Abstract
We have developed a novel model system in Drosophila melanogaster to study chemotherapy-induced neurotoxicity in adult flies. Neurological deficits were measured using a manual geotactic climbing assay. The manual assay is commonly used; however, it is laborious, time-consuming, subject to human error and limited to observing one sample at a time. We have designed and built a new automated fly-counting apparatus that uses a “video capture-particle counting technology” to automatically measure 10 samples at a time, with 20 flies per sample. Climbing behavior was assessed manually, as in our previous studies, and with the automated apparatus within the same experiment yielding statistically similar results. Both climbing endpoints as well as the climbing rate can be measured in the apparatus, giving the assay more versatility than the manual assay. Automation of our climbing assay reduces variability, increases productivity and enables high throughput drug screens for neurotoxicity.
Keywords: automated, Drosophila, geotactic, climbing, neurotoxicity, apparatus, chemotherapy, cisplatin
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a continuing problem for many patients with cancer. Medical care has improved the cure and survival rates for patients; however, many drugs used in cancer treatment can permanently damage neurons.1 For example, cisplatin is a very effective drug used to treat ovarian, testicular and breast cancers with an 85% cure rate against testicular cancer. However, it induces peripheral neuropathy in 20–30% of patients.1-3 The symptoms range from acral dysesthesias to severe gait instability. These symptoms often persist throughout a patient’s lifetime.
Rodent models have helped us to better understand the mechanisms involved in CIPN, however, we have found cancer cells and neurons die by similar mechanisms. In dorsal root ganglion neurons, in vitro, cisplatin binds both nuclear and mitochondrial DNA inducing DNA damage followed by cell cycle re-entry, p53 upregulation, bax translocation and caspase activation leading to apoptosis.4-7 In addition, direct damage to mitochondrial DNA underlies chronic neuron death.8 Neurons have also been shown to accumulate higher levels of platinum-DNA adducts than other cell types leading us to believe there are other cellular processes involved in cisplatin-induced neurotoxicity we have yet to understand.7
Drosophila melanogaster is an established model system that is inexpensive to use and has a wide variety of genetic tools to investigate basic biological processes. Many basic cellular mechanisms are highly conserved, and Drosophila are currently being used to study neurodegenerative diseases.9 Geotactic climbing is a common behavioral assay to study neurological deficits in Drosophila melanogaster. Drosophila have a natural instinct to climb against gravity and has been used to study several neurological disorders including Parkinson disease10 and Amyotrophic Lateral Sclerosis (ALS).11 We have developed a model of chemotherapy-induced neurotoxicity in Drosophila to investigate mechanisms involved in cisplatin-induced neurotoxicity.12 The phenotype for neurotoxicity for our assay is a deficit in geotactic climbing ability.
The geotactic climbing assay is typically performed manually, one vial at a time using the human eye and a stopwatch.13-15 This manual technique to measure geotactic climbing is time consuming and subject to significant intra- and inter-rater variability. Furthermore, with the manual technique it is difficult to process the large numbers of flies necessary for high throughput screening. A climbing assay has been developed using a tube rack and digital camera to manually tap down the flies and digitally acquire images for counting.16 This allows the user to do multiple vials at a time; however, there is still user variability and the process is clumsy. We have therefore developed and manufactured an automated fly-counting apparatus that greatly increases the numbers of flies that can be analyzed removes variability from the assay and enables high throughput screening for neurotoxicity.
Results
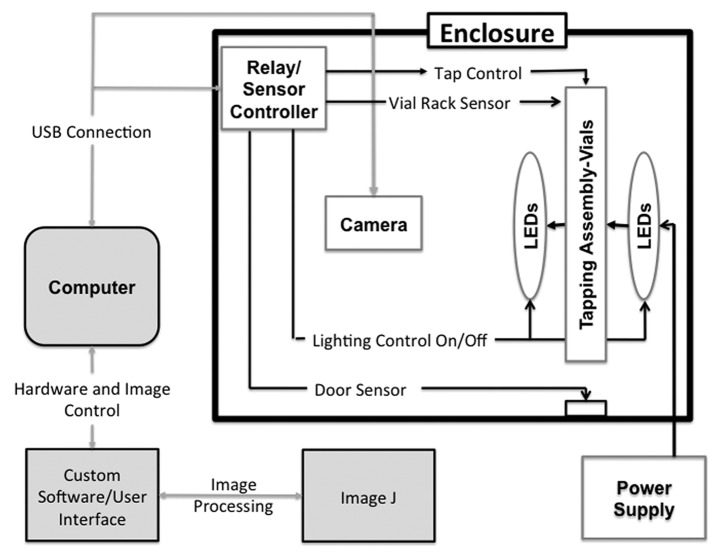
Schematic
A schematic representation of the automated fly-counting apparatus shows the various components of the apparatus (Fig. 1). The user launches the custom software on the computer, which loads the user interface and through a USB connection, turns on the lights, tap control, vial rack and door sensors via the relay/sensor controller. The user interface drives the tapping assembly and camera. The lights are powered by an external power supply. Images are acquired and processed using Image J software and results imported into the user interface.
Figure 1. Schematic drawing of the fly-counting apparatus.
Automated fly-counting apparatus pictures and output
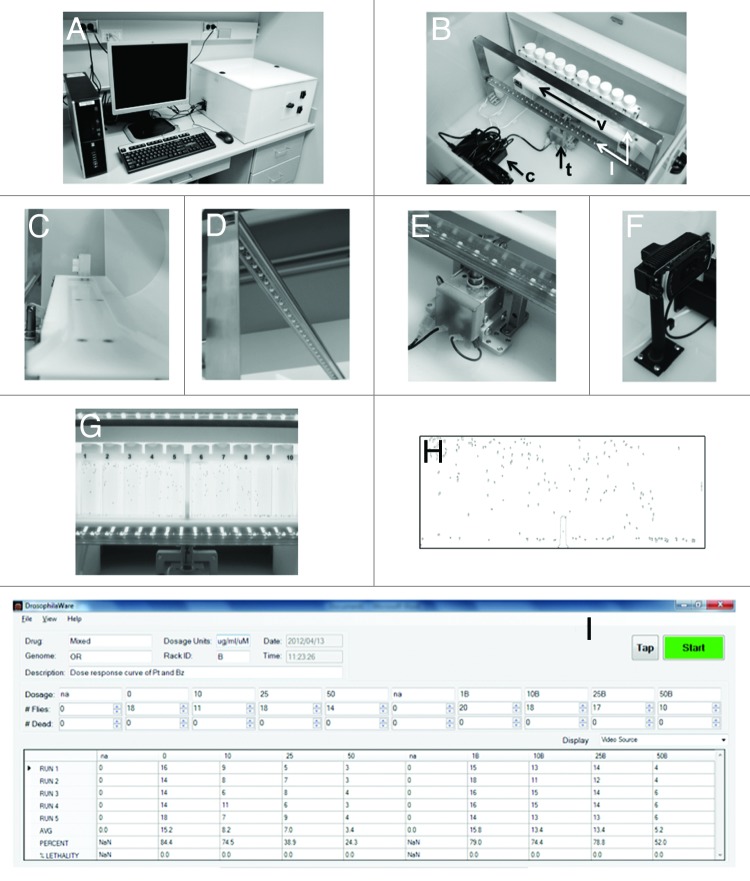
The automated fly-counting apparatus (Fig. 2A) consists of a Plexiglas box that houses all necessary equipment for the geotactic climbing assay, which interfaces with a standard PC computer via USB port. Within the Plexiglas box (Fig. 2B), the 10 vials containing flies are placed in racks and slid into the holder of the tapping mechanism (arrow v, Fig. 2C). Launching of the software initiates the LED lights (optimized via front and backlighting), which remain on throughout the entire assay (arrow l, Fig. 2D). Once the parameters for the program are established, the software automatically drives the tapping mechanism (arrow t, Fig. 2E) and the camera (arrow c, Fig. 3F). The tapping mechanism brings the flies to the bottom of the vials. Next, images are acquired at a user-defined rate, which are then transferred to the computer. Within the software, the (Fig. 2G) images of flies are converted into (Fig. 2H) particles. The particles are counted and calculated as a percent of the total number of flies in the vial and the output is displayed on the computer screen (Fig. 2I). A static, read-only file is saved to a server that contains each of the images acquired, the particle conversion images and a cvi file of the output data.
Figure 2. The fly-counting apparatus is consists of (A) the computer and plexiglass enclosure. Within (B) the enclosure is the (c) camera, the tapping assembly consisting of the (t) tapping mechanism, (v) vial holder and (l) LED lights. Closer examination of the (C) vial holder, (D) lights, (E) tapping mechanism and (F) camera shows the components of the apparatus in more detail. The camera acquires a (G) digital image, which is imported into Image J software and analyzed by (H) particle counting, which is then (I) displayed on the computer screen.
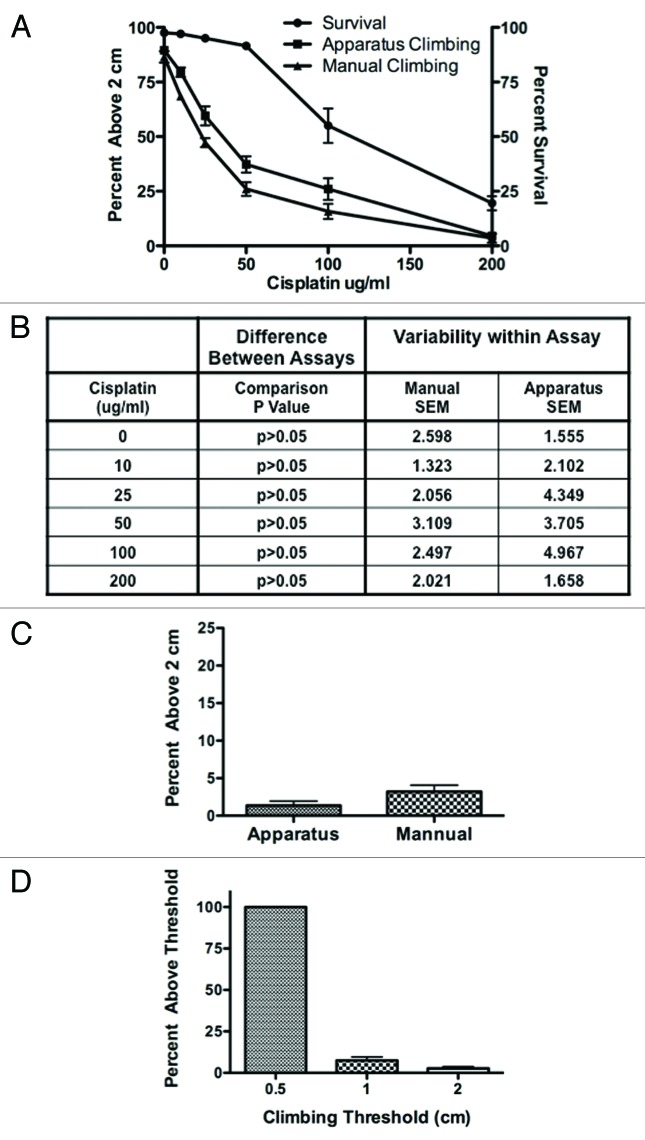
Figure 3. Side-by-side analysis of the geotaxis climbing assay between the fly-counting apparatus and the manual assay was found to be comparable. (A) Percent survival and percent of flies able to climb above 2 cm was performed in the apparatus followed by the manual assay on the same flies. (B) There was no statistical difference in climbing ability of the flies between the apparatus and the manual assay (p > 0.005). Variability within each assay was also similar. (C) Prickle flies were tested for climbing ability in both the manual assay and the apparatus with no statistical difference between the two. (p > 0.05) (D) Climbing ability in prickle flies was assessed in the apparatus with the climbing height (threshold) set at 0.5, 1 and 2 cm. Although we were able measure more flies climbing above 1 cm than 2 cm there was no statistical difference between the two. (p > 0.05) .
To ensure accuracy of our particle counting specific criteria was set up in the software and accuracy of particle counting was verified by high-speed camera (data not shown). All particles counted must be within the size limits for a single fly. If any of the particles is larger than the size limits of a single fly, the areas of all these particles are grouped together either above or below the set climbing height. We then add the areas above the line together and those below the line together and proportionally distribute any remaining flies not counted as individuals. The acceptable error range is 1–3 flies, with 50 flies per vial. If the total flies counted per vial are more than or less than the total number of flies entered into the software, an error message will be displayed.
Comparison of manual vs. automated assay
To validate our automated fly-counting apparatus against our manual assay we performed a side-by-side comparison. Flies were treated with 0, 10, 25, 50, 100 and 200 ug/ml cisplatin for 3 d and observed for survival and climbing behavior both in the apparatus and by hand. (Fig. 3A) Survival in these flies under the above conditions was 97.5%, 97%, 95%, 91.5%, 55% and 19.5%, respectively. The geotactic climbing assay was similar when performed by the automated fly-counting apparatus (89.5%, 79.5%, 59.5%, 37.25%, 26%, 4.5%) vs. the manual counting geotactic climbing assay (86.5%, 68.5%, 47.25%, 26% 15.75% and 3.5%) with 0, 10, 25, 50, 100 and 200 ug/ml cisplatin. (Fig. 3B) Comparing the results of the apparatus and the manual assay, we found no statistical difference between the two methods. The standard error means within each climbing assay method was similar indicating no difference in variability between the assay methods. (p > 0.05)
Besides using Cisplatin treatment to cause climbing defect in wild type flies, a climbing deficient prickle mutant (pkpk-sple-13) strain9 was tested for climbing ability comparing the manual and automatic assay (Fig. 3C). Flies, 2- to 5-d-old, were observed for climbing above 2 cm using the manual method followed by climbing in the apparatus. There was no significant difference in climbing with 3.2% of the flies able to climb above 2 cm in the manual assay with 1.4% in the apparatus. (p > 0.05) Since the climbing deficiency in prickle mutant flies is severe, we lowered the climbing threshold to 1 and 0.5 cm to see how small of a climbing height we can accurately measure (Fig. 3D). Percent climbing was measureable at 1cm but there was no significant difference between 1 and 2 cm with 2.7% at 2 cm and 7.5% at 1 cm. (p > 0.05) Using a 0.5 cm measurement cut-off, 100% of the flies were scored to climb beyond 0.5 cm, indicating that such threshold might be below the lower detection limit of the apparatus.
Measurements of climbing
In addition to the standard geotactic climbing assay that measures numbers of flies that cross a pre-defined height, in some experimental conditions the rate of climbing may provide useful information. We explored this approach to geotactic climbing with our automated fly-counting apparatus. We again treated flies with cisplatin (0, 10, 25, 50 and 100 ug/ml cisplatin for 3 d) and measured the climbing rate. The automated fly-counting apparatus was set up to tap once and acquire pictures every 2 sec for a total of 10 sec. The (Fig. 4A) percent of flies above 4 cm was plotted over time at 2, 4, 6, 8 and 10 sec with cisplatin at 0 ug/ml: 13%, 43%, 61%, 69% and 77%; 10 ug/ml: 6%, 33%, 56%, 68% and 72%; 25 ug/ml: 5%, 20%, 33%, 40% and 42%; 50 ug/ml: 5%, 21%, 27%, 28% and 28%; 100 ug/ml: 2%, 11%, 15%, 16% and 18%. The (Fig. 4B) climbing rate was determined by the percent of flies climbing above 4 cm per minute. The rate of climbing in flies treated with 0, 10, 25, 50 and 100 cisplatin was 8.65%, 8.45%. 5.05%, 3.42% and 1.94%. (Fig. 4C) There was no statistical difference in the maximum climbing or the rate of climbing between 0 and 10 ug/ml cisplatin. There was a significant difference in both the maximum climbing and the rate of climbing when the dose of cisplatin was increased to 25, 50 and 100 ug/ml (p < 0.05).
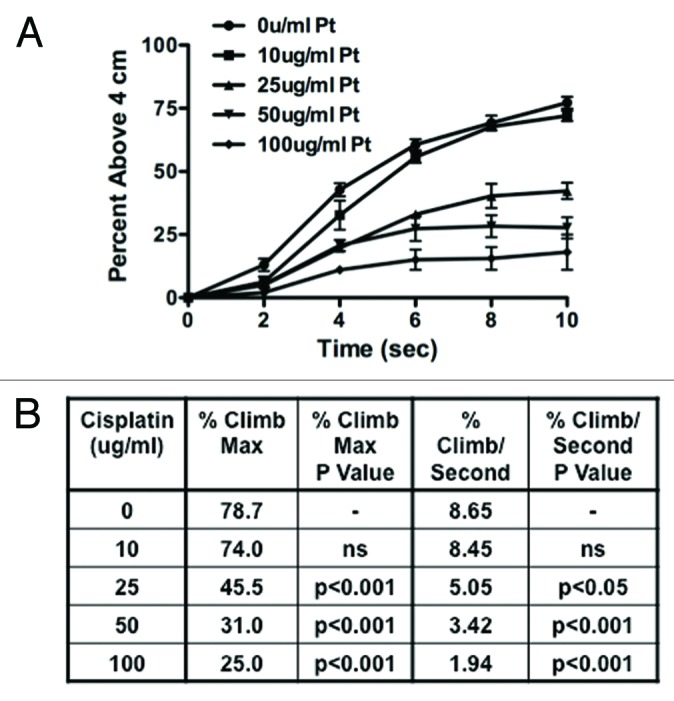
Figure 4. Climbing ability in the fly-counting apparatus can be measure as both a maximum height able to climb as well as the rate of climbing. (A) Cisplatin treated flies were assessed for the percent of flies able to climb above 4 cm at 2, 4, 6, 8 and 10 sec. (B) We were able to determine the maximum percent of flies able to climb above 4 cm as well as the rate of climbing expressed as the percent of flies able to climb above 4 cm per second. There was a significant decrease in maximum climbing and rate of climbing at 25, 50 and 100 ug/ml cisplatin (p < 0.005). No significant difference observed at 10 ug/ml cisplatin.
Discussion
We have developed an automated fly-counting apparatus that improves upon the traditional geotaxis, climbing assay. The apparatus can process a greater number of flies in a shorter amount of time and remove variability introduced by both the user and assay limitations. The apparatus is versatile in that it can measure both the number of flies climbing to a pre-defined height as well as the rate of climbing. The addition of genetic tools available in the Drosophila model system allows us to study signaling pathways involved in chemotherapy-induced neurotoxicity and develop high throughput screens.
Automation of the geotaxis climbing assay eliminates variability introduced by the user. Manual climbing assays rely primarily on human technique and observation. One advantage of our apparatus is the automated tapping down of the flies. There are differences between how hard individual investigators tap the vial of flies. If the tap is too light, the flies do not get all the way to the bottom of the vial. If the tap is too hard, the flies may be physically stressed. The automated system taps the flies by lifting them to a set height and releasing them. This yields a consistent tapping force. A second advantage of the apparatus is better visualization of the climbing flies. Flies move rapidly up and down a vial and the human eye can only keep track of a small number of flies. We found that once the number of flies in a vial exceeded 10 flies, the person counting became less certain of the counts they were getting when doing the assay. Our automated system snaps a picture and can particle count accurately up to 30 flies per vial (data not shown). Manual assays process one vial at a time while the apparatus can process up to 10 vials at a time. Automation of the geotaxis climbing assay allows us to process a larger number of flies with greater accuracy. A side-by-side comparison of our manual and automated assay systems showed no statistical difference between the two methods, and our studies are now exclusively performed using the automated system.
There are some limitations of our climbing assay. The geotaxis climbing assay cannot distinguish between neuronal or motor deficits. In cisplatin treated flies, the deficits in climbing behavior are directly linked to damage of elav positive neurons.12 Climbing deficits in ALS flies, however, is due to motor dysfunction.11 It is a simple assay that is quick and easy to use as a screen for climbing deficits. A change in climbing behavior would need to be studied further to understand what cell types are affected and by what mechanism.
Chemotherapy-induced peripheral neuropathy (CIPN) continues to be a problem for many cancer patients. Rodent model systems have given us a great deal of information as to the general underlying mechanisms involved in neurotoxicity; however, treatments generated from this information has not proven useful in preventing neuropathy in patients.17 Many cellular processes are highly conserved among species, and Drosophila have been shown to be a good model system to study neurological diseases.9 The genetic tools available in Drosophila provide a way to rapidly and inexpensively study the cellular mechanisms involved in CIPN. We have shown that cisplatin inhibits geotactic climbing by inducing apoptosis in brain neurons.12 Using the GAL4-UAS system, we can upregulate or downregulate specific genes in our flies and determine if it involved in cisplatin neurotoxicity using the climbing assay as our phenotype.
Our automated fly-counting apparatus can be expanded to observe changes in climbing behavior in many other model systems. The climbing assay can be used to look at behavior changes related to genetic mutations, environmental and drug toxicity as well as human disease. The apparatus is versatile and can be used for high throughput screens.
Materials and Methods
Oregon Red Drosophila were obtained from Amy Tang, pkpk-sple-13 is a null allele of Pk gene as described in Flybase. Cisplatin obtained from APP Pharmaceuticals LLC (100351). The automated fly-counting apparatus was constructed using a Logitech C910 8 megapixel camera obtained from CDW (2588857). Image analysis software was a modification of ImageJ software (National Institutes of Health).
The automated fly-counting apparatus was developed using Agile Project Management. The project team consisted of experts from Project Management, Biomechanical Development, Software Development, Electronic Development and technical personnel from Anthony Windebank’s Laboratory.
For experiments to compare the manual assay with the apparatus, 7–14 d old flies were placed into empty vial with 50 flies per vial. The flies were starved for 6 h and then given 200 ul of 10% sucrose in Dulbecco’s Phosphate Buffered Saline with and without drug. Flies were treated with 0, 10, 25, 50, 100 and 200 ug/ml cisplatin. Flies were fed every 24 h with 150 ul at 24 and 100 ul at 48 h. At 72 h, the flies were anesthetized by CO2, counted, observed for lethality and placed into vials for the geotactic climbing assay.
Flies were placed into clean, empty vials with 20 flies per vial and allowed to recover from CO2 for 1.5 h. Vials were placed into racks and the climbing assay was performed in the automated fly-counting apparatus. The automated fly-counting apparatus houses a motor that gently taps down all the flies to the bottom of the vials. The apparatus is then set to determine the number of flies above 2 cm from the bottom of the vial at 20 sec. The vials were then removed and a physical 2-cm mark was placed on the vials. The geotactic climbing assay was then performed manually. Each assay had 5 repeats per vial that was averaged for the percent of flies above 2 cm from the bottom of the vial.
prickle mutant flies 2- to 4-d old were placed into vials with 20 flies per vial. A 2-cm mark was marked of the side and the manual assay performed. The mark was then removed and the flies were placed in the apparatus and observed for climbing ability.
To determine the lowest threshold we can measure flies in the apparatus, prickle flies, 20 flies per vial, were measured for climbing ability at 2 cm followed by measurements at 1 cm and 0.5 cm.
To assess the rate of climbing, 20 flies were placed into each vial as previously described. Vials were placed into racks for the automated fly-counting apparatus. The flies were tapped down once and a picture acquired every 2 sec for a total of 10 sec. Each assay had 5 repeats, and the number of flies above 4 cm from the bottom was determined for each image. Climbing was plotted as the percent of flies above 4 cm over time and the rate expressed as percent of flies climbing above 4 cm per minute.
The statistical difference between the manual and automated assay was determined by two-way ANOVA and Bonferroni multiple comparison in cisplatin treated OR flies and t-test for prickle flies. The statistical difference between maximum height of climbing and the rate of climbing per minute was determined by one-way ANOVA and Bonferroni multiple comparison.
Acknowledgments
This manuscript was supported by NIH NS40471.
A special thanks goes to Jane Meyer for her assistance in preparation of this manuscript.
Glossary
Abbreviations:
- Pt
cisplatin
- CIPN
chemotherapy induced neurotoxicity
- ALS
amyotrophic lateral sclerosis
Disclosure of Potential Conflicts of Interest
Mayo Clinic and the authors have protected intellectual property related to the apparatus described. It has not been commercialized and no income has been generated.
References
- 1.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 2.Thompson SW, Davis LE, Kornfeld M, Hilgers RD, Standefer JC. Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer. 1984;54:1269–75. doi: 10.1002/1097-0142(19841001)54:7<1269::AID-CNCR2820540707>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer. 2008;8:1p–, 71, author reply 1p, 71. doi: 10.1038/nrc2167-c1. [DOI] [PubMed] [Google Scholar]
- 4.Fischer SJ, McDonald ES, Gross L, Windebank AJ. Alterations in cell cycle regulation underlie cisplatin induced apoptosis of dorsal root ganglion neurons in vivo. Neurobiol Dis. 2001;8:1027–35. doi: 10.1006/nbdi.2001.0426. [DOI] [PubMed] [Google Scholar]
- 5.McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9:220–33. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- 6.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–13. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41:661–8. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao H, Manak JR, Sowers L, Mei X, Kiyonari H, Abe T, et al. Mutations in prickle orthologs cause seizures in flies, mice, and humans. Am J Hum Genet. 2011;88:138–49. doi: 10.1016/j.ajhg.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botella JA, Bayersdorfer F, Gmeiner F, Schneuwly S. Modelling Parkinson’s disease in Drosophila. Neuromolecular Med. 2009;11:268–80. doi: 10.1007/s12017-009-8098-6. [DOI] [PubMed] [Google Scholar]
- 11.Watson MR, Lagow RD, Xu K, Zhang B, Bonini NM. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J Biol Chem. 2008;283:24972–81. doi: 10.1074/jbc.M804817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podratz JL, Staff NP, Froemel D, Wallner A, Wabnig F, Bieber AJ, et al. Drosophila melanogaster: a new model to study cisplatin-induced neurotoxicity. Neurobiol Dis. 2011;43:330–7. doi: 10.1016/j.nbd.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orso G, Martinuzzi A, Rossetto MG, Sartori E, Feany M, Daga A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J Clin Invest. 2005;115:3026–34. doi: 10.1172/JCI24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol. 2007;67:778–91. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- 15.Bland ND, Robinson P, Thomas JE, Shirras AD, Turner AJ, Isaac RE. Locomotor and geotactic behavior of Drosophila melanogaster over-expressing neprilysin 2. Peptides. 2009;30:571–4. doi: 10.1016/j.peptides.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Nichols CD, Becnel J, Pandey UB. Methods to assay Drosophila behavior. J Vis Exp. 2012 doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nangaku M, Izuhara Y, Takizawa S, Yamashita T, Fujii-Kuriyama Y, Ohneda O, et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler Thromb Vasc Biol. 2007;27:2548–54. doi: 10.1161/ATVBAHA.107.148551. [DOI] [PubMed] [Google Scholar]