Abstract
Skin lesions from mycosis fungoides (MF) patients display an increased expression of interleukin-15 (IL-15), IL-17F, and other cytokines implicated in inflammation and malignant cell proliferation in cutaneous T-cell lymphoma (CTCL). In the leukemic variant of CTCL, Sézary syndrome (SS), IL-2 and IL-15 trigger activation of the Jak-3/STAT3 pathway and transcription of IL17A gene, whereas it is unknown what causes IL-15 expression, Jak3/STAT3 activation, and production of IL-17F in MF. Here, we studied the expression and regulation of IL-15 and its relation to IL-17F in MF cell lines and skin lesions from 60 MF patients. We show that: (1) the spontaneous IL-15 mRNA expression is resistant to Jak3 and STAT3 inhibitors at concentrations that profoundly inhibit STAT3 activation and IL-17F mRNA expression; (2) anti-IL-15 antibody blocks STAT3 activation induced by exogenous IL-15 in non-malignant MF T cells, whereas the spontaneous STAT3 activation and IL-17F expression in malignant T cells is not inhibited; (3) patients display heterogeneous IL-15/IL-17F mRNA expression patterns in skin lesions; and (4) IL-15 expression (in contrast to IL-17F) is not associated with progressive disease. Taken together, these findings indicate that IL-15 and IL-17F are differentially regulated and expressed in MF. We propose that IL-15 and IL-17F are markers for different inflammatory environments and play distinct roles in the development and progression of MF.
Keywords: IL-17F, IL-15, CTCL, MF, STAT3, Jak3
Introduction
Cutaneous T-cell lymphoma (CTCL) is characterized by the expansion of malignant T cells in a chronic inflammatory environment. In the predominant clinical variant, mycosis fungoides (MF), skin lesions initially present as flat erythematous patches or plaques. In the advanced stages of the disease, the lesions develop into overt intradermal tumors, and the malignant T cells may spread to the lymph nodes and, even, internal organs and bone marrow.1 Patients diagnosed in early stages often experience an indolent disease course and have a favorable prognosis, with a life expectancy similar to that of a control population. However, in a subgroup of patients diagnosed with early CTCL, the disease progresses to a more aggressive and occasionally fatal course.1,2
Skin lesions from the early MF share many histological and pathological features with lesions from patients with benign chronic inflammatory skin disorders such as chronic dermatitis and psoriasis, making early diagnosis difficult in many cases. Given the histological similarities between MF and benign inflammatory conditions, it is not surprising that overlapping sets of cytokines and angiogenic and inflammatoryfactors are expressed in both MF and the benign inflammatory skin conditions. For instance, IL-15, IL-21, and members of the IL-17 cytokine family (such as IL-17A and IL-17F) are expressed in skin lesions from both psoriatic and MF patients.3-10 Importantly, IL-17A plays a direct role in the pathogenesis of psoriasis, and anti-IL-17A antibodies have therapeutic effects in many patients.11 In CTCL, an increased expression of IL-17F is associated with progressive disease,8 which also indicates a putative role of IL-17 family cytokines in its pathogenesis. Likewise, psoriasis and CTCL display increased levels of angiogenic factors such as VEGFs, disturbances in apoptotic and cytokine signaling pathways, and partly overlapping, but distinct miRNA expression profiles.12-25 Similarly, T helper cell type 2 (TH2) cytokines and IL-17 family cytokines are elevated in MF and benign conditions such as chronic dermatitis (reviewed in ref. 26).
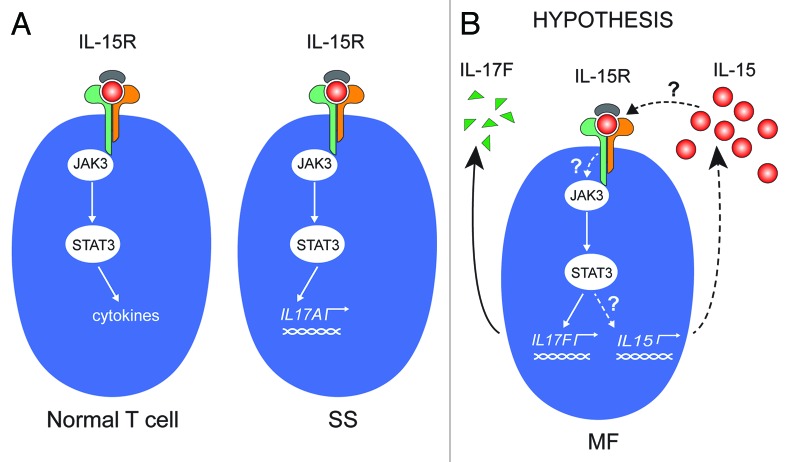
At present, it is not known whether cytokines expressed in CTCL skin lesions: (1) have a direct role in the pathogenesis of the disease; (2) are signs of an overall ineffective anti-lymphoma response; or (3) are secondary events (epiphenomena) to the underlying genuine lymphomagenesis. However, several lines of evidence indicate that the malignant T cells orchestrate the production of cytokines and inflammatory and angiogenic factors. Of interest, malignant MF T-cell lines have been shown to spontaneously produce IL-15 and IL-17F, both of which are expressed in situ in MF lesions and are associated with progressive or advanced disease.8,27-30 In SS cells, IL-15 triggers enhanced IL-17A production via activation of the Jak3/STAT3 signaling pathway,7 and IL-15 has been proposed to function as an autocrine survival and activation factor in these cells (Fig. 1A).3 The present study was undertaken to investigate the expression and regulation of IL-15 in MF. In particular, we strived to address whether IL-15 is an autocrine factor driving the spontaneous STAT3 activation and IL-17F synthesis in malignant MF cell lines, and whether IL-15 and IL-17F are co-regulated and expressed in tandem in situ in the MF lesions (Fig. 1B).
Figure 1. IL-15 signaling in CTCL (A) Left: In normal T cells, IL-15R activates the Jak3/STAT3 pathway, which can result in specific cytokine secretion. The specific cytokine(s) expressed depends on the activation and differentiation status of the T-cell type in question. Right: In malignant T cells from Sézary syndrome patients, IL-15 activates the Jak3/STAT3 signaling pathway, which, in turn, initiates transcription of the gene encoding IL-17A. (B) In MF it is unknown whether (1) the constitutive activation of the Jak3/STAT3 is induced by IL-15; and (2) STAT3 drives the constitutive transcription of IL-15. In other words, whether IL-15 is part of an autocrine stimulation loop involving the Jak3/STAT3 pathway and downstream target genes IL-17F and IL-15.
Results
Although malignant MF lesions and cell lines display a constitutive IL-15 expression in situ and in vitro,3,27 the underlying mechanisms remain unclear. Because STAT3 is a key regulator of the expression of numerous cytokines (IL-5, IL-10, IL-13, and IL-17A and IL-17F) in malignant T cells, we investigated whether the Jak3/STAT3 pathway induces also expression of IL-15. As shown in Figure 2A, malignant T cells display a high, spontaneous expression of IL-15 mRNA when compared with non-malignant T cells (Fig. 2A; Table 1). To determine whether IL-15 expression was driven by STAT3, we examined the effect of Jak3 and STAT3 inhibitors on the expression of IL-15, with IL-17F serving as a positive control.8 Whereas Jak3 and STAT3 inhibitors triggered a profound inhibition of IL-17F expression, the spontaneous expression of IL-15 remained largely unaffected (Fig. 2B), indicating that the Jak3/STAT3 pathway does not regulate IL-15, and that IL-15 and IL-17F expression is regulated differently in malignant T cells. As malignant MF cells also display a constitutive activation of other signaling pathways, including the scr kinase BLK,39,40 NFkB,41,42 and the COX2/prostaglandin E2 (PGE2),43,44 we examined whether any of these pathways was involved in the induction of IL-15 expression. However, inhibition of scr kinase, COX-2, and PGE2 using their respective inhibitors, or inhibition of NFκB via siRNA-mediated knockdown of components of the NFκB pathway (Rel-A and Rel-B), had no inhibitory effect on IL-15 expression (data not shown) indicating that IL-15 expression is driven by a yet-unidentified signaling pathway in malignant MF cells.
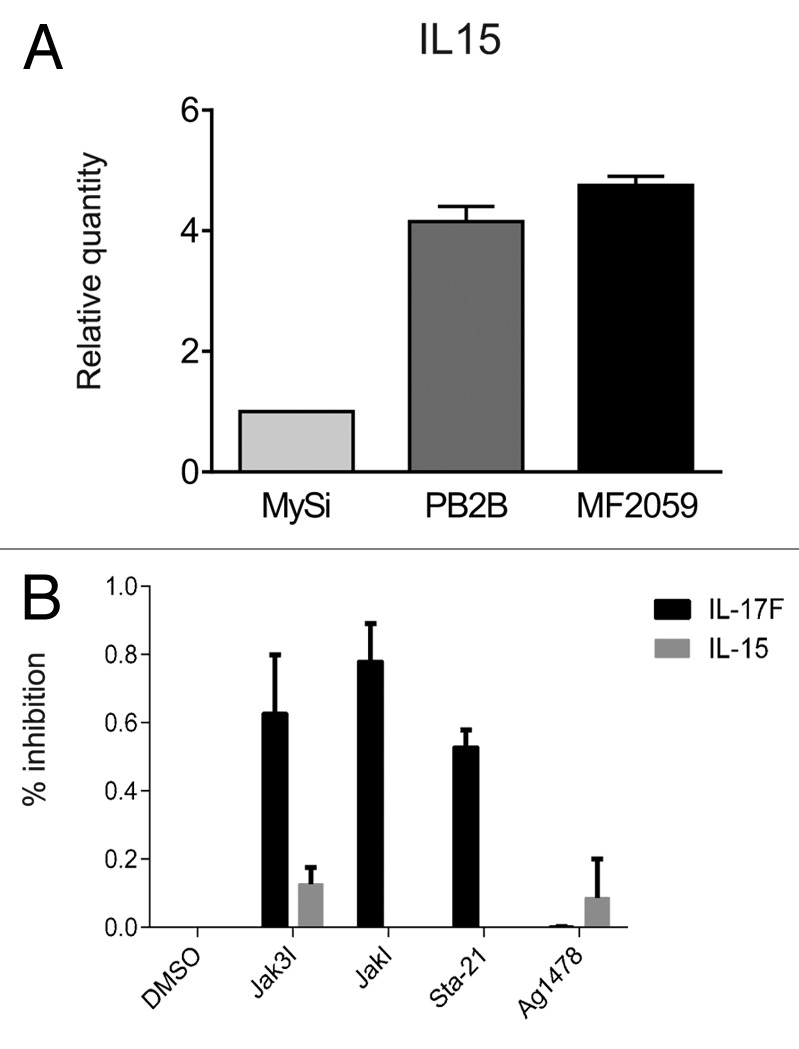
Figure 2. IL-15 expression in malignant T cells. (A) Non-malignant (MySi) and malignant (PB2B, MF2000) T cells were cultured for 24 h. RNA was purified from the cells and reversely transcribed to cDNA and analyzed by quantitative PCR to determine the relative level of IL-17F and GAPDH mRNA. In each sample, the level of IL-17F mRNA was normalized to the amount of GAPDH mRNA and depicted as fold change when compared with non-malignant T cells (MySi). (B) Malignant (PB2B) T cells were cultured with either DMSO, Jak3 inhibitor (40 ug/mL), Jak inhibitor (1 mM), Sta-21 (40 uM), or Ag1478 (200 ng/mL). Cells were harvested after 4 h, and RNA was purified and reverse transcribed to cDNA and analyzed by quantitative PCR to determine the relative level of IL-15, IL-17F, and GAPDH mRNA. In each sample, the level of IL-15 and IL-17F mRNA was normalized to that of GAPDH mRNA and depicted as percent inhibition when compared with cells cultured with DMSO.
Table 1. Characteristics and phenotypes of cell lines.
| Cell line Id# | Malignant/non-malignant | Constitutive pY-STAT3 | IL-15 expression | IL-17F expression |
|---|---|---|---|---|
| MySi | Non-malignant | None (IL-15 induced) | None | None |
| MF1850 | Non-malignant | None (IL-15 induced) | None | None |
| MyLa2059 | Malignant MF | Yes | Yes | Yes |
| MyLa2000 | Malignant MF | Yes | Yes | Yes |
| PB2B | Transformed MF/CTCL | Yes | Yes | Yes |
Adapted from Woetmann et al.31
Dummer and coworkers were the first to describe IL-15 expression in situ in CTCL, and based on studies in SS cell lines, they proposed that IL-15 functions as an autocrine and paracrine activation and survival factor in SS.3 Since IL-15 induces IL-17A expression through the Jak3/STAT3 pathway in SS cells,7 we asked whether IL-15, via an autocrine loop, also induced STAT3-mediated IL-17F expression in MF T cells. To achieve this, we incubated malignant and non-malignant MF cell lines with a neutralizing IL-15 antibody prior to analysis of STAT3 tyrosine phosphorylation pY). Non-malignant T cells did not spontaneously express pY-STAT3, but expressed pY-STAT3 following exposure to exogenous IL-15 (Fig. 3A, lane 3 vs. lane 1). Pre-treatment of non-malignant T cells with anti-IL-15 antibody almost completely blocked IL-15-induced STAT3 activation (Fig. 3A, lane3 vs. lane 4), confirming the specificity of the response and the efficacy of the antibody. In contrast, blocking anti-IL-15 antibodies had no effect on the constitutive STAT3 activation in malignant MF T cells (Fig. 3A, right part, and data not shown). In parallel, we examined the effect of IL-15 inhibition on IL-17F expression in malignant MF cells, and, as shown in Figure 3B, blocking concentrations of IL-15 mAb had no inhibitory effect on IL-17F expression. siRNA-mediated inhibition of IL-15 strongly inhibited IL-15 expression (Fig. 4A), whereas STAT3 activation and IL-17F expression were unaffected (Fig. 4B and C). Taken together, these findings show that in malignant MF cells IL-15 and IL-17F are regulated differentially, and that STAT3 phosphorylation and IL-17F expression are in these cells independent of IL-15.
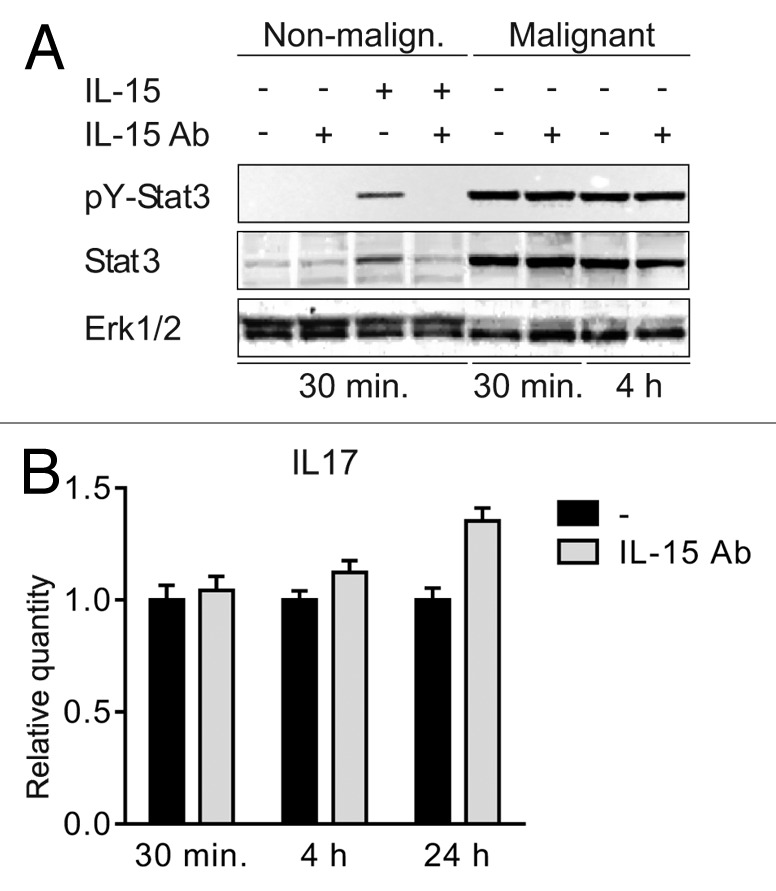
Figure 3. IL-15 drives STAT3 activation in non-malignant but not in malignant T cells (A) Non-malignant (MySi) and malignant (MF2000) T cells were cultured with or without IL-15 neutralizing antibody (IL-15 Ab, 2 μg/mL) for 30 min. Then, IL-15 (10 ng/mL) was added as given and the cells cultured for 30 min or 4 h further. Finally, the cells were lysed and the lysates analyzed by western blotting using antibodies against pYStat3, Stat3, and Erk1/2. (B) Malignant T cells (MF2000) were cultured with and without IL-15 Ab (2 μg/mL) for 30 min, 4 h, or 24 h. Subsequently RNA was purified from the cells and reverse transcribed to cDNA that was subjected to quantitative PCR analysis to determine the relative level of IL-17F and GAPDH mRNA. In each sample, the level of IL-17F mRNA was normalized to that of GAPDH mRNA and depicted as fold change when compared with cells cultured without IL-15Ab for the same period of time.
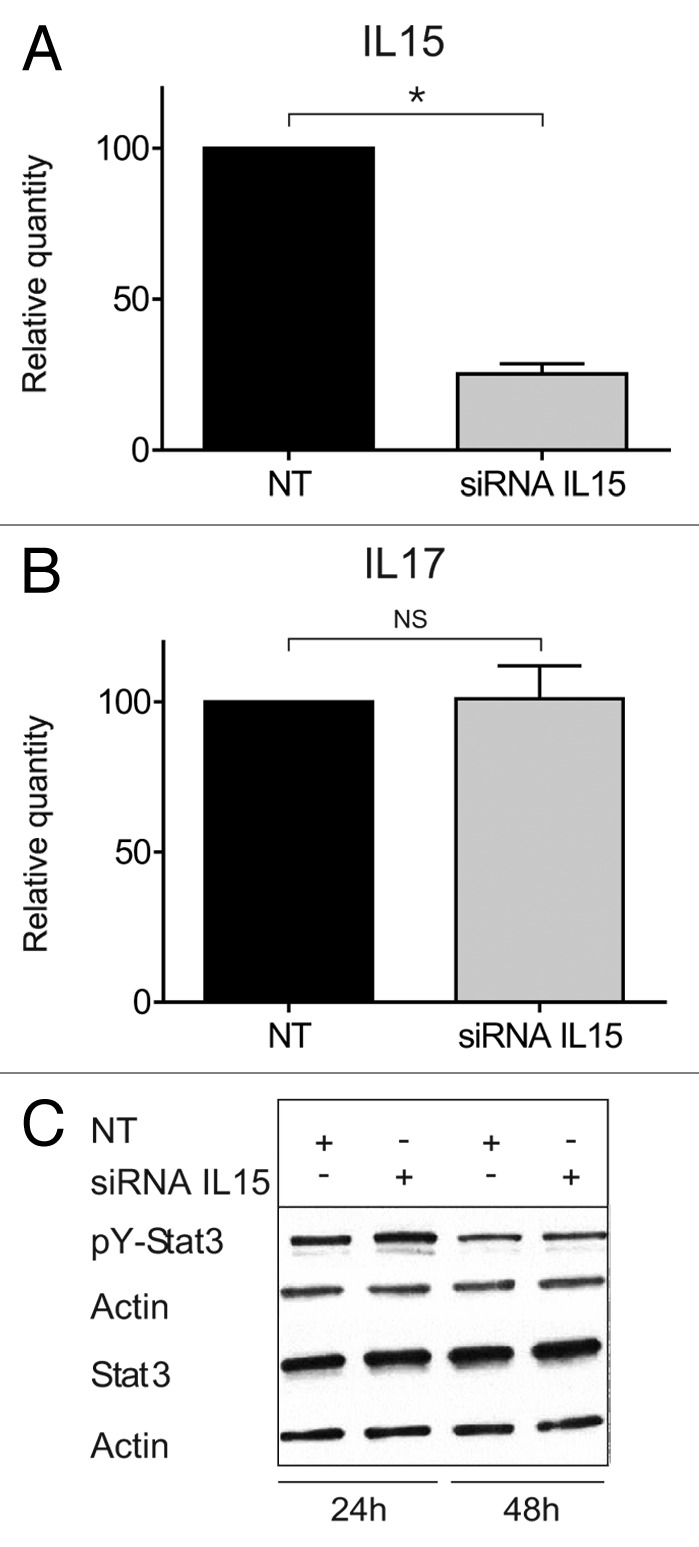
Figure 4. STAT3/IL-17F expression is driven by an IL-15-independent pathway. (A and B) Malignant (MF2059) T cells were transiently transfected with small interfering RNA against IL-15 or non-target control (NT) and cultured for 24 h. After incubation, RNA was purified from the cells and reverse transcribed to cDNA and analyzed by quantitative PCR to determine the relative level of IL15, IL17F, and GAPDH mRNA. In each sample, the level of IL15 mRNA or IL17F mRNA was normalized to the amount of GAPDH mRNA and depicted as fold change when compared NT control. There was a significant difference in IL-15 expression (P value = 0,002). (C) Malignant (MF2059) T cells were transiently transfected with small interfering RNA against IL-15 or non-target control (NT). Twenty-four hours after transfection, cells were washed and cultured for another 24 or 48 h. The cells were lysed and the lysates analyzed by western blotting using antibodies against pY-STAT3, STAT3, and actin.
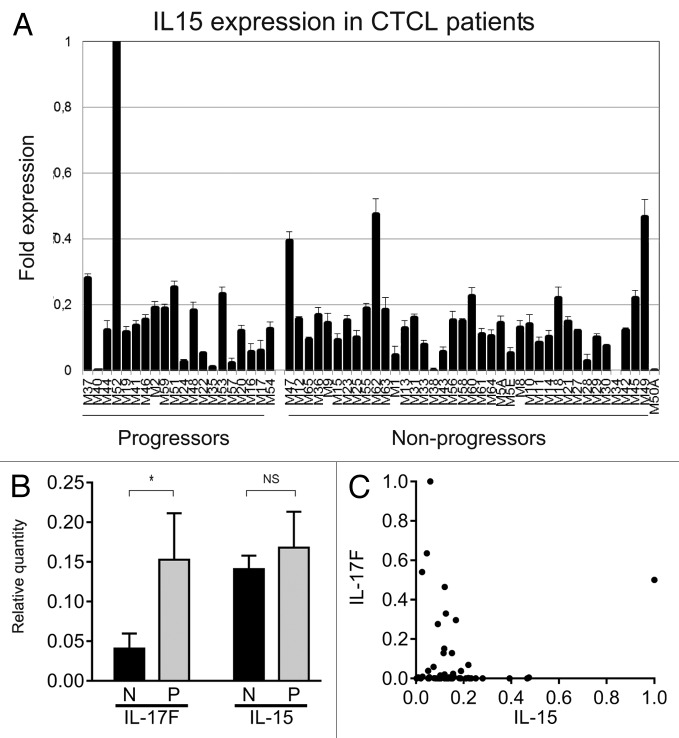
Next, we studied IL-15 mRNA expression in skin lesions from 60 MF patients. A majority of patients expressed IL-15 in situ as judged from the mRNA expression in skin lesion (Fig. 5). Importantly, IL-15 expression was observed in both indolent and progressive disease and, hence, may not be limited to the advanced disease stage (Fig. 5A ), as proposed in the original study on IL-15 in MF.27 Because IL-15 and IL-17F are co-expressed in malignant MF cells in vitro (cf. above), and IL-17F correlates with progressive disease in the present cohort of patients,8 we asked whether IL-15 and IL-17F were co-expressed in skin lesions. However, IL-15 expression was not associated with IL-17F expression in skin lesions (Fig. 5B), although IL-17F predominated in lesions from progressive CTCL patients. On the contrary, a high expression of IL-17F seemed to be associated with a low expression of IL-15, and, reversely, a high IL-15 expression seemed (with one exception) to be associated with a low expression of IL-17F (Fig. 5C), suggesting a possible inverse relationship between IL-15 and IL-17F. The apparent inverse correlation was, however, not statistically significant. Taken together, these findings show that IL-15 and IL-17F are not co-expressed in situ in MF skin, provide further evidence that these cytokines are regulated via different mechanisms, and suggest that the 2 cytokines play different roles in the pathogenesis and progression of the disease. (Fig. 1B);)
Figure 5. IL-15 mRNA in skin lesions from MF patients. (A) IL-15 mRNA expression from skin lesions in 60 MF patients. The patients, the purification of RNA from the skin biopsies, and the method of determination of the relative cytokine expression by QPCR have been described extensively previously .8,30,37–38 (B) The average relative expression of IL-17F and IL-15 mRNA in skin biopsies from CTCL patients with non-progressive (N) and progressive (P) disease. Bars represent mean + SEM of the 60 patients. *Denotes a significant difference (P < 0,05) whereas NS indicates no significant change. (C) Scatter plot of the relative mRNA expression of IL-17F and IL-15 in skin biopsies from 60 CTCL patients as above. (B and C) The depicted IL-17F expressions are based on RT-PCR data obtained in a previous study,8 whereas the IL-15 expressions are novel RT-PCR data.
Discussion
IL-15 was initially assumed to promote disease progression—partly because IL-15 was believed to be predominantly expressed in tumor stage of MF and in Sézary syndrome (SS),3,27 and, to a lesser degree, because IL-15 was identified as a survival factor in primary malignant T cells and cell lines from SS patients.3,25,28 However, in a later study, Leroy et al.45 confirmed IL-15 expression in situ in MF but did not find a correlation with advanced or progressive disease. In contrast, Tamaki and his colleagues46 found virtually no IL-15 mRNA expression in CTCL patients, questioning the relevance of IL-15 in CTCL. In the present study, we addressed this controversy by studying IL-15 mRNA expression in 60 MF patients. In our cohort, the majority of patients expressed IL-15, confirming the original observations in CTCL. However, we did not observe a correlation with progressive disease. Thus, our data are in keeping with the findings by Leroy et al.45 that IL-15 is expressed in MF skin lesions, but not associated with the progressive or advanced disease.
It is unclear why several studies identified IL-15 expression,3,27,45,47 whereas a single study did not find any support for a link between IL-15 and CTCL.46 Once identified, the frequency of IL-15 presence ranged from a fraction of patients27 to a large majority of patients.45 Toll-like receptor (TLR) ligands act synergistically with IL-15 to modulate the immune response and, possibly, disease development in CTCL patients,48,49 and bacterial toxins such as staphylococcal enterotoxins, which are common in CTCL lesions, influence proliferation and cytokine production in CTCL (reviewed in ref. 50). It is, therefore, possible that differences in treatment regimens and microbial infections could, at least in part, explain the observed differences in IL-15 expression and association with disease stage between the studies. Moreover, the studies on IL-15 cited above used different techniques and generally enrolled relatively few patients. In a larger cohort of patients, we now report that IL-15 expression is seen in a majority of CTCL. Importantly, IL-15 expression was not uniform among the patients. Instead, some patients expressed highly elevated IL-15, whereas others expressed the cytokine at low levels, comparable to those found in normal skin. Therefore, the present findings may not be contradictory to the previous studies. On the contrary, all expression profiles (none, intermediate, and high) described previously, were represented in our cohort of patients, suggesting that the apparent discrepancies between the aforementioned studies were possibly due to a small sample size.
Given the role of IL-15 as a growth and survival factor in malignant T cells from SS patients,3,28 it was hypothesized that IL-15, produced by malignant T cells and/or stromal cells such as DCs and macrophages, promotes tumor progression via autocrine and paracrine stimulation of malignant T cells.3 However, IL-15 may play a far more complex role in the pathogenesis of CTCL than originally appreciated. Thus, in addition to its role as a survival and growth factor for malignant T cells, IL-15 is also a growth and activation factor for non-malignant T cells, including CD8 cytotoxic T cells and NK cells. Accordingly, IL-15 and IFNa synergistically potentiate cytotoxicity of CD8 T cells and NK cells,51 and TLR agonist and IL-15 together promote growth inhibition of malignant T cells in CTCL.48,49 Therefore, it is likely that IL-15 has both an anti-tumor function—via its effect on cytotoxic T cells and NK cells—as well as a tumor-promoting activity—via induction of survival and anti-apoptotic signals in malignant T cells. Thus, the net effect of IL-15 expression is likely to depend on the specific cytokine environment and cellular composition of the skin lesion in question.
Because STAT3 in malignant T cells promotes transcription of IL-5, IL-13, IL-10, and IL-17A and IL-17F, all of which are implicated in the pathogenesis in CTCL, we examined whether IL-15 was also induced via the Jak3/STAT3 pathway. However, this was not the case, as inhibitors of Jak3 and STAT3, used at concentrations which profoundly inhibited IL-17F, had little effect on IL-15 expression in malignant cells. Notably, none of the other signaling pathways examined, Scr kinases, NFkB, and COX2/PGE2, appeared not to be involved either;39-44 studies are in progress to determine whether other novel pathways are involved in IL-15 expression in CTCL.
Given the observation that IL-15 induces IL-17A expression via activation of STAT3 in malignant SS cell lines,7 and that STAT3 drives IL-17F in malignant MF T cells,8 we questioned whether the spontaneous IL-17F production in malignant MF cell lines was driven through an autocrine activation loop involving IL-15, as originally proposed by Dummer et al.3 Our observations that a blocking anti-IL-15 mAb had no effect on STAT3 activation and IL-17F expression did not support this hypothesis. This conclusion was further supported by our observation that siRNA-mediated knockdown of IL-15 had no effect on either STAT3 activation or IL-17F expression, strongly arguing against an autocrine role of IL-15 in the activation of STAT3 and expression of IL-17F. This conclusion is also consistent with the observation that STAT3 drives spontaneous IL-17F expression in malignant MF cells.8
In contrast to IL-15, IL-17F is associated with the progressive disease,8 which indicates a possible key role of IL-17F in the pathogenesis of the advanced stages of CTCL. A recent study by Ferrara and coworkers52 found that IL-17 stimulates angiogenesis and modulates the function of stromal cells. Since tumor progression is associated with angiogenesis, and malignant T cells produce angiogenic factors in situ and in vivo,14-16 it is possible that IL-17 cytokines facilitate disease progression through the promotion of angiogenesis and lymph-angiogenesis. Indeed, our preliminary findings show that IL-17F released by malignant T cells stimulate angiogenesis in vitro.
Taken together, our study shows that (1) IL-15 is expressed in skin lesions in a majority of patients; (2) IL-15 and IL-17F are differentially regulated in malignant MF cells; and (3) IL-15 and IL-17F are differentially expressed in CTCL skin lesions. We propose that IL-15 and IL-17F participate, and likely contribute to, different inflammatory environments and, consequently, have different roles in the pathogenesis of CTCL.
Materials and Methods
Antibodies and reagents
Anti-Erk1/2 (Santa Cruz Biotechnology #sc-153), anti-actin (Sigma-Aldrich #A4700), anti-Stat3 (Cell Signaling Technology #9132), anti-pY-Stat3 (Y705) (NanoTools #0036-100/STAT3-9E12), IL-15 neutralizing antibody (R&D Systems #MAB205). Jak inhibitor I (JakI; P6 #420099) and Jak3 inhibitor II (Jak3I; WHI-P154 #420104) (Merck Millipore), Sta-21 (#BML-EI351) and Tyrphostin Ag1478 (#ALX-270-036) (Enzo Life Sciences). Dimethyl sulfoxide (DMSO) (Sigma-Aldrich #D2438), Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich #16561-29-8) and ionomycin (Sigma-Aldrich #56092-81-0). Recombinant human IL-15 (Peprotech, #200-15).
Cell lines
The malignant T-cell lines MyLa2000 (MF2000) and PB2B as well as the nonmalignant T-cell line MySi were obtained from patients with CTCL.31-33 Table 1 summarizes the characteristic features of malignant and non-malignant T cells used in the present study. MF2000 and PB2B were grown in conditional media (CM) (RPMI 1640, 2 mM L-glutamine, and 100 mg/ml penicillin/streptomycin, all from Sigma) supplemented with 10% fetal bovine serum (Life Technologies). MySi were cultured in CM supplemented with 10% pooled human serum (Blood Bank, State University Hospital) and 103 U ml–1 IL-2 (Proleukin, Chiron).
ELISA
The concentrations of IL-17A, IL-17F, and IL-17A/F heterodimers in cell culture supernatants were measured using human DuoSet enzyme-linked immunosorbent assay (ELISA) development kits from R&D Systems in accordance with the manufacturer’s instructions.
Transient transfections
Transient transfections were performed as described previously34 using 0.25 µmol IL15 or the corresponding amount of nontargeting ON-TARGETplus SMARTpool siRNA (Dharmacon) and 2 × 106 cells.
Protein extraction and western blotting
Protein extraction and western blotting were performed as described earlier.35,36 To ensure equal loading, the total protein concentration of each lysate was determined by Bio-Rad protein Assay (Bio-Rad).
RNA purification, cDNA synthesis, and QPCR on cell lines
Total cellular mRNA was purified and reverse transcribed into cDNA (cDNA) as described previously.8,25 Quantitative polymerase chain reaction (QPCR) was subsequently performed using the Brilliant II SYBR Green quantitative PCR kit from Stratagene in accordance with the manufacturer’s instructions and the samples analyzed on a Mx3005P (Stratagene). For amplification, the following primers were used: IL-17F-forward 5′-TTCCAAAAGC CTGAGAGTTG-3′, IL-17F-reverse 5′-GCCCAAGTTC CTACACTGG-3′, IL-15-forward 5′-GTGCAGGGCT TCCTAAAACA-3′, IL-15-reverse 5′-TGCAACTGGG GTGAACATC-3′, GAPDH-forward 5′-AAGGTGAAGG TCGGAGTCAA-3′ and GAPDH-reverse 5′-AATGAAGGGG TCATTGATGG-3′.
Patients
mRNA expression of IL-17F and IL-15 was assessed by RT-PCR in the historic cohort of patients from Boston (n = 60) that has been described previously in multiple publications.8,30,37,38 For these patients, 6-year clinical follow-up data are available for disease progression and for disease-specific mortality, and they were accordingly used to analyze if there was a correlation between IL-17F or IL-15 expression and disease progression or survival. Disease progression was defined as the advancement toward a higher clinical stage and/or incidence of a disease-related death.13 In all cases, the data was subjected to Kaplan–Meier analyses and significance determined using the Log-rank test.
Statistics
P values were obtained by Student t test. “*” indicates P < 0.05. “NS” indicates P > 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by research funding from the Carlsberg Foundation, the Danish Cancer Society, Dansk Kræftforsknings Fond, the Danish Research Councils, the Danish National Advanced Technology Foundation, the Copenhagen Cluster of Immunology, the Lundbeck Foundation, the Novo Nordic Foundation, the Neye Foundation, the Beckett Foundation (Beckett-Fonden), the University of Copenhagen, and the National Cancer Institute (grant CA89194). This work was also supported by the Canadian Dermatology Foundation and the Le Fonds de Recherche du Québec-Santé (research grants to Dr Sasseville). We thank Dr Thomas Kupper from Harvard University for generously providing cDNA samples from CTCL patients for RT-PCR analysis. Finally, we thank K Kaltoft for providing us with the MyLa and SeAx cell lines.
References
- 1.Willemze R. Thirty years of progress in cutaneous lymphoma research. G Ital Dermatol Venereol. 2012;147:515–21. [PubMed] [Google Scholar]
- 2.Imam MH, Shenoy PJ, Flowers CR, Phillips A, Lechowicz MJ. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma. 2013;54:752–9. doi: 10.3109/10428194.2012.729831. [DOI] [PubMed] [Google Scholar]
- 3.Döbbeling U, Dummer R, Laine E, Potoczna N, Qin JZ, Burg G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood. 1998;92:252–8. [PubMed] [Google Scholar]
- 4.Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22:160–8. doi: 10.1684/ecn.2011.0294. [DOI] [PubMed] [Google Scholar]
- 5.Elder JT. IL-15 and psoriasis: another genetic link to Th17? J Invest Dermatol. 2007;127:2495–7. doi: 10.1038/sj.jid.5700855. [DOI] [PubMed] [Google Scholar]
- 6.Qin JZ, Dummer R, Burg G, Döbbeling U. Constitutive and interleukin-7/interleukin-15 stimulated DNA binding of Myc, Jun, and novel Myc-like proteins in cutaneous T-cell lymphoma cells. Blood. 1999;93:260–7. [PubMed] [Google Scholar]
- 7.Krejsgaard T, Ralfkiaer U, Clasen-Linde E, Eriksen KW, Kopp KL, Bonefeld CM, Geisler C, Dabelsteen S, Wasik MA, Ralfkiaer E, et al. Malignant cutaneous T-cell lymphoma cells express IL-17 utilizing the Jak3/Stat3 signaling pathway. J Invest Dermatol. 2011;131:1331–8. doi: 10.1038/jid.2011.27. [DOI] [PubMed] [Google Scholar]
- 8.Krejsgaard T, Litvinov IV, Wang Y, Xia L, Willerslev-Olsen A, Koralov SB, Kopp KL, Bonefeld CM, Wasik MA, Geisler C, et al. Elucidating the role of interleukin-17F in cutaneous T-cell lymphoma. Blood. 2013;122:943–50. doi: 10.1182/blood-2013-01-480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caruso R, Costanzo A, Monteleone G. Pathogenic role of interleukin-21 in psoriasis. Cell Cycle. 2009;8:3629–30. doi: 10.4161/cc.8.22.9964. [DOI] [PubMed] [Google Scholar]
- 10.Kasprzycka M, Zhang Q, Witkiewicz A, Marzec M, Potoczek M, Liu X, Wang HY, Milone M, Basu S, Mauger J, et al. Gamma c-signaling cytokines induce a regulatory T cell phenotype in malignant CD4+ T lymphocytes. J Immunol. 2008;181:2506–12. doi: 10.4049/jimmunol.181.4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiricozzi A, Krueger JG. IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs. 2013;22:993–1005. doi: 10.1517/13543784.2013.806483. [DOI] [PubMed] [Google Scholar]
- 12.Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999;141:1054–60. doi: 10.1046/j.1365-2133.1999.03205.x. [DOI] [PubMed] [Google Scholar]
- 13.Chua RA, Arbiser JL. The role of angiogenesis in the pathogenesis of psoriasis. Autoimmunity. 2009;42:574–9. doi: 10.1080/08916930903002461. [DOI] [PubMed] [Google Scholar]
- 14.Krejsgaard T, Vetter-Kauczok CS, Woetmann A, Lovato P, Labuda T, Eriksen KW, Zhang Q, Becker JC, Ødum N. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia. 2006;20:1759–66. doi: 10.1038/sj.leu.2404350. [DOI] [PubMed] [Google Scholar]
- 15.Karpova MB, Fujii K, Jenni D, Dummer R, Urosevic-Maiwald M. Evaluation of lymphangiogenic markers in Sézary syndrome. Leuk Lymphoma. 2011;52:491–501. doi: 10.3109/10428194.2010.517877. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen IH, Willerslev-Olsen A, Vetter-Kauczok C, Krejsgaard T, Lauenborg B, Kopp KL, Geisler C, Bonefeld CM, Zhang Q, Wasik MA, et al. Vascular endothelial growth factor receptor-3 expression in mycosis fungoides. Leuk Lymphoma. 2013;54:819–26. doi: 10.3109/10428194.2012.726720. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi L, Farrace MG, Nini G, Piacentini M. Abnormal Bcl-2 and “tissue” transglutaminase expression in psoriatic skin. J Invest Dermatol. 1994;103:829–33. doi: 10.1111/1523-1747.ep12413590. [DOI] [PubMed] [Google Scholar]
- 18.Reefman E, Limburg PC, Kallenberg CG, Bijl M. Apoptosis in human skin: role in pathogenesis of various diseases and relevance for therapy. Ann N Y Acad Sci. 2005;1051:52–63. doi: 10.1196/annals.1361.046. [DOI] [PubMed] [Google Scholar]
- 19.Eriksen KW, Lovato P, Skov L, Krejsgaard T, Kaltoft K, Geisler C, Ødum N. Increased sensitivity to interferon-alpha in psoriatic T cells. J Invest Dermatol. 2005;125:936–44. doi: 10.1111/j.0022-202X.2005.23864.x. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen KW, Woetmann A, Skov L, Krejsgaard T, Bovin LF, Hansen ML, Grønbaek K, Billestrup N, Nissen MH, Geisler C, et al. Deficient SOCS3 and SHP-1 expression in psoriatic T cells. J Invest Dermatol. 2010;130:1590–7. doi: 10.1038/jid.2010.6. [DOI] [PubMed] [Google Scholar]
- 21.Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C, Wasik M, Ødum N. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia. 2005;19:209–13. doi: 10.1038/sj.leu.2403610. [DOI] [PubMed] [Google Scholar]
- 22.Meisgen F, Xu N, Wei T, Janson PC, Obad S, Broom O, Nagy N, Kauppinen S, Kemény L, Ståhle M, et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol. 2012;21:312–4. doi: 10.1111/j.1600-0625.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 23.Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clin Exp Dermatol. 2008;33:312–5. doi: 10.1111/j.1365-2230.2008.02804.x. [DOI] [PubMed] [Google Scholar]
- 24.Ralfkiaer U, Hagedorn PH, Bangsgaard N, Løvendorf MB, Ahler CB, Svensson L, Kopp KL, Vennegaard MT, Lauenborg B, Zibert JR, et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL) Blood. 2011;118:5891–900. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopp KL, Ralfkiaer U, Gjerdrum LM, Helvad R, Pedersen IH, Litman T, Jønson L, Hagedorn PH, Krejsgaard T, Gniadecki R, et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle. 2013;12:1939–47. doi: 10.4161/cc.24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–82. doi: 10.1111/all.12184. [DOI] [PubMed] [Google Scholar]
- 27.Asadullah K, Haeussler-Quade A, Gellrich S, Hanneken S, Hansen-Hagge TE, Döcke WD, Volk HD, Sterry W. IL-15 and IL-16 overexpression in cutaneous T-cell lymphomas: stage-dependent increase in mycosis fungoides progression. Exp Dermatol. 2000;9:248–51. doi: 10.1034/j.1600-0625.2000.009004248.x. [DOI] [PubMed] [Google Scholar]
- 28.Qin JZ, Zhang CL, Kamarashev J, Dummer R, Burg G, Döbbeling U. Interleukin-7 and interleukin-15 regulate the expression of the bcl-2 and c-myb genes in cutaneous T-cell lymphoma cells. Blood. 2001;98:2778–83. doi: 10.1182/blood.V98.9.2778. [DOI] [PubMed] [Google Scholar]
- 29.Leroy S, Dubois S, Tenaud I, Chebassier N, Godard A, Jacques Y, Dréno B. Interleukin-15 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome) Br J Dermatol. 2001;144:1016–23. doi: 10.1046/j.1365-2133.2001.04192.x. [DOI] [PubMed] [Google Scholar]
- 30.Litvinov IV, Jones DA, Sasseville D, Kupper TS. Transcriptional profiles predict disease outcome in patients with cutaneous T-cell lymphoma. Clin Cancer Res. 2010;16:2106–14. doi: 10.1158/1078-0432.CCR-09-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woetmann A, Lovato P, Eriksen KW, Krejsgaard T, Labuda T, Zhang Q, Mathiesen AM, Geisler C, Svejgaard A, Wasik MA, et al. Nonmalignant T cells stimulate growth of T-cell lymphoma cells in the presence of bacterial toxins. Blood. 2007;109:3325–32. doi: 10.1182/blood-2006-04-017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krejsgaard T, Gjerdrum LM, Ralfkiaer E, Lauenborg B, Eriksen KW, Mathiesen AM, Bovin LF, Gniadecki R, Geisler C, Ryder LP, et al. Malignant Tregs express low molecular splice forms of FOXP3 in Sézary syndrome. Leukemia. 2008;22:2230–9. doi: 10.1038/leu.2008.224. [DOI] [PubMed] [Google Scholar]
- 33.Søndergaard H, Frederiksen KS, Thygesen P, Galsgaard ED, Skak K, Kristjansen PE, Odum N, Kragh M. Interleukin 21 therapy increases the density of tumor infiltrating CD8+ T cells and inhibits the growth of syngeneic tumors. Cancer Immunol Immunother. 2007;56:1417–28. doi: 10.1007/s00262-007-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauenborg B, Kopp K, Krejsgaard T, Eriksen KW, Geisler C, Dabelsteen S, Gniadecki R, Zhang Q, Wasik MA, Woetmann A, et al. Programmed cell death-10 enhances proliferation and protects malignant T cells from apoptosis. APMIS. 2010;118:719–28. doi: 10.1111/j.1600-0463.2010.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisler C, Dietrich J, Nielsen BL, Kastrup J, Lauritsen JP, Odum N, Christensen MD. Leucine-based receptor sorting motifs are dependent on the spacing relative to the plasma membrane. J Biol Chem. 1998;273:21316–23. doi: 10.1074/jbc.273.33.21316. [DOI] [PubMed] [Google Scholar]
- 36.Theander TG, Kharazmi A, Pedersen BK, Christensen LD, Tvede N, Poulsen LK, Odum N, Svenson M, Bendtzen K. Inhibition of human lymphocyte proliferation and cleavage of interleukin-2 by Pseudomonas aeruginosa proteases. Infect Immun. 1988;56:1673–7. doi: 10.1128/iai.56.7.1673-1677.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litvinov IV, Kupper TS, Sasseville D. The role of AHI1 and CDKN1C in cutaneous T-cell lymphoma progression. Exp Dermatol. 2012;21:964–6. doi: 10.1111/exd.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litvinov IV, Zhou Y, Kupper TS, Sasseville D. Loss of BCL7A expression correlates with poor disease prognosis in patients with early-stage cutaneous T-cell lymphoma. Leuk Lymphoma. 2013;54:653–4. doi: 10.3109/10428194.2012.717695. [DOI] [PubMed] [Google Scholar]
- 39.Krejsgaard T, Vetter-Kauczok CS, Woetmann A, Kneitz H, Eriksen KW, Lovato P, Zhang Q, Wasik MA, Geisler C, Ralfkiaer E, et al. Ectopic expression of B-lymphoid kinase in cutaneous T-cell lymphoma. Blood. 2009;113:5896–904. doi: 10.1182/blood-2008-09-181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Kester MS, Borg MK, Zoutman WH, Out-Luiting JJ, Jansen PM, Dreef EJ, Vermeer MH, van Doorn R, Willemze R, Tensen CP. A meta-analysis of gene expression data identifies a molecular signature characteristic for tumor-stage mycosis fungoides. J Invest Dermatol. 2012;132:2050–9. doi: 10.1038/jid.2012.117. [DOI] [PubMed] [Google Scholar]
- 41.Thakur S, Lin HC, Tseng WT, Kumar S, Bravo R, Foss F, Gélinas C, Rabson AB. Rearrangement and altered expression of the NFKB-2 gene in human cutaneous T-lymphoma cells. Oncogene. 1994;9:2335–44. [PubMed] [Google Scholar]
- 42.Chang TP, Vancurova I. NFκB function and regulation in cutaneous T-cell lymphoma. Am J Cancer Res. 2013;3:433–45. [PMC free article] [PubMed] [Google Scholar]
- 43.Kopp KL, Kauczok CS, Lauenborg B, Krejsgaard T, Eriksen KW, Zhang Q, Wasik MA, Geisler C, Ralfkiaer E, Becker JC, et al. COX-2-dependent PGE(2) acts as a growth factor in mycosis fungoides (MF) Leukemia. 2010;24:1179–85. doi: 10.1038/leu.2010.66. [DOI] [PubMed] [Google Scholar]
- 44.Braun FK, Al-Yacoub N, Plötz M, Möbs M, Sterry W, Eberle J. Nonsteroidal anti-inflammatory drugs induce apoptosis in cutaneous T-cell lymphoma cells and enhance their sensitivity for TNF-related apoptosis-inducing ligand. J Invest Dermatol. 2012;132:429–39. doi: 10.1038/jid.2011.316. [DOI] [PubMed] [Google Scholar]
- 45.Leroy S, Dubois S, Tenaud I, Chebassier N, Godard A, Jacques Y, Dréno B. Interleukin-15 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome) Br J Dermatol. 2001;144:1016–23. doi: 10.1046/j.1365-2133.2001.04192.x. [DOI] [PubMed] [Google Scholar]
- 46.Sugaya M, Nakamura K, Tamaki K. Serum interleukin-15 levels are not elevated in patients with stage I and II mycosis fungoides. Acta Derm Venereol. 2000;80:455. doi: 10.1080/000155500300080455. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka K, Clark R, Rich B, Dowgiert R, Hirahara K, Hurwitz D, Shibata M, Mirchandani N, Jones DA, Goddard DS, et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;107:2440–5. doi: 10.1182/blood-2005-03-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wysocka M, Benoit BM, Newton S, Azzoni L, Montaner LJ, Rook AH. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104:4142–9. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- 49.Wysocka M, Dawany N, Benoit B, Kossenkov AV, Troxel AB, Gelfand JM, Sell MK, Showe LC, Rook AH. Synergistic enhancement of cellular immune responses by the novel Toll receptor 7/8 agonist 3M-007 and interferon-γ: implications for therapy of cutaneous T-cell lymphoma. Leuk Lymphoma. 2011;52:1970–9. doi: 10.3109/10428194.2011.582202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, Bonefeld CM, Wasik MA, Koralov SB, Geisler C, Kilian M, Iversen L, Woetmann A, et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins (Basel) 2013;5:1402–21. doi: 10.3390/toxins5081402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen ML, Woetmann A, Krejsgaard T, Kopp KL, Søkilde R, Litman T, Straten PT, Geisler C, Wasik MA, Odum N, et al. IFN-α primes T- and NK-cells for IL-15-mediated signaling and cytotoxicity. Mol Immunol. 2011;48:2087–93. doi: 10.1016/j.molimm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–23. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]