Abstract
The bacterial microbiota of the human large bowel is a complex ecosystem consisting of several hundred, mostly anaerobic, species. To maintain colonization of the gut lumen and maximize growth in the presence of nutritional competitors, highly diverse metabolic pathways have evolved, with each microbe utilizing a different “winning strategy” for nutrient acquisition and utilization. Conditions and diseases leading to intestinal inflammation are accompanied by a severe disruption the microbiota composition characterized by an expansion of facultative anaerobic Enterobacteriaceae. Here, we review evidence that the local inflammatory response creates a unique nutritional environment that is conducive to a bloom of bacterial species whose genomes encode the capability of utilizing inflammation-derived nutrients.
Keywords: inflammatory bowel disease, irritable bowel syndrome, necrotizing entercolitis, enteric pathogens, Enterobacteriaceae, dysbiosis
The human large bowel harbors a diverse community of microbes, termed the gut microbiota. The collective metabolic activity of a balanced gut microbiota confers benefit by providing nutrients, maintaining immune homeostasis, and granting niche protection (reviewed in refs. 1 and 2). Understanding processes that can lead to a disruption of this microbial community during episodes of disease would represent a significant step forward in the development of treatment strategies. However, this task is not trivial because the make-up of the gut microbiota is complex, exhibiting great diversity between individuals from different families on the level of bacterial species, which makes it difficult to pinpoint the identities of beneficial microbes.
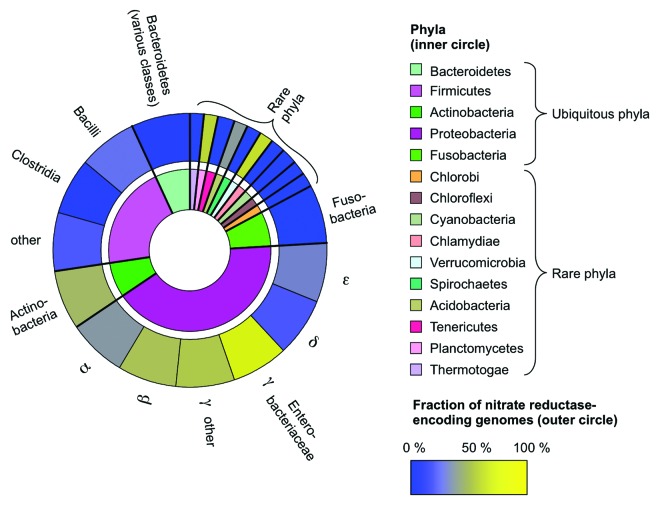
Nonetheless, there are some conserved features that characterize a balanced gut microbiota, including a dominance of obligate anaerobic bacteria belonging to the classes Bacteroidia (phylum Bacteroidetes) and Clostridia (phylum Firmicutes).3 Minor constituents commonly found within a balanced community include members of the phyla Actinobacteria, Proteobacteria, and Fusobacteria, although members of other phylogenetic groupings can be present occasionally (Fig. 1, inner circle).
A disruption of this balanced community structure during episodes of disease is termed dysbiosis. Dysbiosis is often characterized by the rise to prominence of bacteria that do not belong to the classes Bacteroidia or Clostridia. The most robust ecological pattern observed during inflammation in the distal gut is an expansion of facultative anaerobic Enterobacteriaceae (class Gammaproteobacteria, phylum Proteobacteria) within the community.
Enterobacteriaceae normally comprise only a small fraction (approximately 0.1%) of the microbiota in the large bowel,3 however a bloom of this family can be observed in various settings of gut inflammation. For example, the relative luminal abundance of Enterobacteriaceae is elevated dramatically in mouse models of inflammatory bowel disease, in which colitis is induced by a chemical trigger or by genetic predisposition.4-6 An increased prevalence of Enterobacteriaceae is also observed in patients with Crohn's disease, a inflammatory bowel disease of unknown etiology.7-10 Antibiotic treatment raises the inflammatory tone of the intestinal mucosa of mice, which is accompanied by a luminal bloom of Escherichia coli or Citrobacter rodentium (both members of the family Enterobacteriaceae).11,12 Similarly, repeated courses of antibiotics are associated with the development of irritable bowel syndrome in humans, a condition characterized by low-level intestinal inflammation, diarrhea, and a gut microbiota containing a heightened abundance of Proteobacteria belonging to the families Enterobacteriaceae, Pasteurellaceae, and Pseudomonadaceae.13-17 Enterobacteriaceae dominate the gut microbiota in preterm infants with necrotizing enterocolitis.18 Finally, intestinal inflammation induced in mice by bacterial enteric pathogens or parasite infection results in an uncontrolled expansion of Enterobacteriaceae within the community inhabiting the lower gastrointestinal tract.4,5,19-22
One of the mechanisms responsible for a bloom of Enterobacteriaceae in the lumen of the large bowel during inflammation is that the host response changes the growth conditions encountered in this largely anaerobic environment. Specifically, respiratory electron acceptors generated as a by-product of the inflammatory host response support bacterial growth by anaerobic respiration.23-25 For example, reactive oxygen and nitrogen species produced by host enzymes during inflammation can react to form peroxynitrite (ONOO–),26,27 a potent antimicrobial that is rapidly converted to nitrate (NO3–) in a reaction catalyzed by carbon dioxide (CO2).28 In turn, host-derived nitrate supports luminal growth of commensal E. coli or pathogenic Salmonella enterica (family Enterobacteriaceae) by nitrate respiration.12,24,25
The presence of nitrate in the inflamed distal gut is predicted to enhance growth of any bacterial species able to perform nitrate respiration. Thus, the question arises why the ecological pattern observed most consistently during inflammation is an uncontrolled expansion of Enterobatceriaceae, rather than other groupings. To address this conundrum, we searched 2476 genomes representing the phylogenetic groupings that have been described within gut-associated microbial communities for the predicted presence of nitrate reductase activity. While this activity was predicted to be largely absent in obligate anaerobic bacteria belonging to the Bacteroidia and Clostridia, it was predicted to be present in the majority of genomes belonging to the classes Betaproteobacteria and Gammaproteobacteria (Fig. 1, outer circle). Strikingly, the highest frequency of representatives predicted to encode this activity was found within the family Enterobacteriaceae (class Gammaproteobacteria). The observation that Enterobacteriaceae are the group most commonly observed to bloom during inflammation thus might merely reflect the fact that the ability to respire nitrate is more highly conserved within this family than in any other phylogentic group commonly inhabiting the large bowel. In other words, the generation of host-derived nitrate during inflammation in the lower gastrointestinal tract might favor Enterobacteriaceae because members of this family happen to be more likely to encode the enzymes for nitrate respiration.
It should be mentioned that in addition to nitrate reductases, the presence of enzymes for the utilization of other electron acceptors, such as for S-oxides, N-oxides, and tetrathionate, might also provide an advantage in the environment of the inflamed large bowel.23,25 Nonetheless, the example of nitrate reductases illustrates that despite the complexity of communities in the distal gut, an understanding of the mechanisms that control their relative composition is starting to provide a straightforward explanation for dysbiosis in various disease settings. Importantly, these findings point to the development of drugs that prevent or inhibit anaerobic respiration as a possible alternative to microbiota transplants for improving disease outcome by restoring a balanced microbial community structure.
Figure 1. Schematic representation of the prevalence of nitrate reductase-encoding genomes within major phyla. 2476 genome sequences deposited in the Kyoto Encyclopedia of Genes and Genomes (Release 67.1; http://www.kegg.jp/)29,30 were searched for the predicted presence of respiratory nitrate reductase activity (E.C.1.7.99.4). Phyla comprising thermophilic organisms (representing a total of 61 genomes) were excluded from this analysis, as these bacteria are unlikely to be present in the mammalian gut. Phyla commonly present in the gut-associated microbial community (ubiquitous phyla) and phyla present occasionally (rare phyla) are indicated in the inner circle, but the size of each sector is not proportional to their relative abundances. The prevalence of putative nitrate reductase activity (coloring of the outer circle) was calculated as the fraction of nitrate reductase-encoding genomes per total number of genomes within a given phylogenetic group.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
Work in S.E.W.’s laboratory is supported by Public Health Service Grant AI103248. A.J.B.’s laboratory is supported by Public Health Service grants AI107393 and AI096528.
References
- 1.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14:668–75. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–84. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–29. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–89. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–41. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1:403–18. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 10.Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52:237–42. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun. 2011;79:1536–45. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spees AM, Wangdi T, Lopez CA, Kingsbury DD, Xavier MN, Winter SE, Tsolis RM, Bäumler AJ. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. MBio. 2013;4:4. doi: 10.1128/mBio.00430-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–22. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–91. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerckhoffs AP, Ben-Amor K, Samsom M, van der Rest ME, de Vogel J, Knol J, Akkermans LM. Molecular analysis of faecal and duodenal samples reveals significantly higher prevalence and numbers of Pseudomonas aeruginosa in irritable bowel syndrome. J Med Microbiol. 2011;60:236–45. doi: 10.1099/jmm.0.022848-0. [DOI] [PubMed] [Google Scholar]
- 17.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–30, e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Normann E, Fahlén A, Engstrand L, Lilja HE. Intestinal microbial profiles in extremely preterm infants with and without necrotizing enterocolitis. Acta Paediatr. 2013;102:129–36. doi: 10.1111/apa.12059. [DOI] [PubMed] [Google Scholar]
- 19.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–15. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7:e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, et al. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-γ-dependent elimination of Paneth cells. Nat Immunol. 2013;14:136–42. doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S, Quinones M, Dzutsev AK, Gao JL, Trinchieri G, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. 2013;14:318–28. doi: 10.1016/j.chom.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–9. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez CA, Winter SE, Rivera-Chávez F, Xavier MN, Poon V, Nuccio SP, Tsolis RM, Bäumler AJ. Phage-mediated acquisition of a type III secreted effector protein boosts growth of salmonella by nitrate respiration. MBio. 2012;3:e00143–12. doi: 10.1128/mBio.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–11. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–7. doi: 10.1016/0003-9861(92)90434-X. [DOI] [PubMed] [Google Scholar]
- 27.De Groote MA, Granger D, Xu Y, Campbell G, Prince R, Fang FC. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci U S A. 1995;92:6399–403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 29.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–14. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]