Abstract
The successful transformation of uterine spiral arteries by invasion trophoblasts is critical for the formation of the human hemochorial placenta. Placental trophoblast migration and invasion are well regulated by various autocrine/paracrine factors at maternal–fetal interface. Human placental multipotent mesenchymal stromal cells (hPMSCs) are a subpopulation of villous mesenchymal cells and have been shown to produce a wide array of soluble cytokines and growth factors including HGF (hepatocyte growth factor). The function of hPMSCs in placental villous microenvironment has not been explored. The interaction between hPMSCs and trophoblasts was proposed in vitro in a recent article. HGF produced by hPMSCs was able to engage c-Met receptor on trophoblast and induced the trophoblast cAMP expression. The cAMP activated PKA, which in turn, signaled to Rap1 and led to integrin β1 activation. The total integrin β1 protein expression by trophoblasts was not affected by HGF stimulation. Hypoxia downregulated HGF expression by hPMSCs. HGF and PKA activator 6-Bnz-cAMP increased trophoblast adhesion and migration that were inhibited by PKA inhibitor H89 or Rap1 siRNA. Thus, hPMSCs-derived paracrine HGF can regulate trophoblast migration during placentation. These findings provided insight revealing at least one mechanism by which hPMSCs implicated in the development of preeclampsia.
Keywords: Rap1, hepatocyte growth factor, integrin β1, placental multipotent mesenchymal stromal cells, protein kinase A
Comment
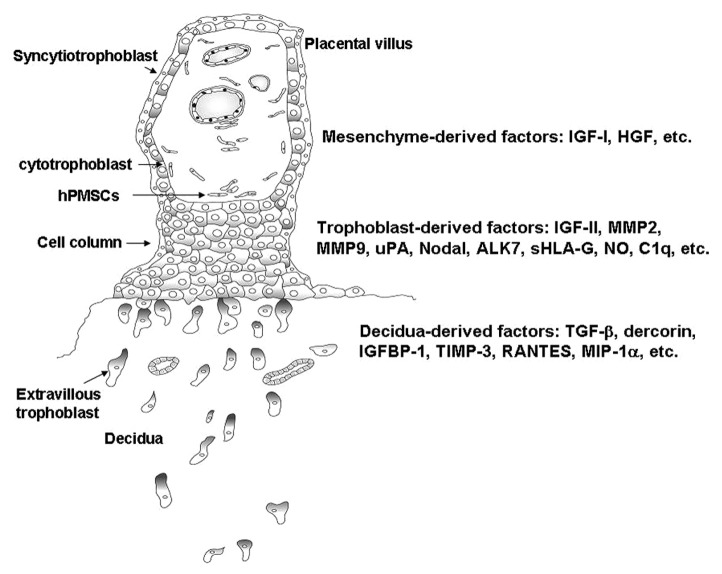
At the human maternal–fetal interface, placental villous trophoblasts form the cell column and differentiate toward extravillous trophoblasts (EVTs). EVTs acquire invasive capability and begin to migrate into the maternal decidua. The maternal uterine spiral arteries, which have high resistance before pregnancy are subjected to remodeling through replacement of the vascular endothelium and smooth muscle cells by invasion of trophoblasts. EVT invasion leads to the transformation of the uterine spiral arteries into large diameter with high-flow and low-resistance vessels that are capable of mediating efficient gas and nutrient exchange between mother and fetus. These steps are critical events for the successful formation of the hemochorial placenta in humans. Faulty vascular remodeling of uterine spiral arteries impairs placental blood flow resulting in placental hypoxia, which in turn, leads to release of placental factors into the maternal circulation causing systemic inflammatory reaction and endothelial activation with subsequent development of preeclampsia.1 Trophoblast invasion of decidua is regulated by a series of signaling events that include the cross talk between trophoblast and decidual at the maternal–fetal interface. Previous studies have suggested that migratory and invasive functions of EVT cells are well regulated in an autocrine/paracrine manner by a variety of factors in the EVT cell and decidual microenvironment, which include growth factors, growth factor-binding proteins, proteoglycans, and the extracellular matrix components that interact with receptors and their ligands on the EVT cell surface.2,3 These factors that regulate EVT migration and invasion can further be classified into three groups. The first group contains the autocrine factors derived from trophoblasts, which include IGF-II (insulin-like growth factor-II), metalloproteinase (MMP)2, MMP9,4 urokinase-type plasminogen activator,5 Nodal and ALK7 (activin receptor-like kinase 7),6 sHLA-G (soluble human leukocyte antigen-G),7 NO (nitric oxide),8 and C1q,9 etc. These factors expressed by EVT may drive the trophoblast migration or interact with the factors either secreted by deciduas or expressed on the cell surface of deciduas. Factors derived from deciduas include TGF-β (transforming growth factor-β), dercorin,10 IGFBP-1 (insulin-like growth factor-binding protein-1), TIMP-3 (tissue inhibitors of matrix metalloproteinase-3),4 RANTES (regulated on activation, normally T-expressed, and presumably secreted), and MIP-1α (macrophage inflammatory protein-1α),11 etc. These factors may either restrain or promote EVT migration through the ligands produced by EVT. The third group contains the factors derived from placental villous mesenchyme that include IGF-I12 and hepatocyte growth factor (HGF),8 etc. These factors play as paracrine factors that trigger the EVT migration and modulate the distance of their invasion12 (Fig. 1). Such a signal diminishes with distance as the EVT migrate away the anchoring villus and is self-limiting when the in situ decidual factor eventually inhibit the stimulatory signal.12
Figure 1. Schematic representation of placental anchoring villus on maternal decidua. The extravillous cytotrophoblasts migrate from cell column and infiltrate the deciduas through the factors derived either from villous mesenchyme, trophoblast, or decidua. ALK7, activin receptor-like kinase 7; HGF, hepatocyte growth factor; hPMSC, human placental multipotent mesenchymal stromal cell; IGF-I/II, insulin-like growth factor-I/II; IGFBP-1, insulin-like growth factor-binding protein-1; MIP-1α, macrophage inflammatory protein-1α; MMP, metalloproteinase; NO, nitric oxide; RANTES, regulated on activation, normally T-expressed, and presumably secreted; sHLA-G, soluble human leukocyte antigen-G; TGF-β, transforming growth factor-β; TIMP-3, tissue inhibitors of matrix metalloproteinase-3; uPA, urokinase-type plasminogen activator.
The placental villous stroma expresses HGF and its receptor c-Met is present in the trophoblasts. HGF is known to stimulate trophoblast motility via phosphatidylinositide 3-kinase and mitogen-activated protein–kinase signaling cascades.13,14 In a recent report, we set up an in vitro model to examine HGF-c-Met signaling in SSEA4-positive human placental mulitipotent mesenchymal stromal cell (hPMSC) and trophoblast interaction. hPMSCs are a subpopulation of villous stromal cells. We have demonstrated that hPMSCs express mesenchymal stem cell markers such as SSEA4, CD44, CD54, CD73, CD90, CD105, and CD166, Oct4, and are abundant in the villous stroma. Because of the energy demand and increasing metabolism, reactive oxygen species are increased during pregnancy. The hPMSCs can secret paracrine factors that protect endothelium from oxidative injury via interleukin 6 signal transducer/STAT3 and manganese superoxide dismutase activation.15 Manganese superoxide dismutase scavenges oxidative stress-induced cellular reactive oxygen species that may function as signaling intermediaries. This mechanism reveals that villous endothelial cells can be protected against oxidative damage by paracrine factors conferred by hPMSCs.15 Our previous data revealed the paracrine factors contain various cytokines and growth factors.15 Furthermore, hPMSCs can be induced to differentiate into endothelial cells and involve in vascularization through integrin α5β1 in vitro and in vivo.16 These observations indicate that hPMSCs may participate in the vascularization of placental villus. In addition to contributing to the vessel formation through differentiation and integration into vascular structure, we further observed these SSEA4-positive hPMSCs that adjacent to the trophoblasts of cell columns can modulate trophoblast migration by paracrine mechanisms during placentation.17 In that report, we proposed hPMSCs secreted HGF, which modulated trophoblast migration and increased MMP9 expression through c-Met receptor ligation and production of the second messenger 3′,5′-cyclic adenosine monophosphate (cAMP), leading to protein kinase A (PKA)-induced Rap1 and integrin β1 activation of trophoblasts (Fig. 2). Thus, villous mesenchymal stromal cells, especially hPMSCs, have a novel function to induce lining trophoblast migration in placental villous microenvironment.
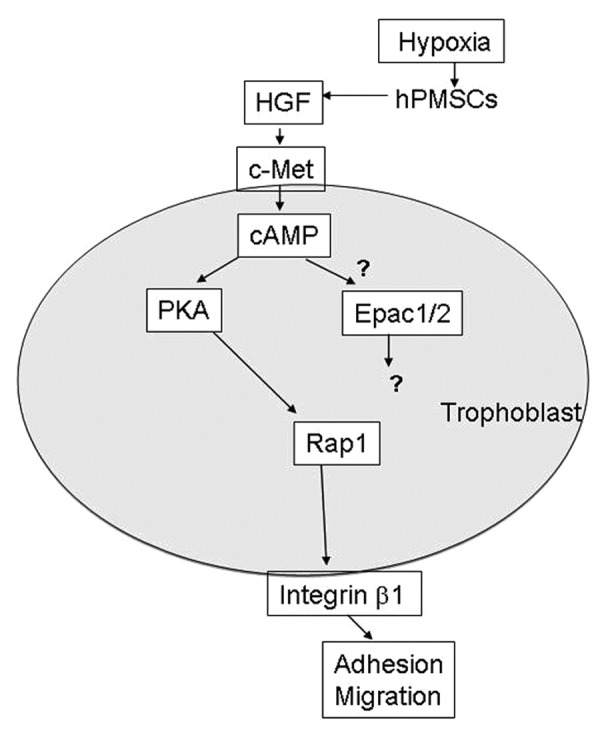
Figure 2. Proposed scheme of mechanisms leading to trophoblast invasion into the decidua by HGF (hepatocyte growth factor) produced by human placental multipotent mesenchymal stromal cell (hPMSC) in villous mesenchyme. Hypoxia reduced HGF expression by hPMSCs. HGF produced by hPMSCs were able to interact with c-Met receptor on trophoblast and induce the trophoblast 3′,5′-cyclic adenosine monophosphate (cAMP) expression. The cAMP activates PKA (protein kinase A), which in turn, activates Rap1 leading to integrin β1 activation. However, the response of Epac-1 and Epac-2 (exchange protein directly activated by cAMP-1 and -2) to HGF stimulation in trophoblasts is not clear.
Rap1 belongs to the Ras subgroup of small GTPases. It cycles between the active GTP-bound and inactive GDP-bound forms, which is regulated by a diverse family of guanine nucleotide exchange factors and GTPase-activating proteins. Various extracellular signals activate Rap1 and its functions ranged from the influence of cell proliferation and differentiation to endocrine secretion, integrin-mediated cell adhesion, and morphogenesis.18 The common second messengers such as cAMP, calcium, and diacylglycerol are involved in transducing the extracellular signal to Rap1. Exchange protein directly activated by cAMP-1 and -2 (Epac-1 and Epac-2) are activated both in vitro and in vivo by direct binding of cAMP. They have been shown to increase Rap1 activity and promote Rap1-dependent cell adhesion via integrin β1. PKA is a target for cAMP and Rap1 is activated by PKA.18 However, in several cell types, the Rap1 downstream signaling may be regulated through different effectors of the cAMP transduction cascade. For example, Epac signaling pathway is associated with the cAMP-mediated functional differentiation (hCG production) and syncytialization of human BeWo trophoblasts.19 Further report reveals that cAMP/Epac1/Rap1 signaling cascade stimulates BeWo trophoblast fusion through promoting placental transcription factor glial cell missing 1 phosphorylation, which is PKA-independent.20 The Epac/Rap1 signaling pathway involves in cAMP-mediated decidualization of human endometrial stromal cells.21 In the recent report, we observed the extravillous trophoblast cell line (HTR-8/SVneo) expressed PKA and c-Met. HGF and PKA-specific agonist 6-Bnz-cAMP activated PKA. Stimulation of HTR-8 trophoblasts with HGF or 6-Bnz-cAMP had no effect on total integrin β1 protein, but significantly enhanced the expression of activated form of integrin β1, suggesting PKA-dependent inside-out signaling.17 The HTR-8 trophoblasts expressed Epac-1 and Epac-2, but their effects on Rap1 need further clarification (Fig. 2).
Shallow trophoblast invasion and defective maternal spiral artery remodeling are common to both intrauterine growth restriction and preeclampsia.22-24 Although the mechanism of defective trophoblast invasion is unknown, abnormal trophoblast integrin expression and hypoxia have been implicated.22-24 Supported by the previous findings that HGF expression was decreased in preeclamptic placentas,25 in the recent article we further demonstrated the number of hPMSCs was reduced in preeclamptic placentas and they produced less HGF than comparable cells isolated from gestational age-matched control placentas.17 Additionally, cAMP expression in preeclamptic placentas was lower than that of controls. Hypoxic treatment of hPMSCs resulted in a significant decrease of HGF production compared with normoxic controls.17 However, the molecular mechanisms of the downregulation of HGF in response to hypoxia in hPMSCs are not known. In the study of vascular smooth muscle cells, TGF-β and cAMP are reported to involve in the reduction of HGF production in hypoxia.26,27 Stimulated vascular smooth muscle cells with cAMP analog enhance HGF production.27 Thus, cAMP seems to have a reciprocal function in HGF signaling transduction.
In conclusion, we suggest trophoblast migration depends on signals acquired from villous stromal hPMSCs during placentation. Through paracrine interplay of signaling and effector molecules, HGF produced by hPMSCs mediates mesenchymal–epithelial interactions via cAMP/Rap1/integrin β1 pathway. Here, we provide a novel mechanism that hypoxia downregulates HGF expression by hPMSCs and reduces trophoblast migration are relevant to the development of preeclampsia.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–18. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 3.Lala PK, Hamilton GS. Growth factors, proteases and protease inhibitors in the maternal-fetal dialogue. Placenta. 1996;17:545–55. doi: 10.1016/S0143-4004(96)80071-3. [DOI] [PubMed] [Google Scholar]
- 4.Irwin JC, Suen LF, Faessen GH, Popovici RM, Giudice LC. Insulin-like growth factor (IGF)-II inhibition of endometrial stromal cell tissue inhibitor of metalloproteinase-3 and IGF-binding protein-1 suggests paracrine interactions at the decidua:trophoblast interface during human implantation. J Clin Endocrinol Metab. 2001;86:2060–4. doi: 10.1210/jcem.86.5.7451. [DOI] [PubMed] [Google Scholar]
- 5.Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Low oxygen concentrations inhibit trophoblast cell invasion from early gestation placental explants via alterations in levels of the urokinase plasminogen activator system. Biol Reprod. 2006;74:403–9. doi: 10.1095/biolreprod.105.047332. [DOI] [PubMed] [Google Scholar]
- 6.Nadeem L, Munir S, Fu G, Dunk C, Baczyk D, Caniggia I, Lye S, Peng C. Nodal signals through activin receptor-like kinase 7 to inhibit trophoblast migration and invasion: implication in the pathogenesis of preeclampsia. Am J Pathol. 2011;178:1177–89. doi: 10.1016/j.ajpath.2010.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick J, Whitley GS, Le Bouteiller P, Cartwright JE. Soluble HLA-G regulates motility and invasion of the trophoblast-derived cell line SGHPL-4. Hum Reprod. 2009;24:1339–45. doi: 10.1093/humrep/dep026. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999;128:181–9. doi: 10.1038/sj.bjp.0702757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostinis C, Bulla R, Tripodo C, Gismondi A, Stabile H, Bossi F, Guarnotta C, Garlanda C, De Seta F, Spessotto P, et al. An alternative role of C1q in cell migration and tissue remodeling: contribution to trophoblast invasion and placental development. J Immunol. 2010;185:4420–9. doi: 10.4049/jimmunol.0903215. [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Guimond MJ, Chakraborty C, Lala PK. Control of proliferation, migration, and invasiveness of human extravillous trophoblast by decorin, a decidual product. Biol Reprod. 2002;67:681–9. doi: 10.1095/biolreprod67.2.681. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y, Higuchi T, Yoshioka S, Tatsumi K, Fujiwara H, Fujii S. Trophoblasts acquire a chemokine receptor, CCR1, as they differentiate towards invasive phenotype. Development. 2003;130:5519–32. doi: 10.1242/dev.00729. [DOI] [PubMed] [Google Scholar]
- 12.Lacey H, Haigh T, Westwood M, Aplin JD. Mesenchymally-derived insulin-like growth factor 1 provides a paracrine stimulus for trophoblast migration. BMC Dev Biol. 2002;2:5. doi: 10.1186/1471-213X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdie KJ, Whitley GS, Johnstone AP, Cartwright JE. Hepatocyte growth factor-induced endothelial cell motility is mediated by the upregulation of inducible nitric oxide synthase expression. Cardiovasc Res. 2002;54:659–68. doi: 10.1016/S0008-6363(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright JE, Tse WK, Whitley GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279:219–26. doi: 10.1006/excr.2002.5616. [DOI] [PubMed] [Google Scholar]
- 15.Liu SH, Huang JP, Lee RK, Huang MC, Wu YH, Chen CY, Chen CP. Paracrine factors from human placental multipotent mesenchymal stromal cells protect endothelium from oxidative injury via STAT3 and manganese superoxide dismutase activation. Biol Reprod. 2010;82:905–13. doi: 10.1095/biolreprod.109.081828. [DOI] [PubMed] [Google Scholar]
- 16.Lee MY, Huang JP, Chen YY, Aplin JD, Wu YH, Chen CY, Chen PC, Chen CP. Angiogenesis in differentiated placental multipotent mesenchymal stromal cells is dependent on integrin alpha5beta1. PLoS One. 2009;4:e6913. doi: 10.1371/journal.pone.0006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CP, Huang JP, Chu TY, Aplin JD, Chen CY, Wu YH. Human placental multipotent mesenchymal stromal cells modulate trophoblast migration via Rap1 activation. Placenta. 2013;34:913–23. doi: 10.1016/j.placenta.2013.06.311. [DOI] [PubMed] [Google Scholar]
- 18.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2:369–77. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 19.Yoshie M, Kaneyama K, Kusama K, Higuma C, Nishi H, Isaka K, Tamura K. Possible role of the exchange protein directly activated by cyclic AMP (Epac) in the cyclic AMP-dependent functional differentiation and syncytialization of human placental BeWo cells. Hum Reprod. 2010;25:2229–38. doi: 10.1093/humrep/deq190. [DOI] [PubMed] [Google Scholar]
- 20.Chang CW, Chang GD, Chen H. A novel cyclic AMP/Epac1/CaMKI signaling cascade promotes GCM1 desumoylation and placental cell fusion. Mol Cell Biol. 2011;31:3820–31. doi: 10.1128/MCB.05582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusama K, Yoshie M, Tamura K, Kodaka Y, Hirata A, Sakurai T, Bai H, Imakawa K, Nishi H, Isaka K, et al. Regulation of decidualization in human endometrial stromal cells through exchange protein directly activated by cyclic AMP (Epac) Placenta. 2013;34:212–21. doi: 10.1016/j.placenta.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 23.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–67. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laresgoiti-Servitje E, Gomez-Lopez N. The pathophysiology of preeclampsia involves altered levels of angiogenic factors promoted by hypoxia and autoantibody-mediated mechanisms. Biol Reprod. 2012;87:36. doi: 10.1095/biolreprod.112.099861. [DOI] [PubMed] [Google Scholar]
- 25.Kauma SW, Bae-Jump V, Walsh SW. Hepatocyte growth factor stimulates trophoblast invasion: a potential mechanism for abnormal placentation in preeclampsia. J Clin Endocrinol Metab. 1999;84:4092–6. doi: 10.1210/jcem.84.11.6120. [DOI] [PubMed] [Google Scholar]
- 26.Nakano N, Morishita R, Moriguchi A, Nakamura Y, Hayashi SI, Aoki M, Kida I, Matsumoto K, Nakamura T, Higaki J, et al. Negative regulation of local hepatocyte growth factor expression by angiotensin II and transforming growth factor-beta in blood vessels: potential role of HGF in cardiovascular disease. Hypertension. 1998;32:444–51. doi: 10.1161/01.HYP.32.3.444. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, Taiji M, Noguchi H, Matsumoto K, Nakamura T, et al. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation. 1999;100(Suppl):II301–8. doi: 10.1161/01.CIR.100.suppl_2.II-301. [DOI] [PubMed] [Google Scholar]