Abstract
Clostridium perfringens causes enteritis and enterotoxemia in humans and livestock due to prolific toxin production. In broth culture, C. perfringens uses the Agr-like quorum sensing (QS) system to regulate production of toxins important for enteritis/enterotoxemia, including beta toxin (CPB), enterotoxin, and epsilon toxin (ETX). The VirS/VirR two-component regulatory system (TCRS) also controls CPB production in broth cultures. Both the Agr-like QS and VirS/VirR systems are important when C. perfringens senses enterocyte-like Caco-2 cells and responds by upregulating CPB production; however, only the Agr-like QS system is needed for host cell-induced ETX production. These in vitro observations have pathophysiologic relevance since both the VirS/VirR and Agr-like QS signaling systems are required for C. perfringens strain CN3685 to produce CPB in vivo and to cause enteritis or enterotoxemia. Thus, apparently upon sensing its presence in the intestines, C. perfringens utilizes QS and TCRS signaling to produce toxins necessary for intestinal virulence.
Keywords: Clostridium perfringens, enterotoxin, beta toxin, epsilon toxin, quorum sensing, Agr, two component regulatory system, VirS/VirR, intestinal infection
Introduction
Clostridium perfringens is a major pathogen of humans and domestic animals.1,2 This Gram-positive, anaerobic spore-forming bacterium causes a range of diseases spanning from histotoxic infections, such as gas gangrene (clostridial myonecrosis),1,2 to infections originating in the intestines.1,2C. perfringens intestinal infections manifest as (1) an enteritis/enterocolitis that, depending upon the toxins produced by the infecting C. perfringens strain, results in a hemorrhagic or nonhemorrhagic diarrhea and/or (2) an enterotoxemia, which develops when this bacterium grows in the intestines and produces toxins that are absorbed into the circulation and then damage organs such as the brain, lungs or kidneys.1,2
C. perfringens pathogenicity is largely due to its abundant toxin production. Studies are now revealing that the C. perfringens strains causing mammalian enteritis or enterotoxemia apparently sense the presence of enterocytes and then respond, via signaling systems, by upregulating their toxin production. After a brief introduction to C. perfringens diseases originating in the intestines, this review will summarize the current knowledge of these signaling systems and their contributions to diseases originating in the mammalian intestines.
Clostridium perfringens Diseases Originating in the Intestines
As a species, C. perfringens produces a massive arsenal of 16 toxins.1,2 However, there is considerable variation in the repertoire of toxins produced by different strains of this bacterium. These strain-to-strain differences in toxin production form the basis for a commonly used typing system that assigns C. perfringens isolates to types A-E, based upon their expression of four typing toxins (Table 1). While this historic typing system was devised before the identification of some C. perfringens toxins, it remains useful since different types, and even subtypes, of this bacterium are associated with certain diseases (Table 2).
Table 1.C. perfringens Toxinotyping.
| Type | Toxin produced: | |||
|---|---|---|---|---|
| Alpha | Beta | Epsilon | Iota | |
| A | + | − | − | − |
| B | + | + | + | − |
| C | + | + | − | − |
| D | + | − | + | − |
| E | + | − | − | + |
Table 2. Diseases associated with Clostridium perfringens.
| Type of C. perfringens* | Major toxins | Most significant diseases** |
|---|---|---|
| A | CPA*** | Human and animal myonecrosis (gas gangrene); |
| CPA, CPE*** | Human food poisoning and non-foodborne gastrointestinal disease; canine gastrointestinal disease | |
| CPA, NetB*** | Necrotic enteritis of poultry | |
| B | CPA, CPB, ETX | Human biodefense concerns (ETX); necro-hemorrhagic enteritis of sheep (lamb dysentery) |
| C | CPA, CPB*** | Human necrotic enteritis (enteritis necroticans, pigbel); necrotic enteritis of neonatal individuals of several animal species (horse, cattle, sheep, pigs) |
| D | CPA, ETX*** | Human biodefense concerns (ETX); enterotoxemia of sheep and goats |
| E | CPE, ITX | No known association with human disease; suspected, but not confirmed association with gastrointestinal disease of cattle, sheep and rabbits. |
All types of C. perfringens may also produce several other toxins, including, but not limited to, CPB2, PFO, and TpeL. **Only diseases that have been confirmed to be associated with each type of C. perfringens, and that are significant in terms of prevalence, are included in this table. ***Critical toxin for virulence. PFO also contributes to virulence during myonecrosis (see text). Some type C, D, and E strains also produce CPE.
It is now well-established that C. perfringens relies heavily upon production of toxins encoded by mobile genetic elements to cause most cases of enteritis or enterotoxemia.2 Specifically, the toxins clearly implicated in enteritis or enterotoxemia are mainly encoded by genes carried on large plasmids.2 An exception is the cpe gene encoding C. perfringens enterotoxin (CPE), which can be carried by either a large plasmid or a putative transposon that has apparently inserted onto the chromosome.2
The C. perfringens types/subtypes causing most cases of mammalian enteritis and/or enterotoxemia, and the toxins involved in those illnesses, will now be briefly reviewed.
CPE-positive C. perfringens type A strains
The most important human intestinal infection caused by this bacterium is C. perfringens type A food poisoning.3 This syndrome currently ranks as the second most common bacterial foodborne illness in the USA, where 1 million cases/year occur and economic losses exceed $310 million/year.4,5 C. perfringens type A food poisoning is typically a self-limiting enteritis, but fatalities do occur in the elderly or in people receiving medications that reduce intestinal motility, possibly due to an enterotoxemia.6
The CPE-positive type A strains responsible for ~75% of C. perfringens type A food poisoning cases carry a chromosomal enterotoxin gene (cpe) that may be part of an integrated transposon.3 These typical food poisoning strains are also genetically distinct in other respects from most other C. perfringens isolates. For example, the typical type A food poisoning strains with a chromosomal cpe gene lack the pfoA gene encoding perfringolysin O (PFO) and generally produce a small acid soluble protein-4 (Ssp4) variant with an Asp at residue 36.7,8 This Asp36 Ssp4 variant binds tightly to DNA, which helps to confer exceptional spore resistance properties against food environment stresses, such as heat.8 Their exceptional spore heat resistance likely enhances the survival of these typical food poisoning strains in incompletely heated foods, thus facilitating their disease transmission.3
The remaining ~25% of C. perfringens type A food poisoning cases are caused by type A strains carrying a plasmid-borne cpe gene.2 These type A plasmid cpe food poisoning strains genetically resemble most other C. perfringens strains by carrying the pfoA gene and producing a Ssp4 variant with a Gly at residue 36.7,8 Compared against the Asp36 Ssp4 variant, the Gly36 Ssp4 variant binds more loosely to DNA, rendering spores containing the Gly36 Ssp4 variant less resistant to food environment stresses.8 Therefore, foodborne illness caused by type A plasmid cpe strains may involve less heated or unheated foods.3
After consumption of contaminated foods containing vegetative cells of a CPE-positive type A strain, the ingested bacteria multiply before sporulating in the intestines.3 During this in vivo sporulation, C. perfringens produces CPE, which is a two domain protein of 35 kDa that belongs to the aerolysin pore-forming toxin family.9,10 CPE accumulates cytoplasmically until it is released into the intestines when the mother cell lyses at the completion of sporulation. Once present in the intestinal lumen, CPE binds to enterocytes via receptors that include certain members of the claudin tight junction protein family.11,12 Bound CPE then oligomerizes into a hexameric prepore on the host cell membrane surface.13 This prepore rapidly inserts into membranes to form an active pore that increases Ca2+ influx, which then kills enterocytes via either oncosis or apoptosis.14,15 Typically, CPE-induced death of enterocytes results in villus damage that culminates in an enteritis involving diarrhea and abdominal cramps. However, evidence suggests that CPE food poisoning occasionally develops into a lethal enterotoxemia when people receive medications that reduce their intestinal motility.6,16
CPE-producing type A strains also cause 5–15% of all cases of nonfoodborne human gastrointestinal (GI) diseases, including antibiotic-associated diarrhea.17 Like the type A plasmid cpe strains causing some food poisoning cases, these nonfoodborne GI disease strains carry their cpe gene on a conjugative plasmid, possess the pfoA gene, and produce the Gly36 Ssp4 variant.7,8 Not surprisingly, the spores of these antibiotic-associated diarrhea isolates are relatively sensitive to heat and other stresses.8
Molecular Koch postulate analyses and human volunteer feeding experiments have established a central role for CPE in the pathogenesis of both C. perfringens type A food poisoning and CPE-associated nonfoodborne GI diseases.18
Type C strains
These C. perfringens strains producing beta toxin (CPB) represent another important cause of disease originating in the intestines.19 In domestic animals, particularly neonatal lambs, piglets, foals and calves, type C strains cause both hemorrhagic necrotic enteritis and enterotoxemias. In humans, these bacteria cause enteritis necroticans (EN), which was first reported in malnourished people in post-World War II Germany, where it was locally known as Darmbrand.20 EN was subsequently recognized as the leading cause of death in children during the 1960s-70s in the Papua New Guinea (PNG) highlands, where it was named PigBel.21 EN still occurs in PNG and, sporadically, in malnourished people in other developing countries. Occasional cases of this illness are observed in developed countries, mainly in people with pancreatic disease.22
A recent study20 showed that many Darmbrand strains produce both CPB and CPE, sometimes from toxin genes carried on the same plasmid. In addition, Darmbrand strains were found to closely resemble the type A chromosomal cpe strains causing most cases of C. perfringens type A food poisoning, i.e., Darmbrand isolates also lack a pfoA gene and produce the Asp36 Ssp4 variant,20 which likely helps to explain why these type C strains also produce very heat resistant spores and cause foodborne illness (see below). Multilocus sequencing typing of housekeeping genes further supported a close genetic relationship between the type A chromosomal cpe food poisoning strains and the type C Darmbrand strains.20 Whether PigBel strains share a close genetic relationship with Darmbrand strains and type A food poisoning strains has not yet been evaluated.
To cause EN, type C strains are transmitted via contaminated foods. However, most people ingesting these bacteria never develop disease because of a hallmark predisposing condition for EN, i.e., reduced intestinal trypsin activity. Because CPB is exquisitely sensitive to inactivation by trypsin, low trypsin levels in the intestines facilitate the persistence of this toxin.21 In people developing EN, reduced trypsin activity can result from several conditions: a protein poor diet, pancreatic disease affecting trypsin production, co-infection with the trypsin inhibitor-producing intestinal parasite Ascaris lumbricoides, and/or a diet rich in foods (e.g., sweet potato, soy beans, colostrum) containing a trypsin inhibitor.21
CPB is a pore-forming toxin that uses a still unidentified receptor for binding to host cells.23 Once bound, this toxin forms a heptameric or hexameric pore to cause cell death.24,25 CPB shares 20–28% identity with some staphylococcal pore-forming toxins and 38% identity with C. perfringens NetB toxin, which is important for the development of avian necrotic enteritis.26
CPB plays a critical role during type C infections of humans or domestic animals. Molecular Koch postulate analyses demonstrated that CPB production is important for type C strain CN3685 to cause either hemorrhagic necrotic enteritis in rabbit small intestinal loops or lethal enterotoxemia in mouse and goat type C challenge models.27,28 Furthermore, CPB alone is sufficient to cause hemorrhagic necrotic enteritis or lethal enterotoxemias in rabbit and mouse models, respectively.29,30
Type D strains
When the microbial balance in the intestines of domestic animals becomes disrupted by dietary or other poorly understood changes, C. perfringens type D strains sometimes proliferate and produce toxins, including epsilon toxin (ETX).1 These toxins cause a classic enterotoxemia in lambs (and occasionally adult sheep) that involves mostly neurologic clinical signs or sudden death. In goats, type D disease often involves necrotizing enterocolitis or colitis, usually along with lethal enterotoxemia.
Recent studies fulfilling Molecular Koch postulates established that ETX is required for type D strains to cause lethal enterotoxemias in sheep or goats or to produce intestinal damage in goats.31 Like CPE, ETX is a member of the aerolysin pore-forming toxin family.32 However, ETX is initially produced as a prototoxin that becomes activated by removal of critical C-terminal amino acids, which can be meditated by host intestinal proteases or by proteases produced by C. perfringens itself.33-35 The identity of the ETX receptor remains unclear, although this toxin can bind in vitro to hepatitis A virus cellular receptor 1.36 After binding, ETX forms a heptameric prepore that rapidly inserts into membranes to form an active pore.33 This pore quickly causes loss of intracellular K+ and increased cytoplasmic levels of Cl- and Na+.37 The ETX-induced K+ loss results in rapid cell death due to a necrosis process involving ATP depletion.37
Other strains
Type B strains, which produce the greatest variety of toxins among C. perfringens types, cause mainly enterotoxemias in young animals, particularly lambs.1 There are no reports of type B infections of humans. Interestingly, for unknown reasons, type B disease in animals only occurs in highly restricted geographic locations, e.g., type B infections have not been diagnosed in North America. The pathological role of individual toxins during C. perfringens type B-associated infections is still undefined.
Type E strains are the only C. perfringens producing iota toxin. Iota toxin is an ADP-ribosyltransferase that targets host cell actin, causing cell rounding.38 Type E strains have been suggested to cause enterotoxemias in neonatal calves, lambs and possibly rabbits.1 There has not yet been an association between these bacteria and human disease.
Introduction to the VirS/VirR and Agr-like C. perfringens Regulatory Systems
The VirS/VirR two-component system
Two-component regulatory systems (TCRS) are signal transduction systems with a wide distribution among bacteria.39 They typically consist of a membrane sensor and a cytoplasmic response regulator. Upon detecting environmental signals such as light, temperature, nutrients, osmotic stress or cell density, the sensor usually undergoes autophosphorylation and then transfers the phosphate to the response regulator. The activated response regulator then typically causes gene expression changes that can affect processes such as virulence, biofilm formation, competence, protein degradation, osmoregulation, bioluminescence, antibiotic resistance, and development.
The C. perfringens genome encodes approximately 18 sets of TCRS, along with several apparent orphan response regulators or sensor kinases.40 Of those TCRS, most have received little study except for VirS/VirR. This TCRS, encoded by the virS/virR operon, was first identified nearly 20 y ago in type A strain 13 and consists of the VirS membrane sensor histidine kinase and the VirR response regulator.41,42 VirS is predicted to contain six or seven transmembrane domains in the N-terminal region, while its C-terminal region has several conserved motifs typical of histidine kinases, including the autophosphorylation site. Upon detection of a still undefined signal by the N-terminal sensor region, VirS is autophosphorylated and then transfers the phosphate to a conserved aspartate residue located in the N-terminal region of VirR.
Once phosphorylated, the activated VirR binds via its C-terminal domain to specific DNA binding sites, known as VirR boxes.43 Five C. perfringens genes, including pfoA, vrr (the RNA regulator VR-RNA), ccp (encoding α-clostripain), virT (RNA regulator) and virU (RNA regulator) have two VirR boxes located upstream of their promoters and are directly regulated by the VirS/VirR TCRS system.44 The VirS/VirR TCRS indirectly regulates the expression of > 100 other genes, which include some toxin genes (e.g., the cpa gene encoding α toxin, CPA) and housekeeping genes that lack VirR boxes44; VirR regulates expression of these genes indirectly via regulatory RNA intermediates such as VR-RNA.44,45
The Agr-like quorum sensing (QS) system
Many bacteria regulate gene expression in response to their population density, a phenomenon known as “quorum sensing” (QS). QS signaling in Gram-positive bacteria is often mediated by the production of extracellular molecules named autoinducing peptides (AIPs).46-48 Typically, AIPs signal bacteria by activating a classical TCRS.46-48
The best-studied AIP-dependent QS system is the accessory gene regulator (Agr) QS system of S. aureus.46,47 In this bacterium, the chromosomal agr locus encodes the RNAIII regulatory RNA and four genes linked in the agr operon. This operon encodes AgrD, which is the AIP precursor peptide, and AgrB, which is an integral membrane endopeptidase. The AgrD peptide is produced in the cytoplasm and then processed and exported through AgrB action at the cytoplasmic membrane to form an active AIP. The operon also encodes the AgrA/AgrC TCRS, where AgrA is the response regulator and AgrC the membrane histidine kinase. AIP binding to the proper AgrC histidine kinase results in phosphorylation of the AgrA response regulator and increased transcription of i) the agr system promoter (P2), leading to more transcription of the agr operon, and ii) the RNAIII promoter (P3). RNAIII represents a major downstream effector of the agr system that regulates the expression of many virulence factors in S. aureus, including toxins. RNAIII acts at the post-transcriptional level, where it can affect message stability and promote or inhibit translation, including blocking translation of a major transcriptional regulator named Rot.
In 2009, two research groups independently identified the presence of an Agr-like QS system in C. perfringens type A strain 13 and showed that this QS system regulates PFO and CPA production when this strain is growing vegetatively in TGY broth.49,50 The deduced strain 13 AgrB amino acid sequence (214aa) has 29% identity and 50% similarity with the AgrB protein of S. aureus,49 while the deduced strain 13 AgrD amino acid sequence (44aa) has 32% identity and 46% similarity with the AgrD propeptide of S. aureus. All other examined C. perfringens strains carry ORFs encoding a nearly identical (> 93% identity) AgrB and AgrD. However, no TCRS similar to AgrA/AgrC in S. aureus is identifiable in the agr locus of C. perfringens. It has been suggested that, instead, VirS/VirR binds AIP to mediate Agr-like QS signaling in C. perfringens. This hypothesis may prove to be generally true but, if so, there is already at least one apparent exception, i.e., as discussed later, the etx gene of CN3718 is regulated by the Agr-like QS system, but a virR mutant of this strain still expresses wild-type levels of ETX.
Interestingly, the C. perfringens agr regulon does contain two other ORFs encoding “hypothetical proteins,” which are co-transcribed together with agrB and agrD in the C. perfringens agr operon. Some results suggest that these two ORFs are important when complementing agrB knockout mutants to restore toxin production,49-52 although the mechanism behind this contribution remains unclear. Also absent from strain 13 are sequences with identifiable homology to the RNAIII-encoding gene in S. aureus.
S. aureus makes four different AIPs, named AIP-I, AIP-II, AIP-III and AIP-IV, with varying amino acid sequences.46,47 All four S. aureus AIPs contain a central cysteine and a five residue thiolactone macrocyclic ring formed between the cysteine sulfhydryl side chain and the C-terminal carboxylate. In contrast, bioinformatic analysis of sequenced C. perfringens strains determined that only two, very similar AgrD peptides are encoded by C. perfringens type A-E isolates. An eight amino acid synthetic peptide corresponding to this putative AIP sequence, with the last five residues constrained in a thiolactone ring, can activate the Agr-like system in C. perfringens type C strain CN3685.51
The Agr-like QS System and VirS/VirR TCRS Control Toxin Production in C. perfringens Broth Cultures
As mentioned, the Agr-like QS system and VirS/VirR TCRS were first discovered in strain 13, where they were shown to regulate both PFO and CPA production by vegetative broth cultures.42,49,50 While CPA and PFO are important toxins for the pathogenesis of gas gangrene,53 strain 13 does not produce CPB, CPE or ETX, which are critical toxins when C. perfringens causes most cases of mammalian enteritis and enterotoxemia.18,27,31,52 Therefore, a series of recent studies used null mutants and complementing strains to evaluate the roles of the Agr-like QS system and VirS/VirR TCRS in regulating production of those three toxins by C. perfringens growing in broth culture.
The Agr-like QS regulates CPB, CPE and (sometimes) ETX production by C. perfringens growing in broth culture
To address whether the Agr-like QS system controls toxin production by CPE-positive type A strain F5603, type C strain CN3685, Type D strain CN3718 and Type B strains CN1793 and CN1795, the agrB gene in each of those strains was inactivated by Targetron insertional mutagenesis.50,52,54,55 Additionally, complementing strains were prepared using a shuttle plasmid carrying the agr locus. No differences were noted in broth culture growth rates between any agrB mutant or complementing strain vs. its wild-type parent.
As summarized in Table 3, results from these studies first confirmed that, as reported earlier for strain 13, production of PFO and CPA during vegetative growth in TGY broth is regulated by the Agr-like QS system for these five other C. perfringens strains. These agrB mutants and complementing strains were then used to evaluate if the Agr-like QS system controls ETX or CPB production when C. perfringens grows vegetatively in broth culture (Fig. 1 and Table 3). These studies established that CPB production by type C strain CN3685, or type B strains CN1793 and CN1795, is strongly regulated by the Agr-like QS system during growth in TGY broth.51,55 Similarly, during growth in TGY broth, ETX production by type D strain CN3718 is also controlled by the Agr-like QS system.54 However, inactivating the Agr-like QS system in either of two type B strains, i.e., CN1793 or CN1795, did not affect their ETX production levels during growth in TGY broth.55 Collectively, since CPB and ETX are both plasmid-encoded,2 these results indicate that the Agr-like QS system can regulate plasmid-borne genes, as well as chromosomal genes such as pfoA or cpa, during vegetative growth in TGY broth. Furthermore, the finding that ETX expression is controlled by the Agr-like QS system in CN3718, but not in CN1793 or CN1795, revealed that the same toxin gene can be regulated differently between C. perfringens strains.
Table 3. Summary of toxin production regulation by the Agr-like QS system and VirS/VirR TCRS for C. perfringens broth cultures.
| Strain: | CPA | PFO | CPE | CPB | ETX |
|---|---|---|---|---|---|
| 13 (CPE-negative type A) |
Agr-like QS + VirS/VirR + |
Agr-like QS + VirS/VirR + |
NR2 | NR | NR |
| F56031 (CPE-positive type A) |
Agr-like QS+ | Agr-like QS+ | Agr-like QS+ | NR | NR |
| CN3685 (type C) |
Agr-like QS+ VirS/VirR+ |
Agr-like QS+ VirS/VirR+ |
NR | Agr-like QS+ VirS/VirR+ |
|
| CN3718 (type D) |
Agr-like QS+ VirS/VirR+ |
Agr-like QS+ VirS/VirR+ |
NR | NR | Agr-like QS+ VirS/VirR- |
| CN17931 (type B) |
Agr-like QS+ | Agr-like QS+ | NR | Agr-like QS+ | Agr-like QS- |
| CN17951 (type B) |
Agr-like QS+ | Agr-like QS+ | NR | Agr-like QS+ | Agr-like QS- |
1 VirS/VirR involvement in toxin regulation by F5603, CN1793 an CN1795 has not yet been evaluated. 2NR is not relevant because that strain does not encode this toxin.
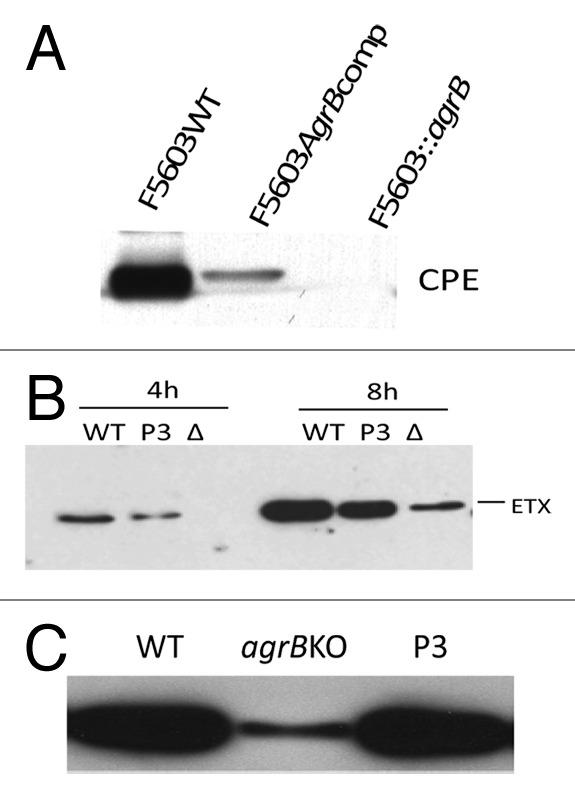
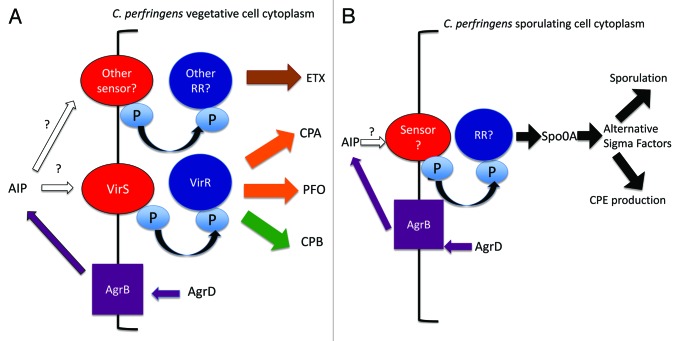
Figure 1. Regulation of C. perfringens toxin production by the Agr-like QS system. (A) western blot analysis of CPE production by 16 h sporulating cultures of CPE-positive type A strain F5603 (F5603WT), an isogenic agrB null mutant (F5603::agrB) or an agr locus complementing strain (F5603AgrBcomp). (B) western blot analysis of ETX production by 4 or 8 h vegetative cultures of type D strain CN3718 (WT), the isogenic agrB null mutant (Δ) or the agr locus complementing strain (P3). (C) western blot of CPB production by overnight vegetative cultures of type C strain CN3685 (WT), an isogenic agrB null mutant (agrBKO) or the agr locus complementing strain (P3). Reproduced with permission from refs. 51, 52, and 54.
Unlike most other C. perfringens toxins, CPE production only occurs during sporulation,3 where it is under the control of the Spo0A and SigF master sporulation regulators.56,57 In Duncan-Strong sporulation medium, an agrB null mutant of CPE-positive type A strain F5603 produced no detectable CPE in sporulating conditions (Fig. 1 and Table 3) and also lost its ability to form spores, i.e., while wild-type F5603 formed refractile spores with an efficiency of 60–70% in this sporulation broth, the agrB gene null mutant had < 1% sporulation efficiency.52 These reductions in sporulation and CPE production were partially reversible by complementation of the F5603 agrB null mutant with a plasmid carrying the wild-type agr locus (Fig. 1). The negative effects of an agrB null mutation on both CPE production and sporulation by F5603 were then shown to involve reduced levels of spo0A and sigF transcripts in the isogenic agrB null mutant.52 Thus, these results revealed that the Agr-like QS system can control toxin and regulatory genes expressed by C. perfringens during in vitro sporulation, as well as regulating genes expressed during vegetative broth culture growth.
For all of the toxin genes regulated by the Agr-like QS system, as described above, RT-PCR analyses showed that, i) lower transcript levels are present in the isogenic agrB mutant vs. the wild-type parent during in vitro growth and ii) this effect is reversible when the mutant is complemented with a plasmid carrying the wild-type agr locus.50,52,54,55
The Agr-like QS system uses a diffusible signal to control toxin production in C. perfringens vegetative broth cultures
Studies next explored whether the regulation of toxin production by the Agr-like QS system during vegetative broth culture growth involves a diffusible signaling molecule, as expected of a QS system. For this purpose, Transwells were used where two different chambers containing TGY broth are separated by a membrane filter that is impermeable to bacteria, yet allows passage of small signaling molecules (if present) between the chambers. Using this culture system, PFO production was readily detectable when strain 13 pfoA or agrB null mutants were grown in different Transwell chambers separated by a membrane filter.50 In contrast, no PFO production was observed when either a strain 13 agrB or pfoA null mutant was grown in a Transwell by itself. Consistent with a QS effect, these physical complementation results indicated that PFO production is regulated by a secreted factor produced by the pfoA null mutant, which has an intact Agr-like QS system; this secreted factor then diffuses into the other Transwell chamber, where it signals the strain 13 agrB mutant, which has a functional pfoA gene, to produce PFO. Similar Transwell studies (Fig. 2) later demonstrated that Agr-like control of CPB production by type C strain CN3685 or ETX production by CN3718 also involve a diffusible signal.51,54
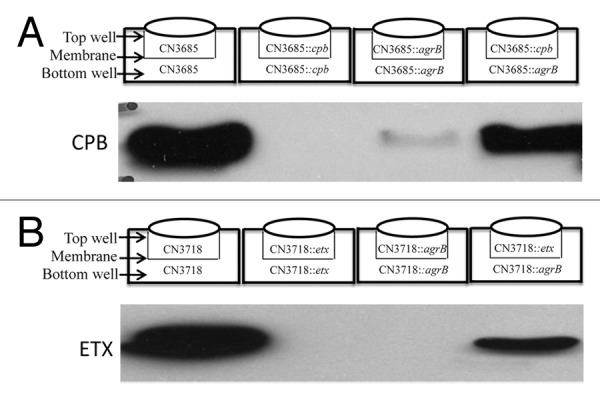
Figure 2. Agr-like QS system regulation of C. perfringens toxin production involves a diffusible signal. (A) Inoculation of Transwell chambers with, as indicated, the type C parent strain CN3685, the isogenic cpb null mutant (CN3685::cpb) or an isogenic agrB null mutant (CN3685::agrB) in two chambers, or CN3685::cpb in one chamber and CN3685::agrB in the other chamber. After 5 h, CPB production in each culture was examined by CPB western blot. (B) Inoculation of Transwell chambers with, as indicated, type D parent strain CN3718, an isogenic etx null mutant (CN3718::etx) or an agrB null mutant (CN3718::agrB) in two chambers, or CN3718::etx in one chamber and CN3718::agrB in the other chamber. After 5 h, ETX production in each culture was examined by ETX western blot. Reproduced with permission from refs. 51 and 54.
As mentioned earlier, insights into the nature of the Agr-like QS signal have been obtained using an eight-mer synthetic peptide that was designed based upon the C. perfringens AgrD sequence and knowledge of the S. aureus AIPs.51 When this peptide contained a pentameric thiolactone ring, it could signal the CN3685 agrB mutant to produce CPB during vegetative growth in TGY broth. However, an eight-mer control peptide lacked any signaling activity.51
The VirS/VirR TCRS regulates CPB production by C. perfringens during growth in vegetative broth culture
As mentioned earlier, studies conducted nearly 20 y ago established that the VirS/VirR system controls production of PFO and CPA by vegetative cultures of strain 13.41,42,58 Similarly, PFO and CPA production were also diminished when virR null mutants were constructed in type C strain CN3685 or type D strain CN3718 (Table 3); in TGY broth cultures of both strains, wild-type production levels of these toxins were restored after complementation with a plasmid carrying the wild-type virS/virR operon. No differences were noted in vegetative broth culture growth rates of any virR null mutant or complementing strains vs. its wild-type parent.
Using those same virR null mutants and complementing strains, western blot analyses then showed (Fig. 3) that the VirS/VirR TCRS also strongly controls CPB production by CN3685 vegetative cells growing in TGY broth. This finding confirms previous findings by others59 that the VirS/VirR system can regulate plasmid-encoded genes, as well as chromosomal genes, of C. perfringens. Interestingly, while the virR null mutant of CN3718 produced reduced amounts of PFO and CPA in TGY broth, this culture still contained wild-type ETX levels (Fig. 3 and Table 3). Since ETX expression by CN3718 is regulated by the Agr-like QS system, the ability of the virR null mutant of CN3718 to produce wild-type levels of ETX indicates that the Agr-like QS system does not always work through the VirS/VirR TCRS.
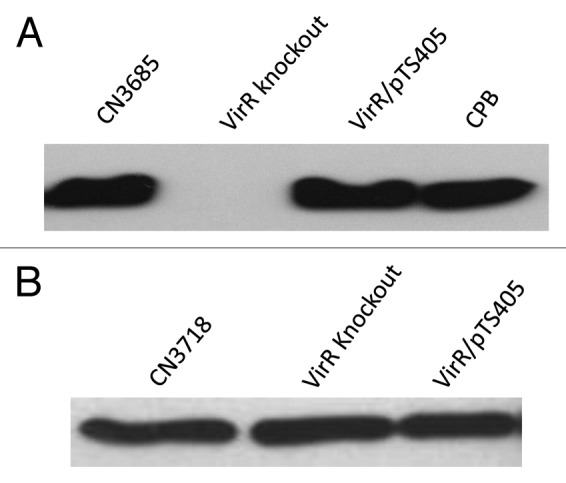
Figure 3. Regulation of C. perfringens toxin production by the VirS/VirR TCRS. (A) western blot analysis of CPB production by overnight vegetative cultures of type C strain CN3685, an isogenic virR null mutant (VirR knockout) or the virS/virR complementing strain (VirR/pTS405). For comparison, the migration of purified CPB is also shown in the left lane of the blot. (B) western blot analyses of ETX production by 4 h vegetative broth cultures of type D strain CN3718, the isogenic virR null mutant (VirR knockout) or the complementing strain (VirR/pTS405). Reproduced with permission from refs. 54 and 61.
C. perfringens Upregulates Toxin Production upon Contacting Cultured Host Cells: The Agr-like QS and VirS/VirR systems can Participate in this Signaling
The broth culture studies of toxin gene regulation described in the preceding section are informative, but it was important to explore more directly the pathophysiologic relevance of those observations. As a first step toward addressing this important issue, recent studies identified host: pathogen cross-talk between C. perfringens and cultured host cells, including enterocyte-like Caco-2 cells. Furthermore, participation of the Agr-like QS system and VirS/VirR TCRS in this process has now been established.
This work began with the discovery that C. perfringens vegetative cells significantly upregulate their production of many toxins when grown in the presence of cultured host cells.60 For type C strain CN3685, host-cell induced toxin upregulation was observed for PFO, CPA, and CPB production (Fig. 4 and data not shown). There were no differences in bacterial numbers when CN3685 was grown in the presence of host cells vs. tissue culture medium alone, supporting host cell-induced cross-talk signaling as causing the upregulation of toxin production.60 This signaling effect was shown to involve increased toxin transcript levels upon host cell contact.60 Several host cell lines, including enterocyte-like Caco-2 cells, were capable of inducing this upregulation of toxin production.60 This signaling to increase toxin production requires close contact between the cultured host cells and CN3685 bacterial cells and could not be produced using host cell culture supernatants.60 This toxin upregulation occurred regardless of whether CN3685-infected Caco-2 cell cultures were grown aerobically or anaerobically.51,60 Together, those observations suggest that direct contact between C. perfringens and enterocytes triggers signaling that leads to increased toxin production.
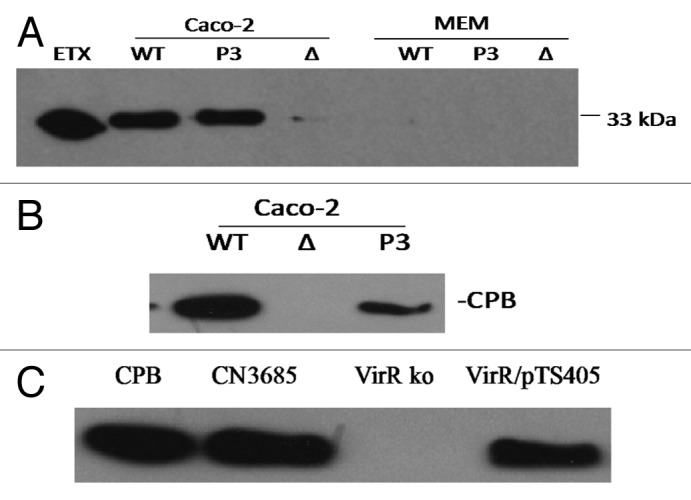
Figure 4. Contact with host Caco-2 cells induces an upregulation of toxin production by C. perfringens. (A) western blot analysis of ETX production by type D strain CN3718 (WT), the isogenic ΔAgrB null mutant (Δ) or the P3 agr locus complementing strain (P3). Shown is ETX production by these strains after 1‒2 h in the presence (Caco-2 cells) or absence (MEM) of enterocyte-like Caco-2 cells. (B) western blot analysis of CPB production by type C strain CN3685 (WT), the isogenic ΔAgrB null mutant (Δ) or the P3 complementing strain (P3) after 2 h in the presence of Caco-2 cells. No CPB production was detected after 2 h growth of these strains in the absence of Caco-2 cells. (C) western blot analysis of CPB production by CN3685, the isogenic virR null mutant (VirR ko) or the VirR/pTS405 complementing strain after 2 h in the presence of Caco-2 cells. Reproduced with permission from refs. 51 and 54.
Similarly, ETX, PFO and CPA production was also shown to increase substantially when vegetative cells of type D strain CN3718 are grown in the presence of Caco-2 cells (Fig. 4, and data not shown).
Supporting the possible relevance of these cross-talk events for pathogenicity, the encounter between C. perfringens and cultured host cells not only increases toxin production levels but also has profound cytotoxic consequences for host cells. For example, when sterile supernatants were removed from CN3685-infected Caco-2 enterocyte-like cells and then applied to fresh Caco-2 cells, they caused much higher cytotoxicity than did comparable supernatants removed from CN3685 grown in tissue culture medium alone.60 Similarly, when sterile supernatants from CN3718 cultures grown in the presence of Caco-2 cells were trypsin-treated to activate their ETX and then applied to MDCK cells, these supernatants were much more cytotoxic than trypsin-activated supernatants removed from CN3718 grown in tissue culture medium alone.54
Involvement of the Agr-like QS system and VirS/VirR TCRS in host cell-induced upregulation of toxin production by C. perfringens was first established when it was demonstrated that inactivating the virS/virR operon abrogates the ability of CN3685 to increase production of toxins, including CPB, in infected Caco-2 cell cultures.51 Again, this increase in toxin production appears to be a cross-talk mediated signaling event since there was no difference in C. perfringens numbers between CN3685 vs. the isogenic virR mutant in the presence of Caco-2 cells. Later studies demonstrated that the Agr-like QS system is similarly required to obtain the host cell-induced increase in CPB production by CN3685. It was also shown that this effect was not attributable to lower bacterial numbers in the agrB mutant vs. wild-type CN3685 during growth in the presence of Caco-2 cells.51 Interestingly, not all QS systems are necessary for this host cell-induced upregulation of toxin production since inactivating the LuxS QS system did not affect the increase in CPB production after CN3685 senses the presence of host cells.51
The Agr-like QS system is also important when type D strain CN3718 upregulates ETX production upon contact with host cells.54 However, as described earlier, production of wild-type ETX levels by CN3718 does not require a functional VirS/VirR system. Consistent with this finding, supernatants removed from cultures of CN3718 or its isogenic virR mutant, grown in the presence of Caco-2 cells, proved equally cytotoxic for MDCK cells after trypsin activation of those supernatants.54
Involvement of the Agr-like QS System and VirS/VirR TCRS in C. perfringens Intestinal Infections of Animal Models
The observation that close contact with enterocyte-like Caco-2 cells causes C. perfringens to increase production of CPB and ETX suggested that vegetative cells of this bacterium may similarly sense contact with enterocytes during intestinal infections and respond by in vivo upregulation of toxin production to increase virulence. If so, our cell culture results described above would strongly suggest that signaling via the Agr-like QS system and VirS/VirR TCRS can play a critical role in the ability of C. perfringens to cause intestinal infections.
To begin testing this hypothesis, pathogenicity was compared after intestinal infection of rabbits and mice with washed vegetative cells of isogenic agrB null mutants, complementing strains and their wild-type CN3685 parent.51 Results from those studies demonstrated that a functional Agr-like QS system is necessary for this type C strain to cause either necrosis in rabbit small intestinal loops (Fig. 5) or enterotoxemic lethality in mice (not shown). These studies further determined that this virulence dependence on the Agr-like QS system does not entail differences in bacterial multiplication between CN3685 and the agrB mutant but instead involves this regulatory system controlling in vivo CPB production by CN3685 during intestinal infection. Furthermore, this work revealed that not all QS systems are required for CN3685 pathogenicity since washed vegetative cells of a luxS null mutant remained fully virulent in both the intestinal necrosis and lethal enterotoxemic animal models.
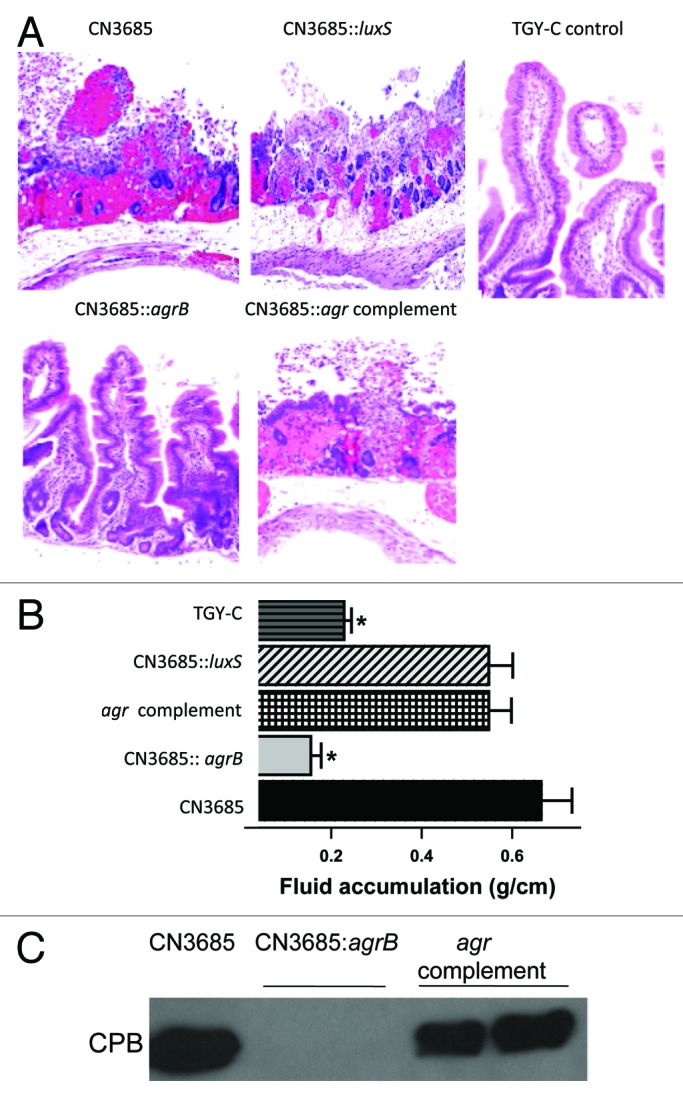
Figure 5. The Agr-like QS system controls the intestinal virulence of type C strain CN3685. (A) Histologic damage in rabbit small intestinal loops treated for 6 h with control (sterile) broth or washed cells of wild-type CN3685, an isogenic luxS null mutant, an isogenic agrB null mutant or the an agr locus complementing strain. Tissues were processed routinely for production of hematoxylin and eosin-stained sections. Sections were photographed at 200 × final magnification. (B) Fluid accumulation in small intestinal loops after challenge with the indicated strains. The asterisk indicates statistically significant (p < 0.05) differences from CN3685 and BMJV13. (C) western blot analysis of in vivo CPB production in luminal fluids recovered from small intestinal loops challenged for 6 h with washed cells of each indicated strain. The migration of purified CPB is indicated to the left of the blot. Similar analyses of luminal fluids collected from loops challenged with washed cells of the luxS null mutant detected wild-type levels of CPB production (not shown). Reproduced with permission from ref. 51.
Similarly, since VirS/VirR is involved when CN3685 senses close contact with Caco-2 cells and responds by upregulating toxin production,61 this TCRS might also be important for the virulence of this type C strain. This hypothesis was confirmed using washed vegetative cells of a virR null mutant and complementing strain of CN3685,61 which revealed that a functional VirS/VirR system is necessary for this type C strain to cause either necrosis in rabbit small intestinal loops (Fig. 6) or lethal enterotoxemias in mice (not shown). The mechanism by which VirS/VirR mediates virulence was shown to involve effects on in vivo CPB production levels rather than affecting in vivo bacterial multiplication.61
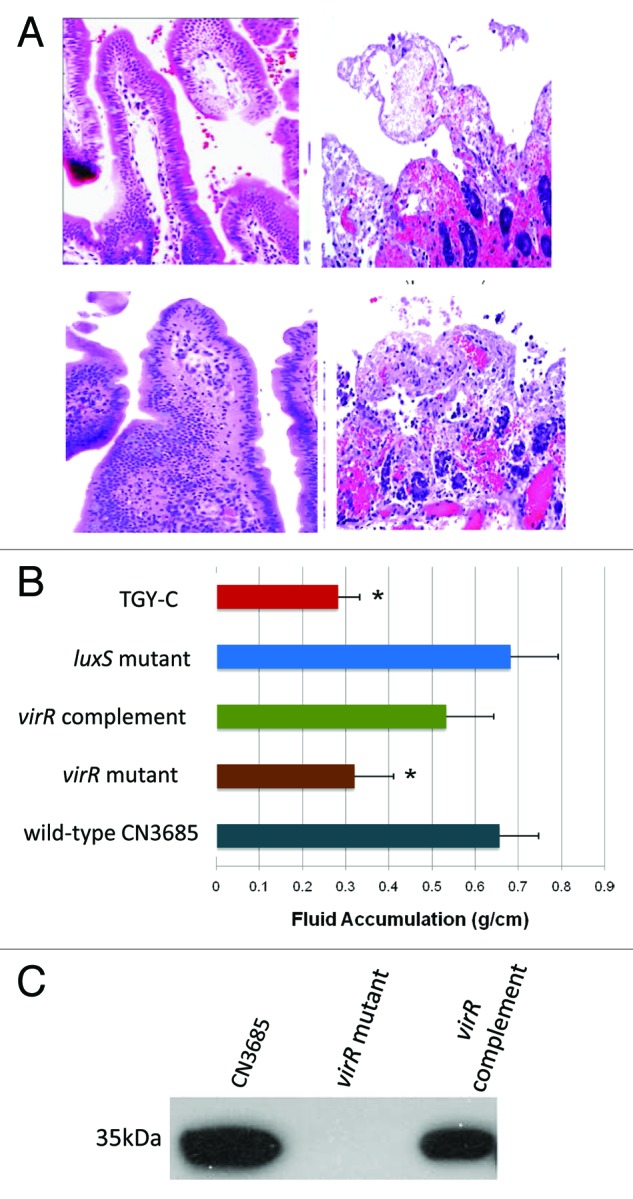
Figure 6. The VirS/VirR TCRS controls the intestinal virulence of type C strain CN3685. (A) Histologic damage in rabbit small intestinal loops challenged for 6 h with sterile control broth (top left), washed cells of wild-type CN3685 (top right), an isogenic virR null mutant (bottom left) or a virS/virR complementing strain (bottom right). Tissues were processed by histology and stained using hematoxylin and eosin. Sections were photographed at 200 × final magnification. (B) Fluid accumulation in small intestinal loops after challenge with the indicated strains. The asterisk indicates statistically significant (p < 0.05) differences from CN3685. (C) western blot analysis of in vivo CPB production in luminal fluids recovered from small intestinal loops challenged for 6 h with washed cells of each indicated strain. The 35 kDa size of purified CPB is indicated to the left of the blot. Reproduced with permission from ref. 61.
Models for C. perfringens Signaling during Intestinal Infections
Type C and D infections
The recent findings described in this review suggest a possible model for how signaling could participate in the mammalian intestinal infections caused by C. perfringens type C and D strains, which require in vivo production of CPB and ETX (respectively) by vegetative cells of those bacteria growing in the intestines. It is likely that at least two steps are involved during Agr-like signaling in the intestines. First, based on Caco-2 cell culture results,51,54 C. perfringens senses the presence of enterocytes and responds by activating the Agr-like QS system to produce an AIP (Fig. 7A). Second, infection by type C and D strains involves the adherence of their vegetative cells to the intestinal epithelium,1,21,62 which locally concentrates the infecting strain. Therefore, together with in vivo multiplication, this locally increased density of adherent C. perfringens cells on the intestinal epithelium likely facilitates the efficacy of activated Agr-like QS signaling, via the secreted AIP.
Figure 7. Models for C. perfringens toxin gene regulation by the Agr-like QS system and VirS/VirR TCRS. (A) Regulation of toxin production in type C and D strains. The Agr-like QS system generates an autoinducing peptide (AIP) that interacts with one or more TCRS, possibly including VirS/VirR and/or for ETX regulation in type D strain CN3718, another still unidentified TCRS (sensor = membrane sensor; RR = response regulator). This signaling results in direct or indirect increased production of CPA and PFO in both type C and D strains, CPB in type C strain CN3685 and ETX in type D strain CN3718 (but not in type B strains). (B) Regulation of sporulation and CPE production in CPE-positive type A strains. The Agr-like QS system generates AIP, which (possibly via a TCRS) increases Spo0A production and, under sporulation-inducing conditions, alternative sigma factor expression levels to promote sporulation and CPE production.
A major unanswered question for understanding C. perfringens signaling concerns the AIP target (Fig. 7A). In S. aureus, the AIPs bind to the AgrC/AgrA TCRS, which C. perfringens lacks. In reponse, it was proposed that the AIP target in C. perfringens is the VirS/VirR TCRS.49 While VirS/VirR involvement in Agr-like QS signaling remains a possibility, it appears that AIP does not only bind to VirS/VirR given that ETX production by CN3718 is controlled by the Agr-like QS, yet wild-type levels of ETX production by this strain do not require a functional VirS/VirR system.54 Those findings suggest that other C. perfringens TCRS may also mediate Agr-like QS signaling.
In any event, the initiation of AIP-signaling triggers increased C. perfringens toxin production (Fig. 7A). In vivo, this upregulation of ETX or CPB production then allows C. perfringens to achieve intestinal toxin levels necessary for causing the necrosis, enteritis, or enterotoxemias associated with type C or D strains.
CPE-positive type A strains
The in vitro results demonstrating that the Agr-like QS system is necessary for both sporulation and CPE production in broth cultures suggest, but do not yet prove, that this QS system is also involved during C. perfringens type A food poisoning. If so, it is interesting that Bacillus subtilis sporulation is also triggered (in part) by population density sensed by QS systems. Together with poor nutrient conditions that remove repression of sporulation by the CodY protein, the resultant QS signaling initiates sporulation.63
By analogy, it can be postulated how the Agr-like QS system might contribute to C. perfringens type A food poisoning.3 This foodborne illness starts with the ingestion of contaminated foods containing large numbers of CPE-positive type A vegetative cells, which then rapidly multiply in the intestines. This high bacterial concentration in the intestines may facilitate accumulation of AIP to concentrations capable of activating pathways that lead to increased levels of master sporulation regulators such as SigF; these regulators, in turn, initiate sporulation and CPE production (Fig. 7B). Whether this process involves CPE-positive type A strains sensing the presence of enterocytes or involves a TCRS has not yet been determined. This in vivo sporulation leads to CPE production,56,64 which (after mother cell lysis) then triggers diarrhea and cramps.
Concluding Remarks
Remarkable progress has recently been achieved in understanding the pathogenesis of mammalian intestinal infections caused by C. perfringens. These advancements2 include dissecting the role of individual toxins during disease, demonstrating the role of toxin plasmids in pathogenesis, identifying potential adherence mechanisms for mammalian intestinal disease strains, and (most recently) evaluating the contributions of the Agr-like QS and VirS/VirR signaling systems to mammalian intestinal disease. It should be mentioned that at least some of these same signaling systems are probably used by C. perfringens strains causing intestinal disease in other animals. Specifically, C. perfringens type A strains producing NetB toxin, which is essential for those strains to cause avian necrotic enteritis, is also regulated by VirS/VirR.65
Despite this progress, many unanswered questions remain. Examples include: Is the Agr-like QS system essential for type A or D isolates to cause mammalian intestinal infections? Do the Agr-like QS system and VirS/VirR system talk directly with each other during intestinal signaling? If so, how? The type D agrB and virR knockout results suggest that other TCRS systems besides VirS/VirR can be involved in regulating ETX production; if so, which ones? What is the host cell factor(s) sensed by C. perfringens that leads to increased toxin production? How does this host cell factor trigger C. perfringens signaling pathways?
Are the Agr-like QS system and VirS/VirR important for the enteropathogenicity of CPE-positive type A strains? What conditions or factor(s), besides high bacterial density signaling by the Agr-like QS system, is necessary for initiating the intestinal sporulation of CPE-positive type A strains? Do type C and D strains also eventually sporulate in vivo? Addressing these, and other important issues, is likely to require substantial additional research effort. However, recent results51,61,65 establishing that many mammalian and avian intestinal infections caused by C. perfringens may involve common signaling systems already opens the possibility of these systems representing potential therapeutic or prophylactic targets.
Lastly, it is notable that other clostridial pathogens, e.g., Clostridium botulinum and Clostridium difficile, can also carry an Agr-like QS system and TCRS. It will be of interest to evaluate whether these signaling systems are also important for the virulence of these bacteria, particularly when they cause intestinal infections such as C. difficile-associated pseudomembranous colitis or infant botulism.
Acknowledgments
Most of the research described in this review was generously supported by US Public Health Service Grants R37AI19844, R01AI056177–09, and a project in MARCE Grant 2U54AI57168–09 (M Levine, overall PI).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. (2006) The Enterotoxic Clostridia. In: Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E, editors. The Prokaryotes. 3rd ed. New York: Springer NY press. pp. 688-752. [Google Scholar]
- 2.Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Uzal FA, Rood JI, McClane BA. Toxin plasmids of Clostridium perfringens. Microbiol Mol Biol Rev. 2013;77:208–33. doi: 10.1128/MMBR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClane BA, Robertson SL, Li J. (2013) Clostridium perfringens. In: Doyle MP, Buchanan RL, editors. Food Microbiology: Fundamentals and Frontiers. 4th ed. Washington D.C.: ASM press. pp. 465-489. [Google Scholar]
- 4.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batz MB, Hoffmann S, Morris JG., Jr. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot. 2012;75:1278–91. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- 6.Caserta JARS, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA. Development and application of a mouse intestinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect Immun. 2011;79:3020–7. doi: 10.1128/IAI.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deguchi A, Miyamoto K, Kuwahara T, Miki Y, Kaneko I, Li J, McClane BA, Akimoto S. Genetic characterization of type A enterotoxigenic Clostridium perfringens strains. PLoS One. 2009;4:e5598. doi: 10.1371/journal.pone.0005598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, McClane BA. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 2008;4:e1000056. doi: 10.1371/journal.ppat.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briggs DC, Naylor CE, Smedley JG, 3rd, Lukoyanova N, Robertson S, Moss DS, McClane BA, Basak AK. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J Mol Biol. 2011;413:138–49. doi: 10.1016/j.jmb.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H, et al. Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J Biol Chem. 2011;286:19549–55. doi: 10.1074/jbc.M111.228478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrestha A, McClane BA. Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. MBio. 2013;4:e00594–12. doi: 10.1128/mBio.00594-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veshnyakova A, Protze J, Rossa J, Blasig IE, Krause G, Piontek J. On the interaction of Clostridium perfringens enterotoxin with claudins. Toxins (Basel) 2010;2:1336–56. doi: 10.3390/toxins2061336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson SL, Smedley JG, 3rd, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell Microbiol. 2007;9:2734–55. doi: 10.1111/j.1462-5822.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Theoret JR, Shrestha A, Smedley JG, 3rd, McClane BA. Cysteine-scanning mutagenesis supports the importance of Clostridium perfringens enterotoxin amino acids 80 to 106 for membrane insertion and pore formation. Infect Immun. 2012;80:4078–88. doi: 10.1128/IAI.00069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti G, McClane BA. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol. 2005;7:129–46. doi: 10.1111/j.1462-5822.2004.00442.x. [DOI] [PubMed] [Google Scholar]
- 16.Bos J, Smithee L, McClane BA, Distefano RF, Uzal F, et al. Fatal necrotizing enteritis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis. 2005;15:78–83. doi: 10.1086/429829. [DOI] [PubMed] [Google Scholar]
- 17.Carman RJ. Clostridium perfringens in spontaneous and antibiotic-associated diarrhoea of man and other animals. Rev Med Microbiol. 1997;8(supplement 1):S43–5. doi: 10.1097/00013542-199712001-00024. [DOI] [Google Scholar]
- 18.Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol. 1999;33:946–58. doi: 10.1046/j.1365-2958.1999.01534.x. [DOI] [PubMed] [Google Scholar]
- 19.Uzal FA, McClane BA. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections. Vet Microbiol. 2011;153:37–43. doi: 10.1016/j.vetmic.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma M, Li J, McClane BA. Genotypic and phenotypic characterization of Clostridium perfringens isolates from Darmbrand cases in post-World War II Germany. Infect Immun. 2012;80:4354–63. doi: 10.1128/IAI.00818-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence GW. (1997) The pathogenesis of enteritis necroticans. In: Rood JI, McClane BA, Songer JG, Titball RW, editors. The Clostridia: Molecular Genetics and Pathogenesis. London: Academic Press. pp. 198-207. [Google Scholar]
- 22.Petrillo TM, Beck-Sagué CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, Dean AG, Lee H, Bueschel DM, Nesheim SR. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med. 2000;342:1250–3. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- 23.Smedley JG, 3rd, Fisher DJ, Sayeed S, Chakrabarti G, McClane BA. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol. 2004;152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 24.Nagahama M, Hayashi S, Morimitsu S, Sakurai J. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J Biol Chem. 2003;278:36934–41. doi: 10.1074/jbc.M306562200. [DOI] [PubMed] [Google Scholar]
- 25.Autheman D, Wyder M, Popoff M, D’Herde K, Christen S, Posthaus H. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS One. 2013;8:e64644. doi: 10.1371/journal.pone.0064644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan XX, Porter CJ, Hardy SP, Steer D, Smith AI, Quinsey NS, Hughes V, Cheung JK, Keyburn AL, Kaldhusdal M, et al. Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens. MBio. 2013;4:e00019–13. doi: 10.1128/mBio.00019-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol. 2008;67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- 28.Garcia JP, Beingesser J, Fisher DJ, Sayeed S, McClane BA, Posthaus H, Uzal FA. The effect of Clostridium perfringens type C strain CN3685 and its isogenic beta toxin null mutant in goats. Vet Microbiol. 2012;157:412–9. doi: 10.1016/j.vetmic.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal JE, McClane BA, Saputo J, Parker J, Uzal FA. Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect Immun. 2008;76:4396–404. doi: 10.1128/IAI.00547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uzal FA, Saputo J, Sayeed S, Vidal JE, Fisher DJ, Poon R, Adams V, Fernandez-Miyakawa ME, Rood JI, McClane BA. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C Enterotoxemias. Infect Immun. 2009;77:5291–9. doi: 10.1128/IAI.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia JP, Adams V, Beingesser J, Hughes ML, Poon R, Lyras D, Hill A, McClane BA, Rood JI, Uzal FA. Epsilon toxin is essential for the virulence of Clostridium perfringens type D infection in sheep, goats, and mice. Infect Immun. 2013;81:2405–14. doi: 10.1128/IAI.00238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole AR, Gibert M, Popoff M, Moss DS, Titball RW, Basak AK. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat Struct Mol Biol. 2004;11:797–8. doi: 10.1038/nsmb804. [DOI] [PubMed] [Google Scholar]
- 33.Miyata S, Matsushita O, Minami J, Katayama S, Shimamoto S, Okabe A. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J Biol Chem. 2001;276:13778–83. doi: 10.1074/jbc.M011527200. [DOI] [PubMed] [Google Scholar]
- 34.Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol. 1997;41:527–35. doi: 10.1111/j.1348-0421.1997.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 35.Harkness JM, Li J, McClane BA. Identification of a lambda toxin-negative Clostridium perfringens strain that processes and activates epsilon prototoxin intracellularly. Anaerobe. 2012;18:546–52. doi: 10.1016/j.anaerobe.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivie SE, McClain MS. Identification of amino acids important for binding of Clostridium perfringens epsilon toxin to host cells and to HAVCR1. Biochemistry. 2012;51:7588–95. doi: 10.1021/bi300690a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popoff MR. Epsilon toxin: a fascinating pore-forming toxin. FEBS J. 2011;278:4602–15. doi: 10.1111/j.1742-4658.2011.08145.x. [DOI] [PubMed] [Google Scholar]
- 38.Stiles BG, Wigelsworth DJ, Popoff MR, Barth H. Clostridial binary toxins: iota and C2 family portraits. Front Cell Infect Microbiol. 2011;1:11. doi: 10.3389/fcimb.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu Rev Microbiol. 2012;66:325–47. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Y, Ohtani K, Yoshizawa S, Shimizu T. Complex transcriptional regulation of citrate metabolism in Clostridium perfringens. Anaerobe. 2012;18:48–54. doi: 10.1016/j.anaerobe.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol. 1994;176:1616–23. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyristis M, Bryant AE, Sloan J, Awad MM, Nisbet IT, Stevens DL, Rood JI. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol Microbiol. 1994;12:761–77. doi: 10.1111/j.1365-2958.1994.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 43.Cheung JK, Dupuy B, Deveson DS, Rood JI. The spatial organization of the VirR boxes is critical for VirR-mediated expression of the perfringolysin O gene, pfoA, from Clostridium perfringens. J Bacteriol. 2004;186:3321–30. doi: 10.1128/JB.186.11.3321-3330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okumura K, Ohtani K, Hayashi H, Shimizu T. Characterization of genes regulated directly by the VirR/VirS system in Clostridium perfringens. J Bacteriol. 2008;190:7719–27. doi: 10.1128/JB.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol Microbiol. 2002;43:257–65. doi: 10.1046/j.1365-2958.2002.02743.x. [DOI] [PubMed] [Google Scholar]
- 46.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–64. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 47.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. Peptide signaling in the staphylococci. Chem Rev. 2011;111:117–51. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray B, Hall P, Gresham H. Targeting agr- and agr-Like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors (Basel) 2013;13:5130–66. doi: 10.3390/s130405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtani K, Yuan Y, Hassan S, Wang R, Wang Y, Shimizu T. Virulence gene regulation by the agr system in Clostridium perfringens. J Bacteriol. 2009;191:3919–27. doi: 10.1128/JB.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidal JE, Chen J, Li J, McClane BA. Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens strain 13. PLoS One. 2009;4:e6232. doi: 10.1371/journal.pone.0006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal JE, Ma M, Saputo J, Garcia J, Uzal FA, McClane BA. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol Microbiol. 2012;83:179–94. doi: 10.1111/j.1365-2958.2011.07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Chen J, Vidal JE, McClane BA. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect Immun. 2011;79:2451–9. doi: 10.1128/IAI.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood JI. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect Immun. 2001;69:7904–10. doi: 10.1128/IAI.69.12.7904-7910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Rood JI, McClane BA. Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system. MBio. 2011;2:e00275–300275. doi: 10.1128/mBio.00275-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Chen J, McClane BA. Role of the Agr-like quorum-sensing system in regulating toxin production by Clostridium perfringens type B strains CN1793 and CN1795. Infect Immun. 2012;80:3008–17. doi: 10.1128/IAI.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, McClane BA. Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect Immun. 2010;78:4286–93. doi: 10.1128/IAI.00528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang IH, Waters M, Grau RR, Sarker MR. Disruption of the gene (spo0A) encoding sporulation transcription factor blocks endospore formation and enterotoxin production in enterotoxigenic Clostridium perfringens type A. FEMS Microbiol Lett. 2004;233:233–40. doi: 10.1111/j.1574-6968.2004.tb09487.x. [DOI] [PubMed] [Google Scholar]
- 58.Ba-Thein W, Lyristis M, Ohtani K, Nisbet IT, Hayashi H, Rood JI, Shimizu T. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J Bacteriol. 1996;178:2514–20. doi: 10.1128/jb.178.9.2514-2520.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtani K, Kawsar HI, Okumura K, Hayashi H, Shimizu T. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol Lett. 2003;222:137–41. doi: 10.1016/S0378-1097(03)00255-6. [DOI] [PubMed] [Google Scholar]
- 60.Vidal JE, Ohtani K, Shimizu T, McClane BA. Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell Microbiol. 2009;11:1306–28. doi: 10.1111/j.1462-5822.2009.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma M, Vidal J, Saputo J, McClane BA, Uzal F. The VirS/VirR two-component system regulates the anaerobic cytotoxicity, intestinal pathogenicity, and enterotoxemic lethality of Clostridium perfringens type C isolate CN3685. MBio. 2011;2:e00338–10. doi: 10.1128/mBio.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Sayeed S, Robertson S, Chen J, McClane BA. Sialidases affect the host cell adherence and epsilon toxin-induced cytotoxicity of Clostridium perfringens type D strain CN3718. PLoS Pathog. 2011;7:e1002429. doi: 10.1371/journal.ppat.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Gestel J, Nowak MA, Tarnita CE. The evolution of cell-to-cell communication in a sporulating bacterium. PLoS Comput Biol. 2012;8:e1002818. doi: 10.1371/journal.pcbi.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harry KH, Zhou R, Kroos L, Melville SB. Sporulation and enterotoxin (CPE) synthesis are controlled by the sporulation-specific sigma factors SigE and SigK in Clostridium perfringens. J Bacteriol. 2009;191:2728–42. doi: 10.1128/JB.01839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheung JK, Keyburn AL, Carter GP, Lanckriet AL, Van Immerseel F, Moore RJ, Rood JI. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect Immun. 2010;78:3064–72. doi: 10.1128/IAI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]