Abstract
Background
Metabolic syndrome (MS) has become a pandemic in Turkey, as is the case globally. Increase in carotid artery intima-media thickness (CIMT) and erectile dysfunction (ED) may be evident before the clinical signs of cardiovascular disease appear. We aimed to investigate the prevalence of increased CIMT and ED as markers of atherosclerotic disease in patients with MS.
Material/Methods
Thirty-two patients with MS and 29 healthy controls were included. Anthropometric and biochemical parameters, along with total testosterone (TT), high sensitive C-reactive protein (hs-CRP), were recorded. Carotid artery intima-media thickness was measured. Erectile dysfunction was assessed with International Index of Erectile Function.
Results
Patients with MS had higher BMI, fasting plasma glucose, post-prandial plasma glucose, insulin, HOMA-IR, total cholesterol, triglycerides, hs-CRP, and CIMT, whereas TT levels were lower (p<0.0001). The prevalence and severity of erectile dysfunction were higher in patients with MS (p<0.0001). Erectile dysfunction scores correlated inversely with CIMT. MS patients with ED were older and had higher CIMT compared to those without ED. Increase in age and HOMA and decrease in TT increased the risk of ED. When KIMT exceeding the 95th percentile of healthy controls was accepted as a risk factor for CVD, presence of ED was the only determinant for this increase.
Conclusions
Erectile dysfunction was more prevalent and severe in patients with MS and correlated with subclinical endothelial dysfunction. Total testosterone deficiency was prominent among MS patients. Presence of ED points to an increased risk of cardiovascular disease when MS is present.
Keywords: Metabolic Syndrome, Erectile Dysfunction, Carotid Intima-Media Thickness
Background
Metabolic syndrome (MS) is characterized by insulin resistance (IR) associated with abdominal obesity, glucose intolerance and diabetes (DM), dyslipidemia, hypertension, and cardiovascular disease (CVD) [1]. Various criteria used the describe the syndrome: World Health Organization (WHO), National Cholesterol Education Programme (NCEP ATP III), and International Diabetes Federation (IDF) [2]. The prevalence is around 25% in the United States and this prevalence increases with age [3]. Metabolic syndrome increases the risk of DM by 5-fold and CVD by 2-fold [4]. Endothelial dysfunction and IR are the early pathogenetic events in MS and they are linked to atherosclerosis and CVD [5].
Erectile dysfunction (ED) is defined as inadequate or non-sustainable penile erection that is required for satisfactory sexual performance [6]. It is not only an alarm sign of CVD, but also has a negative impact on personal relationships, and causes anxiety and loss of self-esteem [7,8]. Neurogenic, hormonal, and psychogenic factors can contribute to ED, but vascular phenomena are thought be most important [9]. Erectile dysfunction is now accepted as an early sign of atherosclerosis and systemic diseases, contrary to the old belief that it is a secondary outcome [10–14]. Even people without evident atherosclerosis show impaired endothelial dysfunction if they have ED [15]. Hence, ED is a preliminary indicator of atherosclerosis and its future complications. Recent research demonstrated an increased prevalence of ED in MS [16–18].
Atherosclerosis is a systemic process that affects the whole arterial system. Carotid arteries can be easily visualized to evaluate the degree of atherosclerosis. Carotid arteria-media thickness (CIMT) has been used to assess the risk of CVD [19].
In this study, we aimed to investigate the prevalence of increased CIMT and ED as markers of atherosclerotic disease in patients with MS.
Material and Methods
The study was carried out in the Endocrinology Outpatient Clinic of a research and training hospital. In this cross-sectional descriptive study, 86 consecutive patients who had MS according to the criteria of International Diabetes Foundation and who had consented to participate were prospectively recruited [20]. Twenty-nine healthy men were included as controls. This study was approved as “Thesis in Endocrinology and Metabolism Residency” by our institution. Exclusion criteria included drug therapy for diabetes and/or dyslipidemia, renal dysfunction, and pelvic trauma, prostate disease, peripheral or autonomous neuropathy, established CVD, psychiatric problems (including depression), and drug or alcohol abuse. Smoking was similar between the groups. Only 1 patient from the healthy controls with ED was a smoker [21]. After exclusion, 32 patients were enrolled in the study.
A thorough physical examination was done and blood samples for laboratory tests were obtained after 12 hours of fasting at 8:00 A.M.. Fasting glucose (FPG), total testosterone (TT), insulin, high-sensitivity C-reactive protein (hs-CRP), total cholesterol (T-chol), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were analyzed. Postprandial plasma glucose (PPPG) was also measured.
Height, weight and body composition were measured by bio-impedance (TANITA, Japan) and Body Mass Index (BMI) was calculated as weight (kg)/height2 (m). Waist circumference (Wc) was measured at the level of the shortest distance below the costal arch while the person is standing and fasting in the morning with a constant tension meter during expiration. Blood pressure (BP) measurement was done while the patient was sitting comfortably in a straight position, from the right arm with a mercury manometer after 5 minutes of rest and without tea, coffee, caffeinated drinks, or cigarettes in the last 30 minutes. Metabolic syndrome was defined according to International Diabetes Foundation (IDF) Criteria [22].
ED was assessed with a validated, multidimensional questionnaire (IIEF-5, International Index of Erectile Function) [23]. Study subjects answered 5 questions by themselves and were assessed on a 25-point scale: Scores ≥22 indicated no ED, 17–21 points indicated mild ED, 12–16 points indicated mild-moderate ED, 8–11 points indicated moderate ED, and 1–7 points indicated severe ED.
Insulin resistance was assessed with homeostasis model assessment- insulin resistance (HOMA-IR): fasting insulin level (μIU/ml) times fasting plasma glucose (mg/dl)/405.
Carotid intima-media thickness (CIMT) was measured by a General Electric LOQIC 400 ultrasonography device and 13 MHz linear probe while the person was in the supine position as described previously [24]. Values less than 0.65 mm were considered normal for the age group of our population [25]. Calculations were done by a single person and CIMT was expressed as the mean of right and left CIMT measurements.
Statistical analysis
Data were analyzed by SPSS 20.0 (SPSS Inc, Chicago, USA). Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as percentages. Two groups were compared with Student’s t test. Pearson correlation tests were used to test for the relation between continuous variables. Chi-square test with continuity correction was used to test for the difference between categorical variables. Statistical significance was accepted as a p value less than 0.05. Binary logistic regression was used to assess the determinants of ED.
Results
Baseline demographic and clinical characteristics of the study groups are summarized in Table 1. Systolic and diastolic BP, BMI, Wc, T-Chol, TG, insulin, plasma glucose levels, and hs-CRP were significantly higher in patients with MS. Total testosterone levels were lower in patients with MS when compared to those in the control group (4.8±1.2 ng/ml vs. 6.4±1.6, respectively, p<0.0001) (Table 1).
Table 1.
Demographic and clinical characteristics of the study groups expressed in mean ± standard deviation.
| Patients with MS (n=32) | Control group (n=29) | P | |
|---|---|---|---|
| Age (years) | 39.6±6 | 37.2±4.8 | 0.092 |
| Systolic BP (mmHg) | 147±16 | 127±13 | <0.0001 |
| Diastolic BP (mmHg) | 92±11 | 83±8 | <0.0001 |
| BMI (kg/m2) | 33.03±5.35 | 23.89±3.01 | <0.0001 |
| Wc (cm) | 112.3±11.2 | 91.2±4.7 | <0.0001 |
| FPG (mg/dl) | 138.7±57.6 | 81.3±10.3 | <0.0001 |
| PPPG (mg/dl) | 204.2±90.4 | 111.1±15.9 | <0.0001 |
| Insulin | 12.1±7.13 | 6.01±2.72 | <0.0001 |
| T-Chol (mg/dl) | 224.4±48.1 | 169.1±36.4 | <0.0001 |
| TG (mg/dl) | 269.6±126.8 | 101.4±50.1 | <0.0001 |
| LDL-C (mg/dl) | 125.4±43.5 | 107.5±27.8 | 0.058 |
| HDL-C (mg/dl) | 40.5±6.4 | 43.2±11.9 | 0.262 |
| hs-CRP | 3.74±2.84 | 0.85±0.76 | <0.0001 |
| CIMT (mm) | 0.76±0.11 | 0.53±0.06 | <0.0001 |
| TT (ng/ml) | 4.8±1.2 | 6.4±1.6 | <0.0001 |
MS – metabolic syndrome; NS – non-significant; BP – blood pressure; BMI – body mass index; Wc – weight circumference; FPG – fasting plasma glucose; PPPG – postprandial plasma glucose; T-chol – total cholesterol; TG – triglyceride; LDL-C – low density lipoprotein cholesterol; HDL-C – high density lipoprotein cholesterol; hs-CRP – high sensitive C reactive protein; CIMT – carotid intima media thickness; TT – total testosterone.
Carotid intima-media thickness was higher in the MS group compared to controls (0.76±0.11 mm vs. 0.53±0.06 mm, respectively, p<0.0001) (Table 1). There was positive correlation with CIMT and hs-CRP, T-Chol, and LDL-c in the control group (Rp=0.577, p=0.001, Rp=0.400, p=0.032 and Rp=0.378, p=0.043, respectively). In contrast, CIMT correlated positively with age and LDL-c, and negatively with TT in the MS group (Rp=0.557 p=0.001, Rp=0.380 p=0.032 and Rp=−0.410 p=0.020, respectively).
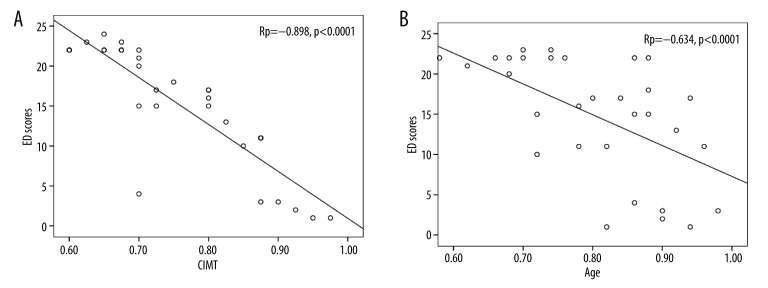
Prevalence of ED was higher in patients with MS (65.6%, n=21) when compared to the healthy men in the control group (13.8%, n=4) (p<0.0001). All the cases in the control group were mild; whereas in MS group 6 had mild, 5 had mild-to-moderate, 4 had moderate, and 6 had severe ED. Scores of ED were lower in the MS group compared to controls (15.2±7.5 vs. 23.8±1.5, respectively, p<0.0001). ED scores correlated inversely with CIMT in both groups (control Rp=−0.864, p<0.0001 and MS Rp=−0.898, p<0.0001) and also with age in the MS group (Rp=−0.634, p<0.0001) (Figure 1).
Figure 1.
Correlation OF ED Scores with CIMT (A) and Age (B) in MS patients.
Mean age was older in MS patients who had ED (41.9±4.8 vs. 35.3±5.9, p= 0.002) and who had higher CIMT (0.82±0.09 vs. 0.65±0.04, p=0.004) when compared to those without MS. Other parameters, including TT levels, were similar between those with ED and without ED in the MS group.
Increase in age and HOMA and decrease in TT increased the risk of ED (OR 1.594 [CI 95% 1.216–2.086 p=0.001]; OR 1.39 (CI 95% 1.058–1.827 p=0.018); OR 0.193 [CI 0.063–0.596 p=0.004], respectively).
When KIMT exceeding the 95th percentile of healthy controls was accepted as a risk factor for CVD, only presence of ED was a determinant for this increase (OR: 0.032 (95%CI: 0.003–0.353 p=0.005).
Discussion
We demonstrated that prevalence of ED was increased in patients with MS when compared to healthy subjects and this correlated with CIMT.
Carotid intima-media thickness and hs-CRP levels were also higher in patients with MS. We demonstrated that TT levels were lower in patients with MS, and CIMT correlated positively with age and LDL-C, and negatively with TT in the MS group. Increase in age and HOMA and decrease in TT increased the risk of ED and the presence of ED was a determinant for the increase in KIMT.
This study confirms the association between MS, ED, and CVD in a Turkish population. Our patients with MS had adverse metabolic profile compared to the control group. hs-CRP, a reliable marker of inflammation, was higher in the MS group, along with increased CIMT [26]. hs-CRP predicts MS and it is used both as a screening tool and to tailor the statin treatment in patients with CVD [27,28].
Our patients with MS had higher CIMT and CIMT correlated with hs-CRP in the control group, LDL-C in both groups, and TT in the MS group. We could not show any relation between hs-CRP and TT, but recent studies have shown that low TT was correlated with inflammatory markers [29].
Carotid intima-media thickness predicts the cardiovascular disease, but the CIMT-MS relationship is rather controversial. Metabolic syndrome per se was not associated with increased CIMT; however, some components of MS are predictive of CIMT [30]. Our patients with MS who had ED were older and had higher CIMT, thus this increase can be attributed to age rather than MS. However, a recent study has documented that men under age 40 years also suffer from ED. In this same study, young patients with ED had higher CIMT compared to controls. The study concluded that ED could be the first sign of endothelial dysfunction and a clinical marker of cardiovascular and metabolic disease in young people [31]. Although age was a determinant, presence of ED was the only factor associated with an increase with CIMT in our study.
In our study group, patients with MS had higher frequency of severe ED. Low testosterone, HOMA-IR, and age were predictors of ED in our study group. A study from China confirmed the role of testosterone in ED [32]. Testosterone deficiency has been blamed for worse cardiometabolic profile [33]. Testosterone treatment is beneficial in some components of MS [34]. Increased fat mass (central adiposity), reduced insulin sensitivity, impaired glucose tolerance, and unfavorable lipid profile had been reported [35]. Testosterone improves glucose, lipid, and cholesterol metabolism at the molecular level thorough different mechanism [36].
Different components of MS may have different effects on the development of ED. Hypertension, elevated fasting blood glucose, and low HDL-C have been found to be correlated with ED [37]. Besides MS, ED may accompany other chronic diseases. Although presence of MS was associated with an increased prevalence of ED, we are unable to demonstrate specific effects of different components of MS on ED. Recently, HOMA-IR determination was reported as a useful screening tool for ED [38]. HOMA-IR was a predictor of MS in our study.
Conclusions
ED was more prevalent in patients with MS in this small study group. There was a positive linear relationship between the degree of ED and CIMT. Increased CIMT might be the common reflection of chronic atherosclerotic diseases and ED, and decreased levels of testosterone along with IR may be the missing link between ED and MS. Presence of ED should alert the physician to the high probability of an impending systemic cardiovascular disease and all patients with MS should be questioned regarding ED.
Footnotes
Source of support: Departmental sources
References
- 1.Lorenzo C, Williams K, Hunt KJ, et al. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 2.Ivezic-Lalic D, Bergman Markovic B, Kranjcevic K, et al. Diversity of metabolic syndrome criteria in association with cardiovascular diseases – a family medicine-based investigation. Med Sci Monit. 2013;19:571–78. doi: 10.12659/MSM.889343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–59. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 5.Wheatcroft SB, Williams IL, Shah AM, et al. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–68. doi: 10.1046/j.1464-5491.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 6.Pohjantahti-Maaroos H, Palomaki A, Hartikainen J. Erectile dysfunction, physical activity and metabolic syndrome: differences in markers of atherosclerosis. BMC Cardiovasc Disord. 2011;11:36. doi: 10.1186/1471-2261-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein I. The mutually reinforcing triad of depressive symptoms, cardiovascular disease, and erectile dysfunction. Am J Cardiol. 2000;86:41F–45F. doi: 10.1016/s0002-9149(00)00892-4. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein I. Screening for erectile dysfunction: rationale. Int J Impot Res. 2000;12(Suppl 4):S147–51. doi: 10.1038/sj.ijir.3900595. [DOI] [PubMed] [Google Scholar]
- 9.Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet. 1985;1:181–84. doi: 10.1016/s0140-6736(85)92023-9. [DOI] [PubMed] [Google Scholar]
- 10.Solomon H, Man J, Wierzbicki AS, et al. Erectile dysfunction: cardiovascular risk and the role of the cardiologist. Int J Clin Pract. 2003;57:96–99. [PubMed] [Google Scholar]
- 11.Bocchio M, Desideri G, Scarpelli P, et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004;171:1601–4. doi: 10.1097/01.ju.0000116325.06572.85. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan ME, Thompson CS, Dashwood MR, et al. Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43:658–65. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 13.Bocchio M, Scarpelli P, Necozione S, et al. Intima-media thickening of common carotid arteries is a risk factor for severe erectile dysfunction in men with vascular risk factors but no clinical evidence of atherosclerosis. J Urol. 2005;173:526–29. doi: 10.1097/01.ju.0000148890.83659.c1. [DOI] [PubMed] [Google Scholar]
- 14.Yao F, Huang Y, Zhang Y, et al. Subclinical endothelial dysfunction and low-grade inflammation play roles in the development of erectile dysfunction in young men with low risk of coronary heart disease. Int J Androl. 2012;35:653–59. doi: 10.1111/j.1365-2605.2012.01273.x. [DOI] [PubMed] [Google Scholar]
- 15.Averbeck MA, Colares C, de Lira GH, et al. Evaluation of endothelial function with brachial artery ultrasound in men with or without erectile dysfunction and classified as intermediate risk according to the Framingham Score. J Sex Med. 2012;9:849–56. doi: 10.1111/j.1743-6109.2011.02591.x. [DOI] [PubMed] [Google Scholar]
- 16.Corona G, Mannucci E, Petrone L, et al. A comparison of NCEP-ATPIII and IDF metabolic syndrome definitions with relation to metabolic syndrome-associated sexual dysfunction. J Sex Med. 2007;4:789–96. doi: 10.1111/j.1743-6109.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Mannucci E, Schulman C, et al. Psychobiologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol. 2006;50:595–604. doi: 10.1016/j.eururo.2006.02.053. discussion 604. [DOI] [PubMed] [Google Scholar]
- 18.Zambon JP, Mendonca RR, Wroclawski ML, et al. Cardiovascular and metabolic syndrome risk among men with and without erectile dysfunction: case-control study. Sao Paulo Med J. 2010;128:137–40. doi: 10.1590/S1516-31802010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin E, Hwang W. Imaging assessment of cardiovascular risk in asymptomatic adults. Am J Roentgenol. 2011;197:W1046–51. doi: 10.2214/AJR.11.6758. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–49. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 21.Sucakli MH, Ozkan F, Inci MF, et al. Effects of smokeless tobacco (Maras powder) use on carotid intima media thickness. Med Sci Monit. 2013;19:859–64. doi: 10.12659/MSM.889654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saely CH, Rein P, Drexel H. The metabolic syndrome and risk of cardiovascular disease and diabetes: experiences with the new diagnostic criteria from the International Diabetes Federation. Horm Metab Res. 2007;39:642–50. doi: 10.1055/s-2007-985822. [DOI] [PubMed] [Google Scholar]
- 23.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 24.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–90. [DOI] [PubMed] [Google Scholar]
- 25.Lim TK, Lim E, Dwivedi G, et al. Normal value of carotid intima-media thickness – a surrogate marker of atherosclerosis: quantitative assessment by B-mode carotid ultrasound. J Am Soc Echocardiogr. 2008;21:112–16. doi: 10.1016/j.echo.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Yeh E. High-sensitivity C-reactive protein as a risk assessment tool for cardiovascular disease. Clin Cardiol. 2005;28:408–12. doi: 10.1002/clc.4960280905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda E. High-sensitivity C-reactive protein and white blood cell count equally predict development of the metabolic syndrome in a Japanese health screening population. Acta Diabetologica. 2013;50(4):633–38. doi: 10.1007/s00592-013-0477-7. [DOI] [PubMed] [Google Scholar]
- 28.Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168(6):5126–34. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]
- 29.Bobjer J, Katrinaki M, Tsatsanis C, et al. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8:e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timóteo A, Carmo M, Ferreira R. Can metabolic syndrome presence predict carotid intima-media thickness? J Clin Hypretens (Greenwich) 2012;14:507–13. doi: 10.1111/j.1751-7176.2012.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao F, Liu L, Zhang Y, et al. Erectile dysfunction may be the first clinical sign of insulin resistance and endothelial dysfunction in young men. Clin Res Cardiol. 2013;102:645–51. doi: 10.1007/s00392-013-0577-y. [DOI] [PubMed] [Google Scholar]
- 32.Liao M, Huang X, Gao Y, et al. Testosterone is associated with erectile dysfunction: a cross-sectional study in Chinese men. PLoS One. 2012;7:e39234. doi: 10.1371/journal.pone.0039234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traish AM, Guay A, Feeley R, et al. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 34.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirabassi G, Gioia A, Giovannini L, et al. Testosterone and cardiovascular risk. Intern Emerg Med. 2013;8(Suppl 1):S65–69. doi: 10.1007/s11739-013-0914-1. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 37.Tan WS, Ng CJ, Khoo EM, et al. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men’s Health Study) Aging Male. 2011;14:231–36. doi: 10.3109/13685538.2011.597463. [DOI] [PubMed] [Google Scholar]
- 38.Yao F, Liu L, Zhang Y, et al. Erectile dysfunction may be the first clinical sign of insulin resistance and endothelial dysfunction in young men. Clin Res Cardiol. 2013;102:645–51. doi: 10.1007/s00392-013-0577-y. [DOI] [PubMed] [Google Scholar]