Abstract
The ability of blood vessels to sense and respond to stimuli such as fluid flow, shear stress, and trafficking of immune cells is critical to the proper function of the vascular system. Endothelial cells constantly remodel their cell–cell junctions and the underlying cytoskeletal network in response to these exogenous signals. This remodeling, which depends on regulation of the linkage between actin and integral junction proteins, is controlled by a complex signaling network consisting of small G proteins and their various downstream effectors. In this commentary, we summarize recent developments in understanding the small G protein RAP1 and its effector RASIP1 as critical mediators of endothelial junction stabilization, and the relationship between RAP1 effectors and modulation of different subsets of endothelial junctions.
Keywords: Rasip1, Rap1, Epac1, effectors, angiogenesis, vasculogenesis, junctions, VE-cadherin, endothelial cells
Endothelial Junctions: Their Composition and Regulation
Similar to epithelial cells, endothelial cell–cell junctions are primarily comprised of two types, tight junctions (TJs) and adherens junctions (AJs), which form through homotypic trans-interaction of molecules on adjacent cells, as well as cis-interactions on the same cell.14 The major constituents of TJs are the transmembrane proteins claudin-5, occludin, nectins, and junctional adhesion molecules (JAMs), whereas AJs are largely composed of calcium-dependent vascular endothelial cadherin (VE-cadherin).14 Other molecules, such as nectins, participate in the establishment of initial cell–cell contacts and organize both TJs and AJs.14,15 In both cases, these classes of junctional proteins are linked to the underlying actin cytoskeleton through various adaptor proteins, such as zonula occludens 1 (ZO1) in the case of TJs and the catenins in the case of AJs. β-catenin interacts directly with the C-terminal tail of VE-cadherin, and α-catenin binds both to actin and to β-catenin,16 although there is still controversy as to whether ternary or higher-order complexes assemble in vitro and in vivo.17,18 Additional catenins, such as p120-catenin, also bind to the juxtamembrane region of VE-cadherin and modulate adhesion strength, largely through prevention of cadherin endocytosis.19,20 AJ integrity is regulated by a number of factors, including linkage of AJs to the actin and microtubule cytoskeleton, which can prevent lateral diffusion and internalization of integral AJ components.
Much recent work has demonstrated that interactions between adjacent cells mediated by junction proteins, as well as between junctions and the actin cytoskeleton, play a key role in control of barrier function and leukocyte diapedesis. Antibodies directed against the extracellular domain of VE-cadherin have been demonstrated to block VE-cadherin-dependent adhesion, and thus reduce barrier function, as assessed by measurement of transendothelial electrical resistance and passage of FITC-dextran across endothelial monolayers.21 Injection of VE-cadherin blocking antibodies in vivo results in increased vascular permeability and neutrophil infiltration.22 Conversely, stabilization of the linkage between adherens junctions and the actin cytoskeleton, achieved through fusion of VE-cadherin with α-catenin, reduced permeability and leukocyte extravasation both in vitro and in vivo.23 Taken together, the data indicate that modulation of AJs, either through disruption of homophilic interactions or control of actin–junction linkages, may have a profound impact on endothelial barrier function. In addition to the role in barrier function, stable VE-cadherin-dependent cell–cell linkages are important for establishment of endothelial apical-basolateral polarity in major arteries.24,25
Recently, interest has grown concerning the precise composition and morphology of distinct subsets of endothelial junctions. In contrast to epithelial junctions, where tight junctions are located at the apical surface of cells and adherens junctions are found at the baso-lateral segment, endothelial AJs and TJs are typically not stratified in most tissues, but instead, intermingle in many junctional contacts.14 Further, endothelial junctions display several distinctive morphologies, at least when examined in confluent cultures of primary endothelial cells from various vascular beds. Linear junctions, which are thought to represent stable cell–cell contacts, display close association with cortical actin (also described as circumferential actin bundles), as shown in numerous immunofluorescence microscopy experiments.26 Reticular junctions, which exhibit a honeycomb-like staining pattern of AJ markers, may represent a specialized type of junction formed by sliding contacts between two or more adjacent cells.27 These contacts were reported to be devoid of actin and associated mechanotransducing proteins, such as myosin, within the reticular network.27 In addition, reticular structures were not rich in TJ markers such as ZO-1.27 This, combined with the fact that PECAM-1 was found within the reticular junctions, yet was interspersed between cadherin–catenin regions, supports the hypothesis that these regions may be a specialized area of junction that facilitates leukocyte extravasation and specific aspects of barrier function under distinct conditions.27
A third type of junctional morphology, variously referred to as “discontinuous” “punctate,” or “focal” adherens junctions (FAJs), is associated both with initial AJ formation and remodeling of AJs.28,29 When two endothelial cells initiate contact with one another, VE-cadherin-containing filopodia form initial adhesions, called focal or spot adherens junctions, which are linked to radial actin stress fibers.28,30 These adhesions subsequently expand laterally to broaden the contact interface, and form linear junctions with associated bundled linear actin. Contraction of endothelial cells, induced by stimuli such as VEGF, thrombin, or TNFα, is able to promote formation of nascent FAJs from linear junctions.28 Interestingly, FAJs contain vinculin, which is recruited to focal adhesions to facilitate integrin clustering and linkage to the actin cytoskeleton.28,31 Recent data indicate that vinculin is recruited to the remodeling FAJs, and may act to maintain cell–cell contacts in the presence of an orthogonal mechanical force.28 α-catenin function is controlled in part by mechanically induced conformational changes, and this may facilitate vinculin recruitment to specific junctional regions that sense local strain.32 Intriguingly, vinculin also binds to talin, a focal adhesion protein that also undergoes force-induced remodeling, and tension is required to maintain vinculin at focal adhesions.33 This suggests that vinculin may be a general means to link the actin cytoskeleton to areas of the cell that are encountering mechanical stress, either at sites of matrix attachment or linkage to other cells.
RAP1 as a Central Mediator of Endothelial Cell–Cell and Cell–Matrix Adhesions
RAP proteins are small monomeric guanosine triphosphatases (GTPases) that are members of the RAS GTPase superfamily.34,35 GTPases function as switches in numerous cellular signaling and trafficking processes.35 This switching behavior is controlled by cycling between GDP (inactive) and GTP-bound (active) forms, processes that are catalyzed by specific exchange factors.35,36 RAP1 is typically bound to GDP, but guanine nucleotide exchange factors (GEFs), such as PDZ-GEF, RAPGEF3/EPAC1, and C3G, promote exchange of GDP for GTP (Fig. 1).36 The GTP-bound form of RAP1 is then primed to specifically interact with its downstream effectors, by binding to their RAS-association (RA) domains. Inactivation of the cycle is achieved through action of GTPase-activating proteins (GAPs), which promote hydrolysis of bound GTP to GDP.35 Although RAS and RAP proteins share significant amino acid sequence identity, RAS signaling functions primarily in the regulation of growth, differentiation, and apoptosis, whereas RAP signaling regulates adhesion of cells to extracellular matrix, as well as formation and possibly stabilization of cell–cell junctions.36
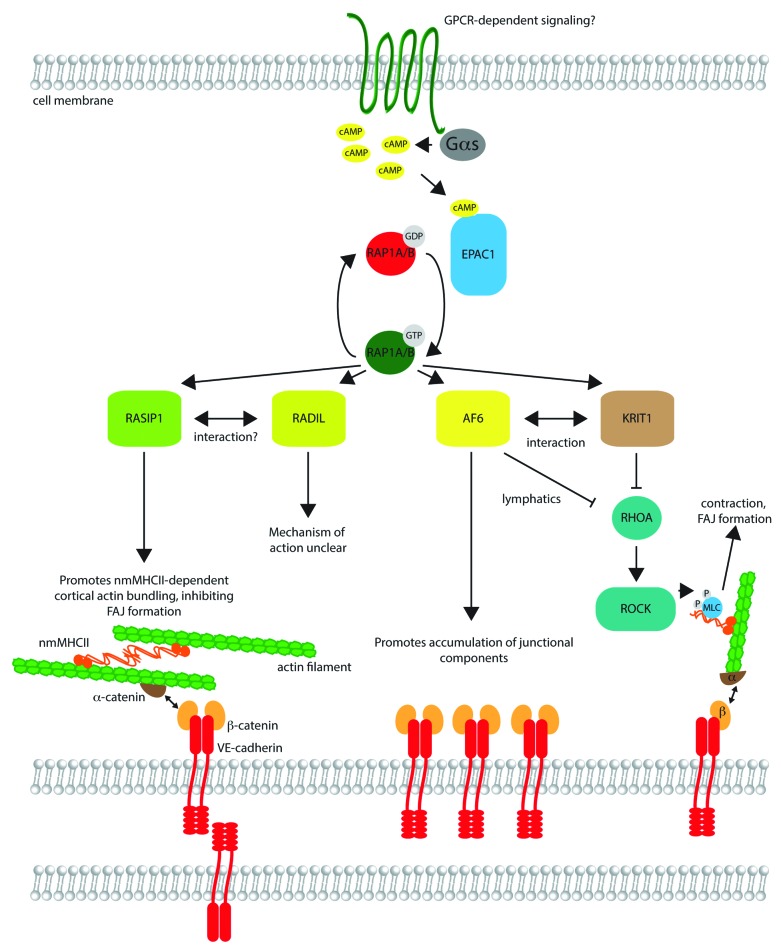
Figure 1. Signaling by RAP1 effectors in endothelial barrier control. Upstream signaling at the cell membrane, potentially mediated by G-protein coupled receptors (GPCRs), triggers activation of adenylyl cyclase via Gαs, resulting in formation of cyclic AMP (cAMP). cAMP binds to EPAC1, a guanine nucleotide exchange factor, which catalyzes exchange of GDP on RAP1A/B for GTP, activating the small G protein. Active RAP1A/B may bind to effectors such as RASIP1, RADIL, AF6, and KRIT1, in many cases triggering their movement to the cell cortex (not shown). RASIP1 promotes bundling of cortical actin, cross-linked by non-muscle myosin heavy chain II (nmMHCII). The cortical actin bundles are linked to transmembrane VE-cadherin molecules through α-catenin and β-catenin, cytosolic adaptor proteins. Assembly of this actin network may inhibit the formation of focal adherens junctions, comprised of cadherin–catenin complexes linked to longitudinal stress fibers. AF6 (also known as afadin) promotes accumulation of junctional components, and binds directly to β-catenin, as well as KRIT1. AF6 and KRIT1 suppress RHOA activation in certain cell types (e.g., AF6 in lymphatic endothelial cells). Inhibition of RHOA reduces phosphorylation of myosin light chain (MLC) by Rho kinase (ROCK), reducing contraction and potentially FAJ formation. The role of RADIL is less clear, but it may partner with RASIP1 and also inhibit RHOA signaling in some contexts.
Two isoforms of RAP1, RAP1A and RAP1B, have been identified, which appear to have overlapping and distinct functions.37 Targeted systemic knockout of murine Rap1a results in defective leukocyte adhesion, altered myeloid cell function, and partially penetrant embryonic and peri-natal lethality resulting from hemorrhage (Table 1), although the severity of these effects is dependent on the genetic background utilized.38-40 Rap1a–/– mice also display reduced neovascularization in hindlimb ischemia models.38 Rap1b knockouts have 50% lethality after E12.5, and also a mild deficiency in platelet function (Table 1).41 Closer examination of postnatal retinas in Rap1b–/– mice indicated a delay in angiogenesis at day 7 (P7), although this delay resolved by P14.42 Ex vivo aortic ring sprouting assays also indicated a reduction in angiogenic sprout outgrowth in response to basic FGF (bFGF) and VEGF.42 Vascular defects and hemorrhage are present in each knockout, and the endothelial cell-autonomous requirements for each Rap1 gene have yet to be clearly defined. Conditional deletion of Rap1b and one allele of Rap1a from endothelial and a small subset of hematopoietic cells using a Tie2-Cre driver causes retinal angiogenesis defects similar to those observed in the single Rap1b knockout.43 The primary data describing any embryonic phenotypes of these animals, as well as the EC-specific double homozygous knockout of Rap1a and Rap1b have not yet been published.43,44 These experiments would shed light on potential redundancies and unique functions of Rap1a and Rap1b in the endothelium.
Table 1. In vitro and in vivo phenotypes of EPAC1-RAP1-effectors.
| Gene name | Loss-of-function endothelial junction phenotype in vitro | Loss-of-function phenotype in vivo | Endothelial junction morphology in vivo | Key references |
|---|---|---|---|---|
| EPAC1/RAPGEF3 | Increased permeability, disorganized junctions, loss of cortical actin | Systemic KO not lethal, EC-specific KO not reported | Not reported | 48 , 73 - 75 |
| RAP1A | Increased permeability, disorganized junctions, loss of cortical actin, increase in gaps between cells, apparent increase in FAJs | Systemic KO displays partially penetrant embryonic/perinatal lethality, hemorrhage, edema | Not reported | 51 , 54 |
| RAP1B | Increased permeability, disorganized junctions, loss of cortical actin, apparent increase in FAJs | Systemic KO shows 50% hemorrhage, lethality after E12.5, transient delay in angiogenesis in neonatal retina | Not reported | 41 , 51 , 54 |
| Afadin/AF6 | Reduction of junctional marker staining at cell periphery, increased actin stress fibers in lymphatic ECs | Systemic KO embryonic lethal by E10.5, EC-specific KO shows mostly penetrant edema and lethality by E16.5 | Punctate VE-cadherin staining in lymphatic ECs | 57 - 60 |
| KRIT1/CCM1 | Increased P-MLC and stress fibers, disrupted β-catenin localization | Systemic KO is embryonic lethal between E10–11; EC-specific neonatal KO displays hemorrhage, vessel dilation | Disorganized VE-cadherin in neonatal cranial vessels | 12 , 63 , 66 |
| RASIP1 | Increased permeability, disorganized junctions, loss of cortical actin, increase in FAJs, compromised barrier function | Embryonic lethality between E9.5–10.5 in systemic knockout, abnormal blood vessel development, hemorrhage | Increase in FAJ proportion in yolk sac | 53 , 67 , 69 , 70 |
| RADIL | Loss of cortical actin, increase in FAJs, compromised barrier function in combination with RASIP1 knockdown | Altered neural crest migration (zebrafish), systemic murine knockout not reported | Not reported | 70 , 72 |
The roles of RAP1A and RAP1B in endothelial cells have been best studied in vitro, primarily through overexpression and gene knockdown studies examining the RAP1 isoforms themselves, or various GAPs and GEFs that regulate RAP1 function. Knockdown of either isoform leads to disruption of integrin-mediated adhesion, with consequences such as impaired VEGF-dependent migration; overexpression of constitutively active RAP1 also inhibits migration, resulting from increased integrin-dependent adhesion.38,45,46 RAP1 and its upstream GEF EPAC1 have been implicated in endothelial barrier control, as activation of RAP1 by EPAC1 impairs induction of permeability by thrombin or VEGF.47-50 Further, loss-of-function studies of RAP1 in vitro result in VE-cadherin disorganization, a reduction in linear junction-associated actin, and an increase in FAJs (Table 1).51-54 There are discrepancies in the literature as to whether RAP1A or RAP1B is the primary contributor to the formation and stabilization of endothelial junctions, as RNAi-mediated knockdown of either isoform leads to disruptions of VE-cadherin staining, increased formation of FAJs and/or gaps between cells, and reduction in transendothelial resistance (Table 1).54 These inconsistencies may be a result of utilization of endothelial cells from different vascular beds, different siRNA sequences with possible off-target effects, or different culture conditions. Nonetheless, recent work indicates that differential localization of RAP1 isoforms may be a contributor to distinct isoform-dependent functions, as RAP1A localizes more strongly to junctions than RAP1B.54
RAP1 Effectors Regulate Actin Linkage and Remodeling at Endothelial Junctions
RAP1 exerts its effects on junctions through control of effectors that impact cytoskeletal and junctional actin organization. The effects of RAP1 on actin remodeling and barrier stabilization may be partially through GEFs for the small G protein RAC1, as RAP1 has been shown to interact with VAV1 and TIAM1, which activate RAC1 and promote actin bundling at the leading edge of migrating cells, and thus, stabilize nascent or remodeling contacts.36 Recent data have implicated RAP1 in direct regulation of actin bundling in epithelial cells, through signaling to non-muscle myosin heavy chain II isoform B (nmMHCIIB), which promotes formation of linear junctions with circumferential actin bundles (Fig. 1).55 In addition, RAP1-specific effectors such as afadin and KRIT1/CCM1 carry out distinct functions at junctions.
Afadin/AF6 is a broadly expressed cytosolic protein that localizes to junctions and interacts with nectins and the actin cytoskeleton, as well as a number of other cytoskeletal regulators.56 The N terminus of afadin contains two Ras superfamily-association (RA) domains, which mediate interaction of afadin with active RAP1.56 RAP1 signaling drives afadin localization, as overexpression of constitutively active RAP1 increases afadin localization at the cell periphery, whereas inhibition of RAP1 signaling through overexpression of RAP1GAP redistributes afadin to the perinuclear region of the cytosol.57 Knockdown of afadin in endothelial cells results in decreased staining of junctional components at cell–cell contacts, although it is unclear if this reflects a reduction in total levels or a reorganization of junctional structures (Fig. 1, Table 1).57 Knockout of afadin is embryonic lethal but endothelial-specific knockout of afadin results in partially penetrant embryonic lethality, resulting from subcutaneous lymphedema (Table 1).58-60 Animals surviving embryogenesis exhibit aberrant retinal angiogenesis and mislocalized junctional VE-cadherin, as well as defective angiogenic sprouting in Matrigel or hindlimb ischemia assays in vivo.56 Thus, afadin appears to be critical for proper vascular function, at different stages of angiogenesis, both in blood vessels and lymphatic vessels.
KRIT1/CCM1 was identified in a yeast two-hybrid screen for Rap1 interactors, and contains a FERM domain responsible for interactions with the actin cytoskeleton and integral membrane proteins.61 Activation of Rap1 signaling stimulates release of KRIT1 from microtubules and promotes its translocation to cell–cell junctions, where it interacts with CCM2, CCM3, and other components of junctions, including afadin and β-catenin, to promote barrier function and endothelial polarity.62-64 KRIT1 is thought to act by suppressing RhoA activation that leads to phosphorylation of myosin light chain (MLC), activating myosin-based contraction and leading to the formation of stress fibers and barrier disruption (Table 1).64 KRIT1 also plays a role in activating integrin-based focal adhesions, as binding of KRIT1 to ICAP-1, a negative regulator of β1 integrin, permits talin binding to integrin and subsequent activation (Figure 1).65 Mouse Krit1 knockouts are embryonic lethal by E11.0, with dilation of both the dorsal aorta and cranial vessels.66 Inducible endothelial-specific knockout of Krit1 in neonatal mice also results in cranial vessel dilation, with accompanied leak, hemorrhage, and mislocalization of VE-cadherin (Table 1).12 The distinct roles of KRIT1 and afadin in promoting vascular barrier function through disparate mechanisms suggests that Rap1 effectors may cooperate, either additively or through coordination, of different signaling cascades.
A Role for the RAP1 Effector RASIP1 in Junctional Actin Assembly and Stabilization
Ras-interacting protein 1 (RASIP1) was also identified through a yeast two-hybrid screen as a protein that preferentially interacts with H-RAS, K-RAS, as well as RAP1.67 It was predicted to be a RAS/RAP effector and member of the afadin protein family on the basis of an identified N-terminal RA domain, as well as forkhead-associated and dilute domains also shared with afadin.67 Subsequent work demonstrated that Rasip1 is highly expressed in embryonic and adult vasculature and is below detectable levels in non-vascular tissue in vertebrates.53,68,69 We and others have shown that knockout of Rasip1 in mice results in pericardial edema, multifocal hemorrhage, and mid-gestational embryonic lethality, resulting from malformation of vasculogenic blood vessels and disruption of circulation (Table 1).53,69 In addition, morpholino-mediated knockdown of rasip1 expression in the developing zebrafish embryo causes blood vessel defects, including irregularly shaped axial vessels and hemorrhage around cranial and intersomitic vessels.53 Taken together, the data from multiple vertebrate species support a critical role of Rasip1 in embryonic blood vessel development.53,68,69
Biochemical and fluorescent resonance energy transfer studies demonstrated that RASIP1 preferentially interacts with GTP-bound RAP1.53,70 Knockdown of RASIP1 in human umbilical vein endothelial cells (HUVEC) results in increased permeability of cell monolayers to FITC-dextran, indicating functional disruption of the endothelial barrier.53 These effects have also been observed with knockdown of RAP1,49 and support the hypothesis that RASIP1 is a downstream RAP1 effector that promotes endothelial barrier function. Loss of RASIP1 further increased monolayer permeability when endothelial cells were exposed to thrombin, which induces cell contraction, and counteracted the effect of Angiopoietin 1, which signals through Tie2 to strengthen barrier function.53 Physical evidence of disrupted cell–cell contacts was shown, as nascent junctions in RASIP1-deficient ECs displayed a greater distance between adjacent membranes when examined via electron microscopy.53 The compromised barrier function in response to physiological stimuli in RASIP1 knockdown HUVEC suggests that RASIP1 may play a key role in controlling how vessels behave in pathological angiogenesis and in response to inflammatory stimuli, but this hypothesis awaits examination in preclinical in vivo disease models.
The reduction in barrier function in RASIP1-deficient cells is likely a consequence of a loss of linear junctions and associated circumferential actin bundles, as actin no longer efficiently assembles at endothelial junctions, but rather is found shifted away from cell-cell contacts (Table 1).53,70 Interestingly, this loss of circumferential actin upon disruption of RASIP1 is accompanied by an increase in FAJs, which appear more rapidly after endothelial junction formation and are found at a higher frequency both in vitro and in embryonic vascular beds in vivo.53,70 The inhibition of FAJ formation in ECs overexpressing a constitutively active form of RAP1A (G12V) was also shown to be RASIP1-dependent.53 RAP1A-G12V suppressed FAJ formation, but was unable to do so in RASIP1-deficient ECs.53 The mechanism of RASIP1 action on FAJ formation remains elusive, but we envision two potential mechanisms. First, RASIP1 may act directly to promote circumferential actin bundling, through regulation of cross-linking molecules such as nmMHCIIB or other as-yet-unidentified partners. Second, RASIP1 might suppress FAJ formation or junction remodeling directly, and its loss would therefore alter equilibrium between linear and FA junctions. We note that RASIP1 knockdown affects actin bundling at “free edges” or lamellipodia of cells, and also disrupts cell spreading shortly after initial adhesion to tissue culture plastic, processes which rely on coordinated actin remodeling.53,70 Thus, RASIP1 may play a fundamental role in the coordination of EC actin dynamics, which could have distinct outcomes in either an isolated cell or an EC in contact with its neighbors.
Many intriguing questions remain concerning RASIP1 function, possible interplay with other RAP1 effectors, and upstream and downstream binding partners. Upstream activators remain somewhat elusive, although the involvement of cAMP suggests a role for Gαs coupled GPCRs. The RAP1 effectors RASIP1, afadin, KRIT1, and RADIL promote circumferential actin bundling to some extent (Fig. 1, Table 1), and their loss disrupts function of the endothelial barrier. However, comparison of RASIP1 and KRIT1 knockdown HUVEC showed a subtle yet distinct change in actin organization, and a difference in activation of RHOA signaling.53 The relationship of RASIP1 to RHOA signaling is still unclear, as RASIP1 is reported to signal to ARHGAP29 and inhibit RHOA-mediated activation of myosin light chain phosphorylation (and thus actin contraction), yet there are conflicting data as to whether loss of RASIP1 results in global RHOA activation and MLC2 phosphorylation.53,69,70 Perhaps, RAP1 effectors act at distinct stages of circumferential actin bundling, and provide specific signals to activate or suppress regulators of actin remodeling such as RHOA, RAC1, and CDC42. Recent data indicates that RAP1 suppresses RHOA-dependent activation of nmMHCII, yet promotes circumferential actin bundling through activation of myotonic dystrophy kinase-related CDC42-binding kinase, but it is unknown which, if any RAP1 effector functions in this pathway.71 Finally, the differing effects of afadin, RASIP1, and RADIL, which is a third member of the afadin protein family, on vascular development in vivo remain to be fully understood. As mentioned, loss of Rasip1 in mice and fish causes early defects in embryonic blood vessels, whereas afadin knockout only affects embryonic lymphatic vessels. Radil-knockout mice have not yet been described, but knockdown of radil in the zebrafish has no overt defect in vascular development (Table 1).72 We hypothesize that these three family members may share some redundant functions, and gene duplication followed by subsequent subfunctionalization and/or localization has contributed to the distinct phenotypes reported. Further in vivo examination is required, especially EC-specific combinatorial knockouts, to fully address this question.
Disclosure of Potential Conflicts of Interest
The authors are employees of Genentech, Inc., a member of the Roche Group.
Acknowledgments
The authors thank J Burton and P Vitorino for comments and suggestions regarding the manuscript.
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–84. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 3.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–26. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konisti S, Kiriakidis S, Paleolog EM. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:153–62. doi: 10.1038/nrrheum.2011.205. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012;320:130–7. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Schlingemann RO, Witmer AN. Treatment of retinal diseases with VEGF antagonists. Prog Brain Res. 2009;175:253–67. doi: 10.1016/S0079-6123(09)17517-9. [DOI] [PubMed] [Google Scholar]
- 10.Vestweber D. Novel insights into leukocyte extravasation. Curr Opin Hematol. 2012;19:212–7. doi: 10.1097/MOH.0b013e3283523e78. [DOI] [PubMed] [Google Scholar]
- 11.Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19:218–23. doi: 10.1097/MOH.0b013e3283523e1c. [DOI] [PubMed] [Google Scholar]
- 12.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–6. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 13.Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17:860–71. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–21. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–15. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1:a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oas RG, Nanes BA, Esimai CC, Vincent PA, García AJ, Kowalczyk AP. p120-catenin and β-catenin differentially regulate cadherin adhesive function. Mol Biol Cell. 2013;24:704–14. doi: 10.1091/mbc.E12-06-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–32. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–84. doi: 10.1182/blood.V97.6.1679. [DOI] [PubMed] [Google Scholar]
- 22.Gotsch U, Borges E, Bosse R, Böggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110:583–8. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 23.Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–70. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strilić B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–15. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–80. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 26.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci. 2013;126:2545–9. doi: 10.1242/jcs.124529. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Martín L, Marcos-Ramiro B, Bigarella CL, Graupera M, Cain RJ, Reglero-Real N, Jiménez A, Cernuda-Morollón E, Correas I, Cox S, et al. Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2012;32:e90–102. doi: 10.1161/ATVBAHA.112.252080. [DOI] [PubMed] [Google Scholar]
- 28.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–52. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millán J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernández-Martín L, Correas I, Ridley AJ. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol Biol Cell. 2012;23:310–23. doi: 10.1091/mbc.E11-08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci. 2013;126:403–13. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 32.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 33.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–17. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frische EW, Zwartkruis FJ. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 35.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 36.Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788:790–6. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Chrzanowska-Wodnicka M. Regulation of angiogenesis by a small GTPase Rap1. Vascul Pharmacol. 2010;53:1–10. doi: 10.1016/j.vph.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol. 2008;28:5803–10. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Yan J, De P, Chang HC, Yamauchi A, Christopherson KW, 2nd, Paranavitana NC, Peng X, Kim C, Munugalavadla V, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–31. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–53. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–7. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111:2647–56. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ₃. Blood. 2011;118:2015–26. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrzanowska-Wodnicka M. Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction. Exp Cell Res. 2013;319:2350–9. doi: 10.1016/j.yexcr.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmona G, Göttig S, Orlandi A, Scheele J, Bäuerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler S, et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113:488–97. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- 46.Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang WJ, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem. 2007;282:19781–7. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]
- 47.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 48.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–72. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 49.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–46. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–82. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 51.Pannekoek WJ, van Dijk JJ, Chan OY, Huveneers S, Linnemann JR, Spanjaard E, Brouwer PM, van der Meer AJ, Zwartkruis FJ, Rehmann H, et al. Epac1 and PDZ-GEF cooperate in Rap1 mediated endothelial junction control. Cell Signal. 2011;23:2056–64. doi: 10.1016/j.cellsig.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–20. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson CW, Parker LH, Hall CJ, Smyczek T, Mak J, Crow A, Posthuma G, De Mazière A, Sagolla M, Chalouni C, et al. Rasip1 regulates vertebrate vascular endothelial junction stability through Epac1-Rap1 signaling. Blood. 2013;122:3678–90. doi: 10.1182/blood-2013-02-483156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittchen ES, Aghajanian A, Burridge K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases. 2011;2:65–76. doi: 10.4161/sgtp.2.2.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–42. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 57.Tawa H, Rikitake Y, Takahashi M, Amano H, Miyata M, Satomi-Kobayashi S, Kinugasa M, Nagamatsu Y, Majima T, Ogita H, et al. Role of afadin in vascular endothelial growth factor- and sphingosine 1-phosphate-induced angiogenesis. Circ Res. 2010;106:1731–42. doi: 10.1161/CIRCRESAHA.110.216747. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, et al. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–32. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Majima T, Takeuchi K, Sano K, Hirashima M, Zankov DP, Tanaka-Okamoto M, Ishizaki H, Miyoshi J, Ogita H. An Adaptor Molecule Afadin Regulates Lymphangiogenesis by Modulating RhoA Activity in the Developing Mouse Embryo. PLoS One. 2013;8:e68134. doi: 10.1371/journal.pone.0068134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhadanov AB, Provance DW, Jr., Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr Biol. 1999;9:880–8. doi: 10.1016/S0960-9822(99)80392-3. [DOI] [PubMed] [Google Scholar]
- 61.Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA. Association of Krev-1/rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21-22. Oncogene. 1997;15:1043–9. doi: 10.1038/sj.onc.1201268. [DOI] [PubMed] [Google Scholar]
- 62.Béraud-Dufour S, Gautier R, Albiges-Rizo C, Chardin P, Faurobert E. Krit 1 interactions with microtubules and membranes are regulated by Rap1 and integrin cytoplasmic domain associated protein-1. FEBS J. 2007;274:5518–32. doi: 10.1111/j.1742-4658.2007.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–54. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010;207:881–96. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Clatterbuck RE, Rigamonti D, Chang DD, Dietz HC. Interaction between krit1 and icap1alpha infers perturbation of integrin beta1-mediated angiogenesis in the pathogenesis of cerebral cavernous malformation. Hum Mol Genet. 2001;10:2953–60. doi: 10.1093/hmg/10.25.2953. [DOI] [PubMed] [Google Scholar]
- 66.Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–48. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- 67.Mitin NY, Ramocki MB, Zullo AJ, Der CJ, Konieczny SF, Taparowsky EJ. Identification and characterization of rain, a novel Ras-interacting protein with a unique subcellular localization. J Biol Chem. 2004;279:22353–61. doi: 10.1074/jbc.M312867200. [DOI] [PubMed] [Google Scholar]
- 68.Xu K, Chong DC, Rankin SA, Zorn AM, Cleaver O. Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev Biol. 2009;329:269–79. doi: 10.1016/j.ydbio.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell. 2011;20:526–39. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci U S A. 2013;110:11427–32. doi: 10.1073/pnas.1306595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol. 2013;202:901–16. doi: 10.1083/jcb.201301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smolen GA, Schott BJ, Stewart RA, Diederichs S, Muir B, Provencher HL, Look AT, Sgroi DC, Peterson RT, Haber DA. A Rap GTPase interactor, RADIL, mediates migration of neural crest precursors. Genes Dev. 2007;21:2131–6. doi: 10.1101/gad.1561507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, Patrikeev I, Hao D, Stutz SJ, Dineley KT, et al. Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Mol Cell Biol. 2013;33:918–26. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kai AK, Lam AK, Chen Y, Tai AC, Zhang X, Lai AK, Yeung PK, Tam S, Wang J, Lam KS, et al. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop β-cell dysfunction and metabolic syndrome. FASEB J. 2013;27:4122–35. doi: 10.1096/fj.13-230433. [DOI] [PubMed] [Google Scholar]
- 75.Sehrawat S, Cullere X, Patel S, Italiano J, Jr., Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–70. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]