Abstract
Facultative heritable bacterial endosymbionts can have dramatic effects on their hosts, ranging from mutualistic to parasitic. Within-host bacterial endosymbiont density plays a critical role in maintenance of a symbiotic relationship, as it can affect levels of vertical transmission and expression of phenotypic effects, both of which influence the infection prevalence in host populations. Species of genus Drosophila are infected with Spiroplasma, whose characterized phenotypic effects range from that of a male-killing reproductive parasite to beneficial defensive endosymbiont. For many strains of Spiroplasma infecting at least 17 species of Drosophila, however, the phenotypic effects are obscure. The infection prevalence of these Spiroplasma vary within and among Drosophila species, and little is known about the within-host density dynamics of these diverse strains. To characterize the patterns of Spiroplasma density variation among Drosophila we used quantitative PCR to assess bacterial titer at various life stages of three species of Drosophila naturally-infected with two different types of Spiroplasma. For naturally infected Drosophila species we found that non-male-killing infections had consistently lower densities than the male-killing infection. The patterns of Spiroplasma titer change during aging varied among Drosophila species infected with different Spiroplasma strains. Bacterial density varied within and among populations of Drosophila, with individuals from the population with the highest prevalence of infection having the highest density. This density variation underscores the complex interaction of Spiroplasma strain and host genetic background in determining endosymbiont density.
Keywords: Spiroplasma, Drosophila, vertically-transmitted endosymbiont, endosymbiont density
Introduction
Well over half of all insect species harbor maternally transmitted bacterial endosymbionts that can have dramatic effects on their host.1,2 Dependent on their host for their own survival, some of these endosymbionts act as mutualists increasing their own fitness by increasing that of the host. Alternatively, as parasites, these bacteria can manipulate their host's reproduction to enhance their own transmission by increasing the proportion of infected females.3,4 These facultative endosymbionts are not required for host survival, and as such their prevalence, which plays a large role in determining their population level impacts, can vary greatly among host species and populations.1,2,5 Endosymbiont prevalence is greatly affected by its within-host density dynamics6 as infection density affects both the fidelity of vertical transmission as well as the strength of expression of fitness effects, 2 key parameters maintaining bacteria in host populations. For many endosymbionts, bacterial strains with higher titers have greater vertical transmission fidelity and stronger phenotypic effects, including both reproductive manipulation phenotypes and fitness benefits.7-9
Spiroplasma is 1 of only 2 bacterial endosymbionts found thus far in species of the genus Drosophila.10 It can act as both a reproductive manipulator, causing male-killing in certain species of Drosophila,11 and as a defensive endosymbiont, affording D. neotestacea protection against a nematode parasite12 and conferring resistance to wasp parasitism in D. hydei.13 Although the role of bacterial density in the defensive strains is unclear, it has been hypothesized that a certain bacterial density is necessary for expression of its male-killing phenotype, as studies have shown that male-killing Spiroplasma have had a higher density than non-male-killing Spiroplasma.14,15Spiroplasma density also affects the developmental stage at which male-killing occurs, with a higher infection density causing male-killing at an earlier stage.16
The dynamics of endosymbiont titer are dependent on bacterial strain,7,17,18 host genotype,19,20 host age,17,21 and temperature,22-24 although the full role of these factors in Spiroplasma infections is unclear. Spiroplasma density in certain Drosophila species increases with age14,15,25 and decreases at lower temperatures.26,27 Lower density, either at young ages or lower temperatures, is correlated with either loss of the male-killing phenotype or loss of the Spiroplasma altogether.14,26 Spiroplasma density dynamics, however, have been explored in only a few strains in a limited number of Drosophila species, namely in relation to the male-killing phenotype. These strains are identical or closely related to the first characterized Spiroplasma endosymbiont infecting D. nebulosa, S. poulsonii.15,28 Furthermore, many of the strains studied were artificial infections; Spiroplasma strains from different Drosophila species transferred to D. melanogaster. While useful for exploring the mechanisms of male-killing, these artificial infections give only limited insight into density variation in natural populations. Furthermore, the phenotypic effects of Spiroplasma in Drosophila, including male-killing as well as defense against parasites, have been only investigated in the poulsonii-type Spiroplasma strains,13,29,30 a limited subset of the diversity of those Spiroplasma now known to infect Drosophila.5,31
At least 17 species of Drosophila are infected with four genetically distinct types of Spiroplasma (Fig. 1), most of which do not cause male-killing and, for many still, their fitness effects are unknown. Screening of natural populations of Drosophila revealed that Spiroplasma infection prevalence varies not only among, but within species, with infection prevalence in D. mojavensis ranging from 15 to 85% in geographically distinct populations.5 Drosophila mojavensis is infected with one of the newly discovered strains of Spiroplasma that is more closely related to S. citri, a well-known plant pathogen,32 than to the poulsonii-type Spiroplasma. These citri-type Spiroplasma infect seven species in the Drosophila repleta group, which have some of the highest infection prevalences of Spiroplasma screened to date.5
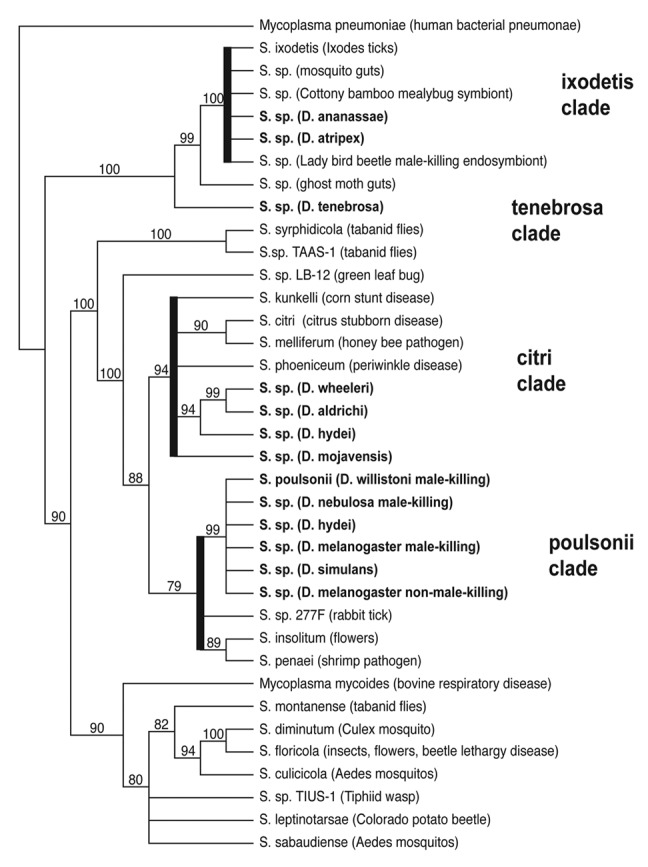
Figure 1. The diversity of Spiroplasma infecting Drosophila. A Neighbor-Joining cladogram based on partial 16s rDNA sequences illustrating the different strains of Spiroplasma that infect Drosophila (shown in bold type). One thousand bootstrap replicates were run to assess support on clades.
Characterizing the density dynamics of various Spiroplasma strains among different Drosophila species can lend insight into factors driving its distribution and prevalence. In this study we have described the variation in Spiroplasma density among different Spiroplasma strains infecting three species of Drosophila. We asked the following questions: (1) Do the non-male-killing Spiroplasma have lower densities than male-killing Spiroplasma? We addressed this by measuring the bacterial titers of a natural male-killing infection and a natural non-male-killing infection in D. melanogaster and evaluating the consistency of these results with bacterial threshold density hypothesis. (2) How do densities of two different types of Spiroplasma differ in naturally occurring infections? Here we examined 2 common Spiroplasma types, poulsonii and citri, and assessed whether they exhibit different densities across different Drosophila life stages. Variation in bacterial titers among bacterial strains and Drosophila species will begin to provide insight into maintenance and effects of these newly discovered and prevalent infections. (3) Do different populations of Drosophila that are characterized by contrasting incidence of infection in nature also exhibit different Spiroplasma densities? We addressed this question using individuals from two D. mojavensis populations with different infection incidences to determine a correlation between bacterial titer and population prevalence.
Results
Male-killing vs. non-male-killing strains
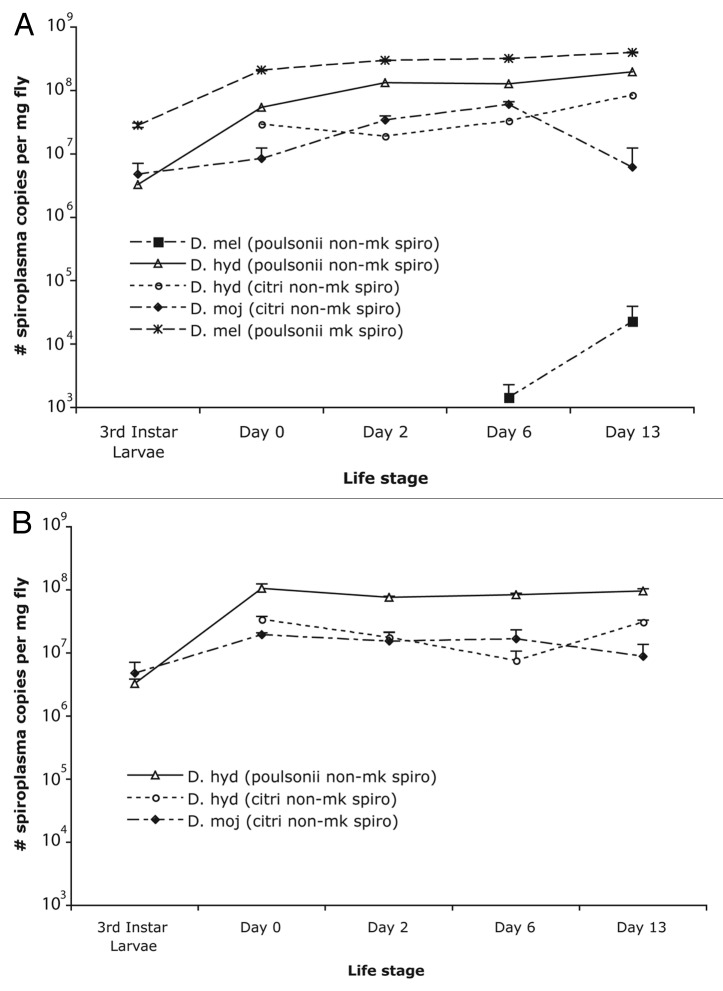
The male-killing Spiroplasma infecting D. melanogaster (UGA) had the highest density at all life stages (p <0.05), followed by the non-male killing poulsonii-type Spiroplasma infecting D. hydei (p <0.05). Lower densities were found in the non-male-killing Spiroplasma of the citri-type infecting D. mojavensis and D. hydei (Fig. 2). This observation was consistent when calculating Spiroplasma density either as number of copies per milligram dry weight or number of Spiroplasma copies per ef1-α gene (data not shown). The citri-type Spiroplasma in both D. hydei and D. mojavensis had lower densities at all life stages.
Figure 2. Density of Spiroplasma in different Drosophila (per mg fly) across life stages. Values for females (A) and males (B) are an average density of six biological replicates, and the error bars represent standard error. Drosophila species measured include D. mojavensis infected with citri-type non-male-killing Spiroplasma, D. hydei infected with citri-type non-male-killing Spiroplasma, D. hydei infected with non-male-killing poulsonii-type Spiroplasma, and D. melanogaster infected with poulsonii-type male-killing Spiroplasma. The male-killing Spiroplasma infecting D. melanogaster have the highest densities across all life stages, whereas the citri-type Spiroplasma are lower.
The non-male-killing Spiroplasma infecting D. melanogaster had the lowest titers (Fig. 2), undetectable using these quantitative PCR methods at eclosion and at 2 weeks in males. Low titers, around 1000 copies, of Spiroplasma were detected only in 1 female at eclosion, and one male at day 6. Infection levels could consistently be detected in one-week-old females (5/6 biological replicates amplified), though titers were quite low. While Spiroplasma titer, on average, was higher in 2-week-old females, density measurements were highly variable and infections were not detected in 3 females. Even at the highest titer levels (2.0 x104 Spiroplasma copies per milligram fly), these non-male-killing D. melanogaster infections were still several orders of magnitude below that of the male-killing Spiroplasma infecting D. melanogaster (4.0 x 108 Spiroplasma copies per milligram fly). Thus, for all Spiroplasma strains measured, the non-male-killing Spiroplasma strains had lower densities, at all life stages, than the male-killing strain.
Bacterial titer change with age
In general, the bacterial titers increased as the flies aged, although not at the same rate among Drosophila species or Spiroplasma types (Fig. 2). The female D. melanogaster infected with the male-killing Spiroplasma showed a pattern of increasing Spiroplasma density from the 3rd instar larval stage (2.8 x 107 Spiroplasma copies per mg fly) to 2-week-old females (4.0 x 108 Spiroplasma copies per mg fly). A similar trend was seen for the female D. hydei infected with the non-male-killing poulsonii Spiroplasma strain. Titers in male D. hydei infected with this poulsonii-type Spiroplasma strain increased from the 3rd instar larval stage to eclosion, but remained the same at the 1-week and 2-week-old stages. In the D. hydei females infected with the citri-type Spiroplasma, the increase in bacterial titer did not occur until the two-week-old stage. Similar to the pattern observed for D. hydei poulsonii-type Spiroplasma infected males, the D. hydei citri-type Spiroplasma infected males did not increase in bacterial titer with fly age. A different pattern, however, was seen with D. mojavensis, infected with the citri-type Spiroplasma. This Spiroplasma had lower titers and several individuals had no detectable Spiroplasma by day 13. In 2-week-old females, 4 out of 6 individuals had no detectable Spiroplasma, while in 2-week-old males, 3 out of 6 had no detectable Spiroplasma. Individuals with measurable levels of Spiroplasma had titers much lower than those at day 6 (D6 females 6.0 x 107 vs. D13 females 6.2 x 106; D6 males 1.6 x 107 vs. D13 males 8.8 x 106). Patterns of Spiroplasma titer change during aging varied among Drosophila species infected with different Spiroplasma strains.
Spiroplasma citri-type density variation
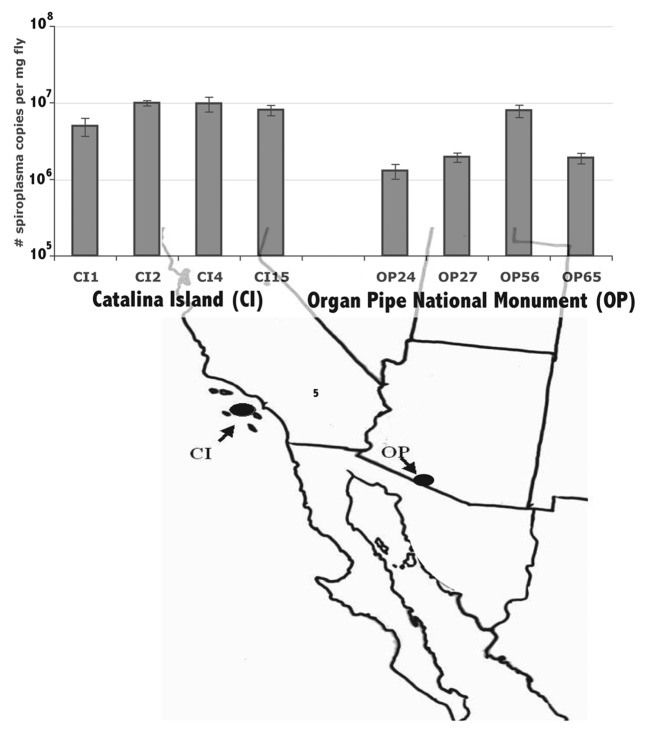
The citri-type Spiroplasma infecting D. hydei had a consistently lower density than did the poulsonii-type Spiroplasma across all life stages (p <0.05) (Fig. 2). Within the citri-type Spiroplasma infecting D. mojavensis, the Spiroplasma density also varied among isofemale lines (Fig. 3). Three of the 4 isofemale lines of D. mojavensis from the Sonoran Desert (OP24, OP27, and OP65) had statistically significant lower densities than those from Catalina Island, though there was variation in the flies from Catalina Island as well. Despite this variation, the densities of the Spiroplasma from Catalina Island were, on average, higher than that of the Sonoran Desert Spiroplasma (p <0.05). Both line and location had a statistically significant effect on density (Table 3A), with 48% of variance explained by location and 28% explained by line (Table 3B). Thus, even among closely related strains within a particular type of Spiroplasma there was large density variation.
Figure 3.D. mojavensis Spiroplasma density variation among 8 fly lines (measured as number of Spiroplasma copies per milligram fly) from 2 different populations. Values are an average density of 6 biological replicates, and the error bars represent standard error.
Table 3. Variation among lines and between populations of D. mojavensis.
| A. Analysis of Variance | ||||
|---|---|---|---|---|
| Source | DF Num | Sum of Sqrs | F Ratio | Prob > F |
| Model | 7 | 274015555 | 12.7047 | < 0.0001 |
| location | 1 | 1.34E+08 | 7.49 | 0.0334 |
| line[location] | 6 | 1.11E+08 | 6.0145 | 0.0002 |
| B. Variance Component Estimates | ||
|---|---|---|
| Component | Var Comp Est | % of Total |
| location | 5436760 | 48.151 |
| line[location] | 2773153 | 24.561 |
| Residual | 3081143 | 27.288 |
| Total | 11291057 | 100 |
Discussion
This study represents the first characterization of Spiroplasma density among several naturally infected Drosophila species harboring diverse Spiroplasma strains. Much of what is known about Spiroplasma density in Drosophila is from studies on artificially infected fly strains. While a few naturally infected flies have been measured, the variation in Spiroplasma density among Drosophila has remained largely obscure.
The density dynamics of Spiroplasma in Drosophila clearly vary among Drosophila species and Spiroplasma strains. Non-male-killing Spiroplasma strains have lower densities than the male-killing Spiroplasma strain in all Drosophila species examined in this study. Previous work had only compared an artificial male-killing infection (NSRO: the D. nebulosa Spiroplasma transferred to D. melanogaster), an artificial non-male-killing infection (NSRO-A: a lab variant of NSRO that lost its male-killing ability), and a single D. hydei non-male-killing Spiroplasma isolated in Japan.14,15 Our finding of similar patterns in naturally infected strains of different Spiroplasma types in additional Drosophila species is consistent with the bacterial threshold density hypothesis for the expression of the male-killing phenotype. Although, only a single male-killing strain was examined in this study, and the extent of Spiroplasma density variation among male-killing strains in different species of Drosophila remains to be determined.
Furthermore, the D. melanogaster with the non-male-killing Spiroplasma strain was an extremely low titer infection, with less than 1000 copies detectable in males and females under 2 weeks of age. This strain is genetically identical to the male-killing strain at the three loci for which it was sequenced;31 however, undetected genetic variation may exist at other loci. The extremely low titer of this infection could be an inherent property of this bacterial strain, which may have lost either the ability to replicate quickly, or the ability to avoid or suppress the host immune system. Effects of host genetic background, however, cannot be ruled out. This strain could possibly cause male-killing, but may not be able to produce enough effector molecule to have any effect, due to its low titer, in accordance with the bacterial threshold density hypothesis.
The consistently lower density of Spiroplasma of the citri-type, along with the lower density of the citri-type Spiroplasma compared with the poulsonii-type Spiroplasma in D. hydei suggest that this may be a general property of the Spiroplasma of this type, and that bacterial strain itself plays a role in density regulation. Although, host factors such as immune response also may be involved. Variation in Spiroplasma densities among D. mojavensis isofemale lines implies the involvement of host genotype in density regulation. There are high levels of genetic variation in both the Sonoran Desert and Catalina Island populations of D. mojavensis.33 Sequencing of these Spiroplasma strains at six different loci revealed no genetic variation in the Spiroplasma that infect D. mojavensis.31 Though undetected variation in Spiroplasma strains cannot be ruled out, genetic variation in host immune response or another host factor that affects Spiroplasma density is more likely to be responsible for the variation in bacterial titer across isofemale lines.
The variation in Spiroplasma dynamics as the flies age may reflect differences in immune response and/or differences in the age of reproductive maturity. When Spiroplasma titer was measured as number of copies per milligram fly, females consistently had higher Spiroplasma titers than males from day 2 onwards. This pattern could reflect Spiroplasma proliferation in the ovaries, as is necessary for vertical transmission, and the timing may correlate to when flies are most likely to reproduce in the wild. Both D. hydei and D. mojavensis females mature later (3–5 d of age)34 than D. melanogaster (1–2 d), therefore the lower bacterial titer, particularly before sexual maturity, may not be as detrimental to the ability to be vertically transmitted. In D. mojavensis, though, the decrease in Spiroplasma titer in 2-week-old females as compared with 1-week-old females is striking. Whether this is a general trend of decrease with aging in this species, as bacterial titer decreases with age in males as well, is unclear. In any case, this trend indicates that infection prevalence in natural populations is likely underestimated, depending on the age of the flies that are sampled.
Spiroplasma infection prevalence varies not only among species, but also among populations within species. In D. mojavensis populations, Spiroplasma infection prevalence varies dramatically.5 Populations at Organ Pipe National Monument in the Sonoran Desert have an infection prevalence of ~15%, whereas on Catalina Island the infection prevalence is greater than 85%. Given that within-host symbiont densities are often correlated with factors that affect populations prevalence, namely the fidelity of vertical transmission and the strength of phenotypic effects,6 we may expect a correlation between infection prevalence and bacterial density. This expectation certainly is observed in the Spiroplasma density differences between the Sonoran Desert D. mojavensis and the Catalina Island D. mojavensis.
The higher densities of the D. mojavensis Spiroplasma from Catalina Island compared with those from the Sonoran Desert may reflect either a higher fidelity of vertical transmission, or the expression of a fitness benefit that explains the higher prevalence of infection on Catalina Island. It remains to be seen if the citri-type Spiroplasma can act as a defensive endosymbiont, and whether or not the lower density of this Spiroplasma would affect the expression of such a phenotype. Of course, environmental factors, such as temperature, also likely play a role in the infection prevalence. The Sonoran Desert population of D. mojavensis experiences more temperature extremes, which could lower infection prevalence if the citri-type Spiroplasma are effected by temperature in a similar manner as the poulsonii-type Spiroplasma.26,27 Measuring Spiroplasma density variation in flies collected directly from the field will lend insight into how environmental variation influences the bacterial density dynamics of this endosymbiont. Since numerous cactophilic Drosophila are infected with Spiroplasma from the citri-type, further exploration of the factors affecting Spiroplasma density dynamics will lend critical insight into this symbiosis.
Methods
Fly lines and symbionts
Thirteen naturally infected fly lines were measured in this experiment, 5 of which were measured across different life stages. Flies were examined under controlled laboratory conditions to minimize any potential effects of temperature and other environmental factors on bacterial density. The Drosophila species and line designation, Spiroplasma strain, and origin of isofemale line are shown in Table 1.
Table 1.Spiroplasma infected Drosophila lines.
| Fly ID | Drosophila species | Spiroplasma type | Phenotype (mk = male-killing) |
Collection details |
| D. melUGA | D. melanogaster | poulsonii | mk | Africa 2005 (Pool et al. 2005) |
| D. melSC | D. melanogaster | poulsonii | non-mk | San Carlos, Mexico 2008 |
| D. hyd (TEN104–102) | D. hydei | poulsonii | non-mk | Mexico 2002 |
| D. moj (OP10) | D. mojavensis | citri | non-mk | Organ Pipe National Monument 2007 |
| D. hyd (ABH5) | D. hydei | citri | non-mk | Anza Borrego Desert 2009 |
| D. moj (CI1) | D. mojavensis | citri | non-mk | Catalina Island 2008 |
| D. moj (CI2) | D. mojavensis | citri | non-mk | Catalina Island 2008 |
| D. moj (CI4) | D. mojavensis | citri | non-mk | Catalina Island 2008 |
| D. moj (CI15) | D. mojavensis | citri | non-mk | Catalina Island 2008 |
| D. moj (OP8) | D. mojavensis | citri | non-mk | Organ Pipe National Monument 2007 |
| D. moj (OP27) | D. mojavensis | citri | non-mk | Organ Pipe National Monument 2007 |
| D. moj (OP56) | D. mojavensis | citri | non-mk | Organ Pipe National Monument 2007 |
| D. moj (OP65) | D. mojavensis | citri | non-mk | Organ Pipe National Monument 2007 |
Fly rearing conditions
Spiroplasma-infected D. melanogaster (UGA), D. melanogaster (SC), D. hydei (TEN104-102), and D. mojavensis (OP-10) were reared on standard banana food at room temperature in summer 2008. D. hydei (ABH5) and the D. mojavensis (CI and OP) isolines were reared in spring 2010. All fly lines were in the laboratory for at least 10 generations prior to collection. Approximately one hundred females were placed in an egg-laying chamber, and allowed to oviposit for three hours to control for the age of offspring. Oviposition plates were replaced multiple times until we obtained a plate with an estimated ~1000 eggs per plate for each species, which resulted in relatively consistent, uncrowded larval densities for each species. Flies were collected at various time points over the course of development, including 3rd instar larvae, day of eclosion (Day 0), 2nd day after eclosion (Day 2), at 1 week (Day 6) and 2 weeks (Day 13). D. mojavensis isolines were collected only at 1 week of age. After eclosion, male and female flies were separated and held as virgins for the later time points. At each time point, for each species, 6 flies were frozen at -80°C for quantitative PCR analysis, and another 6 flies were frozen to obtain dry weight measurements. Flies were dried at 55 °C for 72 h and weighed individually to obtain average dry weights.
DNA extraction
Each biological replicate for each species at each time point was extracted individually using a Qiagen DNA extraction kit. DNA was eluted in 100–200 µl of buffer AE, and quantified using a Nanodrop. DNA samples were diluted to 25 ng/µl for quantitative PCR analysis.
Quantitative PCR standard curve construction
We measured bacterial density using quantitative real-time PCR with the bacterial dnaA gene. To estimate Spiroplasma titer, absolute dnaA copy number was determined using a standard curve. To generate this standard curve, a 500 base pair region containing the quantitative PCR DNA amplicon was amplified using primers SRdnaAF1 and SRdnaAR114 (Table 2) from Spiroplasma from each Drosophila species, sequenced, and cloned using Invitrogen's Topo-TA pCR 2.1 topo vector cloning kit. Quantitative PCR primers were verified and redesigned as necessary for the different Spiroplasma strains (Table 2). Plasmids containing the larger cloned fragment were used to construct two separate standard curves, one for poulsonii-type Spiroplasma and one for citri-type Spiroplasma, using dilutions of 109, 108, 107, 106, 105, 104, 103, and 102 dnaA copies per four microliters. An internal DNA standard, designed in the Drosophila single copy nuclear gene elongation factor 1 α (ef1-α), was constructed for each Drosophila species in a similar manner. Specific ef1-α primers for each Drosophila species are listed in Table 2.
Table 2. Quantitative PCR primers.
| Primer name | Locus | Drosophila species | Direction | Sequence | |
| EF23F | ef1 α | D. melanogaster | Forward | TTAACATTGTGGTCATTGGCCA | |
| EF123R | Reverse | CTTCTCAATCGTACGCTTGTCG | |||
| EF1hydF | ef1 α | D. hydei | Forward | TTAACATCGTTGTTATTGGCCA | |
| EF1hydR | Reverse | CTTCTCAATCGTACGCTTATCG | |||
| EF1mojF | ef1 α | D. mojavensis | Forward | TTAACATCGTTGTGATTGGCCA | |
| EF1mojR | Reverse | CTTCTCAATTGTACGCTTATCG | |||
| Spiroplasma type | |||||
| 109F | dna-A | poulsonii | Forward | CCGATTTTGAAACTGCTCTTAA | |
| 246R | Reverse | TGAAAAAAACAAACAAATTGTTATTACTTC | |||
| 270MJF | dna-A | citri | Forward | TGAAAAAAATAAACAAATTGTAATTACTTC | |
| 407MJR | Reverse | TTA AGTGCTGTTTCA AAATCTGG | |||
| SRdnaAF1 | dna-A | all | Forward | GGAGAYTCTGGAYTAGGAAA | |
| SRdnaAR1 | Reverse | CCYTCTAWYTTTCTRACATCA | |||
Quantitative PCR
Quantitative PCR was performed using Aplied Biosystems (ABI) Power Sybrgreen PCR mix on an ABI 7000 machine. Twenty-five microliter PCR reactions, using 4 µl of DNA, were run on a program of 95 °C for 10 min, then 95 °C for 15 sec, 55 °C for 45 sec, 60 °C for 45 sec for 45 cycles. Primer concentrations were 300 nm each per reaction. Technical replicates were performed for each biological replicate, and if there was a discrepancy of greater than 0.5 amplification cycles, then the sample was run again. Otherwise, the 2 amplification cycle values were averaged and used for copy number calculations. Dissociation curves were constructed for verification of the target amplicon.
Statistical analyses
Spiroplasma titer was calculated at all Drosophila life stages as dnaA Spiroplasma copy equivalents per milligram fly weight. For a subset of life stages (D0, D6, and D13), the internal standard ef1-α was also amplified, and Spiroplasma titer was calculated as number of dnaA Spiroplasma copy equivalents per number of ef1-α copies. DnaA copy numbers were square root transformed to fit a normal distribution. All statistical analyses were performed using JMP v.6 (SAS). T-tests and a one-way analysis of variance were used to compare densities among species at different life stages. For comparisons of Spiroplasma density among D. mojavensis lines from Catalina Island (CI) and Organ Pipe National Monument (OP) a fully nested analysis of variance was performed with line nested within location, with location set as a random factor.
Acknowledgments
Funding for this work was provided by National Science Foundation grant DEB-0315815 to Nancy A. Moran and TAM. TSH was supported by a University of California, San Diego NIH Cell and Molecular Genetics training grant. We thank Nancy Moran for useful discussions on experimental design and comments on the manuscript, Luciano Matzkin for technical and statistical advice and Sarah Johnson for invaluable laboratory assistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstädter J, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?--A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–20. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–90. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill S, Hoffmann A, Werren JH. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press: New York 1997. [Google Scholar]
- 5.Watts T, Haselkorn TS, Moran NA, Markow TA. Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS One. 2009;4:e5703. doi: 10.1371/journal.pone.0005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaenike J. Coupled population dynamics of endosymbionts within and between hosts. Oikos. 2009;118:353–62. doi: 10.1111/j.1600-0706.2008.17110.x. [DOI] [Google Scholar]
- 7.Dutton TJ, Sinkins SP. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol. 2004;13:317–22. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 8.Dyer KA, Minhas MS, Jaenike J. Expression and modulation of embryonic male-killing in Drosophila innubila: opportunities for multilevel selection. Evolution. 2005;59:838–48. [PubMed] [Google Scholar]
- 9.Kittayapong P, Baisley KJ, Sharpe RG, Baimai V, O’Neill SL. Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. Am J Trop Med Hyg. 2002;66:103–7. doi: 10.4269/ajtmh.2002.66.103. [DOI] [PubMed] [Google Scholar]
- 10.Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA. Heritable endosymbionts of Drosophila. Genetics. 2006;174:363–76. doi: 10.1534/genetics.106.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson DL, Poulson DF. Sex Ratio Organisms (Spiroplasmas) of Drosophila in The Mycoplasmas III: Plant and Insect Mycoplasmas (eds Whitcomb, RF and Tully JG) pp 175-208 Academic Press, New York 1979. [Google Scholar]
- 12.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329:212–5. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Vilchez I, Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS One. 2010;5:e12149. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anbutsu H, Fukatsu T. Population dynamics of male-killing and non-male-killing spiroplasmas in Drosophila melanogaster. Appl Environ Microbiol. 2003;69:1428–34. doi: 10.1128/AEM.69.3.1428-1434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kageyama D, Anbutsu H, Watada M, Hosokawa T, Shimada M, Fukatsu T. Prevalence of a non-male-killing spiroplasma in natural populations of Drosophila hydei. Appl Environ Microbiol. 2006;72:6667–73. doi: 10.1128/AEM.00803-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kageyama D, Anbutsu H, Shimada M, Fukatsu T. Spiroplasma infection causes either early or late male killing in Drosophila, depending on maternal host age. Naturwissenschaften. 2007;94:333–7. doi: 10.1007/s00114-006-0195-x. [DOI] [PubMed] [Google Scholar]
- 17.Duron O, Fort P, Weill M. Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity (Edinb) 2007;98:368–74. doi: 10.1038/sj.hdy.6800948. [DOI] [PubMed] [Google Scholar]
- 18.Mouton L, Henri H, Bouletreau M, Vavre F. Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol Ecol. 2003;12:3459–65. doi: 10.1046/j.1365-294X.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- 19.Kondo N, Shimada M, Fukatsu T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol Lett. 2005;1:488–91. doi: 10.1098/rsbl.2005.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGraw EA, Merritt DJ, Droller JN, O’Neill SL. Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A. 2002;99:2918–23. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tortosa P, Charlat S, Labbé P, Dehecq JS, Barré H, Weill M. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS One. 2010;5:e9700. doi: 10.1371/journal.pone.0009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst GDD, Johnson AP, Schulenburg JH, Fuyama Y. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics. 2000;156:699–709. doi: 10.1093/genetics/156.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouton L, Henri H, Bouletreau M, Vavre F. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology. 2006;132:49–56. doi: 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- 24.Mouton L, Henri H, Charif D, Boulétreau M, Vavre F. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett. 2007;3:210–3. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herren JK, Lemaitre B. Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol. 2011;13:1385–96. doi: 10.1111/j.1462-5822.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 26.Anbutsu H, Goto S, Fukatsu T. High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts. Appl Environ Microbiol. 2008;74:6053–9. doi: 10.1128/AEM.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osaka R, Nomura M, Watada M, Kageyama D. Negative effects of low temperatures on the vertical transmission and infection density of a spiroplasma endosymbiont in Drosophila hydei. Curr Microbiol. 2008;57:335–9. doi: 10.1007/s00284-008-9199-4. [DOI] [PubMed] [Google Scholar]
- 28.Williamson DL, Sakaguchi B, Hackett KJ, Whitcomb RF, Tully JG, Carle P, et al. Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int J Syst Bacteriol. 1999;49:611–8. doi: 10.1099/00207713-49-2-611. [DOI] [PubMed] [Google Scholar]
- 29.Bentley JK, Hinds G, Hurst GD. The male-killing Spiroplasmas of Drosophila nebulosa and Drosophila willistoni have identical ITS sequences. Drosoph Inf Serv. 2002;85:63–5. [Google Scholar]
- 30.Jaenike J, Stahlhut JK, Boelio LM, Unckless RL. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol. 2010;19:414–25. doi: 10.1111/j.1365-294X.2009.04448.x. [DOI] [PubMed] [Google Scholar]
- 31.Haselkorn TS, Markow TA, Moran NA. Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol Ecol. 2009;18:1294–305. doi: 10.1111/j.1365-294X.2009.04085.x. [DOI] [PubMed] [Google Scholar]
- 32.Bové JM. Spiroplasmas: infectious agents of plants, arthropods and vertebrates. Wien Klin Wochenschr. 1997;109:604–12. [PubMed] [Google Scholar]
- 33.Machado CA, Matzkin LM, Reed LK, Markow TA. Multilocus nuclear sequences reveal intra- and interspecific relationships among chromosomally polymorphic species of cactophilic Drosophila. Mol Ecol. 2007;16:3009–24. doi: 10.1111/j.1365-294X.2007.03325.x. [DOI] [PubMed] [Google Scholar]
- 34.Pitnick S, Markow TA, Spicer GS. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc Natl Acad Sci U S A. 1995;92:10614–8. doi: 10.1073/pnas.92.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]