto1 and Neto2 do not associate with AMPA-type receptors at PSDs. In contrast, C. elegans Neto/SOL-2 protein associates with GLR-1, which shares some characteristics with both AMPA- and KA-type receptors.25 Likewise, the iGluRs of Drosophila NMJ are complexes of AMPA/KA-like subunits: conserved residues known to favor AMPA binding are present in GluRIIA, GluRIIB and GluRIIC, while GluRIID and GluRIIE are predicted to favor KA binding. Second, the intracellular domains of all known Netos are rich in putative phosphorylation sites suggesting modulatory roles for these domains. These domains may facilitate synaptic trafficking of specific receptors by differentially engaging the receptor subunits and/or connecting the receptor complexes with motors and scaffold proteins. While it was shown that Neto1 binds directly to PSD-95 via its PDZ binding domain and Neto 2 to the scaffold protein GRIP,23,48 characterization of such regulatory functions awaits further experimentation. Third, Netos may also mediate stabilization of the iGluRs at the synapses. In C. elegans, GLR-1 is delivered to the cell surface without Neto/SOL-2, but the stability and/or function of the complex appears compromised.25 Interestingly, Neto1/2 null mice displayed significant reductions (40–50%) in GluK2 receptor subtype at cerebellum and hippocampal PSDs without detectable changes in total receptor levels.29,48 These double knockout mice have defects in long-term potentiation, learning and memory, but they are viable.29 More importantly, Neto1 and Neto2 are not essential for iGluR clustering. In contrast, Drosophila Neto is essential for iGluR clustering and formation of functional NMJ. The requirement for large receptor aggregates at NMJ may account for the difference between the essential roles for Neto at Drosophila NMJ but not in other systems, including fly CNS synapses. Clustering of neurotransmitter receptors at other NMJs is also essential for viability.
Finally, Netos appear to directly modify the properties of the iGluR complexes. Vertebrate Neto1 and Neto2 slow the decay kinetics of KA receptors expressed in Xenopus oocytes (reviewed in ref. 31). Glutamate-gated currents recorded from heterologous cells that express C. elegans GLR-1 appeared faster and smaller with coexpression of Neto/SOL-2.25 Additional auxiliary subunits are required for the function of GLR-1: when C. elegans GLR-1 is expressed alone in heterologous cells, little or no glutamate-gated current is detected. Expression of STG-1, a stargazin-like protein, together with GLR-1 and the CUB-domain protein SOL-1 reconstitutes glutamate-gated currents in Xenopus oocytes.49 Attempts to reconstitute Drosophila iGluRs in heterologous systems have failed so far,50 suggesting that other components or auxiliary proteins are required for functional receptors. This limits our current abilities to investigate a role for Drosophila Neto in the modulation of iGluR channel properties. Along this line, one of the most important limitations appears to be the surface delivery of functional complexes. Our preliminary results suggest that Neto does not modulate the surface delivery of iGluRs. The lack of contribution for Neto proteins to surface presentation of iGluR channels may be a shared feature of Netos from worms to humans.22,25,31
Conclusions
In summary, Netos appear to (1) bind to the iGluRs at extrajunctional locations and remain engaged with the receptors, (2) regulate their trafficking to synaptic locations, (3) mediate stable incorporation of iGluRs and/or their stabilization at PSDs and (4) modulate the properties of the channels. Before innervation, Neto/iGluR complexes could form on the muscle surface and traffic to synaptic locations at Drosophila NMJ. Without innervation, aggregation of receptor complexes at junctional locations cannot occur and presumably the complexes will be free to diffuse away. After innervation, Neto/iGluR complexes stably incorporate at the PSDs and form functional synapses (Fig. 4). During development, Neto mediates further recruitment of iGluRs and promotes the growth and stabilization of postsynaptic structures. How Neto performs all these postsynaptic activities will be the focus of future research.
A remaining question is what mediates the surface delivery of iGluRs. In vertebrates, native AMPA-type receptors were shown to contain transmembrane AMPA receptor regulatory proteins (TARPs) and Cornichon-like proteins (CNIHs) as auxiliary subunits that modulate the surface delivery, trafficking and channel properties of the receptor complexes (reviewed in ref. 21). TARPs include molecules from the stargazin family and appear to associate with nascent AMPA receptor complexes after subunit tetramerization but before their export from the ER. In heterologous systems, TARPs greatly enhanced the surface expression of AMPA receptors but did not affect the traffic of structurally related KA receptors. TARPs appear to function as chaperones to facilitate the trafficking of receptors through secretory compartments and to direct their distribution to specific membrane compartments. In addition, TARPs modulate AMPA receptor gating and pharmacological properties. Drosophila genome contains a stargazin-like protein (Stg1) encoded by CG33670. Previous studies showed that Drosophila Stg1 is functionally homologous with other stargazin-like molecule from worms and vertebrates and they can partially substitute for one another to reconstitute glutamate-gated currents.51 Similarly, the C. elegans SOL-1, a more distantly related TARP, and Drosophila Sol-1 homolog, encoded by CG34402, have been shown to functionally substitute for each other.49,52
Recent studies have shown that AMPA receptors primarily associate with CNIHs and only a fraction associate with TARPs.53 In heterologous systems, CNIHs enhanced the surface expression of AMPA receptors and affected the channel properties to a greater extent than TARPs. Judged by their high conservation with Drosophila Cornichon (Cni) and yeast Erv14p, proteins that aid in the trafficking of the TGFα-related proteins to the cell surface, CNIHs are bonafide chaperones.54-57 The molecular mechanisms underlying the common effects of the structurally distant TARPS and CNIHs on AMPA receptors are under intense investigation. No role in the modulation of iGluRs has been defined for the Drosophila Cornichon (encoded by CG5855) and Cornichon-related (CG17262). Whether TARPs or CNIHs could function as auxiliary subunits for Drosophila iGluRs remains to be determined. But there is no doubt that learning about the dynamic and multi-molecular complexes that iGluRs form with auxiliary subunits will greatly influence our understanding of synapse assembly and function. Building a synapse is after all a complex matter.
Aknowledgments
We are grateful to Kendal Broadie for Drosophila stocks. We thank Mik Sulkowski and members of the Serpe lab for help and suggestions. This work was supported by the Intramural Research Program at NIH, NICHD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Broadie K, Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993;11:607–19. doi: 10.1016/0896-6273(93)90073-Z. [DOI] [PubMed] [Google Scholar]
- 2.Keshishian H, Chiba A, Chang TN, Halfon MS, Harkins EW, Jarecki J, et al. Cellular mechanisms governing synaptic development in Drosophila melanogaster. J Neurobiol. 1993;24:757–87. doi: 10.1002/neu.480240606. [DOI] [PubMed] [Google Scholar]
- 3.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 4.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 5.Schmid A, Qin G, Wichmann C, Kittel RJ, Mertel S, Fouquet W, et al. Non-NMDA-type glutamate receptors are essential for maturation but not for initial assembly of synapses at Drosophila neuromuscular junctions. J Neurosci. 2006;26:11267–77. doi: 10.1523/JNEUROSCI.2722-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–9. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 7.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–42. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- 9.Fambrough DM. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979;59:165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- 10.Burden SJ. The formation of neuromuscular synapses. Genes Dev. 1998;12:133–48. doi: 10.1101/gad.12.2.133. [DOI] [PubMed] [Google Scholar]
- 11.Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/S0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 12.McMahan UJ. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–18. doi: 10.1101/SQB.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 14.DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–12. doi: 10.1016/S0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 15.Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 16.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–42. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–97. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Coldefy AS, Hubbard SR, Burden SJ. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK) J Biol Chem. 2011;286:40624–30. doi: 10.1074/jbc.M111.279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–33. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zong Y, Zhang B, Gu S, Lee K, Zhou J, Yao G, et al. Structural basis of agrin-LRP4-MuSK signaling. Genes Dev. 2012;26:247–58. doi: 10.1101/gad.180885.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–99. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YJ, Bao H, Bonanno L, Zhang B, Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev. 2012;26:974–87. doi: 10.1101/gad.185165.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng D, Pitcher GM, Szilard RK, Sertié A, Kanisek M, Clapcote SJ, et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–96. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R, Mellem JE, Jensen M, Brockie PJ, Walker CS, Hoerndli FJ, et al. The SOL-2/Neto auxiliary protein modulates the function of AMPA-subtype ionotropic glutamate receptors. Neuron. 2012;75:838–50. doi: 10.1016/j.neuron.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J Neurosci. 2011;31:7334–40. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, et al. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci. 2011;14:866–73. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci. 2011;31:8078–82. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, et al. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci. 2011;31:10009–18. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher JL, Mott DD. The auxiliary subunits Neto1 and Neto2 reduce voltage-dependent inhibition of recombinant kainate receptors. J Neurosci. 2012;32:12928–33. doi: 10.1523/JNEUROSCI.2211-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomita S, Castillo PE. Neto1 and Neto2: auxiliary subunits that determine key properties of native kainate receptors. J Physiol. 2012;590:2217–23. doi: 10.1113/jphysiol.2011.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broadie K, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993;361:350–3. doi: 10.1038/361350a0. [DOI] [PubMed] [Google Scholar]
- 33.Rasse TM, Fouquet W, Schmid A, Kittel RJ, Mertel S, Sigrist CB, et al. Glutamate receptor dynamics organizing synapse formation in vivo. Nat Neurosci. 2005;8:898–905. doi: 10.1038/nn1484. [DOI] [PubMed] [Google Scholar]
- 34.Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–44. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Broadie KS, Bate M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J Neurosci. 1993;13:144–66. doi: 10.1523/JNEUROSCI.13-01-00144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitoe M, Tanaka S, Takata K, Kidokoro Y. Neural activity affects distribution of glutamate receptors during neuromuscular junction formation in Drosophila embryos. Dev Biol. 1997;184:48–60. doi: 10.1006/dbio.1996.8480. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Featherstone DE. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 2005;3:1. doi: 10.1186/1741-7007-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa K, Kidokoro Y. Junctional and extrajunctional glutamate receptor channels in Drosophila embryos and larvae. J Neurosci. 1995;15:7905–15. doi: 10.1523/JNEUROSCI.15-12-07905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganesan S, Karr JE, Featherstone DE. Drosophila glutamate receptor mRNA expression and mRNP particles. RNA Biol. 2011;8:771–81. doi: 10.4161/rna.8.5.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiAntonio A. Glutamate receptors at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:165–79. doi: 10.1016/S0074-7742(06)75008-5. [DOI] [PubMed] [Google Scholar]
- 41.Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, et al. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005;25:3199–208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–15. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–32. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer F, Aberle H. At the next stop sign turn right: the metalloprotease Tolloid-related 1 controls defasciculation of motor axons in Drosophila. Development. 2006;133:4035–44. doi: 10.1242/dev.02580. [DOI] [PubMed] [Google Scholar]
- 45.Serpe M, O’Connor MB. The metalloprotease tolloid-related and its TGF-beta-like substrate Dawdle regulate Drosophila motoneuron axon guidance. Development. 2006;133:4969–79. doi: 10.1242/dev.02711. [DOI] [PubMed] [Google Scholar]
- 46.Rohrbough J, Rushton E, Woodruff E, 3rd, Fergestad T, Vigneswaran K, Broadie K. Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation. Genes Dev. 2007;21:2607–28. doi: 10.1101/gad.1574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rushton E, Rohrbough J, Deutsch K, Broadie K. Structure-function analysis of endogenous lectin mind-the-gap in synaptogenesis. Dev Neurobiol. 2012;72:1161–79. doi: 10.1002/dneu.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang M, Ivakine E, Mahadevan V, Salter MW, McInnes RR. Neto2 interacts with the scaffolding protein GRIP and regulates synaptic abundance of kainate receptors. PLoS One. 2012;7:e51433. doi: 10.1371/journal.pone.0051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker CS, Francis MM, Brockie PJ, Madsen DM, Zheng Y, Maricq AV. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc Natl Acad Sci U S A. 2006;103:10787–92. doi: 10.1073/pnas.0604520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas U, Sigrist SJ. Glutamate receptors in synaptic assembly and plasticity: case studies on fly NMJs. Adv Exp Med Biol. 2012;970:3–28. doi: 10.1007/978-3-7091-0932-8_1. [DOI] [PubMed] [Google Scholar]
- 51.Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, et al. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci U S A. 2006;103:10781–6. doi: 10.1073/pnas.0604482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–7. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
- 53.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–9. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 54.Powers J, Barlowe C. Transport of axl2p depends on erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J Cell Biol. 1998;142:1209–22. doi: 10.1083/jcb.142.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bökel C, Dass S, Wilsch-Bräuninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–70. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- 56.Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–78. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 57.Castro CP, Piscopo D, Nakagawa T, Derynck R. Cornichon regulates transport and secretion of TGFalpha-related proteins in metazoan cells. J Cell Sci. 2007;120:2454–66. doi: 10.1242/jcs.004200. [DOI] [PubMed] [Google Scholar]