Abstract
The normal development and function of photoreceptors is essential for eye health and visual acuity in vertebrates. Mutations in genes encoding proteins involved in photoreceptor development and function are associated with a suite of inherited retinal dystrophies, often as part of complex multi-organ syndromic conditions. In this review, we focus on the role of the photoreceptor outer segment, a highly modified and specialized primary cilium, in retinal health and disease. We discuss the many defects in the structure and function of the photoreceptor primary cilium that can cause a class of inherited conditions known as ciliopathies, often characterized by retinal dystrophy and degeneration, and highlight the recent insights into disease mechanisms.
Keywords: primary cilia, ciliopathy, inherted retinal conditions, photoreceptor development, retina, intraflagellar transport
Introduction: the neurosensory retina and photoreceptor cells
The retina is the internal layer of the eyeball, responsible for converting light signals from the environment (focused by the anterior features of the eye) into neural impulses to be sent to the brain. This thin (0.56 – 0.1mm) membrane can be further divided into two distinct layers: an inner neurosensory layer and an outer pigmented layer, the retinal pigment epithelium (RPE). The most prevalent cell-type of the retina is the neuron, of which there are three main groups responsible for relaying light generated impulses. These are the bipolar cells, the ganglion cells and the photoreceptors. Photoreceptor cells are long and narrow, and are sub-divided into inner segments (IS) and outer segments (OS) connected by a connecting cilium (CC).1 The OS of the photoreceptor develops from a primitive primary cilium, and the OS is widely considered to be a highly modified primary cilium,1-4 with the retinal connecting cilium homologous to the transition zone of the primary cilium.5-7
There are two types of photoreceptor cells, rods (Fig. 1A) and cones, which are named after the shape of their respective OS. These specialized regions of the cell contain high concentrations of the components of the phototransduction cascade such as the G-protein transducin and visual pigments, and low concentrations of proteins involved in other cellular functions.8 Rod OS contain the visual pigment rhodopsin within membrane-bound discs, which stack together to form the rod shape. Cones contain several visual pigments, known as opsins, inserted into the highly invaginated plasma membranes which form the conical shape of these cells. While rod OS tips are phagocytosed daily by the RPE and new discs formed at the OS base, the cone OS are not phagocytosed in this manner. Rods are shed daily on light onset9 while cones are shed during light offset.10
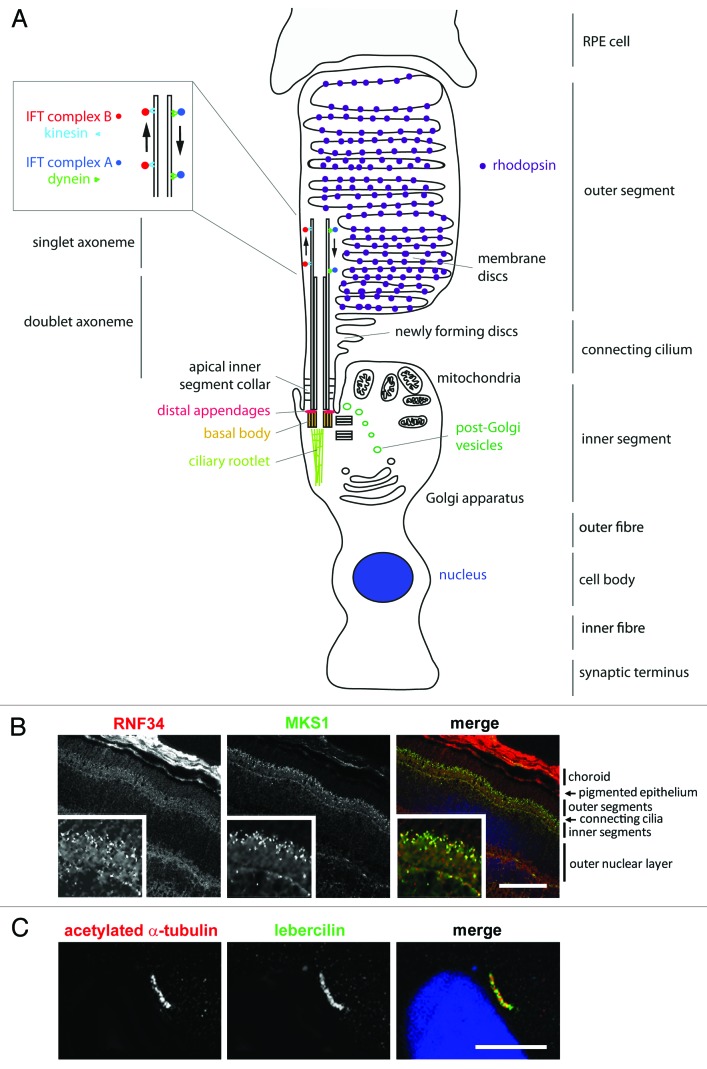
Figure 1. Schematic representation of a rod photoreceptor cell and localization of ciliary proteins.(A) The schematic represents the rod photoreceptor cell outer segment, connecting cilium, inner segment, outer fiber, cell body, inner fiber and synaptic terminus. A number of key components of the ciliary apparatus are color coded and indicated. The IFT complex A (blue) and complex B (red) are represented in the magnified inset. A retinal pigmentary epithelial (RPE) cell is shown in gray at the top. (B) Confocal microscopy images of an immunofluorescent stained P20 mouse retinal cryosection showing the stratified layers of the retina. Cilium transition zone and basal body protein MKS1 is stained in green, and a novel interactant of MKS1, RNF34, is stained in red. These proteins localize to the base of the connecting cilium, as shown by the arrowheads in the enlarged insets. (C) Confocal microscopy image of a human adult retinal pigment epithelium (ARPE19) cell overexpressing enhanced-GFP-tagged lebercilin and immunostained with an antibody against acetylated α tubulin, which marks the axoneme of the cilium. Lebercilin can be seen in a punctuate pattern along the axoneme. Scale bar = 10μm.
The CC connects the OS to the IS and consists of an axoneme of nine microtubule doublets nucleated by a triplet microtubule basal body. This is derived from the mother centriole, at the apical surface of the IS. This axoneme extends into the photoreceptor OS, converting to singlet microtubules toward the distal end (Fig. 1A).11 The axoneme often reaches near the distal tip of cone OS and at least half-way along the rod OS, reaching the distal tip of rod OS in some cases.12 The axoneme is stabilized by post-translational modifications such as glutamylation and acetylation in rods, but is turned over as membranes are replaced at the distal surface of cones.13
The ciliary rootlet extends from the basal side of the basal body and anchors the cilium to the cell, extending deep into IS.14 It is composed of rootletin and is required for the structural stability of the photoreceptor OS (Fig. 1A). It is thought to also act as a docking point for transport motor proteins carrying vesicular cargo which may be destined for the CC or OS. The IS of the photoreceptors is where protein synthesis occurs, and where ATP is produced. The IS are split into two regions. The distal or ellipsoid region is packed with mitochondria, reflecting the high metabolic demands of the cells, whereas the proximal or myoid region is predominantly filled with the Golgi apparatus and the endoplasmic reticulum that enable high rates of protein synthesis in these parts of the cell. The remainder of the photoreceptors consist of the outer fiber connecting the IS to the cell body (containing the cell nucleus), which in turn is connected via the inner fiber to the synaptic terminal, forming junctions with the retinal bipolar cells for transmission of nerve impulses. These cells perform essential roles in phototransduction, and ciliary signaling is a crucial component of this activity that, when disrupted, can lead to retinal disease. The role of ciliary signaling in retinal disease has been reviewed recently15 and the present review will instead focus on photoreceptor assembly, organization and maintenance.
Model Systems for the Study Of Photoreceptor Development and Function
A range of organisms are used as models of retinal development, each with its own individual benefits and drawbacks (Table 1). The interested reader is referred to Table S1 for further details. Diurnal primates are the best model of human retinal development, since they share several important features of the human retina that are absent in other mammals such as the fovea, the region of the central retina populated by cones and with the highest visual acuity.16 Most mammals have dichroic vision, with two opsins (blue-sensitive and green-sensitive) whereas humans and diurnal primates have trichroic vision (with an additional red-sensitive opsin).17 Ethical and practical factors prevent the use of such primates in widespread retinal research, and mice remain the most commonly-used mammalian model. However, mice do not differentiate their photoreceptor OS until P8-P16,18 preventing the study of photoreceptor development and/or degeneration in mouse models of conditions that are embryonic or perinatal lethal, as is the case for many ciliopathies. The use of conditional knockouts by crossing to lines expressing Cre recombinase in the rods and/or cones can help to overcome such lethality. Crossing of conditional knockouts to lines expressing Cre recombinase under the control of a cone-specific gene promoter have been successfully used to develop models of foveal diseases.19
Table 1. The most comprehensively characterized photoreceptor proteins mutated in human disease.
| Gene | Localization | Function | Retinal phenotype in animal model | Human disease (type) |
|---|---|---|---|---|
| AHI1 | connecting cilium | thought to play a role in RAB8-mediated polarized vesicular trafficking | Ahi1 mutant mice do not develop OS, or develop abnormal OS leading to subsequent photoreceptor degeneration, associated with defects in vesicular trafficking of transducin and Rom-1, and a decrease in Rab8 expression. Opsin is also mislocalized throughout the photoreceptor is Ahi mutant mice. Defect in lamination of retina in zebrafish morphants. | JBTS(3) |
| ALMS1 | basal body | thought to play a role in transport along the photoreceptor axoneme, possibly in endosome recycling | gene-trap Alms1 mutant mice accumulate vesicles in their IS and rhodopsin is mislocalized to the OS, leading to retinal degeneration | ALMS(1), LCA |
| ARL6 | IS, OS, ONL | regulates the BBSome | a specific isoform of BBS3 is expressed in the human retina, and when this long isoform is knocked down in zebrafish, green opsin is mislocalized, with functional consequences on vision. Bbs3L mutant mice develop disorganized IS | BBS(3), RP(55) |
| BBS4 | IS and OPL | component of the BBSome, a regulatory submodule of IFT | Bbbs4 null mice develop grossly normal photoreceptors at an early age but exhibit defective IFT. This leads to mislocalization of specific phototransduction proteins (rhodopsin, transducin and arrestin) but not the structural photoreceptor proteins peripherin or rom-1, which subsequently causes photoreceptor degeneration | BBS(4) |
| CC2D2A | connecting cilium | extension of connecting cilium membrane through Rab8-mediated vesicular trafficking | cc2d2a zebrafish morphants develop disorganized photoreceptor OS and accumulation of opsins and vesicles in IS. Cc2d2a mutant mice have microphthalmia | COACH, MKS(6), JBTS(9), RP |
| CEP290 | base of connecting cilium | plays a role in IFT, essential for normal mislocalization of rhodopsin and arrestin but not essential for CC structure | mislocalization of rhodopsin and arrestin in rd16 mouse. rdAc Abyssian cat. cep290 morphant zebrafish reveal no gross lamination defects, but have statistically significant reduction in visual function. | BBS(14), LCA(10), JBTS(5), NPHP(6), MKS(4), SLS(6) |
| LCA5 | connecting cilium | plays a role in connecting the IFT core machinery to proteins involved in selecting and recruiting cargo110 | in mice lacking wild-type lebercilin, cone and rod opsins are mislocalized and the phototransduction G-protein transducin partially mislocalizes in response to light. | LCA(5) |
| MAK | base of connecting cilium | kinase, phosphorylates RP1 to regulate extension of ciliary axoneme to control CC and OS length | loss of Mak in mice leads to increased length of photoreceptors and retinal degeneration | RP(62) |
| MKKS | connecting cilium | chaperonin-like BBS protein, thought to regulate BBSome assembly | late-onset photoreceptor degeneration is observed in Bbs6 mutant mice | MKKS, BBS(6) |
| MYO7A | base of connecting cilium, at the site of disc morphogenesis | plays a role in transport of proteins from the IS to the OS for incorporation into discs | opsin accumulates in the IS of Myo7a mutant mouse | USH(1B), LCA |
| NPHP1 | connecting cilium, especially around the basal body | thought to play role in the transport of specific proteins along the photoreceptor | Nphp1 mutant mice exhibit defects in sorting of proteins between the photoreceptor IS and OS, and compromised IFT | NPHP(1), SLS(1), JBTS(4) |
| NPHP4 | connecting cilium. Proximal to RPGRIP1, RPGR and SDCCAG8 | interacts with CEP290 and RPGRIP1. | Nphp4 mutant mice mislocalize rhodopsin and ROM-1 to the IS and INL, do not develop normal OS despite developing normal CC. The photoreceptors degenerate rapidly. Synaptic ribbons develop normally but degenerate. Mislocalization of synaptic vesicle protein and | NPHP(4), SLS(4), CORS |
| post-synaptic density protein. CORD in Nphp4 wire-haired daschunds. | ||||
| TMEM67 | exact localization in photoreceptors not known | required for membrane disc assembly and rhodopsin transport | dysmorphic, misaligned membrane discs, and mislocalized rhodopsin, arrestin, and transducin, leading to rapid photoreceptor degeneration in bpck mice. CC structurally normal but rhodopsin mislocalized to IS and ONL in bpck mice. | COACH, JBTS(6), MKS(3), NPHP(11) |
| TOPORS | basal body | E3 ubiquitin ligase function. Role in photoreceptors unclear | morpholino silencing in zebrafish affects retinal development, OS fail to form | RP(31) |
| TTC8 | connecting cilium | component of the BBSome, a regulatory submodule of IFT | retinal degeneration and mislocalization of rhodopsin to IS in Bbs8 mutant mice | BBS(8), RP(51) |
| RP1 | photoreceptor axoneme | binds to singlet microtubules of the axoneme, especially at the point of disc membrane formation | In RP1 mutant mice, disc membranes are abnormally organized and discs do not stack correctly. | RP(1) |
| RP1L1 | photoreceptor axoneme | interacts with RP1, thought to play similar role to RP1 | abnormal OS morphology and photosensitivity in RP1L1 mice | OCMD |
| RP2 | basal body | acts as the ARL3 GTPase activating protein. Plays a role in trafficking of vesicles from Golgi to cilium | small eyes and retinal degeneration in rp2 zebrafish morphants | RP(2) |
| RPGR | connecting cilium | contains a domain with homology to the RCC1 guanine nucleotide exchange factor (GEF) for Ran GTPase. This RCC1-like domain in RPGR acts as a GTP/GDP exchange factor for RAB8, a GTPase important for vesicular trafficking to the primary cilium | mislocalization of cone opsins in the cell body and synapses; reduced levels of rhodopsin in rods; leading to photoreceptor degeneration in Rpgr knockout mice | RP(3), CORD(X1) |
| RPGRIP1 | distal part of the photoreceptor axoneme in rod and cone OS in humans, CC in mice. | transprt along the photoreceptor. Recruitment of RPGR, NPHP4 and SDCCAG8 to the CC | mice lacking RPGRIP1 develop grossly oversized OS discs. Rpgrip1nmf247 mice lack RPGR, NPHP4 and SDCCAG8 at the CC. | LCA(6), CORD(13) |
| TTC21B | photoreceptor axoneme | intraflagellar transport A complex protein | Ttc21b mutant mice have defective photoreceptor development | ATD(4), NPHP(12) |
| USH1B (myosin7a) | site of disc morphogenesis. Ribbon synapse. RPE. Apical IS collar. | molecular motor. plays a role in transport of proteins from the IS to the OS for incorporation into discs and RPE65 transport | opsin accumulates in the IS of Shaker1 (Myo7a mutant) mouse. Myo7a mutant mice have lower levels of RPE65, the RPE isomerase that has a key role in the retinoid cycle. Eyes not studied in mariner zebrafish mutant. | USH(1B) |
| USH1C (harmonin) | at the apical IS collar and ribbon synapse | structural protein, functions in the docking and loading of IFT cargos | deaf circular (Dfcr/Ush1c) mice do not undergo retinal degeneration and have normal synaptic ultrastructure and ERGs. Mice with knock-in of c.216G > A cryptic splice site mutation in Exon 3 of Ush1c have progressive loss of rods between 6 and 12 mo of age | USH(1C) |
| USH2A (usherin) | at the apical IS collar, basal body of connecting cilium | structural protein, functions in the docking and loading of IFT cargos, essential for long-term structural maintenance of photoreceptors. Interacts with lebercilin and ninein-like protein | mice lacking Ush2a develop late-onset progressive photoreceptor degeneration | USH(2A) |
| USH2C (GPR98, VLGR1) | G-protein coupled receptor. Exact function in photoreceptors not known | mild, late onset abnormalities in retinal function in Vlgr1/del7TM mice | USH(2C) |
Additionally, mice photoreceptors lack calyceal processes (CPs), finger-like structures that protrude from the apical region of the inner segment and surround the base of the outer segment, further compromising their utility as models of retinal ciliopathies. This is particularly true in the case of mouse models of Usher syndrome (USH), an inherited condition involving sensorineural hearing loss and retinal dystrophy. Mouse knockouts of the USH1 proteins (myosin VIIa, harmonin, cadherin-23, protocadherin-15, SANS) often develop hearing loss and vestibular dysfunction but do not display retinal degeneration due to the lack of CPs.20 Amphibians (especially Xenopus tadpoles) or macaques are a better model of USH1 as they possess CPs, but zebrafish are more commonly used for most models of Usher syndrome.
The retina is fully laminated and light responsive by the third day of embryogenesis in this organism, and zebrafish mutants, morphants and TALEN models provide tractable and versatile resources for the study of retinal ciliopathies. Knockouts and knock-ins by genomic editing with TALENs (or CRISPRs) are a recent exciting addition to the techniques developed for the study of photoreceptor degeneration in zebrafish. Morpholino oligonucleotide knockdown of gene transcript levels is only effective for around three days after microinjection, and so is applicable for studies of retinal development but not slower or later-onset forms of retinal degeneration. Medaka fish are also popular model organisms for studying eye development because the embryos are transparent embryos, allowing simple visual assays of mutant phenotypes, with comparatively simple and affordable animal husbandry.21 However, whole genome duplication in fish can complicate genetic studies in this class of organisms due to the presence of multiple orthologs of genes.22 For example, PCDH15 (protocadherin-15) is mutated in humans with Usher syndrome type 1F23 and both humans and other mammals have a single PCDH15 gene that is expressed in both inner ear and retina. In contrast, the homolog has duplicated and diverged in zebrafish. The two copies of the fish gene have evolved independent, tissue-specific functions: pcdh15a is expressed in inner ear and mediates hearing and vestibular function, whereas pcdh15b is expressed in retina and mediates retinal development and function.24 Zebrafish are therefore not an ideal model for studying this condition. Furthermore, fish and amphibian retinae also have limitations in their use for studying retinal degeneration because—in contrast to mammals—photoreceptors can regenerate in many adult fish and amphibians.25,26 As is the case in most avenues of research, a compromise must be sought when choosing which model organism to use for retinal development and degeneration studies.
Photoreceptor Development and Inherited Retinal Conditions
Mutations in genes encoding proteins involved in photoreceptor development are associated with a broad group of inherited retinal dystrophies (Table 1), often as part of complex multi-organ syndromic conditions termed the ciliopathies.27 Refer to Table S1 for further details. Photoreceptor biogenesis has been suggested to occur in six distinct stages,28 based on the four phases of ciliogenesis in an epithelial cell,29 followed by two stages of OS development. This whole process takes over two weeks to complete in the mouse retina.30 In the mouse, it begins with the docking of paired centrioles (each consisting of nine microtubule triplets) at the apical surface of the undifferentiated progenitor cell early in photoreceptor development.31 The distal end of the mother centriole is surrounded by the primary vesicle and a microtubule ciliary bud begins to grow from this centriole. At the same time, this centriole matures into the basal body by recruitment of appendages, such as ODF2,32 and aligns perpendicular to the plasma membrane. Many proteins implicated in human retinal disease are localized to the basal body (Fig. 1B), and may play a role in photoreceptor biogenesis. Examples include the TOPORS protein, loss of which is associated with failure of OS formation in zebrafish,33 and retinitis pigmentosa (RP), a hereditary retinal degeneration in humans.34 OFD1 is a distal centriole protein that regulates the length of centrioles35 and ciliary axonemes.36 Mutations in OFD1 cause oro-facial-digital syndrome, non-syndromic RP, and RP as a feature of Joubert syndrome (JBTS).37-39 ofd1 morphant zebrafish occasionally develop retinal coloboma.40 RAB28 localizes to the basal body and is suggested to play a role in coordinating ciliogenesis and rhodopsin transport, with mutations in this gene causing cone-rod dystrophy.41
Once the basal body has docked at the apical cell surface, binding of post-Golgi vesicles to the primary vesicle expands the membrane to form the ciliary vesicle, allowing the ciliary bud to extend to form the axoneme. This ciliary vesicle then fuses with the plasma membrane and the CC extends toward the RPE.28,42 The protein CC2D2A plays a role in extension of the CC membrane through RAB8-mediated vesicular trafficking. cc2d2a zebrafish morphants develop disorganized photoreceptor OS and accumulation of opsins and vesicles in IS.43 Mutations in CC2D2A in humans lead to RP, often as part of multi-organ syndromes.44-46 Growth of the microtubule axoneme is dependent on chaperone proteins such as prefoldin-5 that, when mutated in mice, leads to photoreceptor degeneration.47 Other chaperones including HSC70 (Hspa8) have also been found to be associated with photoreceptor axonemal proteins.48 Glutamylation of axonemal tubulin is essential for normal photoreceptor development and function,49 and the stabilization of axonemal microtubules by other post-translational modifications such as acetylation are also presumably required for these normal processes. FAM161A, a microtubule-associated protein, is thought to have a role in this stabilization process since patients with mutations in this gene develop RP.50
Rod OS biogenesis begins as the distal end of the CC, followed by membrane vesicular fusion to form the discs of the rod OS. Disc assembly is an on-going process as the membrane discs of the OS are constantly sloughed off to prevent the accumulation of toxic by-products of phototransduction.51 Normal disc assembly occurs at the base of the OS, near to the ciliary axoneme. OS plasma membrane invaginates to form nascent discs, and disc proteins are then localized to their correct compartment: the OS plasma membrane, disc rim or disc lamellar region. The correct formation of discs is dependent on membrane proteins, including rom-1 and peripherin that localize to disc rims,52,53 and the visual pigment rhodopsin.54 RAB8 mediates transport of vesicles carrying rhodopsin to the base of the CC where they bind to the Qa-SNARE syntaxin 3, or to the IS where they bind the Qbc-SNARE SNAP-25 to fuse with the plasma membrane to deliver their cargo.55 RP1 also plays a role in disc assembly. RP1 binds to singlet microtubules of the axoneme,56 particularly at the point of disc membrane formation. In Rp1 mutant mice, disc membranes are abnormally organized57 and discs do not stack correctly. RP1 is phosphorylated by MAK, and this is thought to regulate extension of the ciliary axoneme to control CC and OS length. Loss of Mak in mice leads to increased length of photoreceptors and retinal degeneration.58 Similar to RP1, RP1L1 binds to the photoreceptor axoneme, with consequences on OS morphology and photosensitivity, leading to progressive photoreceptor degeneration when this protein is lost in mutant mice.59 Loss of this protein in humans is associated with occult macular dystrophy (OMD) and RP.60,61 Myosin 7a, that is mutated in both Leber congenital amaurosis (LCA), an early-onset retinal dystrophy,62 and Usher syndrome type 1B63 is also localized to the site of disc morphogenesis. Myosin 7a plays a role in transport of proteins from the IS to the OS for incorporation into discs, and opsin accumulates in the IS of Myo7a mutant mouse.64 The orphan membrane-bound receptor TMEM67 (meckelin), the protein product of the TMEM67 gene, is also required for membrane disc assembly. Bpck mice, which have a spontaneous deletion of Tmem67, have structurally normal CC but dysmorphic, misaligned membrane discs, and mislocalized rhodopsin, arrestin, and transducin leading to rapid photoreceptor degeneration.65 While the CC are structurally normal in bpck mice, rhodopsin transport is compromised and rhodopsin accumulates in the IS and ONL, likely reflecting a role of TMEM67 in mediating transport along the photoreceptor CC.66 Mutations in human TMEM67 are associated with a suite of ciliopathies, many of which include retinal degeneration phenotypes.67-69
Many retinal proteins also need to be correctly localized to the CC membrane. The cilium membrane is the site of many G-protein coupled receptors (GPCRs), mediating many important roles in cellular signaling. Inositol polyphosphate-5-phosphatase E (INPP5E) is a phosphatase that hydrolyses phosphotidylinositols, membrane-bound intermediate molecules for PI3K signaling. INPP5E localizes to the IS but does not appear to be required for ciliogenesis. Instead, it maintains cilium stability, and loss of the protein results in anophthalmia in mutant mice70 and microphthalmia in morphant zebrafish.71 Human mutations are associated with several syndromic retinal dystrophies including mental retardation, obesity, retinal dystrophy and micropenis (MORM) syndrome, JBTS and CORS.70,72 Trafficking of INPP5E to the cilium depends on PDE6D, in concert with ARL13B and CEP164,73 which when mutated in humans also lead to syndromic retinal dystrophies.74,75
The Tectonic proteins TCTN1 and TCTN2 play a role in targeting GPCRs to the cilium membrane, as well as other components of G-protein signaling such as the downstream effector adenylyl cyclase III (ACIII) and the putative channel protein polycystin-276. Tctn1 mutant mice develop microphthalmia and mutations in humans lead to JBTS.76 Tctn2 mutant mice also develop micropthalmia, and mutations in humans are associated with JBTS77 and Meckel-Gruber syndrome (MKS).78
Transport in the Photoreceptor
All protein synthesis in the photoreceptor occurs in the IS, yet the OS is the region involved in photoreception, with the highest demand for protein. The mouse OS proteome contains almost 2000 proteins,79 yet transcription and translation occur in neither the OS nor CC. An estimated 10% of protein is lost from the rod OS each day as membranes are shed into the RPE to prevent accumulation of toxic by-products of phototransduction.30,80 Thus, transport between the two segments along the CC is of critical importance to photoreceptor development and function.51,81
Some molecules, including even some proteins, move along the CC by passive diffusion. This is perhaps surprising given the presence of the septin “barrier” at the base of the cilium that is thought to regulate the movement of molecules along the cilium.82 However, it has been shown that the CC does not significantly inhibit protein diffusion through the photoreceptor cell.83 There remains a debate over whether transducin and arrestin, proteins involved in phototransduction, move between the OS and IS along the CC in response to light by either active or passive transport mechanisms.84-87 In at least some cases, proteins move by diffusion. For example, after light-induced translocation, transducin forms a stable complex with UNC119 and diffuses back into the OS.88 Movement of transducin is also thought to be regulated by the Ca2+-binding centrin proteins that are localized to the basal body and axoneme of the CC89 and are phosphorylated by CK2.90 UNC119 also binds myristoylated ciliary proteins, such as nephrocystin-3 (NPHP3), to target them to the ciliary membrane and maintain the spatial organization of the cilium. UNC119 releases its protein cargo when it binds to the activated isoform of the small GTP-binding protein ARL3-GTP, but not when it binds ARL2. The Retinitis Pigmentosa 2 protein (RP2) localizes to the Golgi apparatus and the basal body of the photoreceptor, and coordinates vesicle trafficking and docking at the base of the cilium.91 RP2 acts as the ARL3 GTPase activating protein (GAP)92 and ARL2BP is an effector for both ARL2 and ARL3. Correct targeting of such proteins to ciliary membranes is functionally important for photoreceptors, and mutations in RP2, NPHP3 and ARL2BP can all lead to ciliopathies involving retinal degeneration in humans and model organisms.93-97
Intraflagellar Transport in the Photoreceptor Axoneme and Inherited Retinal Conditions
ARL3 is also involved in the main active transport process along the photoreceptor axoneme: the process of intraflagellar transport (IFT).98,99 IFT is required for the entire process of CC and OS development, and IFT proteins are involved at all stages of this development. A pool of IFT proteins is localized in the cytoplasm at the base of the axoneme during the formation of the ciliary vesicle and elongation of the axoneme early in CC and OS development, and IFT proteins are consistently associated with the developed axoneme.28 IFT is also essential for normal photoreceptor function by mediating the transport of proteins of the phototransduction cascade to the OS.87 Cargoes of IFT have been shown to include rhodopsin, chaperone proteins and the photoreceptor-specific membrane protein guanylyl cyclase 1 (GC1, Gucy2e).48
IFT is a bi-directional transport process that moves cargo along the axoneme of the cilium from base to tip (anterograde IFT, from IS to OS in the case of the photoreceptor), and from tip to base (retrograde IFT, from OS to IS). It is likely that retrograde IFT plays a less important role than anterograde IFT in photoreceptor function, because proteins are shed from the distal tip of the OS rather than transported back to the IS for recycling. Nevertheless, retrograde IFT components are found to be associated with the CC, as the cell will always have a minimum requirement for retrograde IFT to return anterograde IFT proteins back to the IS. Kinesins catalyze anterograde IFT,100,101 whereas dyneins (specifically the axonemal dyneins and the DHC1b isoform of cytoplasmic dynein) power retrograde IFT.102 The retrograde IFT motor component cytoplasmic dynein 2 is required for OS biogenesis and function, and is found localized to the photoreceptor axoneme.103 Zebrafish lacking this cytoplasmic dynein develop short, disordered OS which accumulate vesicles.104
Heterotrimeric kinesin-II, composed of subunits of KIF3A in addition to either KIF3B or KIF3C, has been shown to be particularly important for IFT and is considered the most important kinesin motor for powering anterograde IFT. KIF3A localizes to the axoneme of the CC and IS in fish,105 Xenopus, monkey, and humans106 and KIF3B localizes to the CC and IS in Xenopus retina.106 Knockout of Kif3a or Kif3b in mouse results in mid-gestation embryonic lethality, reflecting the general importance of these motor subunits in anterograde IFT in all cilia.107,108 Photoreceptor defects are not seen in Kif3a or Kif3b heterozygote mice, suggesting that variants in these genes in humans do not contribute to degenerative human retinal conditions.109 However, mice with a conditional knockout of Kif3a in rods and cones accumulate vesicles and opsin in the IS preceding photoreceptor death.87 The accumulation of opsin is less severe in the rods yet cell death is more rapid in these cells, suggesting that kinesin-II plays different roles in opsin transport in rods and cones.110 Kif3c knockout mice, however, are viable111 and do not exhibit photoreceptor defects,109 suggesting redundancy with either Kif3a or Kif3b. Consistent with this, kif3b morphant zebrafish embryos develop relatively short but otherwise functionally normal photoreceptor cilia, also suggesting some sort of functional redundancy. Overexpression of kif3c in these kif3b morphants confirmed partial functional redundancy of these subunits.112 Homodimeric kinesin-II, composed of Kif17, is also thought to play a role in anterograde IFT, although its function varies in different cilia. Kif17 is thought to play a role in photoreceptors, as it is found in the axoneme of mouse photoreceptor cells and expression of a dominant negative form of kif17 in zebrafish affects the development of photoreceptor OS.113 Similarly, kif17 morphant zebrafish develop short OS and aberrant discs.114
In association with the motor proteins, two distinct IFT complexes are associated with the bidirectional transport (Fig. 1A). Proteins involved in anterograde IFT are collectively known as IFT complex B proteins, and those involved in retrograde IFT are components of the IFT complex A. IFT complex B proteins IFT20, 52, 57 and 88 localize around the basal body and in discrete puncta along the axoneme of the CC of retinal photoreceptors.115,116 All of these IFT components are required for normal OS development and retinal function. Loss of ift52, 57, or 88 in zebrafish leads to an absence of OS and subsequent photoreceptor degeneration.117 IFT57 is not essential for IFT, but is required for efficient IFT as it plays a specific role in dissociation of kinesin-II from IFT complex proteins.118 IFT57 interacts with DYF-1/Fleer,119 as does IFT74, which helps the IFT particle dock onto the anterograde kinesin motor.120 Ift88 hypomorphic mutant mice develop rod cell photoreceptors with abnormal OS and mislocalized opsin, leading to progressive retinal degeneration.115 Mutations in IFT80, another IFT complex B protein, cause the skeletal ciliopathy asphyxiating thoracic dystrophy (ATD, also known as Jeune syndrome) in humans, a condition that involves retinal degeneration.121 Zebrafish ift80 morphants exhibit defects in photoreceptor OS formation and photoreceptor death122 but hypomorphic Ift80 mutations in mice (human mutations associated with ATD are hypomorphic) have normal retinas at P21, although retinal degeneration may occur later.123
IFT20 is an IFTB component that has both distinct and complementary functions to other IFT proteins since it appears to shuttle between the basal body and the Golgi apparatus,124 yet it is also essential for normal photoreceptor development and function. IFT20 localizes to the cytoplasm around the basal body of the CC of the retinal photoreceptors125 and Ift20 knockout mice do not traffic opsin appropriately and OS develop abnormally.126 Similarly, the IFTB component Ttc26 is required for OS formation, and ttc26 zebrafish morphants have short or absent OS.127 The exact role of IFTB component TRAF3IP1 (IFT54) and IFT172 in photoreceptor development is unclear, but Traf3ip1 mutant mice develop small eyes and Ift172 mutants do not develop eyes at all, suggesting the critical importance of these proteins in ocular development.128,129 It is likely that the other IFTB components are also required for photoreceptor development, but to date their roles in this process have not been studied, in some cases because loss of the protein is embryonically lethal in mice.130
IFT complex A protein IFT140 localizes along the photoreceptor axoneme and is particularly abundant at the tip of the axoneme.125 IFT140 is essential for normal photoreceptor development in humans. Mutations in human IFT140 are a cause of Mainzer-Saldino syndrome (MZSDS)131 and ATD,132 both of which are skeletal ciliopathies that are associated with retinal dystrophy phenotypes. TTC21B, another IFT complex A protein, also localizes to the photoreceptor axoneme, and loss of this protein in mutant mice leads to defective photoreceptor development.133 Mutations in human TTC21B are also associated with ATD, involving photoreceptor degeneration.134 WDR19, which links IFTA to the BBSome (see below), is proposed to be a regulatory sub-module of IFT.135 WDR19 is also mutated in ATD and cranioectodermal dysplasia (CED, also known as Sensenbrenner syndrome), which occasionally involves photoreceptor dystrophy.136 It remains unknown why a skeletal ciliopathy, namely ATD, can be caused by recessive mutations in both an IFTB protein (IFT80) and IFTA proteins, and why they predominately affect the endochondral ossification of the long bones.
Other proteins external to the core A and B complexes regulate IFT, including components of the BBSome that are also localized to the base of the photoreceptor axoneme. The BBSome, comprised of BBS1, 2, 4, 5, 7, 9 and TTC8 (BBS8), is thought to function as a coat complex to sort cilium proteins into membrane domains.137 Another class of BBS proteins, the chaperonin-like proteins MKKS (BBS6), BBS10 and BBS12, are thought to regulate the assembly of the BBSome138 and LZTFL1 (BBS17) regulates trafficking of the BBSome into the cilium.139 The activity of the BBSome is regulated by Arf-like GTPase ARL6 (BBS3)137 which is found throughout the IS, OS and ONL.140 Many BBS proteins have been shown to localize to the photoreceptors,140-143 and loss of most of the BBSome proteins leads to retinal degeneration in model organisms and humans as part of the multi-organ condition Bardet-Biedl syndrome (BBS).141,144-159 For example, Bbs1 knock-in mutant mice develop disorganized OS, and exhibit degeneration of IS and OS.160 Other work has exemplified the disease mechanism of disrupted trafficking that underlies the retinal degeneration in this ciliopathy. Bbbs4 null mice develop grossly normal photoreceptors at an early age but exhibit defective IFT. This leads to mislocalization of specific phototransduction proteins (rhodopsin, transducin and arrestin), but not the structural photoreceptor proteins peripherin or ROM1, which subsequently causes photoreceptor degeneration.142 Bbs2 mutant mice similarly mislocalize rhodopsin, leading to retinal degeneration.161 Late-onset photoreceptor degeneration is observed in Bbs6 mutant mice162 and Bbs8 mutant mice also mislocalize rhodopsin to the IS, leading to retinal degeneration.163 BBS7 is a core component of the BBSome and physically interacts with the BBS chaperonin complex, and Bbs7 mutant mice exhibit IS, OS and ONL degeneration164 due to defects in membrane protein trafficking. However, in contrast, bbs9 morphant zebrafish have an even more severe phenotype and the retina does not develop OS or discrete layers.165
Proteins Interacting with IFT Components in Photoreceptors
Many other proteins, while not core components of IFT particles, are associated with IFT complexes. These proteins include the Arf-like GTPase ARL13, which is thought to promote association between IFT complex A and complex B.98 Arl13B localizes to the CC in mice74 and Arl13bhnn mutant mice develop abnormal eyes, polydactyly and neural tube patterning defects.166 Mutations in ARL13B are a cause of the severe neurodevelopmental condition Joubert syndrome (JBTS) that can include additional clinical features such as RP.74 Lebercilin also interacts with complex A, complex B IFT proteins and retrograde IFT motor proteins, and is found to localize along the ciliary axoneme (Fig. 1C). In photoreceptor cells it is localized at the base of the CC, where it is thought to regulate loading and unloading of IFT cargo. This hypothesis is supported by the finding that in mice lacking wild-type lebercilin, cone and rod opsins are mislocalized and transducin, the G protein mediating phototransduction, partially mislocalizes in response to light. This suggests that lebercilin plays a role in connecting the IFT core machinery to proteins involved in selecting and recruiting cargo.167 Lebercilin is not considered a core component of the IFT machinery because siRNA knockdown of LCA5, the gene which encodes lebercilin in humans, does not affect ciliogenesis or IFT88 localization in ciliated retinal cell lines. These interactions are disrupted when the protein is truncated, leading to disrupted IFT. This results in Leber congenital amaurosis (LCA), an early-onset retinal dystrophy in humans.167,168 In at least some patients with mutations in this gene, photoreceptors in the central retina are structurally normal169 but visual acuity is poor from an early age. This suggests that, while important for IFT along the photoreceptor axoneme, lebercilin is not essential for structural development of the photoreceptor.
Lebercilin interacts with ninein-like protein (NINL) isoform b and usherin (USH2A) isoform b at the basal body of the connecting cilium, where these proteins are thought to play a role in regulating the docking of IFT particles.170 NINL isoform b is a centrosomal protein that functions in nucleation, anchoring and outgrowth of microtubules,170 and usherin (encoded by USH2A) is essential for the long-term structural maintenance of photoreceptors. Mutations in USH2A cause Usher syndrome171 and mice lacking Ush2a develop late-onset progressive photoreceptor degeneration.172 The usherin protein localizes to the apical IS membrane that wraps around the CC, a specialized region also known as the apical inner segment collar or pericilium that is homologous to the periciliary ridge complex of amphibians. Myosin VIIa (USH1B) also localizes to this apical IS collar,173 as does VLGR1 (USH2C), whereas the USH proteins harmonin (USH1C), SANS (USH1G), and whirlin (USH2D) act as structural proteins at the apical IS collar. The apical IS collar is a specialized membrane domain that functions in the docking and loading of IFT cargos, and USH proteins are therefore considered to play important roles at this stage of IFT.174 Their loss or mutation results in deafness and photoreceptor degeneration in humans.63,175-178
CEP290 is also believed to play a role in IFT along the CC because truncating mutations in CEP290 lead to mislocalization of rhodopsin and arrestin.179 CEP290 is necessary for transport along the CC, but is not required for CC development as loss of CEP290 does not compromise normal CC structure.179 CEP290 is localized to the basal body, pericentriolar matrix and axoneme of the CC. Mutations in CEP290 are associated with a suite of ciliopathies with retinal involvement, ranging from LCA180 to multi-organ JBTS.181,182 Mutations in this gene are the single most common cause of non-syndromic LCA, accounting for 21% of cases180,183. CEP290-mutated LCA patients retain normal overall retinal and photoreceptor architecture in the cone-rich central retina, but rod-rich pericentrical and peripheral regions are not conserved in this manner. CEP290 interacts with RAF-1 kinase inhibitory protein (RKIP), and accumulation of Rkip in photoreceptors of Cep290 mutant mice is thought to be one of the causes of photoreceptor degeneration in these mice.184
CEP290 also interacts with RPGR, another protein that localizes to the CC185 and is thought to interact with several protein complexes that mediate transport along the photoreceptor. CEP290 also interacts with the USH protein complex through whirlin,186 and multiple components of the NPHP complex, possibly as two distinct complexes involving NPHP1, 2, 5 and NPHP4, 6, 8.187 However, most of the insights about potential disease mechanisms have come from further study of the complexes containing RPGR. RPGR is mutated in 15% of RP cases in humans188 and loss of this protein in mice leads to ectopic localization of cone opsins and the reduction in levels of rhodopsin in rods, leading to cone-rod degeneration.189 RPGR contains a domain with homology to the RCC1 guanine nucleotide exchange factor (GEF) for Ran GTPase. This RCC1-like domain in RPGR acts as a GTP/GDP exchange factor for RAB8, a GTPase important for vesicular trafficking to the primary cilium.190 Jouberin, the protein encoded by AHI1 and mutated in JBTS,191 localizes to the CC and is thought to play a role in this RAB8-mediated polarized vesicular trafficking. Ahi1 mutant mice either do not develop OS or develop abnormal OS leading to subsequent photoreceptor degeneration, associated with defects in vesicular trafficking of transducin, ROM1 and opsin with a decrease in Rab8 expression.192,193 ahi1 morphant zebrafish develop abnormally shaped eyes with coloboma and defective retinal lamination.194
CEP290 and RPGR also interact with RPGR-interacting protein 1 (RPGRIP1) in rod OS.195 Loss of RPGRIP1 has no effect on central (cone-rich) retinal architecture196 but it is required for rod OS structure197 and mice lacking RPGRIP1 develop grossly oversized OS discs.198 RPGRIP1 is localized to the distal part of the photoreceptor axoneme, and is required for the recruitment of RPGR, NPHP4 and SDCCAG8 to the CC: Rpgrip1nmf247 mutant mice lack these proteins at the CC.199,200 Mutations in RPGRIP1 in humans are associated with RP,201 cone-rod dystrophy (CORD)202 and LCA.203 NPHP4 mutations lead to nephronophthisis (NPHP) and RP,204 and SDCCAG8 is mutated in BBS and a retinal-renal ciliopathy.205-207 Mutations in the homolog of RPGRIP, RPGRIP1L, are also associated with a suite of disorders involving retinal dystrophy, including JBTS,208 MKS and cerebello-oculo-renal syndrome (CORS).209 A specific mutation in RPGRIP1L has been shown to be a modifier of retinal phenotype in these syndromic ciliopathies.210 Mutation of alanine 229 to threonine compromises the interaction of RPGRIP1L with RPGR, with functional consequences on transport within the photoreceptors that leads to retinal degeneration. RPGRIP1L also interacts with MKS and NPHP proteins at the ciliary transition zone/basal body to initiate IFT-driven cilium extension early in ciliogenesis.211 The Ftm mouse mutant (in which Rpgrip1l is mutated) develops microphthalmia, associated with a global reduction in cilia number and Shh signaling defects.212
ALMS1, which is mutated in Alström syndrome (ALMS), a condition often presenting with CORD213,214 and LCA,62 is thought to play a role in transport along the photoreceptor axoneme, as gene-trap Alms1 mutant mice accumulate vesicles in their IS and rhodopsin is mislocalized to the outer nuclear layer.215 ALMS1 has been shown to play a role in endosome recycling in other cell types, which may reflect its role in photoreceptors.216 B9D2, inversin (NPHP2) and IQCB1 (NPHP5) are also thought to play roles in the transport of specific proteins along the photoreceptor. IQCB1 (NPHP5) localizes to the CC, OS and basal bodies of human and mouse photoreceptors, it interacts with RPGR in the CC,217 and mutations in NPHP5 are the most common cause of Senior-Løken syndrome (SLS), a ciliopathy that associates NPHP with RP or LCA.217 Only about 10% of individuals with NPHP also have RP or LCA, constituting SLS, but 100% of NPHP patients with NPHP5 mutations have RP. This emphasizes the essential role of IQCB1 in the photoreceptor,217 but, by contrast, RP is very rarely seen in NPHP2 patients, suggesting that inversin has a high level of functional redundancy in human photoreceptors.218,219 Loss of b9d2 or nphp2 (inversin) in zebrafish leads to mislocalized opsin but not mislocalized peripherin,119 whereas B9D2 mutations in humans are a cause of the lethal ciliopathy MKS that can occasionally present with retinal involvement.220 Nephrocystin (NPHP1) localizes to the CC, especially around the basal body, and may play a similar role to B9D2 and inversin.6 Indeed, Nphp1 mutant mice exhibit defects in the both the sorting of proteins between the photoreceptor IS and OS, and compromised IFT.221 Mutations in NPHP1 cause the ciliopathies NPHP,222 JBTS223 and SLS,224 all of which include retinal degeneration in some patients. NPHP4 localizes to the base of the connecting cilium, proximal to SDCCAG8, RPGRIP1 and RPGR200 and when mutated leads to CORD in dogs,225 NPHP, SLS and CORS in humans.204,226,227 Similar to Nphp1 mutant mice, mice with mutations in Nphp4 exhibit mislocalization of OS proteins (rhodopsin and ROM1) to the IS and inner nuclear layer. These mice do not develop normal OS despite developing normal CC, and their photoreceptors degenerate rapidly leading to early-onset loss of vision.
Interestingly, the synaptic ribbons of Nphp4 mutant mice also degenerate after developing normally, with associated disturbances in the localizations of synaptophysin (a major synaptic vesicle protein) and post-synaptic density protein 95 (PSD95, a presynaptic marker in the outer plexiform layer).228 KIF3A and IFT88 are also localized to the ribbon synapse, and expression of dominant-negative KIF3B results in a complete loss of ribbon synapses,113 suggesting that IFT has an essential functional role at the photoreceptor synapse.115,229 The presence of clarin-1 (USH3A), which is mutated in Usher syndrome, further implicates the role of cilia proteins at the ribbon synapse in inherited disease.230
Future Perspectives and Challenges
Further extending the role of IFT and the cilium in the retina, Ataxia-telangiectasia and Rad3 (ATR) protein has recently been shown to be localized to the photoreceptor CC, and mice with mutations in this gene exhibit early-onset degeneration of rods and cones.231 ATR is a DNA damage sensor, potentially extending the role of photoreceptors, and primary cilia in general, to involvement in the DNA damage response (DDR). Supporting this hypothesis, mutations in other DDR genes (CEP164 and ZNF423) have been found in ciliopathy patients with retinal degeneration. It has been suggested that defective CC could cause postnatal accumulation of UV light-induced DNA damage, subsequently leading to retinal degeneration.75 However, the details of this novel ciliopathy disease mechanism remain unclear, and will undoubtedly form the basis of exciting advances in basic research.
These recent findings highlight our ever-expanding understanding of the importance of primary cilia in retinal development and function, and their role in human disease (Table 1). Refer to Table S1 for further details. Novel uncharacterised proteins are still being discovered to play a role in such processes, such as C8orf37, a ciliary protein which is mutated in CORD and RP232 and C2orf71, a ciliary protein mutated in RP in humans and progressive retinal atrophy in several breeds of dog.233,234 While our understanding of primary cilia and specialized sensory cilia such as the photoreceptor has advanced greatly in the past two decades, there still remains much to discover. With the ever-increasing power and affordability of genetic sequencing technologies, there are now unprecedented opportunities for rapid gene discovery in this group of retinal conditions, providing further insights into disease mechanism. A better understanding of the genetic basis of eye disease provides opportunities for improved diagnostics and disease management, including gene therapy. Retinal disease is a convenient model for the study of gene therapy, since vectors carrying wild-type copies of mutated genes are easily delivered to the affected cells by subretinal injection, and this method has been used to successfully restore partial vision in patients with a variety of gene mutations. Such therapies are currently in development for the treatment of patients with LCA5 mutations (Ronald Roepman, personal communication) because in this ciliopathy, as in many retinal ciliopathies, retinal architecture is not compromised and blindness arises due to the lack of a single specific protein causing defects in protein trafficking. Preliminary gene therapy experiments in Bbs1 mice have given promising results, with improved rhodopsin trafficking, reduced photoreceptor degeneration and improved ERG.235 Similarly, Bbs4 mutant mice have been successfully treated with gene therapy to restore rhodopsin localization, OS structure and prevention of photoreceptor degeneration.236 While treating BBS has its difficulties (particularly in ensuring that the correct stoichiometry of the BBSome complex is restored), retinal gene therapy remains a promising therapeutic approach that needs further research to assess its clinical efficacy and utility.
However, gene therapy is costly and difficult to develop, and can only be used to treat individuals with specific mutations in specific genes. This is a considerable disadvantage for groups of conditions with broad genetic heterogeneity such as the ciliopathies. The wide phenotypic variability, including allelism in different clinical entities, and the genetic heterogeneity of these conditions remain major challenges in the field of research into photoreceptor development and disease, especially in the attempts to develop therapies for these conditions. Broader therapeutic interventions such as treatment with tauroursodeoycholic acid (TUDCA), an anti-apoptotic factor, may offer opportunities for all degenerative retinal ciliopathies in the medium term, and it is promising that this treatment has been used successfully to slow retinal degeneration in Bbs1 mice.237
Summary
The photoreceptor OS is a highly modified primary cilium which has evolved to play a crucial role in vision. Development of the photoreceptor is a complex process that is an elaborate form of the ciliogenesis seen in other cell types. Most of the transport along the photoreceptor is performed by the cilium transport process of intraflagellar transport (IFT). IFT is essential for the movement of proteins involved in visual transduction in this cell type. For these reasons, proteins involved in primary cilium growth, structure, maintenance and function are of critical importance to retinal structure and function. The loss or mutation of these proteins results in a group of conditions known as the retinal ciliopathies that have defects in retinal development or retinal degeneration. Many of these proteins also have functions in the primary cilia of other cell types, so mutations are often associated with other complex multi-organ or developmental phenotypes, and retinal dystrophy forms just part of a syndromic ciliopathy. A range of model organisms and human genetic studies have advanced understanding of the role of the photoreceptor cilium in retinal health and disease, but a number of key challenges still remain in this field of research. Further insights into disease mechanisms in this group of conditions, as well as the identification of mutations in an ever-increasing number of genes, will be essential if gene therapy is to fulfil the early promise of many research studies and become an effective clinical treatment for the retinal ciliopathies.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to acknowledge funding from the European Community's Seventh Framework Programme FP7/2009 under grant agreement no: 241955, SYSCILIA http://syscilia.org/ and a Sir Jules Thorn Award for Biomedical Research (JTA/09).
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/organogenesis/article/26710
Glossary
Abbreviations:
- ACIII
adenylyl cyclase III
- ARL2
ADP-ribosylation factor-like 2 protein
- ARL2BP
ADP-ribosylation factor-like 2 binding protein
- ARL3
ADP-ribosylation factor-like 3 protein
- ATD
asphyxiating thoracic dystrophy
- BBS
Bardet-Biedl syndrome
- CC
connecting cilium
- CED
cranioectodermal dysplasia (Sensenbrenner syndrome)
- CORS
cerebelloocullorenal syndrome
- GC1
guanylyl cyclase 1 protein
- IFT
intraflagellar transport
- IS
inner segment (of a photoreceptor cell)
- JBTS
Joubert syndrome
- LCA
Leber congenital amaurosis
- MKS
Meckel-Gruber syndrome
- MKKS
McKusick-Kaufman syndrome
- MORM
mental retardation, obesity, retinal dystrophy and micropenis
- MZSDS
Mainzer-Saldino syndrome
- NINL
ninein-like protein
- NPHP
nephronophthisis
- OMD
occult macular dystrophy
- ONL
outer nuclear layer
- OS
outer segment (of a photoreceptor cell)
- PKD
polycystic kidney disease
- ROM1
retinal outer segment membrane protein 1
- RP2
Retinitis Pigmentosa 2 protein
- TMEM
transmembrane protein
- SLS
Senior-Løken syndrome
- SNARE
soluble N- ethylmaleimide sensitive factor receptor
- USH
Usher syndrome
References
- 1.De Robertis E. Electron microscope observations on the submicroscopic organization of the retinal rods. J Biophys Biochem Cytol. 1956;2:319–30. doi: 10.1083/jcb.2.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Robertis E. Some observations on the ultrastructure and morphogenesis of photoreceptors. J Gen Physiol. 1960;43:1–13. doi: 10.1085/jgp.43.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tokuyasu K, Yamada E. The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–30. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besharse JC, Forestner DM, Defoe DM. Membrane assembly in retinal photoreceptors. III. Distinct membrane domains of the connecting cilium of developing rods. J Neurosci. 1985;5:1035–48. doi: 10.1523/JNEUROSCI.05-04-01035.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horst CJ, Johnson LV, Besharse JC. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunologic epitope. Cell Motil Cytoskeleton. 1990;17:329–44. doi: 10.1002/cm.970170408. [DOI] [PubMed] [Google Scholar]
- 6.Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Müller D, Thumfart J, Schermer B, Pazour GJ, Neumann HPH, Zentgraf H, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–33. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 7.Besharse JC, Horst CJ. The Photoreceptor Connecting Cilium A Model for the Transition Zone. In: Bloodgood RA, ed. Ciliary and Flagellar Membranes. New York: Springer US, 1990:389-447. [Google Scholar]
- 8.Pugh EN Jr., Lamb TD. Phototransduction in Vertebrate Rods and Cones: Molecular Mechanisms of Amplification, Recovery and Light Adaptation. In: Stavenga DG, Grip WJd, E.N.Pugh J, eds. Handbook of Biological Physics. Amsterdam: Elsevier, 2000:183-254. [Google Scholar]
- 9.LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–4. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 10.Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Exp Eye Res. 1986;43:193–205. doi: 10.1016/S0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg RH, Wood I. Clefts and microtubules of photoreceptor outer segments in the retina of the domestic cat. J Ultrastruct Res. 1975;51:307–403. doi: 10.1016/S0022-5320(75)80102-X. [DOI] [PubMed] [Google Scholar]
- 12.Roof D, Adamian M, Jacobs D, Hayes A. Cytoskeletal specializations at the rod photoreceptor distal tip. J Comp Neurol. 1991;305:289–303. doi: 10.1002/cne.903050210. [DOI] [PubMed] [Google Scholar]
- 13.Eckmiller MS. Renewal of the ciliary axoneme in cone outer segments of the retina of Xenopus laevis. Cell Tissue Res. 1996;285:165–9. doi: 10.1007/s004410050632. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Gao J, Adamian M, Wen X-H, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol. 2005;25:4129–37. doi: 10.1128/MCB.25.10.4129-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yildiz O, Khanna H. Ciliary signaling cascades in photoreceptors. Vision Res. 2012;75:112–6. doi: 10.1016/j.visres.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 17.Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- 18.De Robertis E. Morphogenesis of the retinal rods; an electron microscope study. J Biophys Biochem Cytol. 1956;2(Suppl):209–18. doi: 10.1083/jcb.2.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cideciyan AV, Rachel RA, Aleman TS, Swider M, Schwartz SB, Sumaroka A, Roman AJ, Stone EM, Jacobson SG, Swaroop A. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011;20:1411–23. doi: 10.1093/hmg/ddr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I, Pepermans E, Estivalet A, Carette D, Aghaie A, et al. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol. 2012;199:381–99. doi: 10.1083/jcb.201202012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loosli F, Del Bene F, Quiring R, Rembold M, Martinez-Morales JR, Carl M, Grabher C, Iquel C, Krone A, Wittbrodt B, et al. Mutations affecting retina development in Medaka. Mech Dev. 2004;121:703–14. doi: 10.1016/j.mod.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–4. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 23.Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, et al. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet. 2001;10:1709–18. doi: 10.1093/hmg/10.16.1709. [DOI] [PubMed] [Google Scholar]
- 24.Seiler C, Finger-Baier KC, Rinner O, Makhankov YV, Schwarz H, Neuhauss SC, Nicolson T. Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Development. 2005;132:615–23. doi: 10.1242/dev.01591. [DOI] [PubMed] [Google Scholar]
- 25.Vergara MN, Del Rio-Tsonis K. Retinal regeneration in the Xenopus laevis tadpole: a new model system. Mol Vis. 2009;15:1000–13. [PMC free article] [PubMed] [Google Scholar]
- 26.Hitchcock PF, Raymond PA. The teleost retina as a model for developmental and regeneration biology. Zebrafish. 2004;1:257–71. doi: 10.1089/zeb.2004.1.257. [DOI] [PubMed] [Google Scholar]
- 27.Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–25. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- 28.Sedmak T, Wolfrum U. Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol Cell. 2011;103:449–66. doi: 10.1042/BC20110034. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen LB, Veland IR, Schrøder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 30.LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973;58:650–61. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knabe W, Kuhn HJ. Ciliogenesis in photoreceptor cells of the tree shrew retina. Anat Embryol (Berl) 1997;196:123–31. doi: 10.1007/s004290050085. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer S, Hoyer-Fender S. Mouse Odf2 localizes to centrosomes and basal bodies in adult tissues and to the photoreceptor primary cilium. Cell Tissue Res. 2009;338:295–301. doi: 10.1007/s00441-009-0861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakarova CF, Khanna H, Shah AZ, Patil SB, Sedmak T, Murga-Zamalloa CA, Papaioannou MG, Nagel-Wolfrum K, Lopez I, Munro P, et al. TOPORS, implicated in retinal degeneration, is a cilia-centrosomal protein. Hum Mol Genet. 2011;20:975–87. doi: 10.1093/hmg/ddq543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakarova CF, Papaioannou MG, Khanna H, Lopez I, Waseem N, Shah A, Theis T, Friedman J, Maubaret C, Bujakowska K, et al. Mutations in TOPORS cause autosomal dominant retinitis pigmentosa with perivascular retinal pigment epithelium atrophy. Am J Hum Genet. 2007;81:1098–103. doi: 10.1086/521953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18:410–24. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Angelo A, De Angelis A, Avallone B, Piscopo I, Tammaro R, Studer M, Franco B. Ofd1 controls dorso-ventral patterning and axoneme elongation during embryonic brain development. PLoS One. 2012;7:e52937. doi: 10.1371/journal.pone.0052937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coene KLM, Roepman R, Doherty D, Afroze B, Kroes HY, Letteboer SJF, Ngu LH, Budny B, van Wijk E, Gorden NT, et al. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet. 2009;85:465–81. doi: 10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb TR, Parfitt DA, Gardner JC, Martinez A, Bevilacqua D, Davidson AE, Zito I, Thiselton DL, Ressa JHC, Apergi M, et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23) Hum Mol Genet. 2012;21:3647–54. doi: 10.1093/hmg/dds194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrante MI, Giorgio G, Feather SA, Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F, Woolf AS, et al. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet. 2001;68:569–76. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrante MI, Romio L, Castro S, Collins JE, Goulding DA, Stemple DL, Woolf AS, Wilson SW. Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene. Hum Mol Genet. 2009;18:289–303. doi: 10.1093/hmg/ddn356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roosing S, Rohrschneider K, Beryozkin A, Sharon D, Weisschuh N, Staller J, Kohl S, Zelinger L, Peters TA, Neveling K, et al. European Retinal Disease Consortium Mutations in RAB28, encoding a farnesylated small GTPase, are associated with autosomal-recessive cone-rod dystrophy. Am J Hum Genet. 2013;93:110–7. doi: 10.1016/j.ajhg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greiner JV, Weidman TA, Bodley HD, Greiner CAM. Ciliogenesis in photoreceptor cells of the retina. Exp Eye Res. 1981;33:433–46. doi: 10.1016/S0014-4835(81)80094-2. [DOI] [PubMed] [Google Scholar]
- 43.Bachmann-Gagescu R, Phelps IG, Stearns G, Link BA, Brockerhoff SE, Moens CB, Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum Mol Genet. 2011;20:4041–55. doi: 10.1093/hmg/ddr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noor A, Windpassinger C, Patel M, Stachowiak B, Mikhailov A, Azam M, Irfan M, Siddiqui ZK, Naeem F, Paterson AD, et al. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet. 2008;82:1011–8. doi: 10.1016/j.ajhg.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tallila J, Jakkula E, Peltonen L, Salonen R, Kestilä M. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet. 2008;82:1361–7. doi: 10.1016/j.ajhg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorden NT, Arts HH, Parisi MA, Coene KLM, Letteboer SJF, van Beersum SEC, Mans DA, Hikida A, Eckert M, Knutzen D, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83:559–71. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y, Smith RS, Jordan W, King BL, Won J, Valpuesta JM, Naggert JK, Nishina PM. Prefoldin 5 is required for normal sensory and neuronal development in a murine model. J Biol Chem. 2011;286:726–36. doi: 10.1074/jbc.M110.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhowmick R, Li M, Sun J, Baker SA, Insinna C, Besharse JC. Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic. 2009;10:648–63. doi: 10.1111/j.1600-0854.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–64. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Gioia SA, Letteboer SJF, Kostic C, Bandah-Rozenfeld D, Hetterschijt L, Sharon D, Arsenijevic Y, Roepman R, Rivolta C. FAM161A, associated with retinitis pigmentosa, is a component of the cilia-basal body complex and interacts with proteins involved in ciliopathies. Hum Mol Genet. 2012;21:5174–84. doi: 10.1093/hmg/dds368. [DOI] [PubMed] [Google Scholar]
- 51.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–84. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- 53.Connell G, Bascom R, Molday L, Reid D, McInnes RR, Molday RS. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci U S A. 1991;88:723–6. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee ES, Burnside B, Flannery JG. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47:2150–60. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122:2003–13. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Zuo J, Pierce EA. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J Neurosci. 2004;24:6427–36. doi: 10.1523/JNEUROSCI.1335-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao J, Cheon K, Nusinowitz S, Liu Q, Bei D, Atkins K, Azimi A, Daiger SP, Farber DB, Heckenlively JR, et al. Progressive photoreceptor degeneration, outer segment dysplasia, and rhodopsin mislocalization in mice with targeted disruption of the retinitis pigmentosa-1 (Rp1) gene. Proc Natl Acad Sci U S A. 2002;99:5698–703. doi: 10.1073/pnas.042122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omori Y, Chaya T, Katoh K, Kajimura N, Sato S, Muraoka K, Ueno S, Koyasu T, Kondo M, Furukawa T. Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc Natl Acad Sci U S A. 2010;107:22671–6. doi: 10.1073/pnas.1009437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita T, Liu J, Gao J, LeNoue S, Wang C, Kaminoh J, Bowne SJ, Sullivan LS, Daiger SP, Zhang K, et al. Essential and synergistic roles of RP1 and RP1L1 in rod photoreceptor axoneme and retinitis pigmentosa. J Neurosci. 2009;29:9748–60. doi: 10.1523/JNEUROSCI.5854-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akahori M, Tsunoda K, Miyake Y, Fukuda Y, Ishiura H, Tsuji S, Usui T, Hatase T, Nakamura M, Ohde H, et al. Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am J Hum Genet. 2010;87:424–9. doi: 10.1016/j.ajhg.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davidson AE, Sergouniotis PI, Mackay DS, Wright GA, Waseem NH, Michaelides M, Holder GE, Robson AG, Moore AT, Plagnol V, et al. RP1L1 variants are associated with a spectrum of inherited retinal diseases including retinitis pigmentosa and occult macular dystrophy. Hum Mutat. 2013;34:506–14. doi: 10.1002/humu.22264. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Wang H, Cao M, Li Z, Chen X, Patenia C, Gore A, Abboud EB, Al-Rajhi AA, Lewis RA, et al. Whole-exome sequencing identifies ALMS1, IQCB1, CNGA3, and MYO7A mutations in patients with Leber congenital amaurosis. Hum Mutat. 2011;32:1450–9. doi: 10.1002/humu.21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weil D, Küssel P, Blanchard S, Lévy G, Levi-Acobas F, Drira M, Ayadi H, Petit C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet. 1997;16:191–3. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- 64.Liu X, Udovichenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–74. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collin GB, Won J, Hicks WL, Cook SA, Nishina PM, Naggert JK. Meckelin is necessary for photoreceptor intraciliary transport and outer segment morphogenesis. Invest Ophthalmol Vis Sci. 2012;53:7. doi: 10.1167/iovs.11-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leightner AC, Hommerding CJ, Peng Y, Salisbury JL, Gainullin VG, Czarnecki PG, Sussman CR, Harris PC. The Meckel syndrome protein meckelin (TMEM67) is a key regulator of cilia function but is not required for tissue planar polarity. Hum Mol Genet. 2013;22:2024–40. doi: 10.1093/hmg/ddt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80:186–94. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brancati F, Iannicelli M, Travaglini L, Mazzotta A, Bertini E, Boltshauser E, D’Arrigo S, Emma F, Fazzi E, Gallizzi R, et al. International JSRD Study Group MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum Mutat. 2009;30:E432–42. doi: 10.1002/humu.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otto EA, Tory K, Attanasio M, Zhou W, Chaki M, Paruchuri Y, Wise EL, Wolf MTF, Utsch B, Becker C, et al. Hypomorphic mutations in meckelin (MKS3/TMEM67) cause nephronophthisis with liver fibrosis (NPHP11) J Med Genet. 2009;46:663–70. doi: 10.1136/jmg.2009.066613. [DOI] [PubMed] [Google Scholar]
- 70.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compère P, Schiffmann SN, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–31. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 71.Luo N, Lu J, Sun Y. Evidence of a role of inositol polyphosphate 5-phosphatase INPP5E in cilia formation in zebrafish. Vision Res. 2012;75:98–107. doi: 10.1016/j.visres.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–6. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, Seo S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A. 2012;109:19691–6. doi: 10.1073/pnas.1210916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, et al. International Joubert Syndrome Related Disorders Study Group Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–9. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150:533–48. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–84. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sang LY, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen XH, Scales SJ, Kwong M, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–28. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaheen R, Faqeih E, Seidahmed MZ, Sunker A, Alali FE, AlQahtani K, Alkuraya FSA. A TCTN2 mutation defines a novel Meckel Gruber syndrome locus. Hum Mutat. 2011;32:573–8. doi: 10.1002/humu.21507. [DOI] [PubMed] [Google Scholar]
- 79.Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr., Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007;6:1299–317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson RE, Maude MB, Kelleher PA, Maida TM, Basinger SF. Metabolism of phosphatidylcholine in the frog retina. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1980;620:212–26. doi: 10.1016/0005-2760(80)90203-9. [DOI] [PubMed] [Google Scholar]
- 81.Wolfrum U, Schmitt A. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil Cytoskeleton. 2000;46:95–107. doi: 10.1002/1097-0169(200006)46:2<95::AID-CM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 82.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–9. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calvert PD, Schiesser WE, Pugh EN., Jr. Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol. 2010;135:173–96. doi: 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res. 1988;20:263–70. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- 85.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr., Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/S0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 86.Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225:127–32. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- 87.Marszalek JR, Liu XR, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LSB. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–87. doi: 10.1016/S0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, et al. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci. 2011;14:874–80. doi: 10.1038/nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giessl A, Trojan P, Rausch S, Pulvermüller A, Wolfrum U. Centrins, gatekeepers for the light-dependent translocation of transducin through the photoreceptor cell connecting cilium. Vision Res. 2006;46:4502–9. doi: 10.1016/j.visres.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 90.Trojan P, Rausch S, Giessl A, Klemm C, Krause E, Pulvermüller A, Wolfrum U. Light-dependent CK2-mediated phosphorylation of centrins regulates complex formation with visual G-protein. Biochim Biophys Acta. 2008;1783:1248–60. doi: 10.1016/j.bbamcr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet. 2010;19:1358–67. doi: 10.1093/hmg/ddq012. [DOI] [PubMed] [Google Scholar]
- 92.Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol. 2008;15:373–80. doi: 10.1038/nsmb.1396. [DOI] [PubMed] [Google Scholar]
- 93.Shu X, Zeng Z, Gautier P, Lennon A, Gakovic M, Cheetham ME, Patton EE, Wright AF. Knockdown of the zebrafish ortholog of the retinitis pigmentosa 2 (RP2) gene results in retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52:2960–6. doi: 10.1167/iovs.10-6800. [DOI] [PubMed] [Google Scholar]
- 94.Schwahn U, Lenzner S, Dong J, Feil S, Hinzmann B, van Duijnhoven G, Kirschner R, Hemberger M, Bergen AAB, Rosenberg T, et al. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat Genet. 1998;19:327–32. doi: 10.1038/1214. [DOI] [PubMed] [Google Scholar]
- 95.Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–70. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–9. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 97.Davidson AE, Schwarz N, Zelinger L, Stern-Schneider G, Shoemark A, Spitzbarth B, Gross M, Laxer U, Sosna J, Sergouniotis PI, et al. Mutations in ARL2BP, Encoding ADP-Ribosylation-Factor-Like 2 Binding Protein, Cause Autosomal-Recessive Retinitis Pigmentosa. Am J Hum Genet. 2013;93:321–9. doi: 10.1016/j.ajhg.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189:1039–51. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–92. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Snow JJ, Ou GS, Gunnarson AL, Walker MRS, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 102.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–81. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mikami A, Tynan SH, Hama T, Luby-Phelps K, Saito T, Crandall JE, Besharse JC, Vallee RB. Molecular structure of cytoplasmic dynein 2 and its distribution in neuronal and ciliated cells. J Cell Sci. 2002;115:4801–8. doi: 10.1242/jcs.00168. [DOI] [PubMed] [Google Scholar]
- 104.Krock BL, Mills-Henry I, Perkins BD. Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Invest Ophthalmol Vis Sci. 2009;50:5463–71. doi: 10.1167/iovs.09-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beech PL, Pagh-Roehl K, Noda Y, Hirokawa N, Burnside B, Rosenbaum JL. Localization of kinesin superfamily proteins to the connecting cilium of fish photoreceptors. J Cell Sci. 1996;109:889–97. doi: 10.1242/jcs.109.4.889. [DOI] [PubMed] [Google Scholar]
- 106.Whitehead JL, Wang SY, Bost-Usinger L, Hoang E, Frazer KA, Burnside B. Photoreceptor localization of the KIF3A and KIF3B subunits of the heterotrimeric microtubule motor kinesin II in vertebrate retina. Exp Eye Res. 1999;69:491–503. doi: 10.1006/exer.1999.0724. [DOI] [PubMed] [Google Scholar]
- 107.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–8. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 109.Jimeno D, Lillo C, Roberts EA, Goldstein LS, Williams DS. Kinesin-2 and photoreceptor cell death: requirement of motor subunits. Exp Eye Res. 2006;82:351–3. doi: 10.1016/j.exer.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 110.Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, Marc RE, Frederick JM, Baehr W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–98. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Z, Roberts EA, Goldstein LS. Functional analysis of mouse kinesin motor Kif3C. Mol Cell Biol. 2001;21:5306–11. doi: 10.1128/MCB.21.16.5306-5311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao C, Omori Y, Brodowska K, Kovach P, Malicki J. Kinesin-2 family in vertebrate ciliogenesis. Proc Natl Acad Sci U S A. 2012;109:2388–93. doi: 10.1073/pnas.1116035109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Insinna C, Humby M, Sedmak T, Wolfrum U, Besharse JC. Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev Dyn. 2009;238:2211–22. doi: 10.1002/dvdy.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–70. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–13. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luby-Phelps K, Fogerty J, Baker SA, Pazour GJ, Besharse JC. Spatial distribution of intraflagellar transport proteins in vertebrate photoreceptors. Vision Res. 2008;48:413–23. doi: 10.1016/j.visres.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–16. doi: 10.1016/S0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 118.Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–15. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–44. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ou GS, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 121.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 122.Hudak LM, Lunt S, Chang CH, Winkler E, Flammer H, Lindsey M, Perkins BD. The intraflagellar transport protein ift80 is essential for photoreceptor survival in a zebrafish model of jeune asphyxiating thoracic dystrophy. Invest Ophthalmol Vis Sci. 2010;51:3792–9. doi: 10.1167/iovs.09-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rix S, Calmont A, Scambler PJ, Beales PL. An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Hum Mol Genet. 2011;20:1306–14. doi: 10.1093/hmg/ddr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–92. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J Cell Biol. 2010;189:171–86. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell. 2011;22:921–30. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q, Liu Q, Austin C, Drummond I, Pierce EA. Knockdown of ttc26 disrupts ciliogenesis of the photoreceptor cells and the pronephros in zebrafish. Mol Biol Cell. 2012;23:3069–78. doi: 10.1091/mbc.E12-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Berbari NF, Kin NW, Sharma N, Michaud EJ, Kesterson RA, Yoder BK. Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation. Dev Biol. 2011;360:66–76. doi: 10.1016/j.ydbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev Biol. 2009;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pasek RC, Berbari NF, Lewis WR, Kesterson RA, Yoder BK. Mammalian Clusterin associated protein 1 is an evolutionarily conserved protein required for ciliogenesis. Cilia. 2012;1:20. doi: 10.1186/2046-2530-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perrault I, Saunier S, Hanein S, Filhol E, Bizet AA, Collins F, Salih MA, Gerber S, Delphin N, Bigot K, et al. Mainzer-Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet. 2012;90:864–70. doi: 10.1016/j.ajhg.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmidts M, Frank V, Eisenberger T, Al Turki S, Bizet AA, Antony D, Rix S, Decker C, Bachmann N, Bald M, et al. Combined NGS approaches identify mutations in the intraflagellar transport gene IFT140 in skeletal ciliopathies with early progressive kidney Disease. Hum Mutat. 2013;34:714–24. doi: 10.1002/humu.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Q, Zhang Q, Pierce EA. Photoreceptor Sensory Cilia and Inherited Retinal Degeneration. In: Anderson RE, Hollyfield JG, LaVail MM, eds. Retinal Degenerative Diseases. New York: Springer US, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, et al. NISC Comparative Sequencing Program TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet. 2011;43:189–96. doi: 10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J. The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol. 2012;14:950–7. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbø M, Filhol E, Bole-Feysot C, et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet. 2011;89:634–43. doi: 10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–19. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seo S, Baye LM, Schulz NP, Beck JS, Zhang QH, Slusarski DC, Sheffield VC. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci U S A. 2010;107:1488–93. doi: 10.1073/pnas.0910268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pretorius PR, Baye LM, Nishimura DY, Searby CC, Bugge K, Yang B, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet. 2010;6:e1000884. doi: 10.1371/journal.pgen.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–33. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 142.Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Res. 2007;47:3394–407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim JC, Ou YY, Badano JL, Esmail MA, Leitch CC, Fiedrich E, Beales PL, Archibald JM, Katsanis N, Rattner JB, et al. MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci. 2005;118:1007–20. doi: 10.1242/jcs.01676. [DOI] [PubMed] [Google Scholar]
- 144.Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, et al. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–8. doi: 10.1038/ng935. [DOI] [PubMed] [Google Scholar]
- 145.Nishimura DY, Searby CC, Carmi R, Elbedour K, Van Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, et al. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2) Hum Mol Genet. 2001;10:865–74. doi: 10.1093/hmg/10.8.865. [DOI] [PubMed] [Google Scholar]
- 146.Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, et al. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3) Am J Hum Genet. 2004;75:475–84. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, et al. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–91. doi: 10.1038/88925. [DOI] [PubMed] [Google Scholar]
- 148.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–52. doi: 10.1016/S0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 149.Katsanis N, Beales PL, Woods MO, Lewis RA, Green JS, Parfrey PS, Ansley SJ, Davidson WS, Lupski JR. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat Genet. 2000;26:67–70. doi: 10.1038/79201. [DOI] [PubMed] [Google Scholar]
- 150.Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, Musarella MA, Parfrey PS, Sheffield VC, Biesecker LG. Mutations in MKKS cause Bardet-Biedl syndrome. Nat Genet. 2000;26:15–6. doi: 10.1038/79116. [DOI] [PubMed] [Google Scholar]
- 151.Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet. 2003;72:650–8. doi: 10.1086/368204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, et al. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet-Biedl syndrome gene. Am J Hum Genet. 2005;77:1021–33. doi: 10.1086/498323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–4. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 154.Chiang AP, Beck JS, Yen H-J, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim K-YA, Huang J, et al. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet–Biedl syndrome gene (BBS11) 2006;103:6287–92. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, et al. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muller J, Stoetzel C, Vincent MC, Leitch CC, Laurier V, Danse JM, Hellé S, Marion V, Bennouna-Greene V, Vicaire S, et al. Identification of 28 novel mutations in the Bardet-Biedl syndrome genes: the burden of private mutations in an extensively heterogeneous disease. Hum Genet. 2010;127:583–93. doi: 10.1007/s00439-010-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–8. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 158.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–40. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marion V, Stutzmann F, Gérard M, De Melo C, Schaefer E, Claussmann A, Hellé S, Delague V, Souied E, Barrey C, et al. Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet--Biedl syndrome with situs inversus and insertional polydactyly. J Med Genet. 2012;49:317–21. doi: 10.1136/jmedgenet-2012-100737. [DOI] [PubMed] [Google Scholar]
- 160.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–7. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–93. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, et al. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet. 2005;14:1109–18. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 163.Tadenev AL, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR. Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proc Natl Acad Sci U S A. 2011;108:10320–5. doi: 10.1073/pnas.1016531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, et al. BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci. 2013;126:2372–80. doi: 10.1242/jcs.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Veleri S, Bishop K, Dalle Nogare DE, English MA, Foskett TJ, Chitnis A, Sood R, Liu P, Swaroop A. Knockdown of Bardet-Biedl syndrome gene BBS9/PTHB1 leads to cilia defects. PLoS One. 2012;7:e34389. doi: 10.1371/journal.pone.0034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 167.Boldt K, Mans DA, Won J, van Reeuwijk J, Vogt A, Kinkl N, Letteboer SJF, Hicks WL, Hurd RE, Naggert JK, et al. Disruption of intraflagellar protein transport in photoreceptor cilia causes Leber congenital amaurosis in humans and mice. J Clin Invest. 2011;121:2169–80. doi: 10.1172/JCI45627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.den Hollander AI, Koenekoop RK, Mohamed MD, Arts HH, Boldt K, Towns KV, Sedmak T, Beer M, Nagel-Wolfrum K, McKibbin M, et al. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet. 2007;39:889–95. doi: 10.1038/ng2066. [DOI] [PubMed] [Google Scholar]
- 169.Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EAM, Swider M, Herrera W, Stone EM. Leber congenital amaurosis caused by Lebercilin (LCA5) mutation: retained photoreceptors adjacent to retinal disorganization. Mol Vis. 2009;15:1098–106. [PMC free article] [PubMed] [Google Scholar]
- 170.van Wijk E, Kersten FFJ, Kartono A, Mans DA, Brandwijk K, Letteboer SJF, Peters TA, Märker T, Yan XM, Cremers CW, et al. Usher syndrome and Leber congenital amaurosis are molecularly linked via a novel isoform of the centrosomal ninein-like protein. Hum Mol Genet. 2009;18:51–64. doi: 10.1093/hmg/ddn312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science. 1998;280:1753–7. doi: 10.1126/science.280.5370.1753. [DOI] [PubMed] [Google Scholar]
- 172.Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A. 2007;104:4413–8. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu X, Vansant G, Udovichenko IP, Wolfrum U, Williams DS. Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil Cytoskeleton. 1997;37:240–52. doi: 10.1002/(SICI)1097-0169(1997)37:3<240::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 174.Maerker T, van Wijk E, Overlack N, Kersten FFJ, McGee J, Goldmann T, Sehn E, Roepman R, Walsh EJ, Kremer H, et al. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- 175.Weil D, El-Amraoui A, Masmoudi S, Mustapha M, Kikkawa Y, Lainé S, Delmaghani S, Adato A, Nadifi S, Zina ZB, et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet. 2003;12:463–71. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- 176.Verpy E, Leibovici M, Zwaenepoel I, Liu X-Z, Gal A, Salem N, Mansour A, Blanchard S, Kobayashi I, Keats BJ, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet. 2000;26:51–5. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- 177.Ebermann I, Scholl HP, Charbel Issa P, Becirovic E, Lamprecht J, Jurklies B, Millán JM, Aller E, Mitter D, Bolz H. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet. 2007;121:203–11. doi: 10.1007/s00439-006-0304-0. [DOI] [PubMed] [Google Scholar]
- 178.Weston MD, Luijendijk MW, Humphrey KD, Möller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet. 2004;74:357–66. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–57. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KEJ, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–61. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–81. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 182.Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, et al. International Joubert Syndrome Related Disorders Study Group Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–5. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 183.Cremers FPM, van den Hurk JAJM, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–76. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- 184.Murga-Zamalloa CA, Ghosh AK, Patil SB, Reed NA, Chan LS, Davuluri S, Peränen J, Hurd TW, Rachel RA, Khanna H. Accumulation of the Raf-1 kinase inhibitory protein (Rkip) is associated with Cep290-mediated photoreceptor degeneration in ciliopathies. J Biol Chem. 2011;286:28276–86. doi: 10.1074/jbc.M111.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hong D-H, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, Wright AF, Arshavsky VY, Li T. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–21. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- 186.Wright RN, Hong D-H, Perkins BD. RpgrORF15 connects to the usher protein network through direct interactions with multiple whirlin isoforms. Invest Ophthalmol Vis Sci. 2012;53:1519–29. doi: 10.1167/iovs.11-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murga-Zamalloa CA, Desai NJ, Hildebrandt F, Khanna H. Interaction of ciliary disease protein retinitis pigmentosa GTPase regulator with nephronophthisis-associated proteins in mammalian retinas. Mol Vis. 2010;16:1373–81. [PMC free article] [PubMed] [Google Scholar]
- 188.Meindl A, Dry K, Herrmann K, Manson E, Ciccodicola A, Edgar A, Carvalho MRS, Achatz H, Hellebrand H, Lennon A, et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3) Nat Genet. 1996;12::13. doi: 10.1038/ng0596-35. [DOI] [PubMed] [Google Scholar]
- 189.Hong D-H, Pawlyk BS, Shang J, Sandberg MA, Berson EL, Li T. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3) Proc Natl Acad Sci U S A. 2000;97:3649–54. doi: 10.1073/pnas.97.7.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010;19:3591–8. doi: 10.1093/hmg/ddq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–13. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 192.Westfall JE, Hoyt C, Liu Q, Hsiao Y-C, Pierce EA, Page-McCaw PS, Ferland RJ. Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1. J Neurosci. 2010;30:8759–68. doi: 10.1523/JNEUROSCI.5229-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Gröne HJ, et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–80. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Simms RJ, Hynes AM, Eley L, Inglis D, Chaudhry B, Dawe HR, Sayer JA. Modelling a ciliopathy: Ahi1 knockdown in model systems reveals an essential role in brain, retinal, and renal development. Cell Mol Life Sci. 2012;69:993–1009. doi: 10.1007/s00018-011-0826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Roepman R, Bernoud-Hubac N, Schick DE, Maugeri A, Berger W, Ropers H-H, Cremers FPM, Ferreira PA. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Mol Genet. 2000;9:2095–105. doi: 10.1093/hmg/9.14.2095. [DOI] [PubMed] [Google Scholar]
- 196.Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Schwartz SB, Roman AJ, Stone EM. Leber congenital amaurosis caused by an RPGRIP1 mutation shows treatment potential. Ophthalmology. 2007;114:895–8. doi: 10.1016/j.ophtha.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 197.Won J, Gifford E, Smith RS, Yi H, Ferreira PA, Hicks WL, Li T, Naggert JK, Nishina PM. RPGRIP1 is essential for normal rod photoreceptor outer segment elaboration and morphogenesis. Hum Mol Genet. 2009;18:4329–39. doi: 10.1093/hmg/ddp385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao Y, Hong D-H, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A. 2003;100:3965–70. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hong DH, Yue GH, Adamian M, Li TS. Retinitis pigmentosa GTPase regulator (RPGRr)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J Biol Chem. 2001;276:12091–9. doi: 10.1074/jbc.M009351200. [DOI] [PubMed] [Google Scholar]
- 200.Patil H, Tserentsoodol N, Saha A, Hao Y, Webb M, Ferreira PA. Selective loss of RPGRIP1-dependent ciliary targeting of NPHP4, RPGR and SDCCAG8 underlies the degeneration of photoreceptor neurons. Cell Death Dis. 2012;3:e355. doi: 10.1038/cddis.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Booij JC, Florijn RJ, ten Brink JB, Loves W, Meire F, van Schooneveld MJ, de Jong PT, Bergen AAB. Identification of mutations in the AIPL1, CRB1, GUCY2D, RPE65, and RPGRIP1 genes in patients with juvenile retinitis pigmentosa. J Med Genet. 2005;42:e67. doi: 10.1136/jmg.2005.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hameed A, Abid A, Aziz A, Ismail M, Mehdi SQ, Khaliq S. Evidence of RPGRIP1 gene mutations associated with recessive cone-rod dystrophy. J Med Genet. 2003;40:616–9. doi: 10.1136/jmg.40.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dryja TP, Adams SM, Grimsby JL, McGee TL, Hong D-H, Li T, Andréasson S, Berson EL. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet. 2001;68:1295–8. doi: 10.1086/320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MTF, Schuermann MJ, Becker A, Birkenhäger R, Sudbrak R, et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71:1161–7. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Billingsley G, Vincent A, Deveault C, Héon E. Mutational analysis of SDCCAG8 in Bardet-Biedl syndrome patients with renal involvement and absent polydactyly. Ophthalmic Genet. 2012;33:150–4. doi: 10.3109/13816810.2012.689411. [DOI] [PubMed] [Google Scholar]
- 206.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42:840–50. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schaefer E, Zaloszyc A, Lauer J, Durand M, Stutzmann F, Perdomo-Trujillo Y, Redin C, Bennouna Greene V, Toutain A, Perrin L, et al. Mutations in SDCCAG8/NPHP10 Cause Bardet-Biedl Syndrome and Are Associated with Penetrant Renal Disease and Absent Polydactyly. Mol Syndromol. 2011;1:273–81. doi: 10.1159/000331268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Arts HH, Doherty D, van Beersum SEC, Parisi MA, Letteboer SJF, Gorden NT, Peters TA, Märker T, Voesenek K, Kartono A, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–8. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 209.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 210.Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–45. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–41. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vierkotten J, Dildrop R, Peters T, Wang BL, Rüther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–77. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 213.Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alström syndrome. Nat Genet. 2002;31:74–8. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- 214.Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JFN, Russell-Eggitt I, et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alström syndrome. Nat Genet. 2002;31:79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- 215.Collin GB, Cyr E, Bronson R, Marshall JD, Gifford EJ, Hicks W, Murray SA, Zheng QY, Smith RS, Nishina PM, et al. Alms1-disrupted mice recapitulate human Alström syndrome. Hum Mol Genet. 2005;14:2323–33. doi: 10.1093/hmg/ddi235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Collin GB, Marshall JD, King BL, Milan G, Maffei P, Jagger DJ, Naggert JK. The Alström syndrome protein, ALMS1, interacts with α-actinin and components of the endosome recycling pathway. PLoS One. 2012;7:e37925. doi: 10.1371/journal.pone.0037925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–8. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 218.Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–20. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.O'Toole JF, Otto EA, Frishberg Y, Hildebrandt F. Retinitis pigmentosa and renal failure in a patient with mutations in INVS. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. European Renal Association. 2006;21:1989–91. doi: 10.1093/ndt/gfl088. [DOI] [PubMed] [Google Scholar]
- 220.Dowdle WE, Robinson JF, Kneist A, Sirerol-Piquer MS, Frints SGM, Corbit KC, Zaghloul NA, van Lijnschoten G, Mulders L, Verver DE, et al. Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am J Hum Genet. 2011;89:94–110. doi: 10.1016/j.ajhg.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jiang S-T, Chiou Y-Y, Wang E, Chien Y-L, Ho H-H, Tsai F-J, Lin C-Y, Tsai S-P, Li H. Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Hum Mol Genet. 2009;18:1566–77. doi: 10.1093/hmg/ddp068. [DOI] [PubMed] [Google Scholar]
- 222.Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet. 1997;17:149–53. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 223.Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DWW, McDonald R, Eddy A, Chance PF, Glass IA. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004;75:82–91. doi: 10.1086/421846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Caridi G, Murer L, Bellantuono R, Sorino P, Caringella DA, Gusmano R, Ghiggeri GM. Renal-retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis. 1998;32:1059–62. doi: 10.1016/S0272-6386(98)70083-6. [DOI] [PubMed] [Google Scholar]
- 225.Wiik AC, Wade C, Biagi T, Ropstad EO, Bjerkås E, Lindblad-Toh K, Lingaas F. A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone-rod dystrophy in standard wire-haired dachshund. Genome Res. 2008;18:1415–21. doi: 10.1101/gr.074302.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32:300–5. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- 227.Alazami A, Alshammari M, Baig M, Salih M, Hassan H, Alkuraya F. NPHP4 mutation is linked to cerebello-oculo-renal syndrome and male infertility. Clin Genet. 2013 doi: 10.1111/cge.12160. Forthcoming; [DOI] [PubMed] [Google Scholar]
- 228.Won J, Marín de Evsikova C, Smith RS, Hicks WL, Edwards MM, Longo-Guess C, Li T, Naggert JK, Nishina PM. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum Mol Genet. 2011;20:482–96. doi: 10.1093/hmg/ddq494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci. 1999;19:1027–37. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zallocchi M, Meehan DT, Delimont D, Askew C, Garige S, Gratton MA, Rothermund-Franklin CA, Cosgrove D. Localization and expression of clarin-1, the Clrn1 gene product, in auditory hair cells and photoreceptors. Hear Res. 2009;255:109–20. doi: 10.1016/j.heares.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Valdés-Sánchez L, De la Cerda B, Diaz-Corrales FJ, Massalini S, Chakarova CF, Wright AF, Bhattacharya SS. ATR localizes to the photoreceptor connecting cilium and deficiency leads to severe photoreceptor degeneration in mice. Hum Mol Genet. 2013;22:1507–15. doi: 10.1093/hmg/dds563. [DOI] [PubMed] [Google Scholar]
- 232.Estrada-Cuzcano A, Neveling K, Kohl S, Banin E, Rotenstreich Y, Sharon D, Falik-Zaccai TC, Hipp S, Roepman R, Wissinger B, et al. European Retinal Disease Consortium Mutations in C8orf37, encoding a ciliary protein, are associated with autosomal-recessive retinal dystrophies with early macular involvement. Am J Hum Genet. 2012;90:102–9. doi: 10.1016/j.ajhg.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nishimura DY, Baye LM, Perveen R, Searby CC, Avila-Fernandez A, Pereiro I, Ayuso C, Valverde D, Bishop PN, Manson FDC, et al. Discovery and functional analysis of a retinitis pigmentosa gene, C2ORF71. Am J Hum Genet. 2010;86:686–95. doi: 10.1016/j.ajhg.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Downs LM, Bell JS, Freeman J, Hartley C, Hayward LJ, Mellersh CS. Late-onset progressive retinal atrophy in the Gordon and Irish Setter breeds is associated with a frameshift mutation in C2orf71. Anim Genet. 2013;44:169–77. doi: 10.1111/j.1365-2052.2012.02379.x. [DOI] [PubMed] [Google Scholar]
- 235.Seo S, Mullins RF, Dumitrescu AV, Bhattarai S, Gratie D, Wang K, Stone EM, Sheffield VC, Drack AV. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Invest Ophthalmol Vis Sci. 2013;54:6118–32. doi: 10.1167/iovs.13-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Simons DL, Boye SL, Hauswirth WW, Wu SM. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci U S A. 2011;108:6276–81. doi: 10.1073/pnas.1019222108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Drack AV, Dumitrescu AV, Bhattarai S, Gratie D, Stone EM, Mullins R, Sheffield VC. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Invest Ophthalmol Vis Sci. 2012;53:100–6. doi: 10.1167/iovs.11-8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.