Abstract
Background
Cholinergic signaling via muscarinic acetylcholine receptors (mAChR) is known to influence various physiological functions. In bone, M3 mAChR and M5 mAChR were identified on the membrane of osteoblast-like cells. M3 mAChR seems to be particularly relevant for bone physiology, as signaling via this receptor was reported to increase bone formation and decrease bone resorption. Thus, in the present study we investigated the relative mRNA expression of M3 and M5 mAChR in bones of a rat osteoporosis model.
Material/Methods
Osteoporosis was induced in Sprague-Dawley rats by bilateral ovariectomy and additional feeding of a diet deficient in calcium, vitamins C, D2, D3, and phosphorus, and free of soy and phytoestrogen. After a period of 3, 12, and 14 months, relative mRNA expression of M3 mAChR and M5 mAChR was analyzed in the 11th thoracic vertebra by real-time RT-PCR.
Results
Relative mRNA expression of M3 mAChR was significantly reduced in bones of osteoporotic rats compared to sham operated animals that served as controls. Further, M3 mAChR mRNA expression was significantly down-regulated when comparing 14-month osteoporotic rats to 3-month osteoporotic rats. Relative M5 mAChR mRNA was expressed to a lesser extent than M3 mAChR and did not show significant differences in mRNA expression level between the experimental groups.
Conclusions
M3 mAChR mRNA expression was reduced upon induction of osteoporosis and progression of disease was associated with further decrease of this receptor, indicating that M3 mAChR is involved in the development and regulation of osteoporosis.
Keywords: Rat, Osteoporosis, Muscarinic Acetylcholine Receptors, Bone, Non-Neuronal Cholinergic System
Background
Bone is innervated by autonomic nerve terminals belonging to both the sympathetic and parasympathetic system [1]. Sympathetic activity leads to increased bone resorption and subsequent bone loss as observed in the pathophysiology of osteoporosis [2]. Osteoporosis is a systemic disease characterized by diminished bone mineral density and changes in bone architecture [3]. Inhibition of sympathetic signaling through the beta-adrenergic system by beta-blockers reduces ovariectomy-induced bone loss [2]. Activation of the parasympathetic nervous system decreases bone resorption and increases bone formation. This results in an accrual of bone mass through release of the parasympathetic neurotransmitter acetylcholine (ACh) and binding to muscarinic acetylcholine receptors (mAChR) of the subtype M3 (M3 mAChR) [4]. In addition to having a neuronal origin, ACh can be secreted by a multiplicity of non-neuronal cells like endothelial cells, immune cells, and mesenchymal cells. [5] It binds in an auto/paracrine manner (like a hormone) on acetylcholine receptors and enhances proliferation, differentiation, and maintenance of cell-cell contacts of many cell types, including osteoblasts [6,7]. M3 mAChR is expressed by osteoblasts [6–8]. Besides M3 mAChR, 4 other subtypes of mAChR were identified (M1–M5 mAChR) [9] and expressed in bone tissue, especially in osteoblasts [6–8]. Upon activation, mAChR couple to G-proteins to regulate ion channel permeability and second messenger signaling pathways [9]. M2 and M4 mAChR bind to Gαi/o and M1, M3, and M5 mAChR to Gαq/11, thereby increasing intracellular calcium, whereas M2 and M4 mAChR have only small effects on calcium signaling [9].
Calcium is the main component of the mineralized bone matrix. Bone mineral content is reduced in human postmenopausal osteoporosis and in ovariectomized rats [10]. It is well known that ovariectomy (ovx) induces the state of postmenopausal osteoporosis [11–17]. One cause of postmenopausal osteoporosis is estrogen deficiency, which increases osteoclast formation and therefore supports bone resorption [18]. Shortage of estrogen also induces apoptosis of osteoblasts [18]. This leads to an imbalance between osteoblastic bone formation and osteoclastic bone resorption, favoring bone loss and resulting in a high risk for fractures [3]. Ovariectomy in rats mimics estrogen deficiency in humans. However, ovariectomy alone does not lead to bone loss [19]. Especially vitamin D and calcium are known to contribute to bone mineralization and lack of these factors decreases bone mass [19–23]. Acetylcholine binding to M3 mAChR results in an elevation of cytosolic calcium concentrations and proliferation of osteoblast-like cells, which were inhibited by the muscarinic receptor antagonist, atropine [7,8]. Mice with a homozygous gene deficient in M3 mAChR showed a significant reduction of bone bending stiffness, relative bone volume, and alterations of the trabecular microstructure [4,24]. Since loss of M3 mAChR leads to a decrease in bone mass, we hypothesized that M3 mAChR is involved in the regulation of bone mass during osteoporosis. A regulating role of M1, M2, and M4 mAChR in bone remodeling is somewhat unlikely because mice that are gene-deficient for these receptors do not show any bone phenotype [4]. Bone of M5 mAChR knockout mice has not been investigated until now. M3 mAChR and M5 mAChR belong together to Gαq/11 coupled proteins that enhance the intracellular calcium content and therefore are an interesting target for investigations. Thus, we analyzed the expression of M3 mAChR and M5 mAChR in vertebra of rats with osteoporosis induced by ovariectomy and a deficient diet using real-time RT-PCR. Our results confirmed the hypothesis that M3 mAChR is involved in osteoporotic bone loss, whereas M5 mAChR does not seem to participate in the regulation of bone remodeling under pathological conditions.
Material and Methods
Animal model
The basis for our study was a well-established animal osteoporosis model developed by Heiss et al. [22]
Sprague-Dawley rats were purchased from Charles River at the age of 10 weeks. Experiments started after an acclimatization period of 4 weeks. Rats were held under standard laboratory conditions. Experiments complied with the German Protection of Animals Act and ethical standards laid down in the 1964 Declaration of Helsinki, and were approved by the regional council of Giessen [22,25,26].
Rats were divided into 2 groups. The first group consisted of sham-operated rats (n=30, sham). The second group underwent ovariectomy and was fed a deficient diet (n=27, ovx+diet). The diet was deficient in calcium, vitamins C, D2, and D3, as well as in phosphorus (C1034, Altromin Spezialfutter GmbH, Lage, Germany). It was soy- and phytoestrogen-free [22,25]. The osteoporotic bone status of rats was monitored with bone mineral density (BMD) measurement [22,25]. After 3, 12, and 14 months, rats were sacrificed and bones dissected.
For our study, thoracic vertebra 11 was manually extracted from the spine with a scalpel and surrounding tissue was completely removed. The dissected body of thoracic vertebra 11 was collected in an Eppendorf tube containing RNA Later and stored at −80°C before detecting M3 mAChR and M5 mAChR expressions by real-time RT-PCR.
Real-time RT-PCR
RNA isolation
RNA was isolated by homogenizing ~100 mg of thoracic vertebra 11 in 1 ml Trizol (Invitrogen, Darmstadt, Germany). After 5 min of incubation at room temperature, 200 μl of chloroform were added. Samples were shaken and incubated for 2–3 min at room temperature and afterwards centrifuged for 15 min and 12000 g at 4°C. Supernatants were collected in a fresh tube and 0.5 ml isopropanol added and incubated for 10 min at room temperature before centrifugation at 4°C and 12000 g for 10 min. Supernatants were discarded, pellets washed in 1 ml of 75% ethanol and then centrifuged at 4°C and 7500 g for 5 min. RNA-pellets were dissolved in RNase-free water and the RNA concentration measured by nanodrop at 230 nm.
Reverse transcription
Using the Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany), 500 ng of RNA were reverse transcribed into cDNA in a cycler (TC-3000, Techne, Bibby Scientific, USA). According to the manufacturer’s protocol, 2 μl of gDNA wipeout buffer were added to samples for 5 min at 42°C to remove genomic DNA. Afterwards, 6 μl of the respective RT Master Mix consisting of 1 μl Quantiscript Reverse Transcriptase, 4 μl Quantiscript RT Buffer, 1 μl RT Primer Mix or 1 μl RNase-free water were added to sample tubes and incubated at 42°C for 30 min. Inactivation of reverse transcriptase was achieved after 3 min at 95°C.
Real-time RT-PCR
Real-time RT-PCR was performed using the Quantifast SYBR Green PCR Kit (Qiagen) in an I-Cycler with an IQTM5 detection system (Bio-Rad, Munich, Germany). One μl cDNA was added to 0.5 μl primer (Table 1), 11 μl of RNase- and DNase-free H2O and 12.5 μl Quantifast SYBR Green PCR Master Mix. The cycling procedure started with heating for 5 min at 95°C, followed by 40 cycles of denaturation for 10 sec at 95°C and a combined step of annealing and elongation for 30 sec at 60°C. Finally, a melting curve was conducted to check purity of the RT-PCR product. Samples that were not reverse transcribed or received water instead of cDNA served as negative controls. They were used in the same runs as the probes and remained blank. For evaluation, delta cycle threshold (CT) values were determined using the reference gene beta-2-microglobulin (B2M).
Table 1.
Rat primers used for real-time RT-PCR.
| Primer | Sequence | Length [bp] | Accession | |
|---|---|---|---|---|
| M3* | for | TGCGCAGACAAGACCACGGC | 142 | NM_012527.1 |
| rev | GCGTCTGGGCGGCCTTCTTC | |||
|
| ||||
| M5* | for | TTGCAGTTGTGACTGCGGTG | 197 | NM_017362.4 |
| rev | GAGAACCCAGCGTCCCATGA | |||
|
| ||||
| B2M | for | TGTCTCAGTTCCACCCACCT | 191 | NM_012512.2 |
| rev | GGGCTCCTTCAGAGTGACG | |||
M3 and M5 subtype of the muscarinic acetylcholine receptor.
Statistical analysis
Statistics was performed with the computer program SPSS (version 21.0; SPSS Institute Inc, Chicago, USA), which was also used for the generation of graphs in Figures 1 and 2. Delta CT values were evaluated with Kruskal-Wallis and Mann-Whitney tests and considered to be significant at p≤0.05.
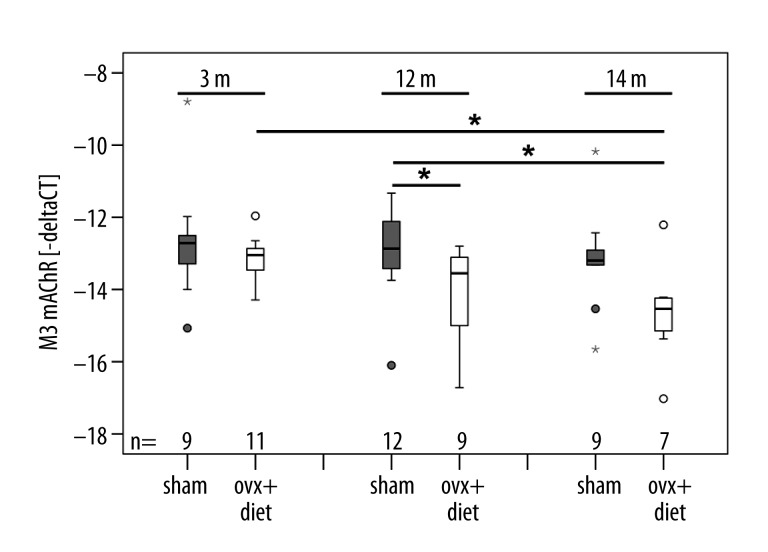
Figure 1.
Expression of muscarinic acetylcholine receptor M3 (M3 mAChR): The x-axis presents the animal groups and the y-axis delta cycle threshold (CT) values shown in minus delta CT values. M3 mAChR mRNA in animals that underwent bilaterally ovariectomy and received a deficient diet (ovx+diet) decreased compared to sham-operated animals (sham). Expression of M3 mAChR decreased with progression of the experiment. m = post-operative months. Box plots present the median of values as a solid line within the box and values that are beyond 3×SD as small stars and circles. Statistical likelihood of p≤0.05 is indicated by 1 star.
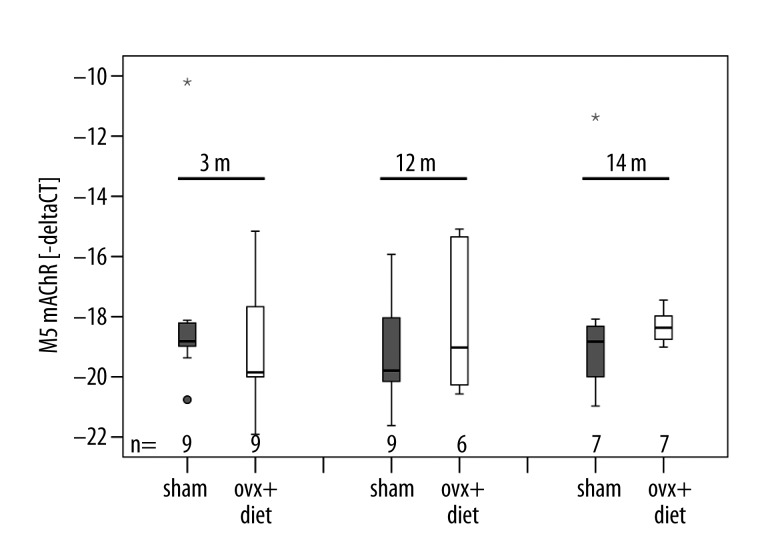
Figure 2.
Expression of muscarinic acetylcholine receptor M5 (M5 mAChR): The x-axis presents the animal groups and the y-axis delta cycle threshold (CT) values shown in minus delta CT values. Expression of M5 mAChR did not differ significantly between sham-operated and ovx+diet animals. m = post-operative months. Box plots present the median of values as a solid line within the box and values that are beyond 3×SD as small stars and circles.
Results
M3 and M5 mAChR were detected in thoracic vertebra 11 of rats. In general, a decrease of M3 mAChR mRNA expression was detectable in thoracic vertebra 11 of rats that were sacrificed at later time points of the investigation (Figure 1). However, M3 mAChR mRNA was generally expressed at higher levels than M5 mAChR mRNA. The mRNA expression of M5 mAChR was barely influenced by progression of the disease, since no overall significant differences in mRNA expression of M5 mAChR within and between the rat groups could be detected (Figure 2).
We found that the ovx+diet group exhibited significantly lower mRNA expressions of M3 mAChR in comparison to the sham group (p=0.014), indicating that the expression of this receptor decreased with severity of osteoporosis. The 3-month sham group showed higher M3 mAChR mRNA expressions than the 3-month ovx+diet group. Rats that underwent ovariectomy and were kept on diet for 3 months (3-month ovx+diet) showed significantly higher M3 mAChR mRNA expressions than the group of ovariectomized rats that received the diet for 14 months (14-month ovx+diet) (p=0.02). M3 mAChR mRNA expression of the 12-month sham group was significantly higher in comparison to 12 (p=0.041) and 14 month ovx+diet groups (p=0.013) (Figure 1).
Results confirming the osteoporotic bone status of rats are shown by Heiss et al. (2012) [22] and Govindarajan et al. (2013) [25]. They demonstrated that the bone mineral density was lower in ovx+diet rats.
Discussion
Our study verified the assumption that M3 mAChR is down-regulated in bone of rats of an osteoporosis model. The decrease in relative M3 mAChR expression correlated with progression of the disease. No impact of M5 mAChR mRNA expression could be determined in bone during osteoporosis. Recently, components of the cholinergic system (e.g., acetylcholine receptors, enzymes, and transporters) were detected in bone [1,7] and it is known that mAChR play an important role in bone physiology [24]. En-Nosse et al. [6] detected M3 and M5 mAChR in osteoblast-like cells and Shi et al. [4] found that M3 mAChR influences bone remodeling. Moreover, it has been shown that M3 mAChR-knockout mice have bone structures similar to systemically diseased bone, as in osteoporosis [24]. Liu et al. [7] concluded that mAChR regulate cell proliferation and induce calcium signaling in bone and thus may be involved in bone remodeling because osteoblast proliferation contributes to this process. It was also shown that mAChR influence bone turnover by increasing osteoblast proliferation [7,8]. Shi et al. [4] demonstrated that M3 mAChR increases bone formation and decreases bone resorption. They reasoned that the parasympathetic system affects bone remodeling by involving the central nervous system and thereby decreases the sympathetic tone of bone [4]. It is well known that sympathetic stimulation of bone leads to increased bone resorption [2]. Hence, osteoporotic bone degradation is reduced through application of beta-blockers [2].
The foundation of our study was a well-established model by Heiss et al. (2012) and Govindarajan et al. (2013) in which osteoporosis was induced in rats by ovariectomy and a diet deficient in calcium and vitamin D. Heiss et al. (2012) and Govindarajan et al. (2013) confirmed the osteoporotic bone status of rats by dual energy X-ray absorptiometry (DEXA) [22,25]. A significant down-regulation in BMD and content was determined for rats that were ovariectomized and received the deficient diet in comparison to the sham group [25]. Apart from the sham group, all rats were osteoporotic, thus demonstrating the basis for our interrogation regarding relative expression of M3 mAChR and M5 mAChR in osteoporotic bone. Although the human situation is more closely represented by large animal osteoporosis models (e.g., sheep, pigs, or goats) because comparable weight ratios and weight-bearing are important for the maintenance of the bone microstructure and mineral density, small animal models offer the advantage of shorter breeding cycles and less expensive animal housing, and thus the possibility to analyze a larger number of animals. Therefore, it was possible to investigate and compare 2 experimental groups: a) sham-operated and b) ovx+diet animal groups at several time points (3, 12, and 14 months). Thereby, a gradual down-regulation was measured for M3 mAChR expression with progression of the experiment and of osteoporosis.
We suppose that the decline in relative M3 mAChR mRNA expression is due to a reduced number of osteoblasts in osteoporosis. It is well-known that M3 mAChR is expressed by osteoblasts [6–8]. Osteoblasts initiate bone mineralization. Since calcium is the main element of the bone matrix, intracellular calcium content of osteoblasts is important for this process. Stimulation of mAChR of the group of Gαq/11-coupled binding proteins is followed by a strong up-regulation of intracellular calcium concentrations [9]. Thus, M3 mAChR might be involved in delayed accumulation of calcium in the mineralized bone matrix in osteoporosis.
Animal models with initial ovariectomy mimic reduction of the estrogen content in postmenopausal women. Estrogens decrease apoptosis of mature osteoblasts and osteocytes [18]. To our best knowledge, the effects of estrogen on mAChR in bone have not been investigated. However, functional studies showed that estrogen treatment increased the responsiveness to M3 mAChR in rat myometrium [27]. In contrast to myometrium, ovariectomy of rats induced an increase in mAChR expression in the hippocampus, which can be prevented with estradiol replacement therapy starting directly after ovariectomy [28]. A delayed start of 15 days had no effect on mAChR expression [28]. Estrogen substitutes showed conducive effects on cholinergic cognitive functions [29]. Thus, there is an organ- and duration-specific connection between estrogen and mAChR, which suggests the hypothesis that loss of estrogen in bone might be followed by a decrease in cholinergic components such as M3 mAChR, which subsequently causes loss of bone mass as shown in M3 mAChR knockout mice [4,24].
Conclusions
In our study, M3 mAChR expression decreased in groups that were ovariectomized and received a diet deficient in calcium, vitamins C, D2, D3, and phosphorus, and that was soy- and phytoestrogen-free. Relative M3 mAChR expression was lower in the ovx+diet group than in the sham group. Since it is known that M3 mAChR is expressed by osteoblasts [6–8], we assume that the decline in M3 mAChR mRNA expression occurs as a result of reduced osteoblast numbers in osteoporosis. We presume that low M3 mAChR expressions indicate bone loss. Decrease of relative M3 mAChR expression correlates with progression of osteoporosis due to estrogen and subsequent osteoblast loss.
Further research is necessary to determine whether the stimulation of M3 mAChR can be used to decrease osteoporotic bone resorption.
Acknowledgements
The authors wish to thank Ivonne Bergen, Ida Oberst, and Annette Stengel for excellent technical support.
Moreover, we thank Dr. Janet Beckmann for correcting the abstract and especially Dr. Edwin L. Cooper (Distinguished Professor at the David Geffen School of Medicine, Department of Neurobiology, University of California Los Angeles, USA) for carefully reading the manuscript and giving useful advice for improving it.
Footnotes
Conflict of interest
None.
Source of support: The study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) SFB/TRR 79 projects B7 and T1
References
- 1.Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proceedings Proc Natl Acad Sci USA. 2012;109(38):15455–60. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togari A, Arai M, Kondo A. The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin Ther Targets. 2005;9(5):931–40. doi: 10.1517/14728222.9.5.931. [DOI] [PubMed] [Google Scholar]
- 3.McNamara LM. Perspective on post-menopausal osteoporosis: establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. J R Soc Interface. 2010;7(44):353–72. doi: 10.1098/rsif.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Oury F, Yadav VK, et al. Signaling through the M(3) muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell Metab. 2010;11(3):231–38. doi: 10.1016/j.cmet.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154(8):1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.En-Nosse M, Hartmann S, Trinkaus K, et al. Expression of non-neuronal cholinergic system in osteoblast-like cells and its involvement in osteogenesis. Cell Tissue Res. 2009;338(2):203–15. doi: 10.1007/s00441-009-0871-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu PS, Chen YY, Feng CK, et al. Muscarinic acetylcholine receptors present in human osteoblast and bone tissue. Eur J Pharmacol. 2011;650(1):34–40. doi: 10.1016/j.ejphar.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Abe T, Chida D, et al. Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS Lett. 2010;584(4):817–24. doi: 10.1016/j.febslet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26(3):219–33. doi: 10.1111/j.1474-8673.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Z, Yao W, Zimmermann EA, et al. Prolonged treatments with antiresorptive agents and PTH have different effects on bone strength and the degree of mineralization in old estrogen-deficient osteoporotic rats. J Bone Miner Res. 2009;24(2):209–20. doi: 10.1359/jbmr.81005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15(3):175–91. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 12.French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis: an assessment of the bone sparing effects of curcumin. Phytomedicine. 2008;15(12):1069–78. doi: 10.1016/j.phymed.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Francisco JI, Yu Y, Oliver RA, Walsh WR. Relationship between age, skeletal site, and time post-ovariectomy on bone mineral and trabecular microarchitecture in rats. J Orthop Res. 2011;29(2):189–96. doi: 10.1002/jor.21217. [DOI] [PubMed] [Google Scholar]
- 14.Lill CA, Gerlach UV, Eckhardt C, et al. Bone changes due to glucocorticoid application in an ovariectomized animal model for fracture treatment in osteoporosis. Osteoporos Int. 2002;13(5):407–14. doi: 10.1007/s001980200047. [DOI] [PubMed] [Google Scholar]
- 15.Omi N, Ezawa I. The effect of ovariectomy on bone metabolism in rats. Bone. 1995;17(4 Suppl):163S–68S. doi: 10.1016/8756-3282(95)00329-c. [DOI] [PubMed] [Google Scholar]
- 16.Kalu DN, Chen C. Ovariectomized murine model of postmenopausal calcium malabsorption. J Bone Miner Res. 1999;14(4):593–601. doi: 10.1359/jbmr.1999.14.4.593. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Nishida A, Nakamura T, et al. Differences of three-dimensional trabecular microstructure in osteopenic rat models caused by ovariectomy and neurectomy. Bone. 2002;30(4):594–98. doi: 10.1016/s8756-3282(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 18.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 19.Melhus G, Solberg LB, Dimmen S, et al. Experimental osteoporosis induced by ovariectomy and vitamin D deficiency does not markedly affect fracture healing in rats. Acta Orthop. 2007;78(3):393–403. doi: 10.1080/17453670710013988. [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Weinerman S. Osteoporosis and gastrointestinal disease. Gastroenterol Hepatol (NY) 2010;6(8):506–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen NR, Schwarz P. Osteoporosis in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2008;14(2):122–27. doi: 10.1097/MCP.0b013e3282f4efb6. [DOI] [PubMed] [Google Scholar]
- 22.Heiss C, Govindarajan P, Schlewitz G, et al. Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by DEXA. Med Sci Monit. 2012;18(6):BR199–207. doi: 10.12659/MSM.882895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlewitz G, Govindarajan P, Schliefke N, et al. Ovariectomy and calcium/vitamin D2/D3 deficient diet as a model of osteoporosis in the spine of Sprague-Dawley Rats. Z Orthop Unfall. 2013;151(1):14–19. doi: 10.1055/s-0032-1327976. [DOI] [PubMed] [Google Scholar]
- 24.Kliemann K, Kneffel M, Bergen I, et al. Quantitative analyses of bone composition in acetylcholine receptor M3R and alpha7 knockout mice. Life Sci. 2012;91(21–22):997–1002. doi: 10.1016/j.lfs.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Govindarajan P, Schlewitz G, Schliefke N, et al. Implications of combined ovariectomy/multi-deficiency diet on rat bone with age-related variation in bone parameters and bone loss at multiple skeletal sites by DEXA. Med Sci Monit Basic Res. 2013;19:76–86. doi: 10.12659/MSMBR.883815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindarajan P, Khassawna T, Kampschulte M, et al. Implications of combined ovariectomy and glucocorticoid (dexamethasone) treatment on mineral, microarchitectural, biomechanical and matrix properties of rat bone. Int J Exp Pathol. 2013;94(6):387–98. doi: 10.1111/iep.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdalla FM, Marostica E, Picarelli ZP, et al. Effect of estrogen on muscarinic acetylcholine receptor expression in rat myometrium. Mol Cell Endocrinol. 2004;213(2):139–48. doi: 10.1016/j.mce.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso CC, Ricardo VP, Frussa-Filho R, et al. Effects of 17ss-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor alpha in rat hippocampus. Eur J Pharmacol. 2010;634(1–3):192–200. doi: 10.1016/j.ejphar.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs RB. Oestrogen and the cholinergic hypothesis: implications for oestrogen replacement therapy in postmenopausal women. Novartis Found Symp. 2000;230:94–107. doi: 10.1002/0470870818.ch8. discussion 107–11. [DOI] [PubMed] [Google Scholar]