Abstract
Drosophila embryo dorsoventral polarity is established by a maternally encoded signal transduction pathway in which three sequentially acting serine proteases, Gastrulation Defective, Snake and Easter, generate the ligand that activates the Toll receptor on the ventral side of the embryo. The spatial regulation of this pathway depends upon ventrally restricted expression of the Pipe sulfotransferase in the ovarian follicle during egg formation. Several recent observations have advanced our understanding of the mechanism regulating the spatially restricted activation of Toll. First, several protein components of the vitelline membrane layer of the eggshell have been determined to be targets of Pipe-mediated sulfation. Second, the processing of Easter by Snake has been identified as the first Pipe-dependent, ventrally-restricted processing event in the pathway. Finally, Gastrulation Defective has been shown to undergo Pipe-dependent, ventral localization within the perivitelline space and to facilitate Snake-mediated processing of Easter. Together, these observations suggest that Gastrulation Defective, localized on the interior ventral surface of the eggshell in association with Pipe-sulfated eggshell proteins, recruits and mediates an interaction between Snake and Easter. This event leads to ventrally-restricted processing and activation of Easter and consequently, localized formation of the Toll ligand, and Toll activation.
Keywords: Drosophila, Easter, Gastrulation Defective, Pipe, Snake, Spätzle, Toll, dorsal-ventral, follicle, perivitelline, sulfation, sulfonation
Introduction
The establishment and orientation of the dorsoventral axis of the Drosophila embryo is determined by the ventrally restricted activation of Toll, a transmembrane receptor that is uniformly distributed in the plasma membrane of the early embryo.1,2 Ventral activation of Toll triggers a signal transduction pathway that results in the nuclear localization of Dorsal, a fly ortholog of the p50 and p65 subunits of mammalian NFκB,3-5 in a concentration gradient from ventral to dorsal.6-8 Dorsal regulates the expression of the zygotic target genes responsible for establishing cellular identity along the dorsal–ventral axis.9-13 Work in our lab has focused on elucidating the mechanism that achieves ventrally-restricted Toll activation. The ligand for Toll is Spätzle (Spz),14,15 which is secreted as an inactive precursor from the embryo into the perivitelline fluid (PVF)16 that fills the perivitelline space (PVS) between the embryonic membrane and the vitelline membrane, the inner layer of the eggshell. To become a functional ligand for Toll, Spz must be processed by the serine protease Easter (Ea),14,15,17 which itself is activated by cleavage by another serine protease, Snake (Snk).18,19 The ventral formation of the Toll ligand, and consequently, the formation of the embryonic dorsoventral axis, is dependent on the prior establishment of dorsoventral polarity in the follicular epithelium that surrounds the developing oocyte during oogenesis, which acts to provide yolk to the developing egg cell and constructs the eggshell. Follicular epithelium polarity is transmitted to the egg/embryo through the expression of the dorsal group gene pipe in a ventral subpopulation of follicle cells.20 We recently showed that Pipe, which encodes a fly ortholog of vertebrate glycosaminoglycan carbohydrate modifying enzymes,21,22 promotes transfer of sulfate groups to several protein components of the vitelline membrane layer of the eggshell,23 presumably added to carbohydrate side chains displayed by those proteins. Pipe-sulfated glycoproteins embedded ventrally within the eggshell constitute a cue that controls the spatial parameters of serine protease activity in the PVS. As we discuss below, the cleavage of both Snk and Ea requires the participation of an additional serine protease, Gastrulation Defective (GD). After secretion into the PVS, GD becomes concentrated in the ventral region of the PVS in a process that depends on the activity of Pipe.24 At this location, GD facilitates a productive interaction between Snk and Ea, leading to Ea and then Spz processing, and ultimately to activation of the Toll receptor on the ventral side of the embryo.
Processing of Ea by Snk Is the Initial Pipe-Dependent, Ventrally-Localized Cleavage in the Dorsal Group Protease Cascade
A serine protease cascade, operating in the egg PVS, accomplishes the ventrally-restricted processing and activation of full-length Spz protein into the active Toll ligand. Which of the protease cleavage events are ventrally restricted and how is this controlled by the sulfated targets of Pipe? By systematically examining the patterns of processing of GFP-tagged versions of GD, Snk and Ea, in the background of mutations affecting each of the genes that act upstream of Toll, we identified the processing of Ea by Snk as the first of the dorsal group proteolytic processing events to be dependent upon the presence of Pipe activity and therefore the first ventrally-restricted cleavage event in the pathway.25 In addition to Ea-GFP, Spz-GFP fails to undergo processing in pipe mutant-derived embryos, presumably because Ea is not activated in that genetic background. In contrast, neither the processing of GD-GFP nor that of Snk-GFP is dependent on Pipe activity. Consistent with these results, overexpression of Pipe leads to increased processing of HA-tagged versions of Ea and Spz, but not to increased processing of Snk, again pointing to the processing of Ea by Snk as the Pipe-dependent, ventrally-restricted proteolytic event, a result that was obtained independently by LeMosy and coworkers.26 An important conclusion of these studies was the recognition that the dorsal group serine proteases do not act in a simple linear progression in which ventrally-restricted cleavage of GD initiates a ventrally localized protease cascade. Rather, the pathway has multiple regulatory inputs that ensure that the activation of Easter is initiated at, and restricted to, the ventral side of the embryo (Fig. 1).
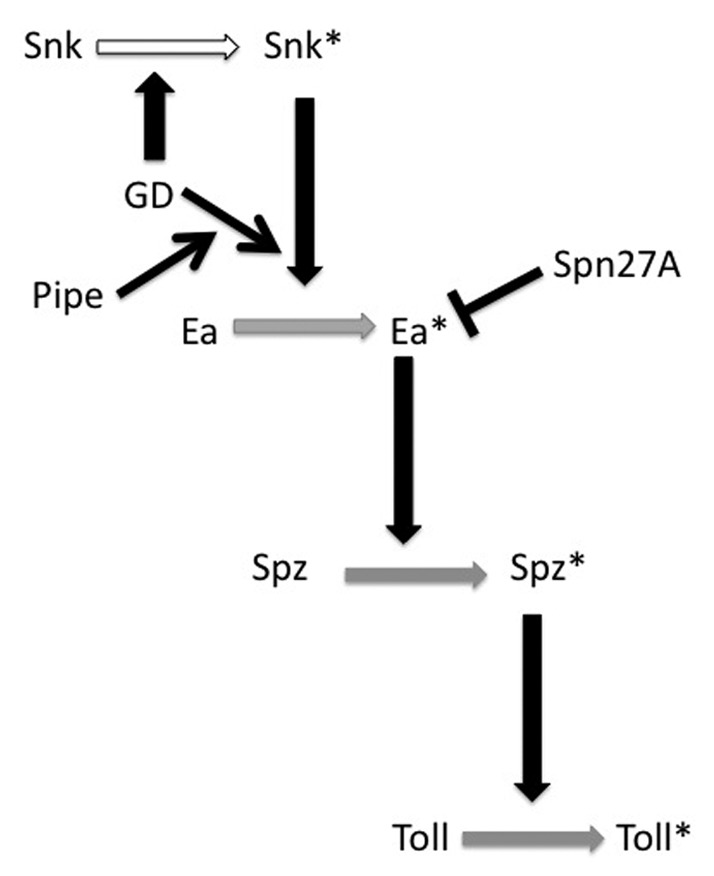
Figure 1. Model for the action of Drosophila dorsal group serine proteases in the PVS of the egg. Horizontal arrows denote the conversion of an inactive version of the respective protein to its activated form, indicated by the presence of an asterisk (*). Vertical black arrows have at their bases, the proteins that directly mediate those activation events. Activation/processing of Snk (white horizonal arrow) occurs uniformly around the egg/embryo DV circumference, while activation of Ea, Spz and Toll (gray horizonal arrows) is restricted to the ventral side of the egg/embryo. Black diagonal arrows denote positive regulatory inputs; the bar originating from Spn27A indicates inhibition. Note that GD directly processes and activates Snk, as well as facilitating Snk*-mediated processing of Ea via a mechanism that requires Pipe activity. Our experiments indicate that processing of Snk can be performed by the GD zymogen, a conclusion that is supported by observations of Steen et al.26 It has not yet been determined whether GD's influence upon Ea processing by Snk requires that GD itself undergo processing. Thus, GD is not marked with an asterisk.
These studies identified Easter activity as the critical target of regulatory control at which DV polarity is imparted to the developing embryo. Not only is it essential that Easter is activated only on the ventral side of the embryo, it is critical that activated Easter not diffuse throughout the PVS. Misra et al.27 showed that following its processing, activated Easter is rapidly converted to a high molecular mass complex suspected to contain a covalently attached serine protease inhibitor. Subsequent studies showed that females homozygous for mutations in the gene encoding Serpin27A (Spn27A) produce ventralized embryos and that Spn27A forms a complex with and inhibits the activity of Easter.28,29 It is presumed that Spn27A is distributed uniformly throughout the PVS and that activated Easter is rapidly bound and inhibited by Spn27A. This ensures that Easter cleaves and converts Spätzle precursor protein into active Toll ligand only near its own point of activation on the ventral side of the PVS.
Our observation that Snk processing is independent of Pipe activity is seemingly at odds with reports that injection of RNA encoding a pre-cleaved form of Snk (SnkΔN) into the progeny of pipe mutant mothers leads to the formation of lateralized progeny.30 Those results implied that Snk function does not require Pipe activity and suggested that during normal development, Pipe acts upstream of Snk in the process leading to cleavage and activation of Snk. However, this is clearly not the case, as Snk-GFP is processed normally in the progeny of pipe mutant mothers.25 This discrepancy is likely explained by the secretion of the activated form of Snk (SnkΔN) directly into the nascent secretory compartment of injected embryos, where it precociously interacts with and processes Ea prior to their secretion into the PVS. This interaction occurs in the absence of spatial cues laid down by Pipe and would therefore be uniform along the dorsal-ventral axis, leading to the lateralized phenotype. In contrast, under normal circumstances, both Snk and Ea transit through the secretory pathway as inactive zymogens. Although Snk is processed into its active form in a Pipe-independent manner, in the PVS its interaction with Ea requires both Pipe and GD activity (see below).
GD Plays a Direct Role in Ea Processing
The GD protease does not itself undergo spatially restricted processing, nor is GD-mediated cleavage of Snk restricted to the ventral side of the embryo. Nevertheless, as we recently demonstrated,24 GD plays a central role in facilitating Pipe-dependent ventral processing of Ea. Our study builds on a report by DeLotto and coworkers31 of interallic complementation between gd mutant alleles, which revealed that gd mutations fall into three classes. Members of the gd[2] and gd[10] classes exhibit allelic complementation with one another in transheterozygous mutant females, while members of the third, noncomplementing group, are not capable of complementing either gd[2] or gd[10] class alleles. Ponomareff et al. identified the lesions associated with 17 gd mutations and found that all of the gd[2] class alleles carry missense mutations in the putative prodomain at the N-terminus of GD, while the gd[10] class alleles correspond to missense mutations that map near the active site serine located within the catalytic domain of the protein. Lesions associated with noncomplementing alleles were identified throughout the GD coding region and, with one exception, either introduce premature stop codons or delete part of the coding region. The existence of two classes of cross-complementing gd alleles led Ponomareff et al.31 to suggest that GD contains two discrete functional domains, one associated with the prodomain of GD and the other with the presumptive catalytic chain. Injection of mRNA encoding a secreted version of the GD protease domain alone is capable of rescuing the dorsalized phenotype of gd[10] class mutant-derived embryos, indicating that the catalytic domain can perform its function independent of the prodomain region. However, injections of RNA encoding the prodomain alone do not rescue the phenotype of gd[2]-derived embryos. Similarly, co-injection of RNAs that express the prodomain and catalytic chains of GD separately do not rescue the dorsalized phenotype of embryos produced by female homozygous for the non-complementing gd9 mutant allele. Finally, the existence of non-complementing mutant alleles of gd bearing nonsense codons near the carboxy terminus of the protein, suggest that the performance of the function associated with the prodomain region of the protein requires the prodomain to be contained within a full-length version of GD.
The analysis by Ponomareff et al.31 indicated that gd[2] class alleles encode proteins with a wild-type catalytic chain, which was surprising, as at that time, the only known function of GD was to cleave Snk. This finding prompted our group to directly examine Snk-GFP processing in the backgrounds of the three different gd mutant classes.24 As expected, Snk processing is perturbed in embryos from females bearing the null allele, gdVM90 or the gd[10] class allele gdVO27. However, normal levels of Snk processing occur in embryos from gd[2] class mutant mothers, indicating that gd[2] class alleles do not affect the ability of the encoded protein to process Snk. In contrast, Ea fails to undergo processing in all gd mutant backgrounds tested. This result suggested that GD plays a role in Ea activation that is independent of its role in cleaving Snk. This idea is supported by complementary experiments in which we overexpressed wild-type or mutant versions of GD that corresponded to GD[2] or GD[10] class proteins. Female germline overexpression of wild-type or of two catalytically inactive versions of GD, (GD[10] class), led to the formation of ventralized progeny embryos in which Ea processing was enhanced relative to wild-type embryos but Snk processing was unchanged. In contrast, no increase in Ea processing was observed in the progeny of females overexpressing either the GD[2] mutant protein or secreted versions of the GD catalytic chain alone, nor was the embryonic phenotype affected. Thus, GD influences Ea processing through a determinant present in the putative proenzyme domain, and the RNA injection results described above suggest that that determinant must be present in a full-length GD molecule to exert its function.
GD is Ventrally Concentrated in the PVS
Although we demonstrated that the cleavage of Ea is dependent on Pipe activity and therefore presumably localized to the ventral region of the PVS,25 it was not clear how this was achieved. At the time of that study it had not been possible to demonstrate localization of any of the components of the dorsal-ventral pathway within the PVS due to the low levels of the endogenous proteins and the need to remove the vitelline membrane to allow penetration by antibodies. Attempts to visualize the spatial distribution of transgenically-expressed GFP-tagged versions of the proteins were hindered by the high levels of GFP-associated fluorescence traversing the embryonic secretory compartment and present throughout the PVS.32 However, the finding that GD plays a role in facilitating the Pipe-dependent processing of Ea by Snk suggested the possibility of a functional interaction between GD and sulfated targets of Pipe, which prompted us to revisit the issue of localization within the PVS. Previous experiments indicated that it is possible to transplant activities contained within PVF from donor to recipient embryos,16,33 and we reasoned that if we transplanted PVF from an embryo expressing GD-GFP into a non-expressing embryo, the greatly reduced levels of GFP fluorescence might permit its spatial distribution to be visualized. We found that regardless of where along the dorsal-ventral axis the transplanted PVF is introduced, injected embryos exhibit a conspicuous enrichment of GD-GFP fluorescence in the ventral PVS.24 Strikingly, no ventral enrichment of GD-GFP is observed following transplantation into embryos from pipe mutant mothers. Instead, the fluorescence is distributed uniformly throughout the PVS, demonstrating that the Pipe-sulfated ventral cue is required for the ventral localization of GD-GFP. Further, although a catalytically inactive GD-GFP (gd[10]class) also becomes concentrated ventrally, GFP-tagged versions of GD bearing each of the gd[2] class mutations gd2, gd3, gdTN124, or gdLu119 do not accumulate on the ventral side. Thus, the gd[2] class mutations disrupt determinants within the prodomain that are necessary for GD localization to the ventral PVS.
Ventral localization of GD is likely to be mediated by the Pipe-sulfated ventral cue, perhaps through a direct interaction between GD and the carbohydrates that undergo sulfation by Pipe. However, as GD is present in complexes with Snk,24 Ea,24 and Spz (Y.S.C. and D.S., unpublished), its spatial distribution in the PVS could instead be a secondary consequence of an interaction between one or more of these proteins and the Pipe-sulfated ventral cue. Accordingly, we tested whether ventral localization of GD-GFP requires the presence of any of these proteins. As seen in Figure 2, GD-GFP was observed to undergo ventral accumulation despite the absence of Snk, Ea or Spz in both the donor and recipient embryos (Fig. 2B–D). To ask whether any of these proteins are themselves localized ventrally, we transplanted PVF from embryos expressing Ea-GFP and Spz-GFP into the PVS of non-expressing recipient embryos. Neither exhibited detectable ventral enrichment within the PVS of the recipient embryos (Fig. 2E–F). We were not able to carry out similar experiments for Snk, as Snk-GFP is not efficiently secreted into the PVS by expressing embryos.
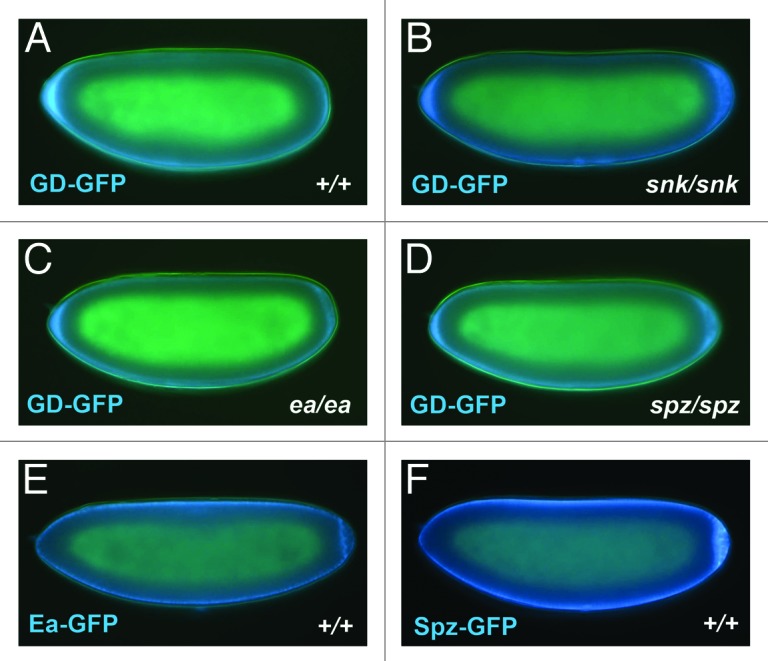
Figure 2. (A–D) Ventral accumulation of GD does not require the activities of Snk, Ea or Spz. PVF was obtained from donor embryos produced by GD-GFP expressing females that were mutant for either snk (snk1/snk2), ea (ea4/Df(3R)ea5022rx1) or spz (spz2/spz4), then transplanted to embryos from females of the same dorsal group genotype lacking GD-GFP expression, as described in Cho et al.24 The two snk mutations have been reported to carry stop codons near the N-terminus of the open reading frame.34 No ea mRNA can be detected in the ea4/Df(3R)ea5022rx1 mutant background.35 Thus the snk and ea mutant backgrounds can be considered null for the respective proteins. While the spz2 and spz4 alleles behave genetically as amorphic alleles, their associated mutant lesions have not been characterized and it is unclear whether they represent protein nulls. Description of the dorsal group mutant alleles used in these studies can be found on Flybase. (E and F) Neither Ea-GFP nor Spz-GFP exhibit ventral accumulation in the egg PVS. PVF from embryos produced by females expressing Ea-GFP or Spz-GFP under the control of the nos-Gal4:VP1636 driver was transplanted into the PVS of wild-type embryos. Details of the generation of the Ea-GFP and Spz-GFP transgenic lines can be found in Cho et al.24 GFP appears blue in all panels.
How Does Ventrally-Localized GD Enhance Snk-Mediated Processing of Ea?
How might GD localization facilitate the processing of Ea by Snk? In the simplest model, a single GD molecule would be bound to either an Ea zymogen or to activated Snk, and concentration of the GD/Ea and GD/Snk complexes in the ventral PVS would serve to bring Ea and activated Snk together. A model in which concentrating GD leads to a productive interaction between Snk and Ea is supported by the observation that overexpression of wild-type GD in a pipe mutant background produces embryos bearing lateral and in some cases ventral, pattern elements,37,38 which are otherwise never seen in the progeny of pipe mutant females. Thus, when it is present at very high levels, GD can facilitate the interaction between Ea and Snk even in the absence of ventral localization. It is also possible that GD may play a more active role by inducing a conformation change in Ea and/or Snk that enhances Ea’s ability to be cleaved and/or Snk’s ability to act as a protease. According to this model, ventral localization of GD under the control of Pipe would bring together Snk and Ea molecules that have been rendered competent to interact with one another. Consistent with these models, we demonstrated the existence of GD/Snk and GD/Ea complexes using co-immunoprecipitation.24 In subsequent studies we found that GD-GFP and Ea-HA co-immunoprecipitated even in embryos lacking Snk protein (Fig. 3), indicating that GD can interact with Ea independently of Snk.
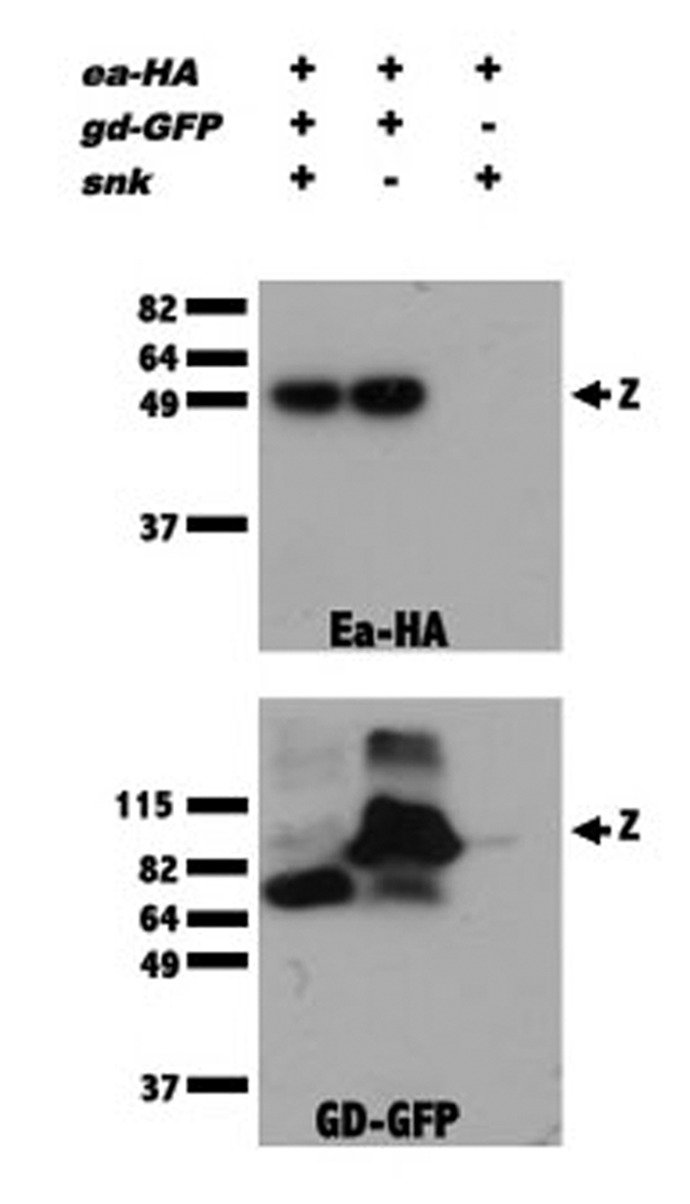
Figure 3. GD complexes with Ea in the absence of Snk. Extracts from wild-type (lanes 1, 3) or snk1/snk2-derived embryos (lane 2) expressing Ea-HA in the presence (lanes 1, 2) or absence (lane 3) of GD-GFP, were subjected to immunoprecipitation with GFP-Trap. Extracts were divided into two portions and western blot analysis was performed with anti-HA (top panel) and anti-GFP (bottom panel) antibodies. “z” indicates the position of the zymogen forms of Ea-HA (top panel) and GD-GFP (bottom panel). The ability of the GFP-Trap to bring down Ea-HA in extracts from both wild-type and snk mutant-derived extracts indicates that the association of GD with Ea does not require Snk protein. When GD-GFP and Ea-HA were co-expressed, all of the GD-GFP was processed (lane 1, bottom panel) due to a feedback mechanism in which high levels of activated Ea process GD.18,19,24 The details of the generation of embryonic extracts, precipitation with GFP-Trap and western blot analysis can be found in Cho et al.24 Details of the generation of the GD-GFP and Ea-HA transgenic lines can be found in Cho et al.25
Another factor that may influence the role of GD in promoting Ea cleavage is the processing of GD itself. As described above, the ability of GD to process Snk is not affected by the gd[2] class mutations that disrupt its own processing. Rather, our data suggest a model in which processing of GD is required for it to become localized ventrally, which is critical to its function in bringing Ea and Snk together when GD is present at its relatively low endogenous levels. Consistent with this idea, when we overexpressed GD[2] in a pipe mutant background, it was capable of inducing the formation of some dorsolateral structures (Fig. 4). Thus, the inability of GD[2] to facilitate the Snk/Ea interaction can be partially overcome when it is present at uniformly high concentrations, supporting the idea that ventral localization is required to bring about locally elevated concentrations of GD. Alternatively, unprocessed GD may have lower affinity for Snk and/or Ea and increasing the concentration of GD[2] may drive their association. A more complete understanding of the role of GD processing in its function awaits the identification of the site at which the protein is cleaved and the generation of mutants in which that site is altered.
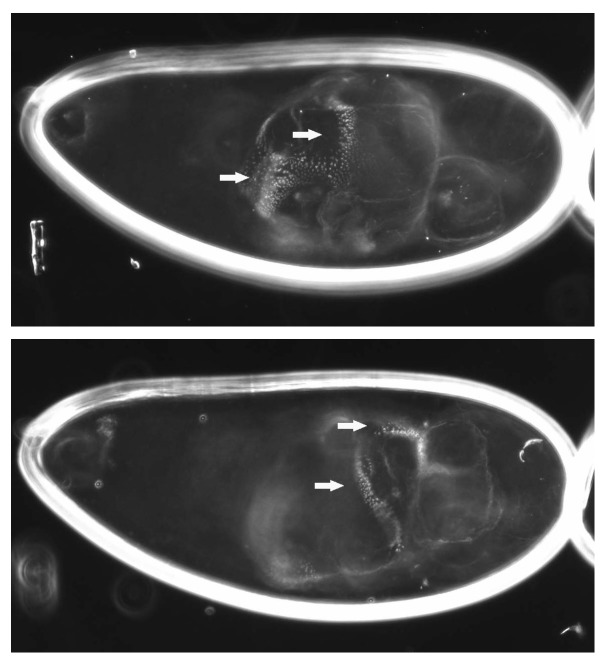
Figure 4. Overexpression of the non-localizing GD[2] mutant version of GD can facilitate the processing of Ea. Cuticle preparation of a progeny embryo from a gdVM90/gdVM90; pipe1/pipe3 mutant female expressing GD[2] under the control of pCOG-Gal4:VP16.36 The embryo is shown at a different focal depth in each panel. Arrows indicate the position of denticle band material encircling the embryonic cuticle.
What is the Role of Nudel in DV Patterning?
The finding that processing of GD is not required for its catalytic activity raises questions about the role of what remains the most enigmatic member of the dorsal group genes, nudel. Like pipe and windbeutel, which encodes a chaperone responsible for transport of Pipe to the Golgi apparatus,39 nudel is required to be expressed in ovarian follicle cells that surround the developing oocyte during oogenesis.33,40,41 Nudel is a very large modular protein of 2616 amino acids that contains a central domain encoding a trypsin-type serine protease.41 Nudel also exhibits features of extracellular matrix proteins that include 11 repeats of a peptide motif found in the Low Density Lipoprotein Receptor (Type A repeats), three amino acid sequence motifs that could act as target sites for glycosaminoglycan addition, 23 potential sites for N-linked glycosylation and two serine/threonine rich regions that might be sites for O-linked glycosylation. Nudel undergoes a complex pattern of processing during oogenesis and embryogenesis, and there is evidence that the Nudel protease acts autocatalytically to carry out the later cleavage events.42
nudel is the only dorsal group gene with mutant alleles that affect the processing of GD,19,25 which led to speculation that the Nudel protease may process GD directly. Processing of Snake also does not occur normally in the progeny of nudel mutant females. This was initially presumed to be the downstream consequence of the failure of GD to undergo proteolytic activation of its own catalytic function. However, our demonstration that the gd2-encoded protein, which does not undergo cleavage, exhibits normal catalytic activity toward Snake,24 indicates that the absence of Snake processing in the nudel mutant background is not due to the failure of GD to undergo processing. In addition to their effect on DV patterning, nudel mutations also cause defects in the VM43,44. Thus, the effect of nudel mutations on GD and Snake cleavage may be indirect and mediated through alterations in the VM or the PVS environment. A more complete understanding of Nudel function will likely lead to novel insights into the regulation of GD and Snake proteolytic activity.
What Does the Future Hold for Studies of Drosophila DV Patterning?
A number of observations made over the last several years have significantly advanced our understanding of the events occurring in the Drosophila egg PVS that lead to the establishment of the embryonic DV axis. At the same time, these findings have highlighted fundamental issues that remain to be addressed. Primary among these are the characterization of the direct target(s) of Pipe-mediated sulfation and an elucidation of the mechanism by which it regulates Ea processing. This pathway provides an excellent opportunity to examine the role of a specific sulfated carbohydrate in regulating the spatial parameters of a proteolytic cleavage event. Does GD bind directly to the Pipe sulfated ventral cue and if so, what are the determinants within GD that mediate this event? What is the molecular mechanism by which GD enhances the processing of Ea by Snk? Is the processing of GD necessary for this event or for the localization of GD? The possibility remains that the processing of GD, while conspicuously correlated with its ventral localization, is not essential for the performance of any of its functions during the establishment of embryonic DV patterning. We are on the verge of a comprehensive picture of the events leading to the localized activation of the Toll receptor in the early Drosophila embryo. The insights obtained are likely to extend beyond the realm of Drosophila embryonic patterning to enhance our general understanding of the regulation of serine proteases in the vast array of processes in which they participate.
Acknowledgments
The work discussed here was supported by a grant from the National Institutes of Health (GM077337).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
These authors contributed equally to this work.
References
- 1.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto C, Gerttula S, Anderson KV. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development. 1991;111:1021–8. doi: 10.1242/dev.111.4.1021. [DOI] [PubMed] [Google Scholar]
- 3.Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987;238:692–4. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Gifford AM, Riviere LR, Tempst P, Nolan GP, Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990;62:1019–29. doi: 10.1016/0092-8674(90)90276-K. [DOI] [PubMed] [Google Scholar]
- 5.Nolan GP, Ghosh S, Liou HC, Tempst P, Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991;64:961–9. doi: 10.1016/0092-8674(91)90320-X. [DOI] [PubMed] [Google Scholar]
- 6.Rushlow CA, Han K, Manley JL, Levine M. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989;59:1165–77. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- 7.Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989;59:1179–88. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- 8.Roth S, Stein D, Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- 9.Ip YT, Kraut R, Levine M, Rushlow CA. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991;64:439–46. doi: 10.1016/0092-8674(91)90651-E. [DOI] [PubMed] [Google Scholar]
- 10.Kosman D, Ip YT, Levine M, Arora K. Establishment of the mesoderm-neuroectoderm boundary in the Drosophila embryo. Science. 1991;254:118–22. doi: 10.1126/science.1925551. [DOI] [PubMed] [Google Scholar]
- 11.Ray RP, Arora K, Nüsslein-Volhard C, Gelbart WM. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development. 1991;113:35–54. doi: 10.1242/dev.113.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Thisse C, Perrin-Schmitt F, Stoetzel C, Thisse B. Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell. 1991;65:1191–201. doi: 10.1016/0092-8674(91)90014-P. [DOI] [PubMed] [Google Scholar]
- 13.Stathopoulos A, Van Drenth M, Erives A, Markstein M, Levine M. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell. 2002;111:687–701. doi: 10.1016/S0092-8674(02)01087-5. [DOI] [PubMed] [Google Scholar]
- 14.Morisato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–88. doi: 10.1016/0092-8674(94)90507-X. [DOI] [PubMed] [Google Scholar]
- 15.Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the Spätzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–50. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- 16.Stein D, Nüsslein-Volhard C. Multiple extracellular activities in Drosophila egg perivitelline fluid are required for establishment of embryonic dorsal-ventral polarity. Cell. 1992;68:429–40. doi: 10.1016/0092-8674(92)90181-B. [DOI] [PubMed] [Google Scholar]
- 17.DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spätzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72:141–8. doi: 10.1016/S0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 18.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 2001;20:2387–93. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeMosy EK, Tan Y-Q, Hashimoto C. Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Natl Acad Sci U S A. 2001;98:5055–60. doi: 10.1073/pnas.081026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen J, Goltz JS, Stevens LM, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–81. doi: 10.1016/S0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Habuchi H, Yoneda M, Habuchi O, Kimata K. Molecular cloning and expression of Chinese hamster ovary cell heparan-sulfate 2-sulfotransferase. J Biol Chem. 1997;272:13980–5. doi: 10.1074/jbc.272.21.13980. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Sugumaran G, Liu J, Shworak NW, Silbert JE, Rosenberg RD. Molecular cloning and characterization of a human uronyl 2-sulfotransferase that sulfates iduronyl and glucuronyl residues in dermatan/chondroitin sulfate. J Biol Chem. 1999;274:10474–80. doi: 10.1074/jbc.274.15.10474. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Stevens LM, Stein D. Sulfation of eggshell components by Pipe defines dorsal-ventral polarity in the Drosophila embryo. Curr Biol. 2009;19:1200–5. doi: 10.1016/j.cub.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YS, Stevens LM, Sieverman KJ, Nguyen J, Stein D. A ventrally localized protease in the Drosophila egg controls embryo dorsoventral polarity. Curr Biol. 2012;22:1013–8. doi: 10.1016/j.cub.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho YS, Stevens LM, Stein D. Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr Biol. 2010;20:1133–7. doi: 10.1016/j.cub.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steen PW, Tian S, Tully SE, Cravatt BF, LeMosy EK. Activation of Snake in a serine protease cascade that defines the dorsoventral axis is atypical and pipe-independent in Drosophila embryos. FEBS Lett. 2010;584:3557–60. doi: 10.1016/j.febslet.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra S, Hecht P, Maeda R, Anderson KV. Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–7. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto C, Kim DR, Weiss LA, Miller JW, Morisato D. Spatial regulation of developmental signaling by a serpin. Dev Cell. 2003;5:945–50. doi: 10.1016/S1534-5807(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 29.Ligoxygakis P, Roth S, Reichhart JM. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr Biol. 2003;13:2097–102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 30.Smith CL, DeLotto R. Ventralizing signal determined by protease activation in Drosophila embryogenesis. Nature. 1994;368:548–51. doi: 10.1038/368548a0. [DOI] [PubMed] [Google Scholar]
- 31.Ponomareff G, Giordano H, DeLotto Y, DeLotto R. Interallelic complementation at the Drosophila melanogaster gastrulation defective locus defines discrete functional domains of the protein. Genetics. 2001;159:635–45. doi: 10.1093/genetics/159.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein D, Charatsi I, Cho YS, Zhang Z, Nguyen J, DeLotto R, et al. Localization and activation of the Drosophila protease easter require the ER-resident saposin-like protein seele. Curr Biol. 2010;20:1953–8. doi: 10.1016/j.cub.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 33.Stein D, Roth S, Vogelsang E, Nüsslein-Volhard C. The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell. 1991;65:725–35. doi: 10.1016/0092-8674(91)90381-8. [DOI] [PubMed] [Google Scholar]
- 34.Smith CL, Giordano H, DeLotto R. Mutational analysis of the Drosophila snake protease: an essential role for domains within the proenzyme polypeptide chain. Genetics. 1994;136:1355–65. doi: 10.1093/genetics/136.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang AJ, Morisato D. Regulation of Easter activity is required for shaping the Dorsal gradient in the Drosophila embryo. Development. 2002;129:5635–45. doi: 10.1242/dev.00161. [DOI] [PubMed] [Google Scholar]
- 36.Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–8. doi: 10.1016/S0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 37.Han JH, Lee SH, Tan YQ, LeMosy EK, Hashimoto C. Gastrulation defective is a serine protease involved in activating the receptor toll to polarize the Drosophila embryo. Proc Natl Acad Sci U S A. 2000;97:9093–7. doi: 10.1073/pnas.97.16.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLotto R. Gastrulation defective, a complement factor C2/B-like protease, interprets a ventral prepattern in Drosophila. EMBO Rep. 2001;2:721–6. doi: 10.1093/embo-reports/kve153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen J, Goltz JS, Konsolaki M, Schüpbach T, Stein D. Windbeutel is required for function and correct subcellular localization of the Drosophila patterning protein Pipe. Development. 2000;127:5541–50. doi: 10.1242/dev.127.24.5541. [DOI] [PubMed] [Google Scholar]
- 40.Konsolaki M, Schüpbach T. windbeutel, a gene required for dorsoventral patterning in Drosophila, encodes a protein that has homologies to vertebrate proteins of the endoplasmic reticulum. Genes Dev. 1998;12:120–31. doi: 10.1101/gad.12.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong CC, Hashimoto C. An unusual mosaic protein with a protease domain, encoded by the nudel gene, is involved in defining embryonic dorsoventral polarity in Drosophila. Cell. 1995;82:785–94. doi: 10.1016/0092-8674(95)90475-1. [DOI] [PubMed] [Google Scholar]
- 42.LeMosy EK, Kemler D, Hashimoto C. Role of Nudel protease activation in triggering dorsoventral polarization of the Drosophila embryo. Development. 1998;125:4045–53. doi: 10.1242/dev.125.20.4045. [DOI] [PubMed] [Google Scholar]
- 43.Hong CC, Hashimoto C. The maternal nudel protein of Drosophila has two distinct roles important for embryogenesis. Genetics. 1996;143:1653–61. doi: 10.1093/genetics/143.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeMosy EK, Hashimoto C. The nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Dev Biol. 2000;217:352–61. doi: 10.1006/dbio.1999.9562. [DOI] [PubMed] [Google Scholar]