Abstract
The small GTPase Rab5 has been extensively studied in the context of endocytic trafficking because it is critical in the regulation of early endosome dynamics. In addition to this canonical role, evidence obtained in recent years implicates Rab5 in the regulation of cell migration. This novel role of Rab5 is based not only on an indirect relationship between cell migration and endosomal trafficking as separate processes, but also on the direct regulation of signaling proteins implicated in cell migration. However, the precise mechanisms underlying this connection have remained elusive. Recent studies have shown that the activation of Rab5 is a critical event for maintaining the dynamics of focal adhesions, which is fundamental in regulating not only cell migration but also tumor cell invasion.
Keywords: Rab5, focal adhesion, cell migration, invasion
Cell migration is a complex process that requires multiple events including cell polarization and extension of protrusions, along with the coordinated assembly and disassembly of cell adhesion complexes.1 Integrin-based focal adhesions (FAs) represent the main sites of cellular contact with the extracellular matrix (ECM), and these are subjected to spatio-temporal regulation during directional migration. Establishment of cellular contact with the ECM is initiated at the so-called nascent adhesions, which differ from FAs in structure and complexity. Nascent adhesions either disassemble or mature into FAs, and the correct balance between these events contributes to the traction forces implicated in cell migration.2 On one hand, the process of FA formation has been extensively studied, and the molecular players have been identified, which include kinases, adaptor and scaffolding proteins, as well as actin-linking proteins that play critical roles in FA maturation (reviewed in refs. 3 and 4). On the other hand, our current understanding of the mechanisms underlying FA disassembly have remained limited, though it is known that this process is not merely a reversal of FA formation (reviewed in refs. 4–6).
Here, I will comment on the recent findings that implicate the small GTPase Rab5 as a relevant regulator of FA disassembly and cell migration,7 which is in addition to its previously demonstrated role in promoting the formation of cellular protrusions and reorganization of the actin cytoskeleton (reviewed in ref. 8). By associating with FA proteins, Rab5 promotes their disassembly in a time-regulated manner, and this contributes not only to sustain cell migration but also to cancer cell invasion and matrix metalloproteinase (MMP) release.7 This evidence, along with previous reports, supports the hypothesis that Rab5 plays a crucial role in regulating the different steps required for cell migration, invasion, and metastasis.
Focal Adhesion Dynamics in Cell Migration
FAs are supramolecular complexes formed by more than 150 different proteins, including kinases, scaffold, and adaptor proteins, as well as actin linking proteins. Formation of these complexes has been extensively studied, and molecular players have been identified (reviewed in ref. 2). A central regulator of FA dynamics is the focal adhesion kinase (FAK), which is phosphorylated on different residues, thus affecting both its interaction with other signaling proteins and its intrinsic kinase activity (reviewed in ref. 3). A critical residue of FAK is Y397, which undergoes phosphorylation during FA maturation and has been shown to participate in both FA assembly and disassembly.9 Aside from the role of FAK and a few other signaling proteins in FA disassembly, the mechanistic details of this process remain poorly understood, although some approaches have been recently developed to dissect this phenomenon. One such approach was initially devised by Kaverina et al., who have shown that microtubules are able to induce FA disassembly via a relaxing factor that is not well understood.10 Using the microtubule-disrupting agent nocodazole, it has been shown that microtubule depolymerization/disruption is accompanied by FA stabilization/synchronization, whereas the removal of this drug is followed by FA disassembly.11 Subsequent studies have demonstrated that microtubule-induced FA disassembly occurs via clathrin-mediated endocytosis, as evidenced by the requirement of proteins, including Dab2, dynamin, clathrin, and FAK in this process.12,13 An intriguing point derived from these studies is that, regardless of the fact that FA components have been visualized en route within Rab5-positive vesicles and early endosomes, no direct connection has been established between these components and Rab5—therefore, the precise role of the early endocytic components in FA disassembly remains unclear. This is intriguing because proteins such as dynamin and Rab5 are known to be relevant in the process of cell migration by mechanisms that have, as yet, remained unclear (reviewed in refs. 8 and 14). For instance, dynamin is known to be recruited into FAs and then mediate the endocytosis of FA proteins. Dynamin is phosphorylated by Src,15 and phosphorylation is required for dynamin-dependent FA disassembly.16 Moreover, phosphorylation of dynamin is enhanced by FAK, which recruits both dynamin and Src, thereby promoting the formation of a ternary complex.16 Dynamin is implicated in both clathrin-dependent and clathrin-independent endocytosis, and hence, the question that arises is which internalization mechanism is required for FA disassembly. Evidence for both scenarios has been provided, whereby clathrin and adaptors, including Dab2, ARH, and AP2, mediate microtubule-induced integrin internalization and FA disassembly,12,13 and evidence for clathrin-independent internalization of integrins has also been presented.17,18 Differences could be due to different experimental approaches used for inducing integrin internalization and FA disassembly. Despite this, the relevance of the endocytic machinery in FA dynamics is becoming better acknowledged, and the identification of new regulators in this process will help to understand the mechanistic details.
Rab5—A New Player in Focal Adhesion Disassembly
The small GTPase Rab5 is a central regulator of vesicle and early endosome dynamics,19 but it is also implicated in other processes, such as cell migration. Early studies have already shown that Rab5 promotes lamellipodia formation,20 and subsequent work indicated that Rab5 is a signaling GTPase required for actin reorganization.21 Rab5 promotes localized activation of Rac via recruitment of the Rac-GEF factor Tiam1 within early endosomes,22 and this leads to lamellipodia and ruffle formation as well as cell migration in vitro and in vivo.22,23 Alternatively, Rab5 associates with β1 integrins, leading to their internalization and increased cell migration.23,24 The latter function calls for attention because β1 integrins are central components of FAs, and thus, it is tempting to speculate that Rab5 integrates in a complex with FA proteins, affecting their dynamics.
Our recent studies have shown that Rab5 promotes FA disassembly in tumor cells, thereby sustaining cell migration, spreading, and invasion.7 Based on immunoprecipitation and co-localization experiments, Rab5 was found to be associated, in a complex, with FA proteins, including Vinculin, Paxillin, β1 integrin, and FAK. These provoking data were obtained at steady-state and suggest that the association between Rab5 and FA proteins is weak and limited to a subset of FAs. Importantly though, this association between Rab5 and FA proteins was substantially increased in certain conditions, such as synchronous stimulation of directional migration in wound assays, and by FA stabilization/synchronization with nocodazole. The question that arises is how this limited interaction affects the more general and complex phenomenon of cell migration. The answer to this question might be drafted from live-cell imaging experiments, which showed that Rab5 co-localizes with Paxillin and Vinculin in a narrow time frame, suggesting that this association is transient. Intriguingly, GFP-Rab5-positive early endosomes were found to collide with mCherry-Paxillin-positive FAs, and this event preceded the collapse of FAs in a short time frame, suggesting that Rab5 plays a role in FA disassembly. This possibility was addressed by two approaches, the first one based on the ability of microtubules to disassemble FAs and the second on tracking mCherry-labeled FAs in live cells induced to migrate. Using both approaches, Rab5 was found to be required for FA disassembly because shRNA-mediated silencing of Rab5 delayed the kinetics of FA disassembly induced by both microtubules and motogenic stimuli. Moreover, Rab5 activity was required for FA disassembly, as shown in reconstitution assays with mutant versions of Rab5. These observations are in agreement with cell spreading and migration data, which indicate a requirement of intact Rab5 activity, and support previous reports indicating a requirement of Rab5-GTP loading for normal and tumor cell migration.22-24 Noteworthy, a recent study by Palamidessi et al. showed that RN-Tre, which is a Rab-GAP, is implicated as a “molecular brake” during cell migration, which is based on its ability to delay FA dynamics. Most importantly, the effect of RN-Tre on FA dynamics is mainly due to its GAP activity toward Rab5.25
The process of FA turnover depends on a balance between FA assembly and disassembly.6 We did not address the requirement of Rab5 in FA assembly, and this remains a relevant question to understand the precise role of Rab5 in the coordination of cell migration. For instance, Rab5 could be activating downstream recycling pathways, such as the Rab11 loop—a pathway known to be implicated in the recycling of β1 integrins26—thus contributing to the recycling of integrins and FA components to the cell surface, providing new supplies of material for newer contacts. This possibility has not been explored thus far, and future studies will be challenging to have a comprehensive model for the regulation of FA dynamics.
These observations have provided insights into a novel mechanism whereby Rab5 promotes cell migration via control of FA disassembly (Fig. 1). However, some questions remain to be addressed, such as the exact nature of the association between Rab5 and FA proteins. Our data suggest that Rab5 integrates in a complex with Vinculin, Paxillin, and integrin β1, but whether this integration is direct or indirect has not been explored. Likewise, earlier studies have shown that Rab5 co-immunoprecipitates with integrin β1, although the identity of specific α subunits present in this complex is unknown.24 We speculate that Rab5 associates indirectly with FA proteins in a complex that requires β1 integrins, but this possibility needs to be assessed by specific in vitro approaches. Intriguingly, the association of β1 integrins and Rab5 was previously reported to be dependent on GTP loading because Rab5/wild-type and the Rab5/Q79L mutant (GTPase deficient, locked in the active conformation), but not Rab5/S34N (high affinity for GDP, locked in the inactive state), were found to co-immunoprecipitate with β1.23 This is in agreement with our observations that Rab5-GTP, but not Rab5-GDP, promotes FA disassembly, and that, upon cell spreading, ectopically expressed Rab5/wild-type and Rab5/Q79L, but not Rab5/S34N, accumulated at FA-enriched fractions.
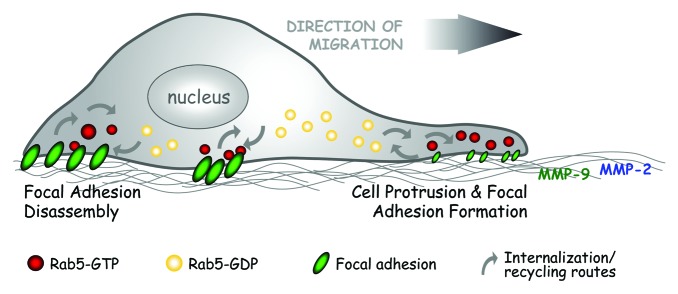
Figure 1. The scheme shows a proposed model for Rab5-driven cell migration and invasion. Both phenomena depend on FA dynamics. By associating with FA proteins such as Vinculin, Paxillin, FAK, and integrin β1 (not detailed in the scheme), Rab5 promotes FA disassembly, leading to sustained and directional cell migration. Although no direct evidence is available regarding the precise role of Rab5 in FA re-assembly, based on data shown in this study and in previous work,8,20,22 Rab5 stimulates cellular protrusions and functional Rab5 is required for cell spreading and the formation of new cell-ECM contacts. Alternatively, by controlling FA dynamics, Rab5 is implicated in MMP-2 and MMP-9 release, which is required for tumor cell invasion.
The Link with Tumor Cell Invasion
An intriguing finding from this study was that Rab5 activity not only promotes tumor cell migration, but also invasiveness. Increasing evidence indicates that FAs represent a “hot spot” for ECM degradation, and hence, that the dynamics of FAs are coupled to cell invasion.27 In addition to other specialized structures, such as invadopodia and podosomes, FA-driven ECM degradation contributes to ECM remodeling and invasion.28 With this in mind, it is tempting to speculate that Rab5 is involved in tumor cell invasion through the modulation of FA dynamics. In fact, our studies showed that Rab5 activation is required for the release of matrix metalloproteinases, MMP-2 and MMP-9, and invasion in 3D matrices. This phenomenon highlights the relevance of Rab5 in tumor cell migration and invasion in vivo because the activity of this small GTPase seems to promote the acquisition of mesenchymal characteristics. The latter is in agreement with previous studies by Palamidessi et al., which suggested that Rab5 influences the migratory switch from an amoeboid to a mesenchymal phenotype in invasive cancer cells.22 Future studies will be needed to provide a better understanding of the mechanisms associated with Rab5-mediated MMP release/activation and ECM remodeling.
Perspectives
With the identification of Rab5 and other Rab GTPases in processes other than intracellular trafficking, the necessity to understand their regulation will be demanding. In the context of intracellular trafficking, several regulators of Rab5 function have been characterized in the last decade (reviewed in ref. 19). Thus, the question that remains unanswered is which mechanisms of regulation are implicated in Rab5 activation during cell migration. Some candidates have been identified, including GEFs29,30 and GAPs,21,31 but this list is likely to increase, which will help provide a better understanding on the role of this small GTPase in tumor cell migration and invasion.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by FONDECYT Initiation #11100287 and CONICYT #79090021 “Insertion of Young Postdoctoral Researchers in the Academy.”
Glossary
Abbreviations:
- ECM
extracellular matrix
- FA
focal adhesion
- FAK
focal adhesion kinase
- shRNA
short hairpin RNA
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol. 2012;24:116–24. doi: 10.1016/j.ceb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 4.Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol. 2012;24:569–81. doi: 10.1016/j.ceb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza P, Ortiz R, Díaz J, Quest AF, Leyton L, Stupack D, Torres VA. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J Cell Sci. 2013;126:3835–47. doi: 10.1242/jcs.119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VA, Stupack DG. Rab5 in the regulation of cell motility and invasion. Curr Protein Pept Sci. 2011;12:43–51. doi: 10.2174/138920311795659461. [DOI] [PubMed] [Google Scholar]
- 9.Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Rondé P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118:4415–25. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- 10.Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–90. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–90. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 12.Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583:1337–43. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–47. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briñas L, Vassilopoulos S, Bonne G, Guicheney P, Bitoun M. Role of dynamin 2 in the disassembly of focal adhesions. J Mol Med (Berl) 2013;91:803–9. doi: 10.1007/s00109-013-1040-2. [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol. 2010;30:781–92. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Cao H, Chen J, McNiven MA. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol Biol Cell. 2011;22:1529–38. doi: 10.1091/mbc.E10-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Echarri A, Del Pozo MA. Caveolae internalization regulates integrin-dependent signaling pathways. Cell Cycle. 2006;5:2179–82. doi: 10.4161/cc.5.19.3264. [DOI] [PubMed] [Google Scholar]
- 18.Fabbri M, Di Meglio S, Gagliani MC, Consonni E, Molteni R, Bender JR, Tacchetti C, Pardi R. Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the alphaL/beta2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell. 2005;16:5793–803. doi: 10.1091/mbc.E05-05-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 20.Spaargaren M, Bos JL. Rab5 induces Rac-independent lamellipodia formation and cell migration. Mol Biol Cell. 1999;10:3239–50. doi: 10.1091/mbc.10.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–14. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 22.Palamidessi A, Frittoli E, Garré M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–47. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Torres VA, Mielgo A, Barbero S, Hsiao R, Wilkins JA, Stupack DG. Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell. 2010;21:369–76. doi: 10.1091/mbc.E09-09-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 2006;173:767–80. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palamidessi A, Frittoli E, Ducano N, Offenhauser N, Sigismund S, Kajiho H, Parazzoli D, Oldani A, Gobbi M, Serini G, et al. The GTPase-activating protein RN-tre controls focal adhesion turnover and cell migration. Curr Biol. 2013;23:2355–64. doi: 10.1016/j.cub.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 26.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–402. doi: 10.1016/S0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–85. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 29.Kunita R, Otomo A, Mizumura H, Suzuki-Utsunomiya K, Hadano S, Ikeda JE. The Rab5 activator ALS2/alsin acts as a novel Rac1 effector through Rac1-activated endocytosis. J Biol Chem. 2007;282:16599–611. doi: 10.1074/jbc.M610682200. [DOI] [PubMed] [Google Scholar]
- 30.Sandri C, Caccavari F, Valdembri D, Camillo C, Veltel S, Santambrogio M, Lanzetti L, Bussolino F, Ivaska J, Serini G. The R-Ras/RIN2/Rab5 complex controls endothelial cell adhesion and morphogenesis via active integrin endocytosis and Rac signaling. Cell Res. 2012;22:1479–501. doi: 10.1038/cr.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres VA, Mielgo A, Barilà D, Anderson DH, Stupack D. Caspase 8 promotes peripheral localization and activation of Rab5. J Biol Chem. 2008;283:36280–9. doi: 10.1074/jbc.M805878200. [DOI] [PMC free article] [PubMed] [Google Scholar]


