Abstract
Antibiotics disturb the gastrointestinal tract microbiota and in turn reduce colonization resistance against Clostridium difficile. The mechanism for this loss of colonization resistance is still unknown but likely reflects structural (microbial) and functional (metabolic) changes to the gastrointestinal tract. Members of the gut microbial community shape intestinal metabolism that provides nutrients and ultimately supports host immunity. This review will discuss how antibiotics alter the structure of the gut microbiota and how this impacts bacterial metabolism in the gut. It will also explore the chemical requirements for C. difficile germination, growth, toxin production and sporulation. Many of the metabolites that influence C. difficile physiology are products of gut microbial metabolism including bile acids, carbohydrates and amino acids. To restore colonization resistance against C. difficile after antibiotics a targeted approach restoring both the structure and function of the gastrointestinal tract is needed.
Keywords: Clostridium difficile, antibiotics, gut microbiota, gut metabolome, colonization resistance
Introduction
Clostridium difficile is an anaerobic, spore-forming, gram-positive bacillus first isolated in 1935 by Hall and O’Toole.1 Attention to this organism as a pathogen developed when C. difficile was recognized as the cause of antibiotic-associated pseudomembranous colitis in the 1970s2. Within the past decade, there has been a renewed focus on C. difficile infection (CDI) due to an increase in morbidity, mortality and health care costs.3,4 In hospitals, CDI accounts for almost all cases of pseudomembranous colitis and 20% of nosocomial diarrhea cases.5 CDI can manifest a range of clinical disease from mild diarrhea to severe pseudomembranous colitis and even death.4
Hospitalization, advanced age (greater than 65 y) and antibiotic treatment are main risk factors for CDI.6,7 Antibiotics associated with CDI include clindamycin, quinolones, cephalosporins, and aminopenicillins.8-10 The key role of antibiotics in the development of CDI has prompted an interest in how these drugs can reduce colonization resistance against pathogens.11,12 Antibiotics, even at sub therapeutic levels, can have significant and long lasting effects on the gut microbiota.13-15 By altering the community structure of the gut microbiome, antibiotics also alter the intestinal metabolome, which is composed of both host- and microbial-derived metabolites.16-18
How an antibiotic-altered microbiome and metabolome facilitates the development of CDI is not well understood. There are multiple chemical queues that C. difficile encounters and reacts to within the host. In vitro studies, and a limited number of in vivo studies, have shed light on chemical requirements for C. difficile germination, outgrowth, and toxin production.19-22 This review will focus on how microbes shape the metabolic environment of the gastrointestinal tract and how this influences C. difficile pathogenesis.
Role of the Microbiome in Intestinal Metabolism
The indigenous gut microbiota is the complex community of microorganisms that populates the gastrointestinal tract. This community composes 70% of the total microbiota found on the human body (total 1014 bacterial cells).23 It plays a critical role in human health by providing resistance to colonization and infection by pathogenic organisms.12,24 It also has profound effects on homeostasis of the host, providing signals for epithelial maturation, shaping the immune response and participating in key metabolic transformations.17 Bacteria carry out multiple metabolic processes that have a profound effect on the chemical composition of the gastrointestinal environment (Fig. 1).
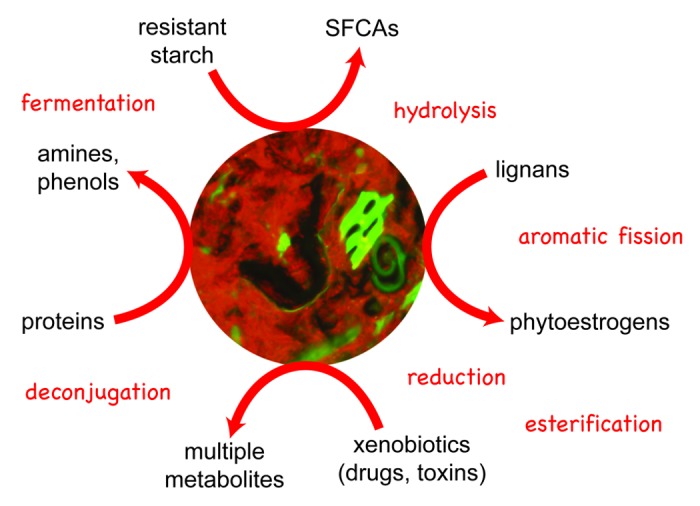
Figure 1. Functional role of the indigenous gastrointestinal tract microbiota. The gastrointestinal tract microbiota provides many metabolic functions that are able to convert luminal compounds into secondary metabolites. The chemical reactions (labeled in red) can produce metabolites that are both beneficial and harmful to the host. Fluorescence in situ hybridization (FISH) of the gastrointestinal tract microbiota in a wild type mouse is at the center (red, hybridized with Cy3-labeled Eub338) and was provided by Christine Bassis, PhD.
Two bacterial phyla that make up the majority of the gut bacterial population are the Firmicutes and Bacteroidetes.25 Much attention has been given to members of the Bacteroidetes phylum for their ability to breakdown host glycans and non-digestible carbohydrates including resistant starches and plant cell wall polysaccharides.26-28 The Firmicutes phylum, specifically members from the Lachnospiraceae and Ruminococcaceae family, makeup 50–70% of the colonic bacterial population and are also important for polysaccharide degradation.27 Additionally, Clostridium species are the most common amino acid fermenting bacteria found in the gut.29
It is estimated that 20 to 60 g of undigested carbohydrates enter the colon each day.30,31 The colonic microbiota plays a major functional role by fermenting these complex carbohydrates and amino acids into short chain fatty acids (SCFAs), which are important for colonic health and secondary bacterial fermenters in the gut.32-34 SCFAs, especially butyrate, are the main energy source for the colonic mucosa. SCFAs also play key roles in the regulation of host gene expression, inflammation, differentiation and apoptosis.35
The gut microbiota also plays a role in many other host and bacterial metabolic reactions including regulating amino acid metabolism and protein digestion.29 Host and bacterial proteases are important for breaking down exogenous protein into smaller peptides and amino acids. Members of the gut microbiota utilize amino acids and peptides as sources of nitrogen.29 End products of amino acid fermentation can be both beneficial and toxic to the host. Beneficial end products include SCFAs (acetate, butyrate, propionate, valerate), BCFAs (isobutyrate and isovalerate), organic acids and gaseous compounds. Toxic end products include phenols, indoles, ammonia, amines, thiols and hydrogen sulfide.29,32
The gut microbiota also plays a role in lipid metabolism. Bile acids are amphipathic lipids that are important in fat and cholesterol metabolism.36 Additionally bile acids modulate lipoprotein, glucose, drug and energy metabolism.36,37 Specific members of the gut microbiota, including some of the spore-forming and anaerobic members of the Clostridium genus, are able to perform two-enzymatic reactions on bile acids: deconjugation and 7α-dehydroxylation.36-39 Deconjugation of the glycine and taurine conjugates yields primary bile acids, which can then undergo 7α-dehydroxylation via gut microbial enzymes, yielding secondary bile acids.36 Members of the gut microbiota are important for shaping metabolism in the gastrointestinal tract.
Effect of Antibiotics on Microbiome Structure and Function
Given the key role of antibiotics in CDI there have been multiple studies in mice and humans examining the relationship between antibiotics and the gut microbiome. Treatment of mice with an antibiotic cocktail consisting of ampicillin, gentamicin, metronidazole, neomycin and vancomycin resulted in a 10-fold reduction in fecal bacterial density.40 Antibiotic treatment was associated with significant alteration of the gut community including decreased abundance of the Firmicutes phylum and increased persistence of Bacteroidetes and Proteobacteria. In agreement with Hill et al., Antonopoulos et al. demonstrated that treatment of mice with a cocktail of antibiotics (amoxicillin, metronidazole and bismuth or AMB) altered the gut microbiota with a persistent decrease in overall diversity.41 After antibiotics the murine gut microbiota was dominated by the Proteobacteria phylum, Enterobacteriaceae family, whereas the Bacteroidetes and Firmicutes phyla only made up a small portion of the total population.41 After AMB-treated animals were given 2 wk to recover off of antibiotics, the gut microbiota was restored to the original structure observed prior to antibiotic treatment. Mice treated with a broader spectrum antibiotic, cefoperazone, showed longer lasting alterations to the gut microbiota up to 6 wk after stopping antibiotics.41
There are fewer studies defining the structure of the gastrointestinal tract after antibiotic treatment in humans. We know that administration of the combination antibiotic amoxicillin-clavulanic acid (antibiotic and β-lactamase inhibitor) altered the gastrointestinal bacterial community structure and was associated with the development of antibiotic-associated diarrhea (AAD),42 including a marked reduction of many butyrate-producing bacterial members from the Clostridiaceae family that are essential for colonic health.42 Resolution of AAD and an increase in Clostridiaceae members was observed after cessation of antibiotic therapy.42 In a separate study, a 5-d ciprofloxacin treatment resulted in a decrease in the richness and diversity of the gut microbial community.43 Four weeks after antibiotic administration, the gut microbiota for one patient returned to the state prior to antibiotic therapy; however, the other patients’ microbiota took up to six months to recover. These studies demonstrate the potentially long-lasting alterations to the structure of the human gastrointestinal tract microbiota following antibiotic use.
By altering the gut microbial communities, antibiotics affect the intestinal metabolome, which is the total number of metabolites in the intestine. Over 87% of murine gut metabolites changed after streptomycin treatment, although the most significantly altered play an important role in sugar, amino acid, fatty acid, steroid, bile acid and eicosanoid metabolism.16 Additionally, streptomycin treatment resulted in an increase of the bile acids glycocholate, taurocholate, and taurochendeoxycholate and a decrease in chendeoxycholate and cholate.16
Zhao et al. demonstrated that gentamycin and ceftriaxone treatment resulted in a significant change to the fecal metabolome of mice.44 This resulted in decreased levels of monosaccharides (glucose, fucose, xylose, and galactose) and SCFAs, as well as increased levels of oligosaccharides (sucrose, cellobiose, raffinose, and stachyose). Shifts in amino acid and bile acid metabolism, specifically cholate, taurocholate, and tauro-β-muricholate to deoxycholate, were observed after antibiotics. Similar results were observed in other studies following treatment with vancomycin or enrofloxacin (fluoroquinolone).45,46 In a separate study, rats given a broad-spectrum β-lactam, imipenem/cilastatin sodium, specific metabolomic changes were observed.47 Amino acids (tryptophan, tyrosine, phenylalanine, histidine, cysteine, methionine, valine, leucine, isoleucine, lysine, arginine, and proline), organic acid (SCFAs), oligopeptide, carbohydrate, purine, pyrimidine, and TCA cycle metabolites were all affected.47
In mice and humans, antibiotics can cause a decrease in the bacterial load, bacterial diversity and a change in the bacterial community dynamics in the gut. Given that the most predominant members of the gut microbiota (Firmicutes and Bacteroidetes) are important for many metabolic processes including fermentation of carbohydrates and amino acids, it is expected that antibiotics will impact bacterial metabolism in the gastrointestinal tract. This was demonstrated in humans where clindamycin and ampicillin treatment decreased fecal SCFAs, functionally reflective of diminished bacterial fermentation in the gut.48 Antibiotics alter bacterial fermentation in the murine and human gut, which results in a decrease in SCFAs and an excess in fermentation substrates including carbohydrates and amino acids. C. difficile can utilize many of the metabolites in the gastrointestinal tract that were altered after antibiotics including bile, carbohydrates and amino acids for germination and growth.19,21
The Influence of the Metabolic Environment of the Gut on C. difficile
C. difficile spores require a germinant for outgrowth into vegetative cells. Much insight on C. difficile germination has been accomplished with in vitro studies. In 1972 Wilson et al. first characterized C. difficile spore germination and discovered that sodium taurocholate supplemented media increased recovery of spores.49,50 Since then, other researchers have revisited germination requirements for C. difficile. In a series of in vitro studies, Sorg and Sonenshein found that bile acids, and analogs made by the host, were able to both inhibit and support C. difficile spore germination and colony formation.51 Based on in vitro germination assays, primary bile acid chendeoxycholate inhibited spore germination and colony formation and was able to out compete other bile acids including taurocholate, cholate and glycocholate.19,52C. difficile spores were able to use taurocholate and glycine as a co-germinant for maximal germination.19 Moreover, the secondary bile acid deoxycholate was able to stimulate germination of C. difficile spores; however, like chendeoxycholate, it also inhibited growth of C. difficile.19,52 Additionally, amino acids have the ability to stimulate germination of spores including histidine, another co-germinant in vitro.53,54 Interestingly, certain C. difficile clinical isolates can utilize a wide range of bile acids for germination while others only require taurocholate and glycine for maximal germination.55,56
There have been a limited number of studies on the requirement for germination in vivo. Recently, Giel et al. demonstrated that filtered gastrointestinal contents from antibiotic treated mice were able to stimulate colony formation of C. difficile spores; however, samples from non-antibiotic treated mice from the small intestine also supported germination.22 Furthermore, Giel et al. demonstrated that primary bile acids were the predominant bile acid in the mouse gut after antibiotic treatment. Additionally, taurocholate, when supplemented into unfiltered, non-antibiotic treated samples, was converted to secondary bile acids, a result not observed when supplemented into antibiotic treated samples. Antibiotics alter the bacterial community capable of deconjugating and 7α-dehydroxylating bile acids in the gastrointestinal tract resulting in a decrease in secondary bile acids and an increase in primary and conjugated bile acids.22,57 C. difficile can use both primary (taurocholate) and secondary bile acids (deoxycholate) for germination, although deoxycholate can inhibit C. difficile growth in vitro.19 Biotransformation of bile acids by the gut microbiota could play an important role in C. difficile germination in vivo.22
Many gaps still exist in our understanding of how C. difficile germinates within a host and how this contributes to disease onset. The physiologically relevant concentration of bile acids that spores encounter in the human and mouse intestine is unknown and most germination assays are done in a pure culture in vitro environment for only a 30 min period, and it is unknown to what extent this mimics that of the host, especially in the gastrointestinal tract. Furthermore, germination is only the first step of the C. difficile lifecycle, and the metabolic environment leading to germination may also contribute to downstream events during disease development, including outgrowth of vegetative cells, toxin production and ultimately sporulation.
C. difficile has a wide repertoire of energy producing pathways. C. difficile is heterotrophic, saccharolytic, proteolytic and has recently been discovered to be autotrophic due to its ability to utilize carbon dioxide and hydrogen.58-60 In a defined minimal media, C. difficile requires amino acids (cysteine, isoleucine, leucine, proline, tryptophan, and valine) and vitamins (biotin, pantothenate, and pyridoxine) for optimal growth.60,61 Additionally, C. difficile is able to ferment many carbohydrates including fructose, glucose, mannitol, mannose, melezitose and sorbitol.21 Expression of toxin A and B, the primary virulence factors of CDI, are induced during the stationary growth phase when nutrients become limited and is affected by amino acids, butyrate, butanol, glucose and other carbon sources.62-66 More specifically, repression of C. difficile toxins has been observed when proline, cysteine, butanol or glucose is supplemented into growth media. Alternatively, toxin is induced when growth media is supplemented with butyrate67 and or limited in biotin68 suggesting a relationship between virulence and metabolism. Many of the nutrients that support C. difficile growth and toxin production were present in the gastrointestinal tract metabolomic studies highlighted in this review.
Taking advantage of the C. difficile genetic system has given researchers insight into C. difficile metabolism and pathogenesis. C. difficile encodes many genes important for regulating toxin expression and sporulation, many of which are directly linked to metabolism and availability of nutrients. CodY, a global regulatory protein that monitors nutrient availability, represses C. difficile toxin gene expression during growth in rich media.69,70 The direct target of CodY is the tcdR gene, which encodes the sigma factor required for the transcription of toxin genes,69 which lies with in the 19.6-kb pathogenicity locus (PaLoc). The PaLoc also includes toxin genes, tcdA and tcdB, as well as tcdR, tcdE, and tcdC genes.71,72 Other genes regulated by CodY are involved in amino acid biosynthesis, nutrient transport, fermentation, membrane components, and surface proteins. Another global regulator in C. difficile that controls transcription in response to carbohydrate availability is CcpA, a carbon catabolite control protein.73 CcpA binds to the regulatory region of the tcdA and tcdB genes.74 CcpA directly regulates genes important for sugar uptake, fermentation, amino acid metabolism, sporulation and toxin, suggesting a link between carbon metabolism and toxin production.
Studies defining the C. difficile transcriptome in two different animal models (germ-free mouse and pig ileal ligated loop) have shed light on what may be required for in vivo colonization and infection.75,76 Recently, Janoir and colleagues defined the C. difficile transciptome during early and late infection in a germ-free mouse model.75 Genes differentially expressed in vivo, compared with in vitro growth, were involved in C. difficile metabolism (fermentation, amino acids, and lipids), regulatory processes, cell process, stress response, pathogenicity and sporulation.75 Expression of genes responsible for degradation of polysaccharides and fermentation of carbohydrates and amino acids increased during infection in vivo. Furthermore, increased expression of genes responsible for butyrate biosynthesis and the production of ethanol and butanol, which are important for fermentation, were observed. Similarly, genes important for the biosynthesis of leucine and d-proline reductase increased in expression, suggesting C. difficile could be using the Stickland reaction to generate ATP in vivo.61 Expression of ethanolamine and N-acetylglucosamine utilization genes were found to have increased expression during late infection, suggesting there are potential carbon sources for C. difficile in the germ-free mouse gut even late in infection.77
The pig ileal loop model has also been used in C. difficile infection studies.76 Gene expression profiles of C. difficile were similar to those seen in the germ-free mouse during infection, and expression of genes encoding amino acid and carbohydrate transport and metabolism significantly increased in vivo compared with in vitro grown cultures.76 The pig ileal loop model also exhibited differences compared with the germ-free mouse model. Of note, toxin expression increased early during infection in the pig ileal loop model. Additionally, the observed decrease in expression of glucose degradation pathways and increase in degradation of mannose, xylose and glycogen was not observed in the germ-free mouse model discussed above.75 These differences likely reflect the ability of C. difficile to adapt to different host environments.
Animal Models and Human Studies of C. difficile/Gut Microbiome Interactions
Several animal models have been developed to study CDI.78-80 Bartlett et al. developed the first rodent model that was used to study pathogenesis of CDI.81 Hamsters were administered clindamycin followed by C. difficile challenge five days later, resulting in pseudomembranous colitis and death within 3 d.81,82 In 2008, Chen et al. developed a mouse model that approximates human CDI using a pretreatment of five antibiotics (gentamicin, kanamycin, colistin, metronidazole and vancomycin), followed by an intraperitoneal injection of clindamycin before challenge with C. difficile.78 This model of CDI is unlike the uniformly fatal hamster model because disease severity was dependent on the bacterial inoculum administered, and treatment with vancomycin prevented death. Additionally, when vancomycin was discontinued, relapse occurred, which resembles human disease. In addition to studying CDI pathogenesis in the hamster model, the mouse model of CDI is a valuable tool to explore the interplay between antibiotics, the gut microbiota, the host and colonization of C. difficile.
Reeves et al. used this murine model of CDI to analyze the microbiome in response to antibiotics and determined that antibiotic pretreatment resulted in a decrease in the relative abundance of Firmicutes and Bacteroidetes phyla, and an increase in Proteobacteria, specifically members of the Enterobacteriaceae family.83 Mice treated with broad-spectrum cephalosporin, cefoperazone, were also susceptible to C. difficile infection.83 In particular, cefoperazone treatment resulted in significant and long-lasting alterations to the mouse gut microbiota.41,83 An increased abundance of the Firmicutes and Proteobacteria phyla (specifically Lactobacillaceae and Pseudomonadaceae family members) was observed in cefoperazone-treated mice.83 Similarly, Buffie et al. demonstrated that clindamycin treatment alone resulted in C. difficile susceptibility, and observed a decrease in microbial diversity and long-lasting effects on the gut microbiota.84 After clindamycin treatment, bacterial members from the Proteobacteria phylum (Enterobacteriaceae family) were dominant. Thus, specific changes to the murine indigenous gut microbiota have been associated with loss of colonization resistance against C. difficile (Table 1)83-87.
Table 1. Animal studies of the gut microbiota and C. difficile infection.
| Host | Antibiotics (route/dose) | Microbiome analysis |
Structural changes to the gut microbiota | Strain of C. difficile |
Reference |
|---|---|---|---|---|---|
| Female CF-1 mice |
Subcutaneous injections saline, tigecycline (0.05 mg/day), clindamycin (1.4 mg/day), or piperacillin-tazobactam (8 mg/day) for 4 d. |
Culture based: plating onto brucella agar and Bacteroides bile-esculin agar | • Tigecycline did not suppress total anaerobes or Bacteroides spp. in comparison to saline controls and did not allow for C. difficile colonization. • Clindamycin and piperacillin-tazobactam did suppress Bacteroides spp.and allowed for C. difficile colonization. |
ATCC 43593 VA 17 VA 11 |
86 |
| Male and female C57BL/6 mice | -Antibiotic cocktail in drinking water: kanamycin (0.4 mg/ml), gentamicin (0.035 mg/ml), colistin (850 U/ml), metronidazole (0.215 mg/ml), vancomycin (0.045 mg/ml) for 5 d followed by clindamycin (10 mg/Kg) intraperitoneal injection. -Cefoperazone (0.5 mg/ml) in drinking water for 10 d. |
Non-culture based: 16S rRNA-encoding gene clone libraries | • Increased abundance of the Proteobacteria phylum (Enterobacteriaceae family) and decreased abundance of the Firmicutes phylum (Lachnospiraceae family) was associated with C. difficile colonization. • Increased abundance of the Firmicutes and Proteobacteria phyla specifically members of the Lactobacillaceae and Pseudomonadaceae family were also associated with C. difficile colonization. |
VPI 10463 | 83 |
| Female C57BL/6 mice | Single dose of clindamycin (200 ug) by intraperitoneal injection. | Non-culture based: Roche-454 pyrosequencing (V1-V3 primers) |
• Loss of Lachnospiraceae family members and Barnesiella populations and expansion of the Enterobacteriaceae species was associated with C. difficile colonization. | VPI 10463 | 84 |
| Female C57BL/6, C57BL/6 p402/2, C3H/HeN and C3H/HeJ mice |
Clindamycin (250 mg/L) in drinking water for 1 wk. | Non-culture based: 16S rRNA-encoding gene clone libraries | • Increased abundance of facultative anaerobes including members of the Enterobacteriaceae family and Enterococci was associated with C. difficile colonization. • Supershedder microbiota contained 16S rRNA gene clones derived from Blautia producta and included 16S rRNA gene sequences of Klebsiella pneumoniae, Escherichia coli, Proteus mirabilis, Parabacteroides distasonis and Enterococcus faecalis. |
BI-7 M68 630 |
85 |
| Male golden syrian hamsters |
Single dose of clindamycin (50 mg/Kg) by subcutaneous injection. |
Non-culture based: Roche-454 pyrosequencing (V1-V2 primers) |
• Reduction in the abundance of Bacteroidetes and Firmicutes and increase in Proteobacteria was associated with C. difficile colonization. • Temporary suppression of Bacteroidales and the fungus Saccinobaculus was also associated with C. difficile colonization. • Inoculation with C. difficile was associated with increases in Clostridiales on days 1 and 2 with a smaller increase in Burkholderiales and Pasteurellales species. |
ATCC 43596 | 87 |
Only a handful of studies have detailed the structure of the human gut microbiota after C. difficile infection, and human samples prior to CDI are limited (Table 2).88-93 The most recent study comes from Antharam et al. 2013 who compared the fecal microbiota of healthy subjects (n = 40) to those with antibiotic-associated diarrhea (AAD, n = 36) and C. difficile infection (CDI, n = 39).88 Decreased microbial diversity and species richness was observed in the fecal microbiota of AAD and CDI cases compared with healthy controls. Additionally, a decrease was seen in butyrate-producing bacteria from the Ruminococcaceae and Lachnospiraceae family and from Clostridia clusters IV and XIVa. Furthermore, CDI cases had a gut microbiota profile enriched in Enterococcus, Veillonella and Lactobacillus, and members from the Gammaproteobacteria class.88
Table 2. Human studies of the gut microbiota and C. difficile infection.
| Sample collection | Microbiome analysis |
Structural changes to the gut microbiota | Reference |
|---|---|---|---|
| Fecal samples were collected from three subject groups: healthy young adults, aged 21–34 y (n = 7); healthy elderly people, aged 67–88 y (n = 4); and elderly patients with C. difficile associated diarrhea (CDAD), aged 67–73 years (n = 4). |
Characterization of cellular fatty acid (CFA) profiles | • CDAD patients had a greater diversity of facultative species, Lactobacilli and Clostridia, and reduced numbers of Bacteroides, Prevotella and Bifidobacteria. • Enterobacteria and Enterococci increased in CDAD patients. |
91 |
| Fecal samples of patients with CDAD (both initial and recurrent episodes) were obtained from 10 individuals—patients with CDAD (n = 7) (initial C. difficile, ICD n = 3 and recurrent C. difficile, RCD n = 4) and control subjects (n = 3). | 16S rRNA-encoding gene clone libraries | • Species richness in the patients with ICD was similar to the controls. • Species richness in the RCD patients was consistently lower than both the patients with ICD and the controls. • RCD is associated with decreased overall diversity of the gut microbiota. |
93 |
| Fecal samples from 599 patients, hospitalized from September 2006 through May 2007 in Montreal, Quebec, were obtained within 72 h after admission. Twenty-five developed CDAD, and 50 matched controls were selected for analysis. | 16S rRNA-gene encoding microarrays | • Probe intensities were higher for Firmicutes, Proteobacteria, and Actinobacteria in CDAD patients, compared with controls, whereas probe intensities for Bacteroidetes were lower. • After epidemiologic factors were controlled for, only Bacteroidetes and Firmicutes remained significantly and independently associated with development of CDAD. |
90 |
| Fecal samples were collected from elderly subjects recruited from the community; including outpatient, short-term respite, and long-term hospital stay subjects. The carriage rate for C. difficile ranged from 1.6% (n = 123) for subjects in the community, to 9.5% (n = 43) in outpatient settings, and increasing to 21% (n = 151) for patients in short- or long-term care in hospital. | Culture-independent Roche/454 pyrosequencing (V4 region) | • C. difficile positive subjects had a decrease in Enterococcaceae but an increase in Lactobacillaceae and Enterobacteriaceae. • The dominant 072 ribotype was carried by 43% (12/28) of subjects, while the hypervirulent strain R027 (B1/NAP1/027) was isolated from 3 subjects (11%), 2 of whom displayed CDAD symptoms at the time of sampling. • Emerging ribotypes (078 and 018) were also isolated from two asymptomatic subjects. |
89 |
| Fecal samples (n = 208), of which 171 were routine samples and 37 were from healthy volunteers were collected. Of the 171 routine samples, 105 were C. difficile positive and 66 were C. difficile negative. From all 105 positive fecal samples C. difficile was isolated and strains were assigned to 22 different C. difficile PCR ribotypes. The five most frequent ribotypes were 027, 014/020, 081, 002 and 023. | Denaturing high-pressure liquid chromatography (DHPLC) and machine learning methods | • C. difficile positive samples showed lower levels of bacterial taxons from Bifidobacterium longum, Prevotella sp. and Bacteroides sp.. • Bifidobacterium longum was the most important predictor for the C. difficile negative status. • C. difficile positive samples had increases in Ruminococcus bromii, the family Peptostreptococcaceae and Streptococcus sp./Enterococcus sp. 2. • Healthy donors had higher frequencies of Methanobrevibacter smithii compared with C. difficile negative samples sent for routine testing and to C. difficile positive samples. |
92 |
| Fecal samples were collected from fecal microbiota transplant patients or FMT (n = 3) and their healthy donors (n = 3). | High-throughput 16S rRNA gene sequencing (V6 region) |
• Post FMT samples from patients showed an increase in the abundance of Firmicutes and Bacteroidetes. Proteobacteria and Actinobacteria were less abundant (< 5%) than that found in patients prior to FMT. • Bacteroidetes phylum was represented by family members Bacteroidaceae, Rikenellaceae and Porphyromonadaceae, and were largely comprised of Bacteroides, Alistipes and Parabacteroides genera. • -Firmicutes phylum was represented by family members Ruminococcaceae, Lachnospiraceae, Verrucomicrobiaceae and unclassified Clostridiales and members of the Firmicutes. |
96 |
| Fecal samples were collected from individuals with C. difficile infection (CDI) (n = 39), subjects with nosocomial diarrhea not attributed to C. difficile (CDN) group (n = 36), and healthy controls (n = 40). | Culture-independent Roche/454 pyrosequencing (V1-V3 primers) | • CDI and CDN subjects were accompanied by a marked decrease in microbial diversity and species richness driven by a decrease in phylotypes within the Firmicutes phylum. • CDI and CDN subjects were depleted of Ruminococcaceae and Lachnospiraceae family members and butyrate-producing C2-C4 anaerobic fermenters. |
88 |
In another study by Manges and colleagues, fecal samples were collected from 599 patients after 72 h of admission to a Montreal hospital.90 Twenty-five patients developed C. difficile associated diarrhea (CDAD) and their fecal DNA was analyzed by 16S rRNA-gene encoding microarrays. CDAD patients had increased probe intensities for the Firmicutes, Proteobacteria and Actinobacteria phylum and decreased for Bacteroidetes; however, only Firmicutes and Bacteroidetes were significantly correlated after accounting for epidemiologic factors. CDAD patients also had an increased abundance of the Lactobacillaceae and Enterococcaceae family members.90
Another study by Rea et al. in 2012 compared the fecal microbiota of elderly subjects who were asymptomatic (n = 20) to patients that were culture negative for C. difficile (n = 252).89 C. difficile positive subjects had a decrease in Bacteroides, Prevotella and Bifidobacteria and an increase in members from the Lactobacillaceae and Enterobacteriaceae family.89 Using a culture-dependent method, Hopkins and MacFarlane observed similar results, including an increase in the diversity of facultative species such as Lactobacilli and Clostridia in four CDI cases.91
Standard treatment of CDI in humans is oral administration of either metronidazole or vancomycin. Unfortunately, after successful treatment more than 20% of patients experience one or more relapses of disease.94 Patients that have failed traditional treatment with severe CDI have had success with fecal bacteriotherapy, which is the restoration of colon homeostasis by reintroducing normal bacterial microbiota from stool obtained from a healthy donor.95 In 2008, Chang et al. found that patients with recurrent CDAD had decreased diversity of the fecal microbiota with highly variable bacterial composition.93 A more recent study following successful fecal bacterial transplantation of three subjects observed an increase in Firmicutes and Bacteroidetes (Bacteroidaceae, Rikenellaceae and Porphyromonadaceae and Ruminococcaceae, Lachnospiraceae, Verrucomicrobiaceae, and unclassified Clostridiale members) and a decrease in Proteobacteria and Actinobacteria members.96 Interestingly, an increased abundance of the Enterobacteriaceae family was observed in one patient requiring antibiotics during the study.96
Structural changes to the gut microbiota in humans resemble changes in mice after antibiotics (as seen in Tables 1 and 2); however no specific structural profile has been correlated with decreased colonization resistance against C. difficile. Commonalities among studies that allow for C. difficile colonization in both Tables 1 and 2 include a decrease in bacterial diversity, a decrease in bacteria from the phylum Bacteroidetes and an increase in Proteobacteria. A decrease in bacterial diversity and a shift in the predominant members of the gut microbiota could alter bacterial metabolism in the gut, potentially allowing for C. difficile colonization. Future studies will need to investigate which microbes inhabit the gastrointestinal tract during different disease states, as well as determine the metabolic role of each specific commensal member.
Targeted Interventions and Treatment Options
Based on the structural studies detailed in Tables 1 and 2, a healthy, diverse gut microbiota is necessary for colonization resistance against C. difficile. Targeted interventions that will preserve or reestablish the structure and more importantly the function of the gut microbiota (ie. non-antibiotics) are needed. In order to restore colonization resistance in the gut after multiple failed courses of antibiotics, patients have turned to fecal transplantation, which is recolonizing the gut with healthy donor stool. Fecal bacteriotherapy has a 90% success rate although the long-term consequences of this treatment are still unclear.97,98 Repopulating the gut with healthy bacteria is not a new concept, and both human and murine models have demonstrated effective recovery of colonization resistance against C. difficile.85,96 In 1989 this approach was applied to patients by Tvede and Rask-Madsen where a cocktail of ten bacteria was given to six patients suffering from recurrent CDI.99 After rectal installation of the bacteria, patients’ stool had decreased levels of C. difficile and toxin and an increase in Bacteroides sp. More recently, Petrof et al. used a synthetically prepared stool transplant comprised of 33 bacterial isolates from a healthy donor stool.100 This cocktail, consisting of a community of bacteria, was able to treat two patients for up to 6 mo post-transplant at which point they were symptom free. In 2012, Lawley et al. designed a bacterial cocktail that was able to reestablish colonization resistance in mice against C. difficile 027/BI strain.85 The cocktail of six bacteria isolated from the mouse gut consisted of Staphylococcus, Enterococcus, Lactobacillus, Anaerostipes, Bacteroidetes, and Enterorhabdus. It will be important to determine what the bacteria are doing in the gut that is creating functional resistance to C. difficile. There has also been some success using individual strains of bacteria in a murine model to prevent or minimize C. difficile infection with non-toxigenic C. difficile, Escherichia coli, Bifidobacterium bifidum, and members of the Lachnospiraceae family.101-103
Alternate ways to manipulate the gut microbiota that could restore colonization resistance against C. difficile include diet changes and the use of pre- or probiotics. Prebiotics are non-digestible food ingredients that promote the growth of beneficial microorganisms in the intestines.104 Probiotics are microorganisms that are believed to counteract disturbances in the gut made by antibiotics, thus restoring colonization resistance against pathogens.105 A recent review examining 20 trials of probiotics for the prevention of CDAD estimated they prevent 33 episodes per 1000 persons.106 However, the mechanism for which probiotics prevent CDAD is unknown at this time and requires further study. A combination of diet, pre- and probiotics represents a promising strategy for reengineering the gastrointestinal tract environment to be functionally resistant to CDI.
Future Directions
It is well documented that antibiotics alter the structure of the gut microbiome,43,83 but it is unknown how this impacts bacterial metabolism in the gut. New “omics” techniques including metagenomics, transcriptomics, proteomics and metabolomics are now available to help define function in the gut. Many things can cause an imbalance in the gut microbiota including antibiotic usage, changes in diet, medications, inflammation, and pathogens. Molecular tools, including metabolomics, will be critical in the future for understanding these imbalances and could contribute to diagnosis, biomarker discovery and aid in personalized medicine. A more targeted approach to alter the gut functionally and potentially restore colonization resistance to C. difficile, such as diet, pre- and probiotics is needed. Unlocking how C. difficile is able to overcome colonization resistance in the gut has major implications for the development of therapeutics for prevention and treatment of human CDI.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Hall JC, O'Toole E. Intestinal flora in new-born infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390–402. doi: 10.1001/archpedi.1935.01970020105010. [DOI] [Google Scholar]
- 2.Bartlett JG, Gorbach SL. Pseudomembranous enterocolitis (antibiotic-related colitis) Adv Intern Med. 1977;22:455–76. [PubMed] [Google Scholar]
- 3.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucado J, Gould C, Elixhauser A. in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs (2006). [PubMed] [Google Scholar]
- 5.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–8. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 6.Freeman J, Wilcox MH. Antibiotics and Clostridium difficile. Microbes Infect. 1999;1:377–84. doi: 10.1016/S1286-4579(99)80054-9. [DOI] [PubMed] [Google Scholar]
- 7.Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, Leblanc M, Rivard G, Bettez M, Primeau V, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–60. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 8.Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29:44–50. doi: 10.1086/524320. [DOI] [PubMed] [Google Scholar]
- 9.Gifford AH, Kirkland KB. Risk factors for Clostridium difficile-associated diarrhea on an adult hematology-oncology ward. Eur J Clin Microbiol Infect Dis. 2006;25:751–5. doi: 10.1007/s10096-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 10.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563–78. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 11.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971;69:405–11. doi: 10.1017/S0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–14. doi: 10.1128/AAC.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009;77:2367–75. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antunes LC, Han J, Ferreira RB, Lolić P, Borchers CH, Finlay BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother. 2011;55:1494–503. doi: 10.1128/AAC.01664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:14. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4523–30. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190:2505–12. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson S, Burman LG, Akerlund T. Induction of toxins in Clostridium difficile is associated with dramatic changes of its metabolism. Microbiology. 2008;154:3430–6. doi: 10.1099/mic.0.2008/019778-0. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S, Nakashio S, Yamakawa K, Tanabe N, Nishida S. Carbohydrate fermentation by Clostridium difficile. Microbiol Immunol. 1982;26:107–11. doi: 10.1111/j.1348-0421.1982.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 22.Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010;5:e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 24.Freter R. In vivo and in vitro antagonism of intestinal bacteria against Shigellaflexneri. II. The inhibitory mechanism. J Infect Dis. 1962;110:38–46. doi: 10.1093/infdis/110.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–9. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 27.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers TE, Pudlo NA, Koropatkin NM, Bell JS, Moya Balasch M, Jasker K, Martens EC. Dynamic responses of Bacteroides thetaiotaomicron during growth on glycan mixtures. Mol Microbiol. 2013;88:876–90. doi: 10.1111/mmi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci (Landmark Ed) 2011;16:1768–86. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443–59. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 31.Silvester KR, Englyst HN, Cummings JH. Ileal recovery of starch from whole diets containing resistant starch measured in vitro and fermentation of ileal effluent. Am J Clin Nutr. 1995;62:403–11. doi: 10.1093/ajcn/62.2.403. [DOI] [PubMed] [Google Scholar]
- 32.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.SGE_Macfarlane. [DOI] [PubMed] [Google Scholar]
- 33.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci (Landmark Ed) 2011;16:1768–86. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 35.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–28. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–59. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Midtvedt T. Microbial bile acid transformation. Am J Clin Nutr. 1974;27:1341–7. doi: 10.1093/ajcn/27.11.1341. [DOI] [PubMed] [Google Scholar]
- 39.Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66:1107–13. doi: 10.1128/AEM.66.3.1107-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–58. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009;77:2367–75. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203–6. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Wu J, Li JV, Zhou NY, Tang H, Wang Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J Proteome Res. 2013;12:2987–99. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 45.Yap IK, Li JV, Saric J, Martin FP, Davies H, Wang Y, Wilson ID, Nicholson JK, Utzinger J, Marchesi JR, et al. Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. J Proteome Res. 2008;7:3718–28. doi: 10.1021/pr700864x. [DOI] [PubMed] [Google Scholar]
- 46.Romick-Rosendale LE, Goodpaster AM, Hanwright PJ, Patel NB, Wheeler ET, Chona DL, Kennedy MA. NMR-based metabonomics analysis of mouse urine and fecal extracts following oral treatment with the broad-spectrum antibiotic enrofloxacin (Baytril) Magn Reson Chem. 2009;47(Suppl 1):S36–46. doi: 10.1002/mrc.2511. [DOI] [PubMed] [Google Scholar]
- 47.Zheng X, Xie G, Zhao A, Zhao L, Yao C, Chiu NH, Zhou Z, Bao Y, Jia W, Nicholson JK, et al. The footprints of gut microbial-mammalian co-metabolism. J Proteome Res. 2011;10:5512–22. doi: 10.1021/pr2007945. [DOI] [PubMed] [Google Scholar]
- 48.Høverstad T, Carlstedt-Duke B, Lingaas E, Midtvedt T, Norin KE, Saxerholt H, Steinbakk M. Influence of ampicillin, clindamycin, and metronidazole on faecal excretion of short-chain fatty acids in healthy subjects. Scand J Gastroenterol. 1986;21:621–6. doi: 10.3109/00365528609003109. [DOI] [PubMed] [Google Scholar]
- 49.Wilson KH, Kennedy MJ, Fekety FR. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol. 1982;15:443–6. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson KH. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol. 1983;18:1017–9. doi: 10.1128/jcm.18.4.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol. 2010;192:4983–90. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol. 2009;191:1115–7. doi: 10.1128/JB.01260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeldon LJ, Worthington T, Lambert PA. Histidine acts as a co-germinant with glycine and taurocholate for Clostridium difficile spores. J Appl Microbiol. 2011 doi: 10.1111/j.1365-2672.2011.04953.x. [DOI] [PubMed] [Google Scholar]
- 54.Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. J Bacteriol. 2011;193:274–82. doi: 10.1128/JB.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heeg D, Burns DA, Cartman ST, Minton NP. Spores of Clostridium difficile clinical isolates display a diverse germination response to bile salts. PLoS One. 2012;7:e32381. doi: 10.1371/journal.pone.0032381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson PE, Jr., Walk ST, Bourgis AE, Liu MW, Kopliku F, Lo E, Young VB, Aronoff DM, Hanna PC. The relationship between phenotype, ribotype, and clinical disease in human Clostridium difficile isolates. Anaerobe. 2013;24:109–16. doi: 10.1016/j.anaerobe.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Köpke M, Straub M, Dürre P. Clostridium difficile is an autotrophic bacterial pathogen. PLoS One. 2013;8:e62157. doi: 10.1371/journal.pone.0062157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poilane I, Karjalainen T, Barc MC, Bourlioux P, Collignon A. Protease activity of Clostridium difficile strains. Can J Microbiol. 1998;44:157–61. [PubMed] [Google Scholar]
- 60.Karasawa T, Ikoma S, Yamakawa K, Nakamura S. A defined growth medium for Clostridium difficile. Microbiology. 1995;141:371–5. doi: 10.1099/13500872-141-2-371. [DOI] [PubMed] [Google Scholar]
- 61.Bouillaut L, Self WT, Sonenshein AL. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J Bacteriol. 2013;195:844–54. doi: 10.1128/JB.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karlsson S, Lindberg A, Norin E, Burman LG, Akerlund T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect Immun. 2000;68:5881–8. doi: 10.1128/IAI.68.10.5881-5888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antunes A, Martin-Verstraete I, Dupuy B. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol Microbiol. 2011;79:882–99. doi: 10.1111/j.1365-2958.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 64.Dupuy B, Sonenshein AL. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol. 1998;27:107–20. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 65.Karasawa T, Maegawa T, Nojiri T, Yamakawa K, Nakamura S. Effect of arginine on toxin production by Clostridium difficile in defined medium. Microbiol Immunol. 1997;41:581–5. doi: 10.1111/j.1348-0421.1997.tb01895.x. [DOI] [PubMed] [Google Scholar]
- 66.Ikeda D, Karasawa T, Yamakawa K, Tanaka R, Namiki M, Nakamura S. Effect of isoleucine on toxin production by Clostridium difficile in a defined medium. Zentralbl Bakteriol. 1998;287:375–86. doi: 10.1016/S0934-8840(98)80174-6. [DOI] [PubMed] [Google Scholar]
- 67.Karlsson S, Lindberg A, Norin E, Burman LG, Akerlund T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect Immun. 2000;68:5881–8. doi: 10.1128/IAI.68.10.5881-5888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamakawa K, Karasawa T, Ikoma S, Nakamura S. Enhancement of Clostridium difficile toxin production in biotin-limited conditions. J Med Microbiol. 1996;44:111–4. doi: 10.1099/00222615-44-2-111. [DOI] [PubMed] [Google Scholar]
- 69.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol. 2007;66:206–19. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 70.Dineen SS, McBride SM, Sonenshein AL. Integration of metabolism and virulence by Clostridium difficile CodY. J Bacteriol. 2010;192:5350–62. doi: 10.1128/JB.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun V, Hundsberger T, Leukel P, Sauerborn M, von Eichel-Streiber C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene. 1996;181:29–38. doi: 10.1016/S0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]
- 72.Hammond GA, Lyerly DM, Johnson JL. Transcriptional analysis of the toxigenic element of Clostridium difficile. Microb Pathog. 1997;22:143–54. doi: 10.1006/mpat.1996.0100. [DOI] [PubMed] [Google Scholar]
- 73.Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 2012;40:10701–18. doi: 10.1093/nar/gks864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antunes A, Martin-Verstraete I, Dupuy B. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol Microbiol. 2011;79:882–99. doi: 10.1111/j.1365-2958.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 75.Janoir C, et al. Insights into the adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infect Immun. 2013 doi: 10.1128/IAI.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scaria J, Janvilisri T, Fubini S, Gleed RD, McDonough SP, Chang YF. Clostridium difficile transcriptome analysis using pig ligated loop model reveals modulation of pathways not modulated in vitro. J Infect Dis. 2011;203:1613–20. doi: 10.1093/infdis/jir112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson KH, Perini F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun. 1988;56:2610–4. doi: 10.1128/iai.56.10.2610-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–92. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes. 2011;2:326–34. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steele J, Feng H, Parry N, Tzipori S. Piglet models of acute or chronic Clostridium difficile illness. J Infect Dis. 2010;201:428–34. doi: 10.1086/649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. Commentary: Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis 1977; 136:701. J Infect Dis. 2004;190:202–9. doi: 10.1086/421470. [DOI] [PubMed] [Google Scholar]
- 82.Fekety R, Silva J, Toshniwal R, Allo M, Armstrong J, Browne R, Ebright J, Rifkin G. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev Infect Dis. 1979;1:386–97. doi: 10.1093/clinids/1.2.386. [DOI] [PubMed] [Google Scholar]
- 83.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145–58. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jump RL, Li Y, Pultz MJ, Kypriotakis G, Donskey CJ. Tigecycline exhibits inhibitory activity against Clostridium difficile in the colon of mice and does not promote growth or toxin production. Antimicrob Agents Chemother. 2011;55:546–9. doi: 10.1128/AAC.00839-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peterfreund GL, Vandivier LE, Sinha R, Marozsan AJ, Olson WC, Zhu J, Bushman FD. Succession in the gut microbiome following antibiotic and antibody therapies for Clostridium difficile. PLoS One. 2012;7:e46966. doi: 10.1371/journal.pone.0046966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, Wang GP. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–92. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rea MC, O’Sullivan O, Shanahan F, O’Toole PW, Stanton C, Ross RP, Hill C. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J Clin Microbiol. 2012;50:867–75. doi: 10.1128/JCM.05176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manges AR, Labbe A, Loo VG, Atherton JK, Behr MA, Masson L, Tellis PA, Brousseau R. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J Infect Dis. 2010;202:1877–84. doi: 10.1086/657319. [DOI] [PubMed] [Google Scholar]
- 91.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–54. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- 92.Skraban J., et al. Gut microbiota patterns associated with colonization of different Clostridium difficile ribotypes. PloS one. 2013;8:e58005. doi: 10.1371/journal.pone.0058005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 94.Maroo S, Lamont JT. Recurrent clostridium difficile. Gastroenterology. 2006;130:1311–6. doi: 10.1053/j.gastro.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 95.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 96.Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4:125–35. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–9. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 98.Mattila E, Uusitalo-Seppälä R, Wuorela M, Lehtola L, Nurmi H, Ristikankare M, Moilanen V, Salminen K, Seppälä M, Mattila PS, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–6. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 99.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156–60. doi: 10.1016/S0140-6736(89)92749-9. [DOI] [PubMed] [Google Scholar]
- 100.Petrof EO, et al. Stool Substitute transplant therapy for the eradication of Clostridium difficile infection: “RePOOPulating” the gut. Microbiome 2013; 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Corthier G, Dubos F, Raibaud P. Modulation of cytotoxin production by Clostridium difficile in the intestinal tracts of gnotobiotic mice inoculated with various human intestinal bacteria. Appl Environ Microbiol. 1985;49:250–2. doi: 10.1128/aem.49.1.250-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun. 2012;80:3786–94. doi: 10.1128/IAI.00647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagaro KJ, Phillips ST, Cheknis AK, Sambol SP, Zukowski WE, Johnson S, Gerding DN. Nontoxigenic Clostridium difficile protects hamsters against challenge with historic and epidemic strains of toxigenic BI/NAP1/027 C. difficile. Antimicrob Agents Chemother. 2013;57:5266–70. doi: 10.1128/AAC.00580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 105.Sullivan A, Nord CE. Probiotics in human infections. J Antimicrob Chemother. 2002;50:625–7. doi: 10.1093/jac/dkf194. [DOI] [PubMed] [Google Scholar]
- 106.Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878–88. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]


