Abstract
Endothelial adherens junctions are critical for physiological and pathological processes such as differentiation, maintenance of entire monolayer integrity, and the remodeling. The endothelial-specific VE-cadherin/catenin complex provides the backbone of adherens junctions and acts in close interaction with actin filaments and actin/myosin-mediated contractility to fulfill the junction demands. The functional connection between the cadherin/catenin complex and actin filaments might be either directly through α-catenins, or indirectly e.g., via linker proteins such as vinculin, p120ctn, α-actinin, or EPLIN. However, both junction integrity and dynamic remodeling have to be contemporarily coordinated. The actin-related protein complex ARP2/3 and its activating molecules, such as N-WASP and WAVE, have been shown to regulate the lammellipodia-mediated formation of cell junctions in both epithelium and endothelium. Recent reports now demonstrate a novel aspect of the ARP2/3 complex and the nucleating-promoting factors in the maintenance of endothelial barrier function and junction remodeling of established endothelial cell junctions. Those mechanisms open novel possibilities; not only in fulfilling physiological demands but obtained information may be of critical importance in pathologies such as wound healing, angiogenesis, inflammation, and cell diapedesis.
Keywords: endothelium, VE-cadherin, ARP2/3 complex, actin, stress fibers, cortical actin
Introduction
Endothelial junctions control the integrity and remodeling of the entire endothelial cell layer. Multiple physiological and pathophysiological processes such as barrier function, inflammation, wound healing, and angiogenesis require both a mechanically stable connection of the cells to each other as well as dynamic plasticity. These features are required for all sheet-forming cell layers of epithelium and endothelium, but there are significant differences in junction organization and dynamics between the two cell types. One of the most impressive differences belongs to the apico-basal constitution of distinguished tight, adherens, and gap junctions in epithelial cells, while these structures are interwoven in endothelium.1,2 Differences in cell junction organization relate to the mesenchymal origin of the endothelium, the flat morphology, and the expression of epithelial and endothelial-specific adhesion receptors, which implicate variations in regulation and dynamics accordingly.
Adherens junction proteins, particularly the cadherin/catenin complex and actin filaments, mediate adhesion of adjacent cells, and this, apart from others, constitutes a precondition for overall junction differentiation and regulation.1-3 Adherens junctions of the endothelium are ubiquitously expressed along the vascular tree and are the predominant structures of the micro-vascular bed where solutes and water are exchanged between the blood and the tissue.4,5 Furthermore, the endothelium of the micro-vascular bed, particularly in post-capillary venules where inflammatory reactions preferentially take place,1,6-8 do not express the tight junctions strands that are usually required for tight barrier function.4,5 These facts make adherens junctions a critical structure for regulating all cell junctions in endothelium. Due to its critical assignments, adherens junction exhibit high plasticity and dynamics under physiological and pathological conditions.1,9-13 The vascular endothelial (VE) cadherin/catenin complex14,15 is the backbone of adherens type junctions in endothelium and is, similar to the E-cadherin/catenin complex in epithelium, associated with actin filaments. However, there are significant differences in molecular organization of the cell junctions. Apart from the differences in morphological appearance, the chain protein γ-catenin/plakoglobin is expressed in both cell types, but it is exclusively localized at desmosomes in epithelium and is associated with keratin intermediate filaments. Desmosomes, on the other hand, are largely absent (there are few exceptions described) in endothelium, and thus, γ-catenin/plakoglobin interacts with vimentin intermediate filaments via association to VE-cadherin.16 This difference might also have an impact in organization of actin filaments. Furthermore, endothelial junction expresses endothelial-specific proteins and proteins not found in epithelium, such as PECAM-117,18 and MUC18 (synonym S-endo; CD 146).19
It is frequently proposed that the dynamics of both the VE-cadherin/catenin complex and actin filaments are interdependently regulated to ensure junction adhesion and barrier function, but at the same time to allow quick remodeling and plasticity. There are a number of controversial issues that have been discussed as to whether the cadherin/catenin complex in general or the VE-cadherin/catenin complex in particular is directly or indirectly linked to actin filaments. However, interdependent dynamics between the VE-cadherin/catenin complex and the actin filaments are of critical importance for regulation. A novel aspect in this discussion came up as recent reports place the actin-related protein-2/3 complex (ARP2/3) and its activating molecules, the nucleation-promoting factors (NPF), in a central role for junction regulation. In this context, α-catenin and p120ctn might mediate the functional connection between the ARP2/3 complex and the VE-cadherin/catenin complex. This includes initial junction formation, but also junction maintenance, dynamics, and plasticity of established cell junctions.11,20-22
Short Overview of the VE-Cadherin/Catenin Complex
The vascular endothelial cadherin (VE-cadherin) is an endothelial-specific type II cadherin that mediates mechanically stable cell adhesion between adjacent endothelial cells.14,15 VE-cadherin consists of an extracellular N-terminal extracellular domain, a trans-membrane domain, and a short C-terminal cytoplasmic domain. The extracellular domain consists of five cadherin repeats (EC1–EC5) of which the terminal EC domains binds like other cadherins in a homophilic and calcium-dependent manner, and thus forming cell–cell adhesion complexes.23 The extracellular domain is highly glycosylated carrying large amounts of terminal sialic acids that might also contribute to adhesion features of the extracellular domain.24 The extracellular (EC1-EC5) domain also binds regulatory molecules in cis as shown for the vascular endothelial (VE) phosphotyrosine phosphatase (VE-PTP)25 that, in turn, is associated with the vascular endothelial growth factor receptor 2, VEGFR2.26 The close association of VE-cadherin via its extracellular domain with other membrane receptors might cause functional clusters that are critical for local signal transduction. For example, downregulation of VE-PTP increased endothelial permeability and compromised VE-cadherin adhesion. Furthermore, binding of certain leucocytes to endothelium dissociated the VE-PTP from VE-cadherin.27 Another example is the transduction of mechanical stimulation into intracellular signals requiring VEGFR2, VE-cadherin, and PECAM-1.28 Furthermore, VE-cadherin comprises a single membrane-spanning domain followed by a short carboxyterminal cytoplasmic domain that connects many regulatory molecules, including p120ctn, β-, and γ-catenin. P120ctn binds to the juxtamembrane cytoplasmic region of VE-cadherin, while β-catenin and γ−catenin binds to the distal part of the cytoplasmic domain. Both β-catenin and γ-catenin bind in turn α-catenin together forming the VE-cadherin/catenin complex.1,9,10 The p120ctn is supposed to possess pleiotropic functions and might be directly involved in controlling actin dynamics (for details, see below). VE-cadherin, β-, and γ-catenin are involved in transcriptional control, and can function as targets for cell signals in inflammation, angiogenesis, and wound healing (for review, see refs. 1, 6, 9, and 29). Beta-catenin also interacts with caveolin-130 and recent reports indicate that phsophocaveolin-1 increasingly binds β-catenin and controls a stimulus-dependent association of β-catenin to VE-cadherin.31,32 Both catenins have been shown to be essential in barrier function regulation.27,33,34 Alpha-catenin that binds to either β- or γ-catenin however is supposed to directly or indirectly regulate and connect the cadherin/catenin complex1,2,23,35,36,37 to actin filaments (for more details, see below).
Short Overview of Actin Filaments in Endothelium
Non-muscle actin, particular β-actin and γ-actin,38 comprises about 10% of the total endothelial protein,39,40 and thus, is a major protein in endothelium. Actin filaments (F-actin) polymerize from actin monomers (globular or G-actin) forming filaments between 5–7 nm. Actin filaments mostly appear in endothelium as components of super-structured protein assemblies, described as actin bundles, as actin networks, and as cortical actin filaments.37,41,42 (Fig. 1).
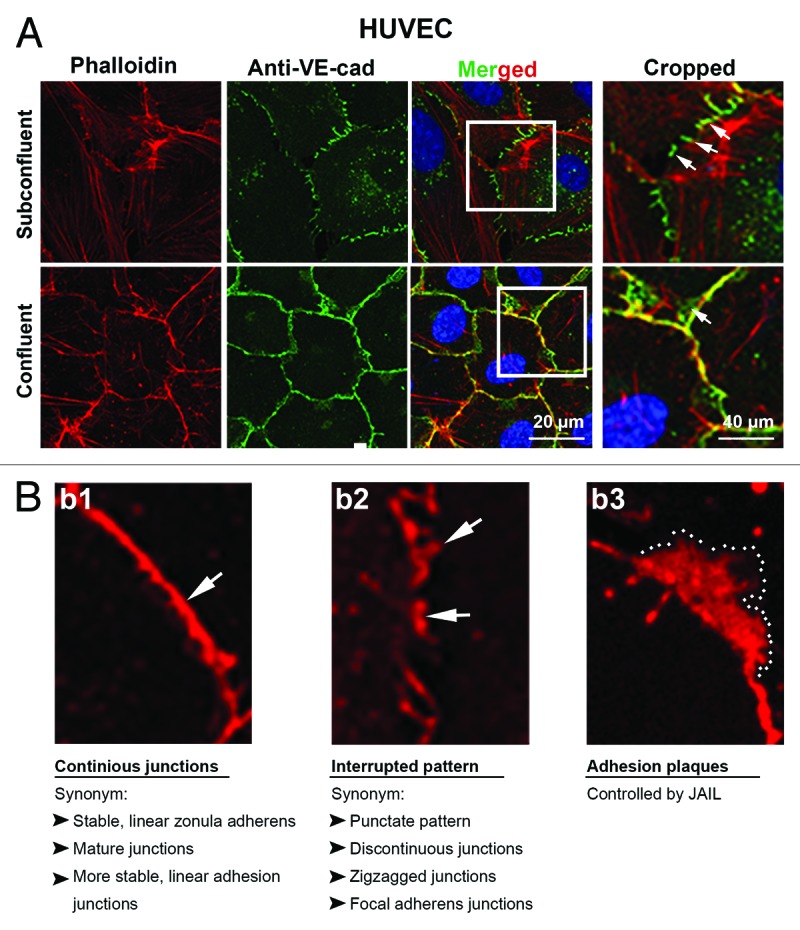
Figure 1. Distribution of VE-cadherin and actin at cell junctions of subconfluent and confluent endothelial cells in culture. Subconfluent and confluent human umbilical vein endothelial cells (HUVEC) cultures were labeled with (a) anti-VE-cadherin (green) and phalloidin-TRITC for filamentous actin (red) or (b1–3) VE-cadherin alone (red). (a) VE-cadherin appears interrupted in the subconfluent cultures (arrows) with large cells and extended perimeter, while cells of confluent cultures are small and polygonal and preferentially exhibits a continuous VE cadherin distribution (arrow). Boxes in the merged images indicated the cropped area as indicated. Actin filaments are incomplete co-localized with the VE-cadherin/catenin complex in both subconfluent and confluent cultures. (b1–3) Higher magnification of HUVEC cultures labeled with anti-VE-cadherin (red). (b1) VE-cadherin appears in confluent cultures in a continuous VE cadherin patterning (arrow) while (b2) in subconfluent cultures, VE-cadherin preferentially appears as an interrupted patterning by which VE-cadherin cluster display different sizes (arrows). (b3) A VE-cadherin adhesion plaques, which is a result of JAIL formation is indicated (dotted line).
Actin bundles consist of polarized short actin filaments that are associated with myosin II and α-actinin; proteins required for development of contractile forces in endothelium.39,43 Actin bundles include both the circumferential actin filaments along the junctions and the cytoplasm traversing stress fibers. The circumferential junction-associated actin bundles are characteristic for intact endothelium in vivo, while stress fibers preferentially appear when endothelium becomes activated e.g., under inflammatory conditions and wound healing; conditions that are generally associated with cell proliferation and migration.1,9,37,44
Actin networks mostly appear, like in other cells, as lammellipodia at the leading edge of migrating cells11,45,46 and as junction-associated intermittent lamellipodia (JAIL) at established cell junctions.11 Those networks are of critical importance in controlling endothelial barrier function and remodeling (for details, see below).
Cortical actin filaments are supposed to be located below the plasma membrane where they associate with the endothelial membrane cytoskeleton.37,42 Since the endothelial membrane cytoskeleton contains spectrin, protein 4.1, and ankyrin, similar to the erythrocyte membrane cytoskeleton,47-49 a comparable organization might exist. However, the organization of the membrane cytoskeleton and its interaction with cortical actin in endothelium is less defined. Particular investigations are required to understand how the membrane cytoskeleton interacts with cortical actin filaments and how these structures connect to cell junctions and focal adhesion sites. The importance of these interactions are supported by the demonstration that α-II-spectrin, a major component of the membrane cytoskeleton, is involved together with the vasodilator-stimulated phosphoprotein (VASP) in endothelial barrier function control.49
Interaction of the VE-Cadherin/Catenin Complex with Actin Filaments
The interaction between the VE-cadherin/catenin complex and actin filaments includes initial junction formation, junction maturation, maintenance of junction integrity, and junction remodeling in physiological and pathological conditions, such as angiogenesis, wound healing, and inflammation. A number of signaling mechanisms modulate the structure and function of both VE-cadherin/catenin complex and the actin filaments. This includes Rho GTPase family, kinases, phosphatases, and junction-regulating molecules such as PECAM-1 ESAM, VE-PTP, and others, which are described in a number of excellent reviews.1,9,50 Most of the current knowledge on interactions between the cadherin/catenin complex and actin filaments arises from studies performed on epithelium,2,10,51 but recent data defines α-catenin, p120ctn, and the ARP2/3 complex as critical molecules that contribute to the coordination between VE-cadherin/catenin complex and actin filaments in endothelium.
Impact of α-catenin in linking actin filaments to the VE-cadherin/catenin complex
The first molecule described to connect the cadherin/β-catenin complex to actin filaments is α-catenin as it binds both β-catenin and actin.52 A large number of further studies supported this concept until two papers appeared in 2005 that challenged this dogma.53,54 A controversial debate during that time increased surrounding the question as to whether α-catenin can mediate a direct or indirect link to actin filaments. The two papers53,54 demonstrated that α-catenin exists as both a monomer that preferentially binds to β-catenin and as a homodimer that preferentially binds actin filaments. Since the α-catenin monomer but not the homodimer can bind to β-catenin, together with the observation that α-catenin cannot bind to both actin and β-catenin at the same time, it was suggested that there is no direct interaction between the cadherin/catenin complex and actin filaments. Furthermore, α-catenin competes with the actin-related protein (ARP) 2/3 complex for binding to actin filament indicating a dynamic and competitive regulation,53,54 rather than a relatively static molecular architecture. Such a mechanistic concept allows a quick remodeling of cell junctions. Further proteins were shown to bind α-catenin as well. This includes α-actinin,55 vinculin,56 ZO-1,57 afadin (AF6),58 ajuba,59 and formins.60 Most of these proteins are also actin-binding proteins and the large diversity of proteins indicates a complicated, and possibly stimulus-specific, differential regulation of this interaction. Alpha-catenin has further been linked to actomyosin filaments61 and can transmit mechanical forces into chemical signals.62 Another molecule that has been demonstrated as a connector between actin filaments and the cadherin/catenin complex is the epithelial protein lost in neoplasms (EPLIN). EPLIN is an actin and α-catenin-binding protein,63 and thus, can mediate a linkage between the VE-cadherin/catenin complex and actin filaments.23 In summary, α-catenin is a suitable target to coordinate the regulation between VE-cadherin/catenin complex and actin filaments. The functional importance of these regulations was recently supported through evidence discovered in vivo, as replacement of VE-cadherin by VE-cadherin-α-catenin fusion construct in mice largely blocked growth factor and histamine-induced permeability.64 Further studies are required to unravel a molecular architecture that regulates the dynamic interplay between α-catenin and actin.
Impact of p120ctn in dynamics of actin filaments at cell junctions
P120ctn, an armadillo family protein, binds to the juxtamembrane region of the cadherin cytoplasmic domain and is supposed to control lateral E-cadherin clustering, E-cadherin recycling, modulation of Rho GTPase and Rho-activated protein kinase (ROCK) activity, cytoskeleton rearrangement, and transcriptional control.65-67 Many of these functions were also demonstrated for regulation of the VE-cadherin in endothelium.68-76 Importantly, recent data indicates the involvement of p120ctn in controlling ARP2/3 complex-mediated actin polymerization at endothelial junctions, by binding to the nuclear-promoting factors Wiskott-Aldrich Syndrome Protein (N-WASP)21 and to cortactin.77,78 Both the ARP2/3 complex11,21 and cortactin79-85 control endothelial integrity, and particularly, N-WASP control ARP2/3 complex-mediated formation of new VE-cadherin adhesion sites, which drive the VE-cadherin dynamics.11
Junction dynamics is controlled by lammellipodia and filopodia
Junction dynamics starts with initial junction formation between adjacent cells. This process requires cell migration to bring plasma membranes of two cells together. Cell migration and junction dynamics are mediated by plasma membrane protrusions that appear as lamellipodia and filopodia; highly dynamic structures that are driven by actin polymerization. Cell migration and initial cell contact formation is thoroughly investigated but the mechanisms that explain the motion of cells within sheet-forming endothelial cell layers, a process that essentially require constitutive junction remodeling, are incompletely understood. Initial junction formation is mediated by lamellipodia formation that overlaps the plasma membranes of adjacent cells. This process led to homophilic engagement of cadherins that are found as mono or multimers at the free plasma membrane.86
In endothelium, the initial VE-cadherin/catenin complex-mediated contact formation is suggested to occur in a two-step process. In the first step, VE-cadherin engagement is mediated by lamellipodia that subsequently disappear and second remodel into filopodia-like structures as evidenced by the presence of fascin and the vasodilatator-stimulated phosphoprotein (VASP).46 Vice versa, cadherin induces actin polymerization as shown for E-cadherin in 2002 and has been demonstrated to be required for junction extension while being controlled by the ARP2/3 complex in epithelium.20 This process was demonstrated to use α-actinin-4 as a bridge molecule between adherens junctions and actin filaments in MDCK cells.87 In this nice study, latrunculin was used to depolymerize actin filaments followed by wash out, which led to actin polymerization at adherens junctions and required the α-actinin-4.87 VE-cadherin engagement also seems to induce actin polymerization in endothelium, as filopodia were positive for VE-cadherin and associated with myosin II-containing stress fibers that radiate perpendicular to cell junctions while also being able to develop contractile force.46 The functional impact of the lamellipodia–filopodia transition in endothelium is currently unclear but may depend on cell density. The overall cell and cell–junction dynamics in confluent endothelium also depend on lamellipodia-like plasma membrane protrusions that appear intermittently and are spatiotemporally restricted at established cell junctions that locally lack VE-cadherin. Those lamellipodia were termed junction-associated intermittent lamellipodia (JAIL) and shown to be of central importance in the maintenance of junction integrity and remodeling.11 Lamellipodia and filopodia are driven by formation of actin networks due to polymerization and branching, processes that are controlled by actin nucleators and nucleation-promoting factors. Actin nucleators include the ARP2/3 complex and its nucleation-promoting factors, such as N-WASP and cortactin, the FH2-domain-containing nucleators of the formin superfamily, and the WH2-domain-containing nucleation factors such as Spir-1 proteins.88-90 In endothelium, the ARP2/3 complex and its nucleation-promoting factors have been recently addressed to play a central role in regulation of endothelial cell junctions.
The ARP2/3 complex, a central player in junction dynamics of the endothelium
The ARP 2/3 complex is a widely expressed protein and controls actin polymerization via nucleation and branching and is thus of critical importance in cell migration, phago-, and endocytosis, and the formation and maintenance of the cadherin/catenin complex-mediated endothelial cell–cell adhesion and integrity. The ARP2/3 complex is a heptamer of approximately 220 kDa and consists of two subunits that have a similar structure to actin (ARP2 and ARP3), in addition to five other subunits; p41-Arc (ArpC1), p34-Arc (ArpC2), p21-Arc (ArpC3), p20-Arc (ArpC4), and p16-Arc (ArpC5).89,91-93 The ARP2/3 complex binds with its ArpC2, ArpC4 to connect with existing actin filaments (mother filament),89 while ARP2 and ARP3 control actin nucleation by mimicking an actin heterodimer that serves as a nucleation template.94 The ARP2/3 complex is usually inactive under resting conditions and needs to be activated by nucleation-promoting factors (NPFs).89,91-93 NPFs are classified in two groups according to their mechanistic activity; class I and class II.89 The Wiskott-Aldrich syndrome protein (WASP) and the WASP-family verprolin-homologous protein (WAVE) are NPFs type I, while cortactin, a class II-promoting factor, were indicated to control junction regulation.89,93
Activation of ARP2/3 complex differs largely between NPFs and several models have been proposed. Both N-WASP and WAVE2 was indicated to control adherens junctions in epithelium and endothelium by triggering ARP2/3-mediated actin nucleation and mediate tension development and stabilization.95,96 However, N-WASP has been indicated to be critical in junction dynamics of both epithelium and endothelium,11,21,96,97 and thus, is briefly discussed here in more detail. N-WASP consists of an N-terminal WASP-homology domain (WH-domain), a central GTPase-binding domain (GBD), and a conserved carboxyterminal veroprolin central acidic domain (VCA-domain). Intramolecular binding of the VCA-domain to the GBD-domain blocks the ability of N-WASP to activate ARP2/3 complex. Activation of ARP2/3 complex and subsequent actin polymerization is supposed to occur by binding of the V-domain of N-WASP to actin and the CA domain to the ARP2/3 complex. For further details, we refer to recent reviews.89,91-93 A novel study indicate that two rather than one VCA domains are required for ARP2/3 activation;98 a discovery that might have an impact in coordination of different signals appearing at the same time. Another protein that controls the ARP2/3 complex activity is cortactin, a class II NPF that has been demonstrated to control endothelial barrier function and leucocyte transmigration.79,80,83,85,99,100
Furthermore, cortactin has multiple functions in cell motility and is able to activate N-WASP.101 For further readings to cortactin, please see recent reviews.102-105 Both WASP and cortactin are targeted and activated by a number of cellular signals. These include the Rho-family GTPases Cdc 42 and Rac, tyrosine kinases, the phosphatidyl (3,4,5) triphosphate, and focal adhesion kinase.93,102,104,106-108 These signals are of critical importance in regulation of endothelial barrier function,50 and thus, might provide a link to ARP2/3 complex-mediated actin polymerization.
Interdependent activity between the ARP2/3-controlled JAIL and the VE-cadherin/catenin complex
The concept of an interactive regulation between VE-cadherin-mediated cell adhesion and actin dynamics to control junction dynamics and barrier function was proposed a long time ago. This includes the dynamics of junction formation, maintenance, and remodeling after stimulation. A clear piece of evidence for the role of the nuclear-promoting factor N-WASP in regulation of endothelial barrier function was recently provided.21 Depletion of N-WASP in cultured endothelium altered the barrier function and delayed the barrier function recovery after thrombin stimulation. Furthermore, it was demonstrated that p120ctn precipitates with N-WASP, and this behavior was dependent on the phosphorylation of tyrosine 256 in N-WASP in endothelial cells.21 It was concluded that actin polymerization is critical in both the maintenance of junction integrity and resealing activity following stimulation in interaction with p120ctn.21 Another study using a fluorescence energy transfer technique indicated spontaneous RhoA activity at plasma membrane protrusions109 under resting conditions. RhoA activity was enhanced after thrombin application, but was also seen during barrier function recovery. This suggests that both the opening and closing of endothelial cell junctions in response to thrombin is accompanied by local RhoGTPase activity at plasma membrane protrusions.109 Furthermore, the ARP2/3 complex-mediated actin polymerization was essential in sphingosine-1-phosphate-induced increase in barrier function.97 Together, this data clearly indicate that actin polymerization is an essential step in endothelial barrier function dynamics. The molecular mechanistic background that constantly controls junction dynamics and endothelial barrier function and integrity was recently demonstrated in a cell culture model when comparing the cell density-dependent dynamics of VE-cadherin and actin.11
VE-cadherin patterning is independent on VE-cadherin expression level but depends on cell size
Endothelial cell cultures display a density-dependent VE-cadherin and actin patterning. This was first described in 1995110 and confirmed later in many other studies (Fig. 1). Subconfluent endothelial cultures display an interrupted VE-cadherin patterning, while highly confluent cultures (we use the term confluent for HUVEC cultures for a cell density > 105 cells/cm2) display a continuous line of VE-cadherin along the junctions. The interrupted VE-cadherin-patterning in subconfluent cells is characterized by the appearance of individual VE-cadherin clusters of various sizes leaving VE-cadherin-free gaps in between themselves (Fig. 1). These subconfluent cultures exhibit a high dynamic remodeling of VE-cadherin which is in contrast to confluent cultures.11,13 Compare movie published in MBoC 2014 (http://www.molbiolcell.org/content/suppl/2013/11/11/mbc.E13-07-0404v1.DC1/mc-E13-07-0404-s05.mp4). VE-cadheirn dynamics coincide with actin patterning and dynamics, as well.11 Cells of subconfluent cultures do not develop a circumferential band of actin filaments at the junctions, and there is less co-localization between VE-cadherin/catenin complex and actin filaments (Fig. 1). This patterning, seen in subconfluent cells, is usually accompanied by the appearance of large amounts of stress fibers that traverse the cytoplasm and can terminate at cell junctions.13,111 These stress fibers are contractile as they consist of polarized actin filaments, myosin II, and α-actinin.39,43 Together, the appearance and dynamics of actin filaments in endothelium critically depend on the physiological and pathological conditions in their environment.37,41,42 Thus, both the VE-cadherin and the actin patterning in growing culture differ from the actin and VE-cadherin patterning seen physiologically in endothelial cells in vivo (see above). The mechanistic background of the cell density-dependent VE-cadherin and actin patterning and dynamics remained enigmatic. Recently, the naturally elegant, and yet simple principle behind this mystery was found to depend on two facts; first, a constant cell density-independent expression of VE-cadherin and second, the cell density-dependent cell size. The cell density-independent expression of VE-cadherin was already shown in 1995 by comparing the total amount of VE-cadherin in subconfluent and confluent human umbilical vein endothelial cultures;110 a result recently confirmed by a detailed quantitative analysis.11 When combining these two facts, it became obvious that a given amount of VE-cadherin distributed along the cell junctions appear interrupted in large cells having an elongated cell perimeter (subconfluent cultures). Cell growth increases cell density, and thus, cells become smaller, and subsequently, possess a shortened cell perimeter (confluent cells). As a consequence, the individual VE-cadherin clusters become narrow and, near the end, (highly confluent cells with > 105 cells/cm2) form a continuous line along the junctions11 (Fig. 1). The mechanistic background of cell density-dependent differences in VE-cadherin dynamics11,13 and junction integrity can be explained by the formation of lamellipodia-like structures that preferentially appear between individual VE-cadherin clusters.11
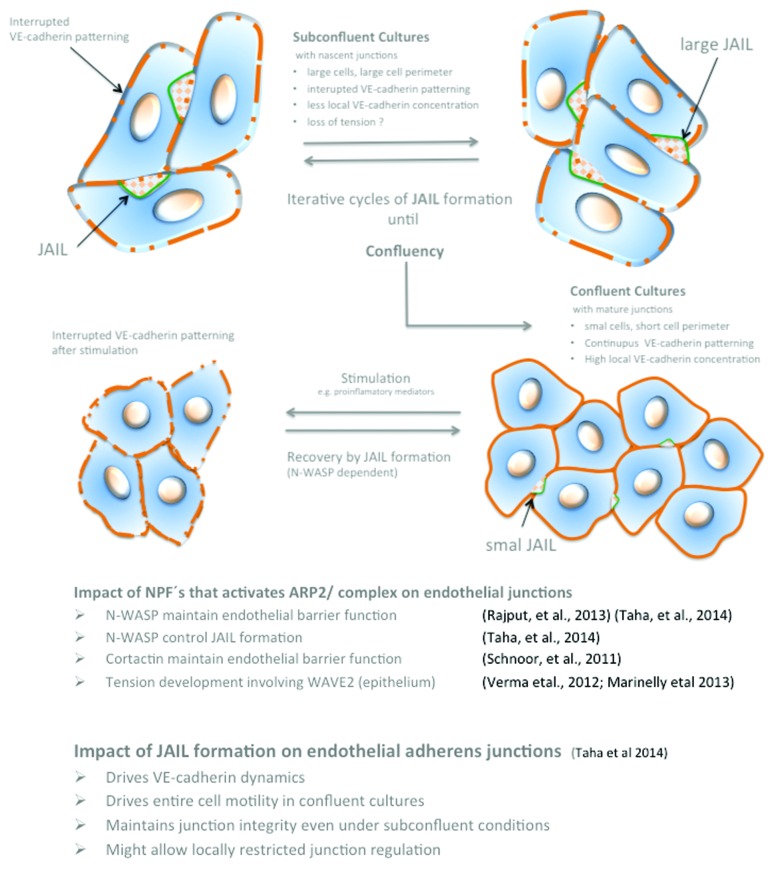
Interdependency between VE-cadherin-mediated cell adhesion and JAIL formation control junction integrity
Junction associated intermittent lamellipodia (JAIL) indicates lamellipodia-like structures that appear at established endothelial junctions and are spatiotemporally restricted.11 Particularly, JAIL are formed at cell junctions where VE-cadherin is locally lacking and controlled by N-WASP-activated ARP2/3 complex that mediates actin polymerization,11 a molecular mechanism comparable to lamellipodia formation at the leading edges of migrating cells. An interdependent regulation between VE-cadherin-mediated cell adhesion and ARP2/3 complex-mediated and actin-driven JAIL formation was proposed to control VE-cadherin dynamics by an iterative cycle (Fig. 4). This was demonstrated by fluorescent life cell imaging of HUVEC expressing both p20, a subunit of the ARP2/3 complex, tagged with EGFP and VE-cadherin-mCherry fusion-protein uncovered the underlying spatiotemporal mechanism.11 It was demonstrated that even at established endothelial cell junctions, JAIL formation occurred and induced an overlap of plasma membranes at which VE-cadherin trans-adhesion plaques were formed (Figs. 2 and 3). Those plaques increasingly clustered during JAIL retraction and incorporated VE-cadherin into the junctions (Fig. 3). VE-cadherin that forms adhesion plaques over the entire JAIL area were proposed to derive from membrane-localized mono- or oligomers, as described for E-cadherin already.86 In this way, JAIL formation change the VE-cadherin pattern and contributes to the observed dynamics. Thus, it becomes clear that subconfluent cultures with interrupted VE-cadherin patterning display a higher dynamics compared with confluent cultures with a continuous VE-cadherin distribution. However, VE-cadherin dynamics constitutively still occur in confluent cultures,11,13 due to the spatiotemporal small interruption of the continuous VE-cadherin line, a phenomenon that might be facilitated by thermodynamic activity in addition to the cell density dependency as described above (Fig. 2B). A recent report showed the importance of the ARP2/3-dependent maintenance of the blood–testis barrier (Lie et al., 2010) and the sphingosine-1-phosphate-induced junction remodeling (Li et al., 2004), results that supports the critical importance of the ARP2/3 complex and JAIL formation in junction regulation. Furthermore, JAIL formation and VE-cadherin dynamics were shown to drive the overall cell motility in sheet-forming endothelial cell layers as shown by a quick adenovirus-mediated overexpression of full-length VE-cadherin-EGFP in subconfluent cultures. Adenovirus-mediated gene transfer can be used to cause a quick protein overexpression, which is in contrast to lentiviral gene transfer that needs at least 48 h.112 Quick VE-cadherin-EGFP overexpression within hours maintained the subconfluency of the cells but changed the VE-cadherin patterning form interrupted into a continuous line. Those cells displayed less JAIL, less VE-cadherin dynamics, and dramatically reduced the overall cell motility within the endothelial monolayer11 (link: http://www.molbiolcell.org/content/suppl/2013/11/11/mbc.E13-07-0404v1.DC1/mc-E13-07-0404-s06.mp4), while inhibition of ARP2/3 complex blocked VE-cadherin dynamics and caused intercellular gap formation.11 Gap formation was also demonstrated using N-WASP mutants11,21 or depletion of cortactin,85 molecules that to the end control the activity of the ARP2/3 complex.113,114
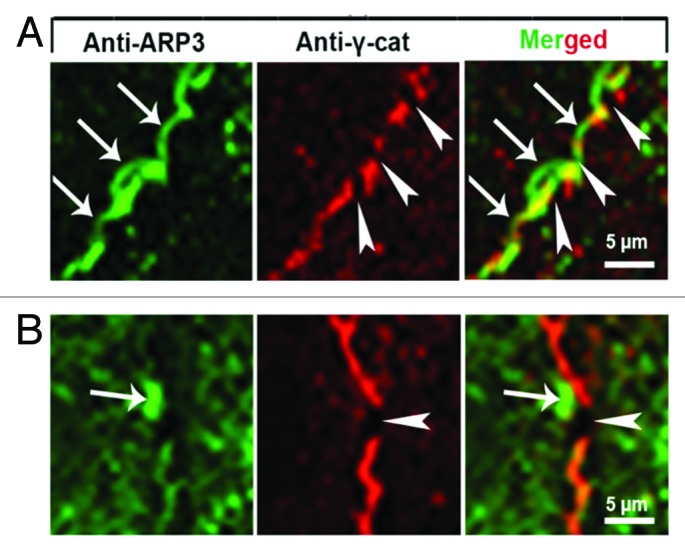
Figure 4. Scheme illustrating the interdependency between JAIL-activity and VE-cadherin dynamics in relation to cell density. For details, please compare text.
Figure 2. High magnification of cell junctions in (A) subconfluent and (B) confluent HUVEC cultures labeled with antibodies as indicated. (A) JAIL (arrows) preferentially appear at spaces close to and between VE-cadherin/catenin clusters (arrowheads). (B) Even in confluent cultures, small interruptions of the continuous VE-cadherin/catenin line appear spatiotemporally restricted followed by formation of small ARP2/3 complex controlled JAIL. Taken from Taha et al., 2014, MBoC.
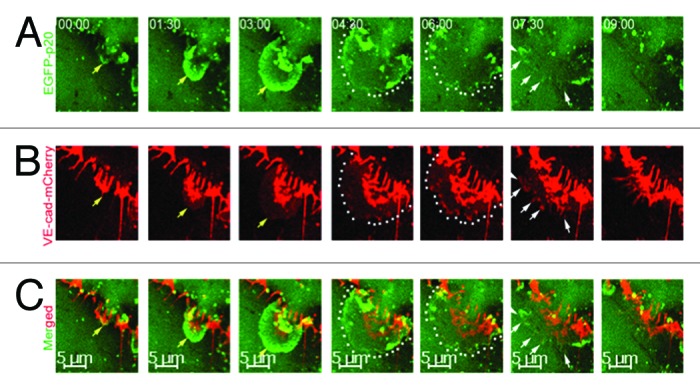
Figure 3. Time-lapse series of ARP2/3 complex-mediated JAIL formation and VE-cadherin dynamics in subconfluent endothelial cell cultures expressing both the fusion protein EGFP-p20 (green) and VE-cadherin-mCherry (red) at high magnification. (A) JAIL developed (yellow arrows) to its maximal extension within 4–5 min (green, dotted lines). JAIL developed close to and between interruptions of VE-cadherin-m-Cherry clusters and caused new VE-cadherin adhesion plaques (B, yellow arrows and dotted lines). (B) VE-cadherin-mCherry plaques (dotted lines) increasingly cluster (white arrows) during JAIL retraction and assemble at cell junctions. This mechanisms change the VE-cadherin pattern, and thus, contributes to VE-cadherin dynamics. Taken from Taha et al., 2014, MBoC.
Although the molecular mechanisms that control interaction between JAIL formation and VE-cadherin-mediated cell adhesion is incompletely understood there is evidence that α-catenin and p120ctn, components of the VE-cadherin/catenin complex, are interaction partners for molecules that control actin polymerization. In particular, vinculin is associated with the cadherin/catenin complex,56 and was indicated to couple actin stress fibers to the VE-cadherin/catenin complex in endothelium.13 Furthermore, α-catenin, a vinculin homolog,115 recruited the ARP2/3 complex to cell junction,116,117 and also becomes activated by α-catenin,118 and thus, might contribute to control actin polymerization at adherens junctions.119 Otherwise, JAIL are not associated with stress fibers, and thus, vinculin might not play a central role in this process. Rather, it seems more reasonable to assume that α-catenin, which competes with the ARP2/3 complex for actin binding,53,54 mediates this process. However, the junction-associated molecules that to the end control activation or blocking of JAIL has to be identified. In addition, a recent paper suggested a tension-dependent recovery of micro-wounds that develop due to leukocyte transmigration or after mechanical wounding by actin-driven and ARP2/3-dependent formation of lamellipodia-like structures that appeared quickly after wounding and were termed ventral lamellipodia.113 Those actin-rich structures were also responsible for closing transcellular wounds, and are mostly independent on cadherin-mediated cell adhesion.113 It has to be investigated if, apart from loss of tension, other mechanisms play a role. It is reasonable to assume that those transcellular wounds disintegrate both the membrane cytoskeleton and cortical actin filaments, and thus, might lead to actin nucleation which in turn might drive wound closure. If loss of tension play a role for the formation of JAIL that also appear in stress fiber-free, highly confluent, endothelial cultures37 might be tested. However, actin-rich structure that move along the junctions has also been shown to maintain the blood brain barrier via G-protein coupled receptor in Drosophila melanogaster.114 In summary, it becomes obvious that actin-driven and ARP2/3-controlled lammellipodia are not restricted to the leading edge of migrating cells but are also of critical importance in formation, maintenance, and dynamics at cell junctions and for cellular repair mechanisms.
Concluding Remarks and Future Directions
Regulation of endothelial adherens junctions, particularly the interaction of the VE-cadherin catenin complex and actin filaments, is central in physiology and pathology but is incompletely understood. This also includes the question how the membrane cytoskeleton and the cortical actin filaments are structurally organized at cell junctions. Recent work addressed the role of actin nucleators, such as ARP2/3 complex and its activators N-WASP and cortactin, in adherens junction regulation. Based on biochemical, morphological, and dynamic analyses, evidence was provided that certain actin-binding actin-nucleators can interact with components of adherens junctions, in particular, with the VE-cadherin/catenin complex in endothelium. The large number of molecular interactions between actin-regulating and VE-cadherin-associated molecules open a very large number of regulatory possibilities. This becomes even more complex when more than one stimuli targets the endothelium at the same time. It remains to be investigated if these interactions are stimulus-specific and whether or not molecules that control VE-cadherin and actin interaction are in balance with each other. It becomes increasingly evident that dynamics of junction regulation occurs at the subcellular level rather than a general response of the entire cell junctions. Therefore, spatiotemporal resolved quantitative experimental approaches are required. Live cell imaging is a suitable method of choice for these challenging questions. The uncovering of these local dynamic regulations and successfully placing them in a local regulatory network involving mathematical modeling may help to design future therapies and treatments for severe diseases such as septic shock, acute and chronic inflammation, and tumor-angiogenesis. We must press forward to find out.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The German Research Council, DFG INST 2105/24-1 and SCHN 430/6-1 to Schnittler H supported this work.
References
- 1.Dejana E, Vestweber D. The Role VE-Cadherin in Vascular Morphogenesis and Permeability Control. In: VanRoy F, ed. Molecular Biology of Cadherins, 2013:119-44. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WJ, Dickinson DJ, Weis WI. Roles of cadherins and catenins in cell-cell adhesion and epithelial cell polarity. Prog Mol Biol Transl Sci. 2013;116:3–23. doi: 10.1016/B978-0-12-394311-8.00001-7. [DOI] [PubMed] [Google Scholar]
- 3.Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s) Curr Opin Cell Biol. 2013;25:39–46. doi: 10.1016/j.ceb.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–85. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976;68:705–23. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci. 2013;126:2545–9. doi: 10.1242/jcs.124529. [DOI] [PubMed] [Google Scholar]
- 7.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–96. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 8.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol Rev. 2002;186:57–67. doi: 10.1034/j.1600-065X.2002.18606.x. [DOI] [PubMed] [Google Scholar]
- 9.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–93. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 10.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91:691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taha AA, Taha M, Seebach J, Schnittler HJ. ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Mol Biol Cell. 2014;25:245–56. doi: 10.1091/mbc.E13-07-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seebach J, Donnert G, Kronstein R, Werth S, Wojciak-Stothard B, Falzarano D, Mrowietz C, Hell SW, Schnittler HJ. Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Cardiovasc Res. 2007;75:596–607. doi: 10.1016/j.cardiores.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–52. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992;118:1511–22. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Sano K, Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 1991;2:261–70. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valiron O, Chevrier V, Usson Y, Breviario F, Job D, Dejana E. Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J Cell Sci. 1996;109:2141–9. doi: 10.1242/jcs.109.8.2141. [DOI] [PubMed] [Google Scholar]
- 17.Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem. 2000;275:21435–43. doi: 10.1074/jbc.M001857200. [DOI] [PubMed] [Google Scholar]
- 18.Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardin N, Anfosso F, Massé JM, Cramer E, Sabatier F, Le Bivic A, Sampol J, Dignat-George F. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98:3677–84. doi: 10.1182/blood.V98.13.3677. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–82. doi: 10.1016/S0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- 21.Rajput C, Kini V, Smith M, Yazbeck P, Chavez A, Schmidt T, Zhang W, Knezevic N, Komarova Y, Mehta D. Neural Wiskott-Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions. J Biol Chem. 2013;288:4241–50. doi: 10.1074/jbc.M112.440396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou K, Muroyama A, Underwood J, Leylek R, Ray S, Soderling SH, Lechler T. Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. Proc Natl Acad Sci U S A. 2013;110:E3820–9. doi: 10.1073/pnas.1308419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geyer H, Geyer R, Odenthal-Schnittler M, Schnittler HJ. Characterization of human vascular endothelial cadherin glycans. Glycobiology. 1999;9:915–25. doi: 10.1093/glycob/9.9.915. [In Process Citation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–95. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellberg S, Dimberg A, Bahram F, Hayashi M, Rennel E, Ameur A, Westholm JO, Larsson E, Lindahl P, Cross MJ, et al. Transcriptional profiling reveals a critical role for tyrosine phosphatase VE-PTP in regulation of VEGFR2 activity and endothelial cell morphogenesis. FASEB J. 2009;23:1490–502. doi: 10.1096/fj.08-123810. [DOI] [PubMed] [Google Scholar]
- 27.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–45. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 31.Kronstein R, Seebach J, Grossklaus S, Minten C, Engelhardt B, Drab M, Liebner S, Arsenijevic Y, Taha AA, Afanasieva T, et al. Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc Res. 2012;93:130–40. doi: 10.1093/cvr/cvr256. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res. 2009;105:676–85, 15, 685. doi: 10.1161/CIRCRESAHA.109.201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnittler HJ, Püschel B, Drenckhahn D. Role of cadherins and plakoglobin in interendothelial adhesion under resting conditions and shear stress. Am J Physiol. 1997;273:H2396–405. doi: 10.1152/ajpheart.1997.273.5.H2396. [DOI] [PubMed] [Google Scholar]
- 34.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–22. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michael M, Yap AS. The regulation and functional impact of actin assembly at cadherin cell-cell adhesions. Semin Cell Dev Biol. 2013;24:298–307. doi: 10.1016/j.semcdb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–55. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnittler HJ, Taha M, Taha AA, Seebach J. Actin filaments dynamics in endothelium The Ying and the Yang between stabilization and motion. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-1856-2. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein PA. The functional importance of multiple actin isoforms. Bioessays. 1990;12:309–15. doi: 10.1002/bies.950120702. [DOI] [PubMed] [Google Scholar]
- 39.Schnittler HJ, Wilke A, Gress T, Suttorp N, Drenckhahn D. Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J Physiol. 1990;431:379–401. doi: 10.1113/jphysiol.1990.sp018335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson CE, Lum H. Update on pulmonary edema: the role and regulation of endothelial barrier function. Endothelium. 2001;8:75–105. doi: 10.3109/10623320109165319. [DOI] [PubMed] [Google Scholar]
- 41.Lampugnani MG. Endothelial adherens junctions and the actin cytoskeleton: an ‘infinity net’? J Biol. 2010;9:16. doi: 10.1186/jbiol232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77:53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drenckhahn D, Wagner J. Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol. 1986;102:1738–47. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mochizuki N. Vascular integrity mediated by vascular endothelial cadherin and regulated by sphingosine 1-phosphate and angiopoietin-1. Circ J. 2009;73:2183–91. doi: 10.1253/circj.CJ-09-0666. [DOI] [PubMed] [Google Scholar]
- 45.Doggett TM, Breslin JW. Study of the actin cytoskeleton in live endothelial cells expressing GFP-actin. J Vis Exp. 2011:3187. doi: 10.3791/3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol Biol Cell. 2012;23:310–23. doi: 10.1091/mbc.E11-08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295:102–18. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Leto TL, Pratt BM, Madri JA. Mechanisms of cytoskeletal regulation: modulation of aortic endothelial cell protein band 4.1 by the extracellular matrix. J Cell Physiol. 1986;127:423–31. doi: 10.1002/jcp.1041270311. [published erratum appears in J Cell Physiol 1986 Sep;128(3):511] [DOI] [PubMed] [Google Scholar]
- 49.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, Walter U, Feller SM, Renné T. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol. 2008;180:205–19. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol. 2006;26:1431–8. doi: 10.1161/01.ATV.0000218510.04541.5e. [DOI] [PubMed] [Google Scholar]
- 51.Lecuit T. “Developmental mechanics”: cellular patterns controlled by adhesion, cortical tension and cell division. HFSP J. 2008;2:72–8. doi: 10.2976/1.2896332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–7. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss EE, Kroemker M, Rüdiger AH, Jockusch BM, Rüdiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755–64. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–92. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem. 2002;277:18868–74. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, Moss SJ, Troyanovsky S, Attwell D, Longmore GD, et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J Biol Chem. 2003;278:1220–8. doi: 10.1074/jbc.M205391200. [DOI] [PubMed] [Google Scholar]
- 60.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–6. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 62.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 63.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–9. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–70. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–32. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pieters T, van Roy F, van Hengel J. Functions of p120ctn isoforms in cell-cell adhesion and intracellular signaling. Front Biosci (Landmark Ed) 2012;17:1669–94. doi: 10.2741/4012. [Landmark Ed] [DOI] [PubMed] [Google Scholar]
- 68.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res. 2012;111:739–49. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation. Am J Physiol Cell Physiol. 2012;303:C385–95. doi: 10.1152/ajpcell.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hatanaka K, Simons M, Murakami M. Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. Am J Physiol Heart Circ Physiol. 2011;300:H162–72. doi: 10.1152/ajpheart.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell. 2009;20:1970–80. doi: 10.1091/mbc.E08-07-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–9. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–51. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–12. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 75.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1143–53. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- 76.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol. 2003;285:L434–42. doi: 10.1152/ajplung.00075.2003. [DOI] [PubMed] [Google Scholar]
- 77.Wang Q, Lu TL, Adams E, Lin JL, Lin JJ. Intercalated disc protein, mXinα, suppresses p120-catenin-induced branching phenotype via its interactions with p120-catenin and cortactin. Arch Biochem Biophys. 2013;535:91–100. doi: 10.1016/j.abb.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, Feinstein E, Geiger B, Bershadsky A. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc Natl Acad Sci U S A. 2007;104:10882–7. doi: 10.1073/pnas.0702731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol. 2006;291:L289–95. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- 80.Kredy-Farhan L, Kotev-Emeth S, Savion N. Involvement of cortactin and phosphotyrosine proteins in cell-cell contact formation in cultured bovine corneal endothelial cells. Histochem Cell Biol. 2008;129:193–202. doi: 10.1007/s00418-007-0357-8. [DOI] [PubMed] [Google Scholar]
- 81.Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem Cell Biol. 2006;126:297–304. doi: 10.1007/s00418-006-0143-z. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Liu J, Zhan X. Tyrosine phosphorylation of cortactin is required for H2O2-mediated injury of human endothelial cells. J Biol Chem. 2000;275:37187–93. doi: 10.1074/jbc.M005301200. [DOI] [PubMed] [Google Scholar]
- 83.Maharjan S, Kim K, Agrawal V, Choi HJ, Kim NJ, Kim YM, Suh YG, Kwon YG. Sac-1004, a novel vascular leakage blocker, enhances endothelial barrier through the cAMP/Rac/cortactin pathway. Biochem Biophys Res Commun. 2013;435:420–7. doi: 10.1016/j.bbrc.2013.04.104. [DOI] [PubMed] [Google Scholar]
- 84.Paradis H, Islam T, Tucker S, Tao L, Koubi S, Gendron RL. Tubedown associates with cortactin and controls permeability of retinal endothelial cells to albumin. J Cell Sci. 2008;121:1965–72. doi: 10.1242/jcs.028597. [DOI] [PubMed] [Google Scholar]
- 85.Schnoor M, Lai FP, Zarbock A, Kläver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med. 2011;208:1721–35. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iino R, Koyama I, Kusumi A. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys J. 2001;80:2667–77. doi: 10.1016/S0006-3495(01)76236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang VW, Brieher WM. α-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol. 2012;196:115–30. doi: 10.1083/jcb.201103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aspenström P. Formin-binding proteins: modulators of formin-dependent actin polymerization. Biochim Biophys Acta. 2010;1803:174–82. doi: 10.1016/j.bbamcr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–26. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 90.Kerkhoff E. Actin dynamics at intracellular membranes: the Spir/formin nucleator complex. Eur J Cell Biol. 2011;90:922–5. doi: 10.1016/j.ejcb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–35. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 93.Rottner K, Hänisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–61. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Robinson RC, Turbedsky K, Kaiser DA, Marchand J-B, Higgs HN, Choe S, Pollard TD. Crystal structure of Arp2/3 complex. Science. 2001;294:1679–84. doi: 10.1126/science.1066333. [DOI] [PubMed] [Google Scholar]
- 95.Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap ASA. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell. 2012;23:4601–10. doi: 10.1091/mbc.E12-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol. 2011;13:934–43. doi: 10.1038/ncb2290. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Uruno T, Haudenschild C, Dudek SM, Garcia JG, Zhan X. Interaction of cortactin and Arp2/3 complex is required for sphingosine-1-phosphate-induced endothelial cell remodeling. Exp Cell Res. 2004;298:107–21. doi: 10.1016/j.yexcr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 98.Padrick SB, Doolittle LK, Brautigam CA, King DS, Rosen MK. Arp2/3 complex is bound and activated by two WASP proteins. Proc Natl Acad Sci U S A. 2011;108:E472–9. doi: 10.1073/pnas.1100236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–93. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 100.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 101.Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- 102.MacGrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. J Cell Sci. 2012;125:1621–6. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011;5:187–98. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren G, Crampton MS, Yap AS. Cortactin: Coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell Motil Cytoskeleton. 2009;66:865–73. doi: 10.1002/cm.20380. [DOI] [PubMed] [Google Scholar]
- 105.Weaver AM. Cortactin in tumor invasiveness. Cancer Lett. 2008;265:157–66. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev Cell. 2002;3:645–58. doi: 10.1016/S1534-5807(02)00324-6. [DOI] [PubMed] [Google Scholar]
- 107.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 108.Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004;279:9565–76. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- 109.Szulcek R, Beckers CM, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJ, Minshall RD, van Hinsbergh VW, van Nieuw Amerongen GP. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc Res. 2013;99:471–82. doi: 10.1093/cvr/cvt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–17. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Millán J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernández-Martín L, Correas I, Ridley AJ. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindemann D, Schnittler H. Genetic manipulation of endothelial cells by viral vectors. Thromb Haemost. 2009;102:1135–43. doi: 10.1160/TH09-10-0724. [DOI] [PubMed] [Google Scholar]
- 113.Martinelli R, Kamei M, Sage PT, Massol R, Varghese L, Sciuto T, Toporsian M, Dvorak AM, Kirchhausen T, Springer TA, et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J Cell Biol. 2013;201:449–65. doi: 10.1083/jcb.201209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hatan M, Shinder V, Israeli D, Schnorrer F, Volk T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J Cell Biol. 2011;192:307–19. doi: 10.1083/jcb.201007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci U S A. 1991;88:9156–60. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Craig SW, Chen H. Lamellipodia protrusion: moving interactions of vinculin and Arp2/3. Curr Biol. 2003;13:R236–8. doi: 10.1016/S0960-9822(03)00160-X. [DOI] [PubMed] [Google Scholar]
- 117.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng X, Maiers JL, Choudhury D, Craig SW, DeMali KA. α-Catenin uses a novel mechanism to activate vinculin. J Biol Chem. 2012;287:7728–37. doi: 10.1074/jbc.M111.297481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chhabra ES, Higgs HN. INF2 Is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem. 2006;281:26754–67. doi: 10.1074/jbc.M604666200. [DOI] [PubMed] [Google Scholar]