Abstract
Background:
Induction of labour is common, and cesarean delivery is regarded as its major complication. We conducted a systematic review and meta-analysis to investigate whether the risk of cesarean delivery is higher or lower following labour induction compared with expectant management.
Methods:
We searched 6 electronic databases for relevant articles published through April 2012 to identify randomized controlled trials (RCTs) in which labour induction was compared with placebo or expectant management among women with a viable singleton pregnancy. We assessed risk of bias and obtained data on rates of cesarean delivery. We used regression analysis techniques to explore the effect of patient characteristics, induction methods and study quality on risk of cesarean delivery.
Results:
We identified 157 eligible RCTs (n = 31 085). Overall, the risk of cesarean delivery was 12% lower with labour induction than with expectant management (pooled relative risk [RR] 0.88, 95% confidence interval [CI] 0.84–0.93; I2 = 0%). The effect was significant in term and post-term gestations but not in preterm gestations. Meta-regression analysis showed that initial cervical score, indication for induction and method of induction did not alter the main result. There was a reduced risk of fetal death (RR 0.50, 95% CI 0.25–0.99; I2 = 0%) and admission to a neonatal intensive care unit (RR 0.86, 95% CI 0.79–0.94), and no impact on maternal death (RR 1.00, 95% CI 0.10–9.57; I2 = 0%) with labour induction.
Interpretation:
The risk of cesarean delivery was lower among women whose labour was induced than among those managed expectantly in term and post-term gestations. There were benefits for the fetus and no increased risk of maternal death.
Labour is induced in 1 of 5 births1,2 for maternal reasons (e.g., preeclampsia, cardiac or renal disease), fetal reasons (e.g., intrauterine growth restriction) or a combination (e.g., poorly controlled diabetes, preterm rupture of the membranes or post-term pregnancy).3 Induction of labour artificially ripens the cervix and initiates uterine contractions in women who are not already in labour, leading to progressive dilation of the cervix to achieve vaginal birth of a baby at any gestation beyond the legal definition of fetal viability.4
Although induction of labour has been criticized for an associated increased risk of cesarean delivery, recent studies have shown that there are fewer cesarean deliveries with induction than without it. However, the findings have not had much impact on practice, in part because the systematic reviews5–8 investigated subsets of induction and included few randomized controlled trials (RCTs), and because observational data in a cohort study9 had risk of confounding. Consumer organizations,10 guidelines11 and textbooks12,13 have given contradictory information about cesarean risk, which can lead to confusion over decision-making, particularly given a desire to support normal birth in the face of increasing cesarean rates worldwide. Cesarean delivery carries multiple risks to mother and baby, including maternal death,14 infection and postnatal depression,15,16 and respiratory distress syndrome in neonates.14 Accurate, precise information about cesarean risk is therefore needed for decision-making regarding labour induction.
We conducted a systematic review and meta-analysis of RCTs to investigate the risk of cesarean delivery associated with labour induction compared with expectant management. We also explored the effects of clinical characteristics and study quality on the overall result using subgroup and meta-regression analyses.
Methods
Data sources and study selection
In April 2012, we searched the electronic databases MEDLINE, Embase, CAB Abstracts, CENTRAL (the Cochrane Central Register of Controlled Trials) and Science Citation Index (accessed via Web of Science) for eligible RCTs using the search terms and word variants for the concept “labour induction” and “cesarean delivery.” The search strategy is outlined in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1). The Cochrane Database of Systematic Reviews was also searched for primary trials. Titles and abstracts of all citations were assessed for inclusion by 2 of 3 reviewers (C.M., E.R. or E.M.). Disagreements were resolved through consensus with input from the third reviewer.
We included RCTs if they recruited pregnant women carrying a viable fetus, they compared an active induction with placebo or expectant management (hereafter referred to as expectant management), and they reported rates of cesarean delivery as one of the outcomes. Methods of active intervention included mechanical methods (amniotomy, membrane sweeping, Foley catheter insertion with or without extra-amniotic saline infusion), use of pharmacologic agents (prostaglandins, oxytocin, corticosteroids, mifepristone, estrogens, relaxin, misoprostol, isosorbide mononitrate) and alternative methods (acupuncture, breast stimulation, sexual intercourse, homeopathic preparations, castor oil, bath, enema). We excluded RCTs that recruited women who had a multiple pregnancy or were in active labour, those in which an active intervention was applied in the control group or within 12 hours after randomization and trials in which the control group comprised women in spontaneous labour. No restrictions were applied to year, language or publication status.
Data extraction and quality assessment
Two of us (E.R. and E.M.) independently extracted the following data from the trials: method of induction, reason for induction, and number of cesarean and noncesarean (vaginal or instrumental) deliveries in the intervention and control groups, and information required to assess methodologic quality. Many of the efficacy or safety studies did not provide reasons for labour induction. If primary trials were inaccessible through online and library databases, we extracted information from the Cochrane Database of Systematic Reviews and contacted the authors of the trial to confirm the results.
For the assessment of methodologic quality, we evaluated the following parameters for risk of bias: sequence generation, allocation concealment, blinding of participants and personnel, and management of incomplete output data.17 We did not assess blinding of participants and personnel in trials that used mechanical interventions because it cannot be applied in such situations.
Disagreements in grouping, coding or quality assessment were resolved through consensus with input from a third author (C.M. or K.S.K.).
Data synthesis
We assessed heterogeneity between trials by inspecting forest plots and using the I2 statistic (I2 values of 25%, 50% and 75% corresponded to cut-off points of low, moderate and high degrees of heterogeneity, respectively).18 For the primary and subgroup analyses, we calculated pooled relative risk (RR) estimates and 95% confidence intervals (CIs) using random-effects models, with each study weighted according to inverse of variance. In a number of trials, some participants were lost to follow-up or were excluded from analyses for other reasons; we therefore used intention-to-treat denominators in the meta-analyses. In the subgroup meta-analyses, we investigated whether risk of cesarean delivery differed according to indication for labour induction, induction method, gestational age (term, preterm or post-term), definition of induction (cervical priming, induction of uterine contractions or both), cervical status (unfavourable or favourable), pregnancy risk (high or low) and parity (nulliparous or parous).
We used meta-regression analysis to explore whether patient characteristics (indication for induction, gestational age at induction, cervical score, pregnancy risk, parity), induction methods (type of intervention and definition of induction [cervical priming v. no cervical priming]) and study quality (random sequence generation, allocation concealment, performance bias and attrition bias) explained the heterogeneity. We also evaluated safety (in terms of maternal and fetal deaths, and admission to neonatal intensive care unit) and methods of induction currently used in clinical practice in the United Kingdom, Canada and the United States.1,2,19 We used the Harbord modified test for small-study effects to examine publication bias in funnel plots.
We used Review Manager software (RevMan version 5.2; Nordic Cochrane Centre, Cochrane Collaboration, 2012) to conduct the meta-analyses and Stata software (Stata Statistical Software, release 11; StataCorp; 2009) to perform the meta-regression analyses.
Results
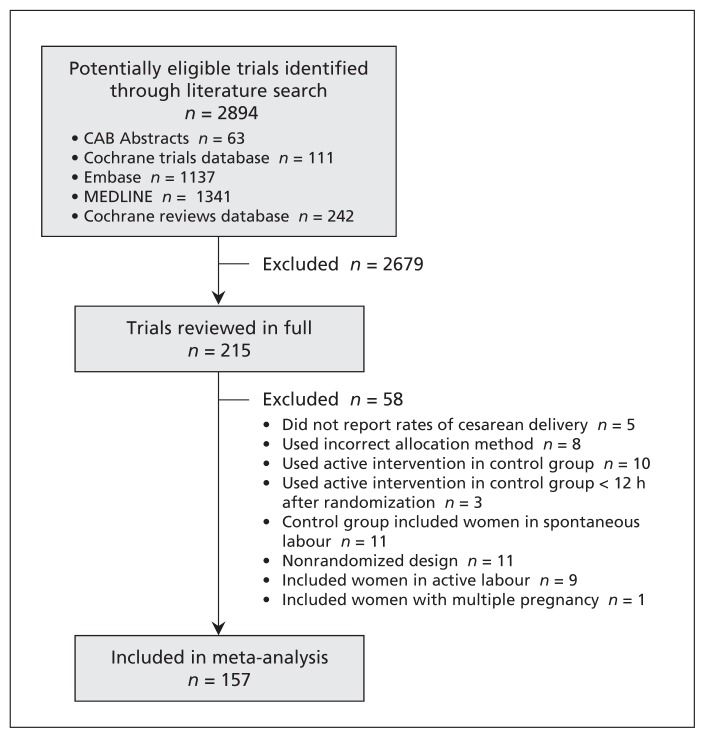
We identified 2894 potentially relevant studies through the literature search. The selection of trials for our meta-analysis is summarized in Figure 1. After the screening of titles and abstracts and removal of duplicate records, 215 articles were reviewed in full. We excluded 58 because they did not report rates of cesarean delivery (5 trials), the active intervention was applied in the control group (10) or within 12 hours after randomization (3), an incorrect allocation method was used (8), the control group included women in spontaneous labour (11), the study had a nonrandomized design (11), women in active labour were included at the time of randomization (9), or the trial included women with a multiple pregnancy (1). The excluded studies, with reason for exclusion, are listed in Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1).
Figure 1:
Selection of randomized controlled trials for the meta-analysis.
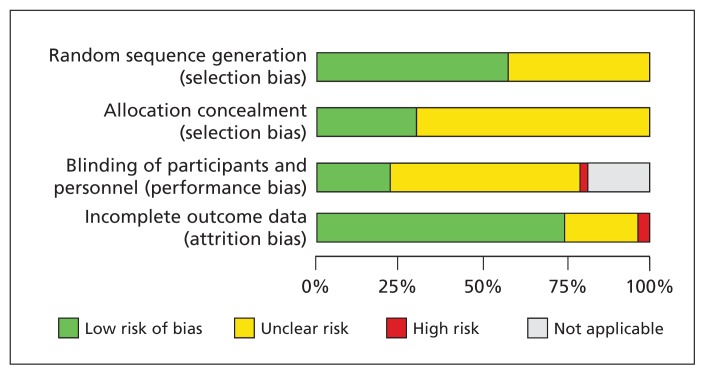
Characteristics of the 157 RCTs (n = 31 085) included in the meta-analysis20–176 are summarized in Appendix 3 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1). Despite extensive searches, we were unable to retrieve the reports of 4 trials; we extracted available clinical results for the trials from the Cochrane Database of Systematic Reviews107,124,125,146 but did not have access to data about the study design to assess their methodologic quality. The results of the quality assessment for the other RCTs are summarized in Figure 2 and shown for each trial in Appendix 4 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1).
Figure 2:
Risk-of-bias assessment of trials included in the meta-analysis. Results for individual trials are shown in Appendix 4 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1).
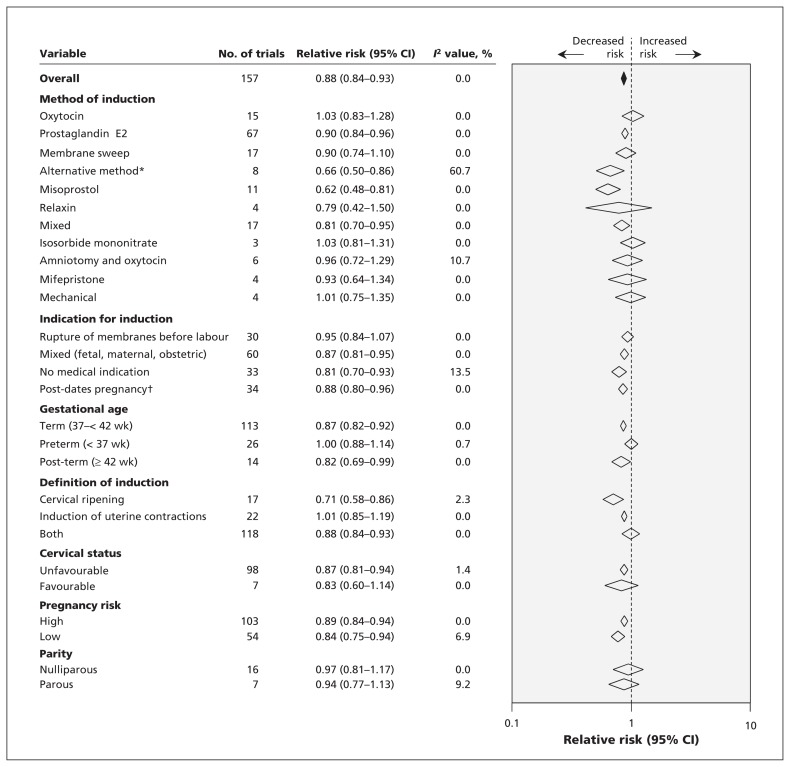
The summary results of the primary and subgroup meta-analyses are shown in Figure 3. Results for the individual trials are shown in Appendix 5 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1). Overall, the risk of cesarean delivery was lower with labour induction than with expectant management (pooled RR 0.88, 95% CI 0.84–0.93). Statistical heterogeneity was low (I2 = 0%). Nevertheless, planned meta-regression analysis to explain heterogeneity did not show a significant impact of variables on the main result (Appendix 6, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1). In particular, initial cervical score (p = 0.4), indication for induction (p = 0.8) and method of induction (p = 0.6) did not alter the main result. The test for publication bias was not significant (p = 0.5) (Appendix 7, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130925/-/DC1).
Figure 3:
Overall and subgroup analyses investigating the effect of induction of labour versus expectant management on the risk of cesarean delivery. Values less than 1 indicate a decreased risk of cesarean delivery. *Acupuncture, breast stimulation, sexual intercourse, homeopathic preparations, castor oil, bath or enema. †Gestation > 40 wk. CI = confidence interval.
In the subgroup analysis by method of induction (Figure 3), 4 methods were associated with a significant reduction in risk of cesarean delivery: prostaglandin E2 (RR 0.90, 95% CI 0.84–0.96; I2 = 0%), misoprostol (RR 0.62, 95% CI 0.48–0.81; I2 = 0%), alternative method (RR 0.66, 95% CI 0.50–0.86; I2 = 60.7%) and mixed method (RR 0.81, 95% CI 0.70–0.95; I2 = 0%). Subgroup analysis by indication for induction showed a universal reduction in risk of cesarean delivery. Induction without a medical indication provided was associated with risk reduction of 19% (RR 0.81, 95% CI 0.70–0.93; I2 = 13.5%). When we looked at risk of cesarean delivery by gestational age, we found statistically significant reductions in risk with labour induction in term and post-term pregnancies, but not in preterm pregnancies. In the analysis by definition of induction, risk of cesarean delivery was significantly lower when the definition included cervical ripening alone or combined with stimulation of uterine contractions than when it included uterine stimulation alone. The analysis by cervical status showed a 13% reduction in risk of cesarean delivery if the cervix was unfavourable at induction (RR 0.87, 95% CI 0.81–0.94; I2 = 1.4%) and no difference in risk if the cervix was favourable (RR 0.83, 95% CI 0.60–1.14; I2 = 0%). The risk of cesarean delivery was reduced in both high- and low-risk pregnancies.
Results from studies with low risk of bias related to allocation concealment were compatible with the main finding (RR 0.89, 95% CI 0.80–0.99, I2 = 9%). Exclusion of induction methods not currently used in clinical practice in the UK, Canada and the US (relaxin, isosorbide mononitrate, mifepristone, corticosteroids and alternative methods described earlier in the methods) showed an effect comparable to the main result (RR 0.89, 95% CI 0.84–0.93; I2 = 0%).
Analysis of adverse outcomes showed a lower risk of fetal death and admission to neonatal intensive care unit associated with labour induction than with expectant management (Table 1). No impact on maternal death was shown.
Table 1:
Risk of adverse outcomes associated with labour induction versus expectant management
| Outcome | Relative risk (95% CI) | I2 value, % | No. of trials |
|---|---|---|---|
| Fetal death | 0.50 (0.25–0.99) | 0 | 60 |
| Admission to NICU | 0.86 (0.79–0.94) | 0 | 55 |
| Maternal death | 1.00 (0.10–9.57) | 0 | 20 |
Note: CI = confidence interval, NICU = neonatal intensive care unit.
Interpretation
Our meta-analysis showed that the risk of cesarean delivery following labour induction was significantly lower than the risk associated with expectant management. This finding supports evidence from systematic reviews5–8 but is contrary to prevalent beliefs and information from consumer organizations, guidelines and textbooks.10–13 Labour induction was associated with benefits for the fetus and no increased risk of maternal death.
Subgroup analysis by method of induction showed a significant reduction in risk of cesarean delivery associated with the use of prostaglandin E analogues (prostaglandin E2 and misoprostol). This is encouraging, since use of prostaglandin E2 is the main method of induction in the UK and the main method of cervical ripening in the US and Canada.1,2,19 However, use of oxytocin and amniotomy, still widely practised induction methods, did not confer a benefit on cesarean delivery. In the subgroup analysis by indication for induction, mixed reason (maternal, fetal and obstetric) and post-dates pregnancy were associated with a reduced risk of cesarean delivery. In uncomplicated term pregnancies with no medical reason for induction provided, the risk of cesarean delivery was reduced by 19% on average. We also observed risk reductions associated with induction in term and post-term pregnancies and in high- and low-risk pregnancies. Our findings are important when selecting candidates for labour induction and when advising women on the risks of induction.
Limitations
Our study has limitations. We could not retrieve and verify 4 primary studies and therefore had to use numeric data obtained from the Cochrane Database of Systematic Reviews. However, the reliance on these data likely did not have much effect on the results because our analysis including the remaining 153 studies was sufficiently powerful for reliable inferences. When we updated our searches in February 2014, we found 336 new citations published since April 2012. Of these, only 2 trials177,178 met our inclusion criteria. The number of participants in the trials was 636, which represented 2% of the total in our current analysis. Both studies concluded that there was no effect on cesarean delivery rates with relative imprecision, so our main conclusion remains robust.
We restricted our analyses to RCTs with appropriate sequence generation, minimizing the risk of bias and confounding. Concealment of allocation was reported unclearly in half of the studies, risking bias in both the clinical management of the women and the assessment of outcomes. However, studies with a low risk of bias in allocation concealment supported the main finding.
We explored many potential confounding factors but did not account for all. For example, body mass index and maternal age may be associated with increased rates of complication. We could not always disentangle reasons for induction and had to lump together maternal, fetal and obstetric indications, leading to some difficulty in interpretation.
We included studies dating from 1975 to 2010; therefore, differences in practice over this time period may have influenced the findings. These issues would be best addressed in a meta-analysis of individual patient data.
Although 2 of us abstracted the study data independently, errors in coding may have occurred and thus are a potential source of bias. This is especially true for the definition of induction and assessment of pregnancy risk, because these parameters were not always defined in detail in the primary studies and had to be judged.
Conclusion
Our meta-analysis has provided a robust answer to the disputed question of risk of cesarean delivery associated with induction of labour. Women whose labour was induced were less likely than those managed expectantly to have a cesarean delivery. In addition, the risk of fetal death and admission to neonatal intensive care unit were decreased in the induction group. Our findings have implications for guidelines and the practice of obstetrics, and are reassuring for mothers, midwifes and obstetricians.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Ekaterina Mishanina conducted the Cochrane database searches, made inclusion and exclusion decisions, extracted data, performed methodologic quality assessment and wrote some of the manuscript. Ewelina Rogozinska conducted the literature search, extracted data, performed methodologic quality assessment, contributed to the analysis and contributed to the writing of the manuscript. Tej Thatthi helped with this project as part of his placement from a medical school in Kenya. He obtained journal articles, helped with inclusion and exclusion decisions and contributed to the writing of the manuscript. Rehan Uddin-Khan contributed to the interpretation of data and to the writing of the manuscript. Khalid Khan conceived the idea of the meta-analysis, provided the overall direction of the project, conducted the meta-regression analysis and revised the manuscript. Catherine Meads supervised Ekaterina Mishanina, Ewelina Rogozinska and Tej Thatthi, conducted data extraction and contributed to the writing of the manuscript. All of the authors approved the final version of the manuscript submitted for publication and agreed to act as guarantors of the work.
References
- 1.ACOG Committee on Practice Bulletins — Obstetrics. ACOG Practice Bulletin no 107: induction of labor. Obstet Gynecol 2009; 114:386–97 [DOI] [PubMed] [Google Scholar]
- 2.Induction of labour [clinical guideline 70]. London (UK): National Institute for Health and Clinical Excellence; 2008 [Google Scholar]
- 3.Liu DTY, Fairweather DVI. Labour ward manual. Philadelphia (PA): Elsevier; 2007 [Google Scholar]
- 4.Induction of labour: evidence-based clinical guideline number 9. London (UK): Royal College of Obstetricians and Gynaecologists Press; 2001 [Google Scholar]
- 5.Caughey AB, Sundaram V, Kaimal AJ, et al. Systematic review: elective induction of labor versus expectant management of pregnancy. Ann Intern Med 2009;151:252–63, W53–63 [DOI] [PubMed] [Google Scholar]
- 6.Gülmezoglu AM, Crowther CA, Middleton P, et al. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2012;(6):CD004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennerholm UB, Hagberg H, Brorsson B, et al. Induction of labor versus expectant management for post-date pregnancy: Is there sufficient evidence for a change in clinical practice? Acta Obstet Gynecol Scand 2009;88:6–17 [DOI] [PubMed] [Google Scholar]
- 8.Wood S, Cooper S, Ross S. Does induction of labour increase the risk of caesarean section? A systematic review and meta-analysis of trials in women with intact membranes. BJOG 2014;121:674–85 [DOI] [PubMed] [Google Scholar]
- 9.Stock SJ, Ferguson E, Duffy A, et al. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ 2012;344:e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willacy H. Labour — active management and induction. Patient.co.uk; 2009. Available: www.patient.co.uk/doctor/Labour-Active-Management-and-Induction.htm (accessed 2013 Feb. 6). [Google Scholar]
- 11.Caesarean section [clinical guideline 132]. London (UK): National Institute for Health and Clinical Excellence; 2004 [PubMed] [Google Scholar]
- 12.Warren R, Arulkumaran S. Best practice in labour and delivery. Cambridge (UK): Cambridge University Press; 2009 [Google Scholar]
- 13.Murray M, Huelsmann G. Labor and delivery nursing: a guide to evidence-based practice. New York (NY): Springer; 2009 [Google Scholar]
- 14.Field MJ, Lohr KN, editors; Committee on Clinical Practice Guidelines, Division of Health Care Services, Institute of Medicine. Guidelines for clinical practice: from development to use. Washington (DC): National Academy Press; 1992 [Google Scholar]
- 15.Edwards DR, Porter SA, Stein GS. A pilot study of postnatal depression following cesarean delivery using two retrospective self-rating instruments. J Psychosom Res 1994;38:111–7 [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb SE, Barrett DE. Effects of unanticipated cesarean section on mothers, infants, and their interaction in the first month of life. J Dev Behav Pediatr 1986;7:180–5 [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.0.2. London (UK): Cochrane Collaboration; 2009. Available: www.cochrane-handbook.org (accessed 2013 Jan. 22). [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane J. Clinical practice guidelines: induction of labour at term. Ottawa (ON): The Society of Obstetricians and Gynaecologists of Canada; 2001:1–12 [Google Scholar]
- 20.Akyol D, Mungan T, Unsal A, et al. Prelabour rupture of the membranes at term — no advantage of delaying induction for 24 hours. Aust N Z J Obstet Gynaecol 1999;39:291–5 [DOI] [PubMed] [Google Scholar]
- 21.Al-Malt A, Ashmead G, Amini S. Cervical ripening: effect of vaginal PGE2 on bishop score. Am J Obstet Gynecol 1995;172:297 [Google Scholar]
- 22.Alcalay M, Hourvitz A, Reichman B, et al. Prelabour rupture of membranes at term: early induction of labour versus expectant management. Eur J Obstet Gynecol Reprod Biol 1996;70:129–33 [DOI] [PubMed] [Google Scholar]
- 23.Alcoseba-Lim, Famador-Juario H. Stripping of the membranes to induce labor at term. Philipp J Surg Spec 1992;47:139–42 [Google Scholar]
- 24.Allott HA, Palmer CR. Sweeping the membranes: a valid procedure in stimulating the onset of labour? Br J Obstet Gynaecol 1993;100:898–903 [DOI] [PubMed] [Google Scholar]
- 25.Asher GN, Coeytaux RR, Chen W, et al. Acupuncture to initiate labor (Acumoms 2): a randomized, sham-controlled clinical trial. Matern Fetal Neonatal Med 2009;22:843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augensen K, Bergsjo P, Eikeland T, et al. Randomised comparison of early versus late induction of labour in post-term pregnancy. BMJ 1987;294:1192–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beigi A, Kabiri M, Zarrinkoub F. Cervical ripening with oral misoprostol at term. Int J Gynaecol Obstet 2003;83:251–5 [DOI] [PubMed] [Google Scholar]
- 28.Bell RJ, Permezel M, MacLennan A, et al. A randomized, double-blind, placebo-controlled trial of the safety of vaginal recombinant human relaxin for cervical ripening. Obstet Gynecol 1993;82:328–33 [PubMed] [Google Scholar]
- 29.Benito Reyes V, Hurtado Medoza R, Rodríguez Rodrígueza F, et al. Elective termination versus expectant management in prolonged pregnancy: a prospective study of 200 pregnant women [article in Spanish]. Progresos de Obstetricia y Ginecología 2010; 53:446–53 [Google Scholar]
- 30.Berghella AM, Pellegrini P, Piancatelli D, et al. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunol Immunother 1994;38:160–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergsjø P, Huang GD, Yu SQ, et al. Comparison of induced versus non-induced labor in post-term pregnancy. A randomized prospective study. Acta Obstet Gynecol Scand 1989;68:683–7 [DOI] [PubMed] [Google Scholar]
- 32.Bernstein P. Prostaglandin E2 gel for cervical ripening and labour induction: a multicentre placebo-controlled trial. [published erratum in CMAJ 1992;146:1290]. CMAJ 1991;145:1249–54 [PMC free article] [PubMed] [Google Scholar]
- 33.Boers KE, Vijgen SM, Bijlenga D, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ 2010;341:c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollapragada S, Mackenzie F, Norrie J, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour — clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy Childbirth 2006;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulvain M, Fraser WD, Marcoux S, et al. Does sweeping of the membranes reduce the need for formal induction of labour? A randomised controlled trial. Br J Obstet Gynaecol 1998;105:34–40 [DOI] [PubMed] [Google Scholar]
- 36.Bréart G, Goujard J, Maillard F, et al. Comparison of 2 obstetrical attitudes vis-a-vis inducing labor at term. Randomized study [article in French]. J Gynecol Obstet Biol Reprod (Paris) 1982;11: 107–12 [PubMed] [Google Scholar]
- 37.Brennand JE, Calder AA, Leitch CR, et al. Recombinant human relaxin as a cervical ripening agent. Br J Obstet Gynaecol 1997; 104:775–80 [DOI] [PubMed] [Google Scholar]
- 38.Buchanan D, Macer J, Yonekura ML. Cervical ripening with prostaglandin E2 vaginal suppositories. Obstet Gynecol 1984; 63:659–63 [PubMed] [Google Scholar]
- 39.Bullarbo M, Orrskog ME, Andersch B, et al. Outpatient vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening and labor induction postterm: a randomized controlled study. A J Obstet Gynecol 2007;196:50 e1–5 [DOI] [PubMed] [Google Scholar]
- 40.Buttino LT, Jr, Garite TJ. Intracervical prostaglandin in postdate pregnancy. A randomized trial. J Reprod Med 1990;35:155–8 [PubMed] [Google Scholar]
- 41.Cammu H, Van Eeckhout E. A randomised controlled trial of early versus delayed use of amniotomy and oxytocin infusion in nulliparous labour. Br J Obstet Gynaecol 1996;103:313–8 [DOI] [PubMed] [Google Scholar]
- 42.Campbell JM. Induction of labour using prostaglandin E2 pessaries. Clin Exp Obstet Gynecol 1984;11:1–5 [PubMed] [Google Scholar]
- 43.Chakravarti S, Goenka B. Conservative policy of induction of labor in uncomplicated postdated pregnancies. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3–8; Washington (DC) [Google Scholar]
- 44.Chang P, Langer O. Premature rupture of membranes at term: a randomized controlled trial. Am J Obstet Gynecol 1997;176: S148 [Google Scholar]
- 45.Chanrachakul B, Herabutya Y. Postterm with favorable cervix: Is induction necessary? Eur J Obstet Gynecol Reprod Biol 2003; 106:154–7 [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee MS, Ramchandran K, Ferlita K, et al. Prostaglandin E2 (PGE2) vaginal gel for cervical ripening. Eur J Obstet Gynecol Reprod Biol 1991;38:197–202 [DOI] [PubMed] [Google Scholar]
- 47.Cheung PC, Yeo EL, Wong KS, et al. Oral misoprostol for induction of labor in prelabor rupture of membranes (PROM) at term: a randomized control trial. Acta Obstet Gynecol Scand 2006;85:1128–33 [DOI] [PubMed] [Google Scholar]
- 48.Chua S, Arulkumaran S, Yap C, et al. Premature rupture of membranes in nulliparas at term with unfavorable cervices: a double-blind randomized trial of prostaglandin and placebo. Obstet Gynecol 1995;86:550–4 [DOI] [PubMed] [Google Scholar]
- 49.Chung T, Rogers MS, Gordon H, et al. Prelabour rupture of the membranes at term and unfavourable cervix; a randomized placebo-controlled trial on early intervention with intravaginal prostaglandin E2 gel. Aust N Z J Obstet Gynaecol 1992;32:25–7 [DOI] [PubMed] [Google Scholar]
- 50.Cole RA, Howie PW, Macnaughton MC. Elective induction of labour. A randomised prospective trial. Lancet 1975;1:767–70 [DOI] [PubMed] [Google Scholar]
- 51.Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30–34 weeks’ gestation. Obstet Gynecol 1995;86:875–9 [DOI] [PubMed] [Google Scholar]
- 52.Crane J, Bennett K, Young D, et al. The effectiveness of sweeping membranes at term: a randomized trial. Obstet Gynecol 1997;89:586–90 [DOI] [PubMed] [Google Scholar]
- 53.Curet LB, Gauger LJ. Cervical ripening with intravaginal prostaglandin E2 gel. Int J Gynaecol Obstet 1989;28:221–8 [DOI] [PubMed] [Google Scholar]
- 54.Dare FO, Oboro VO. The role of membrane stripping in prevention of post-term pregnancy: a randomised clinical trial in Ile-lfe, Nigeria. J Obstet Gynaecol 2002;22:283–6 [DOI] [PubMed] [Google Scholar]
- 55.Darroca RJ, Buttino L, Jr, Miller J, et al. Prostaglandin E2 gel for cervical ripening in patients with an indication for delivery. Obstet Gynecol 1996;87:228–30 [DOI] [PubMed] [Google Scholar]
- 56.Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin E2 gel and membrane stripping. J Matern Fetal Med 1997;6:71–8 [DOI] [PubMed] [Google Scholar]
- 57.Dommisse J, Davey DA, Allerton G. The induction of labour with prostaglandin E2 tablets administered intravaginally. S Afr Med J 1980;58:518–9 [PubMed] [Google Scholar]
- 58.Dyson DC, Miller PD, Armstrong MA. Management of prolonged pregnancy: induction of labor versus antepartum fetal testing. Am J Obstet Gynecol 1987;156:928–34 [DOI] [PubMed] [Google Scholar]
- 59.Egarter C, Kofler E, Fitz R, et al. Is induction of labor indicated in prolonged pregnancy? Results of a prospective randomised trial. Gynecol Obstet Invest 1989;27:6–9 [DOI] [PubMed] [Google Scholar]
- 60.Ek S, Andersson A, Johansson A, et al. Oligohydramnios in uncomplicated pregnancies beyond 40 completed weeks. A prospective, randomised, pilot study on maternal and neonatal outcomes. Fetal Diagn Ther 2005;20:182–5 [DOI] [PubMed] [Google Scholar]
- 61.Elliott JP, Flaherty JF. The use of breast stimulation to prevent postdate pregnancy. Am J Obstet Gynecol 1984;149:628–32 [DOI] [PubMed] [Google Scholar]
- 62.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol 1998;92:804–9 [DOI] [PubMed] [Google Scholar]
- 63.el-Torkey M, Grant JM. Sweeping of the membranes is an effective method of induction of labour in prolonged pregnancy: a report of a randomized trial. Br J Obstet Gynaecol 1992;99:455–8 [DOI] [PubMed] [Google Scholar]
- 64.Fenton DW, Speedie J, Duncan SL. Does cervical ripening with PGE2 affect subsequent uterine activity in labour? Acta Obstet Gynecol Scand 1985;64:27–30 [DOI] [PubMed] [Google Scholar]
- 65.Fletcher HM, Mitchell S, Simeon D, et al. Intravaginal misoprostol as a cervical ripening agent. Br J Obstet Gynaecol 1993;100:641–4 [DOI] [PubMed] [Google Scholar]
- 66.Frydman R, Lelaidier C, Baton-Saint-Mleux C, et al. Labor induction in women at term with mifepristone (RU 486): a double blind, randomized, placebocontrolled study. Obstet Gynecol 1992;80:972–5 [PubMed] [Google Scholar]
- 67.Giacalone PL, Targosz V, Laffargue F, et al. Cervical ripening with mifepristone before labor induction: a randomized study. Obstet Gynecol 1998;92:487–92 [DOI] [PubMed] [Google Scholar]
- 68.Gilad R, Hochner H, Vinograd O, et al. The CIC Trial — castor oil for induction of contractions in post-term pregnancies Am J Obstet Gynaecol 2012;S77–8 [Google Scholar]
- 69.Gilson GJ, Izquierdo LA, Chatterjee MS, et al. Prevention of cesarean section. Does intracervical dinoprostone work? West J Med 1993;159:149–52 [PMC free article] [PubMed] [Google Scholar]
- 70.Gilson GJ, Russell DJ, Izquierdo LA, et al. A prospective randomized evaluation of a hygroscopic cervical dilator, Dilapan, in the preinduction ripening of patients undergoing induction of labor. Am J Obstet Gynecol 1996;175:145–9 [DOI] [PubMed] [Google Scholar]
- 71.Gittens L, Schenkel L, Strassberg S, et al. Vaginal birth after cesarean section: comparison of outpatient use of prostaglandin gel to expectant management. Am J Obstet Gynecol 1996;174(1 pt 2):354 [Google Scholar]
- 72.Golbus MS, Creasy RK. Uterine priming with oral prostaglandin E2 prior to elective induction with oxytocin. Prostaglandins 1977;14:577–81 [DOI] [PubMed] [Google Scholar]
- 73.Goldenberg M, Dulitzky M, Feldman B, et al. Stretching of the cervix and stripping of the membranes at term: a randomised controlled study. Eur J Obstet Gynecol Reprod Biol 1996;66: 129–32 [DOI] [PubMed] [Google Scholar]
- 74.Gonen O, Rosen DJ, Dolfin Z, et al. Induction of labor versus expectant management in macrosomia: a randomized study. Obstet Gynecol 1997;89:913–7 [DOI] [PubMed] [Google Scholar]
- 75.Gower RH, Toraya J, Miller JM., JrLaminaria for preinduction cervical ripening. Obstet Gynecol 1982;60:617–9 [PubMed] [Google Scholar]
- 76.Grant JM, Serle E, Mahmood T, et al. Management of prelabour rupture of the membranes in term primigravidae: report of a randomized prospective trial. Br J Obstet Gynaecol 1992;99:557–62 [DOI] [PubMed] [Google Scholar]
- 77.Graves GR, Baskett TF, Gray JH, et al. The effect of vaginal administration of various doses of prostaglandin E2 gel on cervical ripening and induction of labor. Am J Obstet Gynecol 1985; 151:178–81 [DOI] [PubMed] [Google Scholar]
- 78.Gupta R, Vasishta K, Sawhney H, et al. Safety and efficacy of stripping of membranes at term. Int J Gynaecol Obstet 1998; 60:115–21 [DOI] [PubMed] [Google Scholar]
- 79.Habib SM, Emam SS, Saber AS. Outpatient cervical ripening with nitric oxide donor isosorbide mononitrate prior to induction of labor. Int J Gynaecol Obstet 2008;101:57–61 [DOI] [PubMed] [Google Scholar]
- 80.Hamdan M, Sidhu K, Sabir N, et al. Serial membrane sweeping at term in planned vaginal birth after cesarean: a randomized controlled trial. Obstet Gynecol 2009;114:745–51 [DOI] [PubMed] [Google Scholar]
- 81.Hannah ME, Hannah WJ, Hellmann J, et al. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med 1992;326:1587–92 [DOI] [PubMed] [Google Scholar]
- 82.Hannah ME, Ohlsson A, Farine D, et al. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. TERMPROM Study Group. N Engl J Med 1996;334:1005–10 [DOI] [PubMed] [Google Scholar]
- 83.Harper TC, Coeytaux RR, Chen W, et al. A randomized controlled trial of acupuncture for initiation of labor in nulliparous women. Matern Fetal Neonatal Med 2006;19:465–70 [DOI] [PubMed] [Google Scholar]
- 84.Hauth JC, Cunningham FG, Whalley PJ. Early labor initiation with oral PGE2 after premature rupture of the membranes at term. Obstet Gynecol 1977;49:523–6 [PubMed] [Google Scholar]
- 85.Hayashi R, Keirse MJNC. PGE2 gel (Prepidil gel) for preinduction cervical softening (unpublished). [Google Scholar]
- 86.Heimstad R, Skogvoll E, Mattsson LA, et al. Induction of labor or serial antenatal fetal monitoring in postterm pregnancy: a randomized controlled trial. Obstet Gynecol 2007;109:609–17 [DOI] [PubMed] [Google Scholar]
- 87.Heinzl S, Ramzin MS, Schneider M, et al. Priming of cervix with prostaglandin gel during immature birth situation at term [article in German]. Z Geburtshilfe Perinatol 1980;184:395–400 [PubMed] [Google Scholar]
- 88.Henry GR. A controlled trial of surgical induction of labour and amnioscopy in the management of prolonged pregnancy. J Obstet Gynaecol Br Commonw 1969;76:795–8 [DOI] [PubMed] [Google Scholar]
- 89.Herabutya Y, Prasertsawat PO, Tongyai T, et al. Prolonged pregnancy: the management dilemma. Int J Gynaecol Obstet 1992; 37:253–8 [DOI] [PubMed] [Google Scholar]
- 90.Hidar S, Bibi M, Jerbi M, et al. Contribution of intracervical PGE2 administration in premature rupture of the membranes at term. Prospective randomised clinical trial [article in French]. J Gynecol Obstet Biol Reprod (Paris) 2000;29:607–13 [PubMed] [Google Scholar]
- 91.Hjertberg R, Hammarstrom M, Moberger B, et al. Premature rupture of the membranes (PROM) at term in nulliparous women with a ripe cervix. A randomized trial of 12 or 24 hours of expectant management. Acta Obstet Gynecol Scand 1996;75:48–53 [DOI] [PubMed] [Google Scholar]
- 92.Hoffmann RA, Anthony J, Fawcus S. Oral misoprostol vs. placebo in the management of prelabor rupture of membranes at term. Int J Gynaecol Obstet 2001;72:215–21 [DOI] [PubMed] [Google Scholar]
- 93.Hutchon DJ, Geirsson R, Patel NB. A double-blind controlled trial of PGE2 gel in cervical ripening. Int J Gynaecol Obstet 1980;17:604–7 [DOI] [PubMed] [Google Scholar]
- 94.Incerpi MH, Fassett MJ, Kjos SL, et al. Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol 2001;185:916–9 [DOI] [PubMed] [Google Scholar]
- 95.James C, George SS, Gaunekar N, et al. Management of prolonged pregnancy: a randomized trial of induction of labour and antepartum foetal monitoring. Natl Med J India 2001;14:270–3 [PubMed] [Google Scholar]
- 96.Kashanian M, Akbarian A, Baradaran H, et al. Effect of membrane sweeping at term pregnancy on duration of pregnancy and labor induction: a randomized trial. Gynecol Obstet Invest 2006; 62:41–4 [DOI] [PubMed] [Google Scholar]
- 97.Kjos SL, Henry OA, Montoro M, et al. Insulin-requiring diabetes in pregnancy: a randomized trial of active induction of labor and expectant management. Am J Obstet Gynecol 1993; 169:611–5 [DOI] [PubMed] [Google Scholar]
- 98.Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet 2009;374: 979–88 [DOI] [PubMed] [Google Scholar]
- 99.da Graca Krupa F, Cecatti JG, de Castro Surita FG, et al. Misoprostol versus expectant management in premature rupture of membranes at term. BJOG 2005;112:1284–90 [DOI] [PubMed] [Google Scholar]
- 100.Lackritz R, Gibson M, Frigoletto FD., JrPreinduction use of laminaria for the unripe cervix. Am J Obstet Gynecol 1979;134: 349–50 [DOI] [PubMed] [Google Scholar]
- 101.Ladfors L, Mattsson LA, Eriksson M, et al. A randomised trial of two expectant managements of prelabour rupture of the membranes at 34 to 42 weeks. Br J Obstet Gynaecol 1996;103:755–62 [DOI] [PubMed] [Google Scholar]
- 102.Larmon JE, Magann EF, Dickerson GA, et al. Outpatient cervical ripening with prostaglandin E2 and estradiol. J Matern Fetal Neonatal Med 2002;11:113–7 [DOI] [PubMed] [Google Scholar]
- 103.Laube DW, Zlatnik FJ, Pitkin RM. Preinduction cervical ripening with prostaglandin E2 intracervical gel. Obstet Gynecol 1986;68:54–7 [PubMed] [Google Scholar]
- 104.Lelaidier C, Baton C, Benifla JL, et al. Mifepristone for labour induction after previous caesarean section. Br J Obstet Gynaecol 1994;101:501–3 [DOI] [PubMed] [Google Scholar]
- 105.Levy R, Vaisbuch E, Furman B, et al. Induction of labor with oral misoprostol for premature rupture of membranes at term in women with unfavorable cervix: a randomized, double-blind, placebo-controlled trial. J Perinat Med 2007;35:126–9 [DOI] [PubMed] [Google Scholar]
- 106.Lewis GJ. Cervical ripening before induction of labour with prostaglandin E2 pessaries or a Foley’s catheter. J Obstet Gynaecol 1983;3:173–6 [Google Scholar]
- 107.Leave alone or induce for the big baby (LIBBY). Current Controlled Trials. DOI: 10.1186/ISRCTN98146741 [DOI] [Google Scholar]
- 108.Lien JM, Morgan MA, Garite TJ, et al. Antepartum cervical ripening: applying prostaglandin E2 gel in conjunction with scheduled nonstress tests in postdate pregnancies. Am J Obstet Gynecol 1998;179:453–8 [DOI] [PubMed] [Google Scholar]
- 109.Liggins GC. Controlled trial of induction of labor by vaginal suppositories containing prostaglandin E2. Prostaglandins 1979;18:167–72 [DOI] [PubMed] [Google Scholar]
- 110.MacKenzie IZ, Embrey MP. A comparison of PGE2 and PGF2 alpha vaginal gel for ripening the cervix before induction of labour. Br J Obstet Gynaecol 1979;86:167–70 [DOI] [PubMed] [Google Scholar]
- 111.MacLennan AH, Green RC, Bryant-Greenwood GD, et al. Ripening of the human cervix and induction of labour with purified porcine relaxin. Lancet 1980;1:220–3 [DOI] [PubMed] [Google Scholar]
- 112.MacLennan AH, Green RC, Grant P, et al. Ripening of the human cervix and induction of labor with intracervical purified porcine relaxin. Obstet Gynecol 1986;68:598–601 [PubMed] [Google Scholar]
- 113.Magann EF, Chauhan SP, Nevils BG, et al. Management of pregnancies beyond forty-one weeks’ gestation with an unfavorable cervix. Am J Obstet Gynecol 1998;178:1279–87 [DOI] [PubMed] [Google Scholar]
- 114.Magann EF, McNamara MF, Whitworth NS, et al. Can we decrease postdatism in women with an unfavorable cervix and a negative fetal fibronectin test result at term by serial membrane sweeping? Am J Obstet Gynecol 1998;179:890–4 [DOI] [PubMed] [Google Scholar]
- 115.Mahmood TA, Dick MJ, Smith NC, et al. Role of prostaglandin in the management of prelabour rupture of the membranes at term. Br J Obstet Gynaecol 1992;99:112–7 [DOI] [PubMed] [Google Scholar]
- 116.Mahmood TA, Dick MJ. A randomized trial of management of pre-labor rupture of membranes at term in multiparous women using vaginal prostaglandin gel. Obstet Gynecol 1995;85:71–4 [DOI] [PubMed] [Google Scholar]
- 117.Martin DH, Thompson W, Pinkerton JH, et al. A randomized controlled trial of selective planned delivery. Br J Obstet Gynaecol 1978;85:109–13 [DOI] [PubMed] [Google Scholar]
- 118.Martin JN, Jr, Sessums JK, Howard P, et al. Alternative approaches to the management of gravidas with prolonged-postterm-postdate pregnancies. J Miss State Med Assoc 1989;30:105–11 [PubMed] [Google Scholar]
- 119.McCaul JF, Rogers LW, Perry KG, et al. Premature rupture of membranes at term with an unfavorable cervix: Comparison of expectant management, vaginal prostaglandin, and oxytocin induction. South Med J 1997;90:1229–33 [DOI] [PubMed] [Google Scholar]
- 120.McColgin SW, Patrissi GA, Morrison JC. Stripping the fetal membranes at term. Is the procedure safe and efficacious? J Reprod Med 1990;35:811–4 [PubMed] [Google Scholar]
- 121.McKenna DS, Costa SW, Samuels P. Prostaglandin E2 cervical ripening without subsequent induction of labor. Obstet Gynecol 1999;94:11–4 [DOI] [PubMed] [Google Scholar]
- 122.McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol 2004;104:579–84 [DOI] [PubMed] [Google Scholar]
- 123.McQueen D. A randomized controlled trial comparing expectant with active management in early rupture of the membranes at term (unpublished). [Google Scholar]
- 124.Milasinovi L, Radeka G, Petrovic D, et al. Premature rupture of the fetal membranes—an active or expectant approach in management of this obstetrical problem [article in Croation].Med Pregl 1998;51:346–9 [PubMed] [Google Scholar]
- 125.Nager CW, Key TC, Moore TR. Cervical ripening and labor outcome with preinduction intracervical prostaglandin E2 (Prepidil) gel. J Perinatol 1987;7:189–93 [PubMed] [Google Scholar]
- 126.Natale R, Milne JK, Campbell MK, et al. Management of premature rupture of membranes at term: randomized trial. Am J Obstet Gynecol 1994;171:936–9 [DOI] [PubMed] [Google Scholar]
- 127.Newman M, Newman R. Multiple-dose PGE2 cervical ripening on an outpatient basis: safety and efficacy. Am J Obstet Gynecol 1997;176:S112 [Google Scholar]
- 128.Ngai SW, To WK, Lao T, et al. Cervical priming with oral misoprostol in pre-labor rupture of membranes at term. Obstet Gynecol 1996;87:923–6 [DOI] [PubMed] [Google Scholar]
- 129.A clinical trial of induction of labor versus expectant management in postterm pregnancy. Am J Obstet Gynecol 1994;170: 716–23 [PubMed] [Google Scholar]
- 130.Nielsen PE, Howard BC, Hill CC, et al. Comparison of elective induction of labor with favorable Bishop scores versus expectant management: a randomized clinical trial. J Matern Fetal Neonatal Med 2005;18:59–64 [DOI] [PubMed] [Google Scholar]
- 131.Nicholson JM, Parry S, Caughey AB, et al. The impact of the active management of risk in pregnancy at term on birth outcomes: a randomized clinical trial. Am J Obstet Gynecol 2008; 198:511 e1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nimrod C, Currie J, Yee J, et al. Cervical ripening and labor induction with intracervical triacetin base prostaglandin E2 gel: a placebo-controlled study. Obstet Gynecol 1984;64:476–9 [PubMed] [Google Scholar]
- 133.Noah ML, DeCoster JM, Fraser TJ, et al. Preinduction cervical softening with endocervical PGE2 gel. A multi-center trial. Acta Obstet Gynecol Scand 1987;66:3–7 [DOI] [PubMed] [Google Scholar]
- 134.Nuutila M, Kajanoja P. Cervical ripening prior to labour induction with intracervical prostaglandin E2 gel in patients with preeclampsia — a placebo controlled study. Hypertens Pregnancy 1995;14:313–7 [Google Scholar]
- 135.Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand 2005;84:628–31 [DOI] [PubMed] [Google Scholar]
- 136.O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol 1995;173:1855–9 [DOI] [PubMed] [Google Scholar]
- 137.Ocon L, Hurtado R, Coteron JJ, et al. Prolonged pregnancy: procedure guidelines [article in Spanish]. Progresos de Obstetricia y Ginecologia. 1997;40:101–6 [Google Scholar]
- 138.Ottervanger HP, Keirse MJ, Smit W, et al. Controlled comparison of induction versus expectant care for prelabor rupture of the membranes at term. J Perinat Med 1996;24:237–42 [DOI] [PubMed] [Google Scholar]
- 139.Owen J, Winkler CL, Harris BA, Jr, et al. A randomized, double-blind trial of prostaglandin E2 gel for cervical ripening and meta-analysis. Am J Obstet Gynecol 1991;165:991–6 [DOI] [PubMed] [Google Scholar]
- 140.Peedicayil A, Jasper P, Balasubramaniam N, et al. A randomized controlled trial of extra-amniotic ethinyloestradiol for cervical ripening in multiparas. Aust N Z J Obstet Gynaecol 1990;30: 127–30 [DOI] [PubMed] [Google Scholar]
- 141.Prasad RN, Adaikan PG, Arulkumaran S, et al. Preinduction cervical priming with PGE2 vaginal film in primigravidae — a randomised, double blind, placebo controlled study. Prostaglandins Leukot Essent Fatty Acids 1989;36:185–8 [DOI] [PubMed] [Google Scholar]
- 142.Rabl M, Ahner R, Bitschnau M, et al. Acupuncture for cervical ripening and induction of labor at term — a randomized controlled trial. Wien Klin Wochenschr 2001;113:942–6 [PubMed] [Google Scholar]
- 143.Ray DA, Garite TJ. Prostaglandin E2 for induction of labor in patients with premature rupture of membranes at term. Am J Obstet Gynecol 1992;166:836–43 [DOI] [PubMed] [Google Scholar]
- 144.Rayburn WF, Gittens LN, Lucas MJ, et al. Weekly administration of prostaglandin E2 gel compared with expectant management in women with previous cesareans. Prepidil Gel Study Group. Obstet Gynecol 1999;94:250–4 [DOI] [PubMed] [Google Scholar]
- 145.Roach VJ, Rogers MS. Pregnancy outcome beyond 41 weeks gestation. Int J Gynaecol Obstet 1997;59:19–24 [DOI] [PubMed] [Google Scholar]
- 146.Roberts WE, North DH, Speed JE, et al. Comparative study of prostaglandin, laminaria and mini-dose oxytocin for ripening of the unfavorable cervix prior to induction of labor. J Perinatal 1986;6:16–9 [Google Scholar]
- 147.Rydhström H, Ingemarsson I. No benefit from conservative management in nulliparous women with premature rupture of the membranes (PROM) at term. A randomized study. Acta Obstet Gynecol Scand 1991;70:543–7 [DOI] [PubMed] [Google Scholar]
- 148.Sahraoui W, Hajji S, Bibi M, et al. Management of pregnancies beyond forty-one week’s gestation with an unfavorable cervix [article in French]. J Gynecol Obstet Biol Reprod (Paris) 2005; 34:454–62 [DOI] [PubMed] [Google Scholar]
- 149.Sande HA, Tuveng J, Fonstelien T. A prospective randomized study of induction of labor. Int J Gynaecol Obstet 1983;21:333–6 [DOI] [PubMed] [Google Scholar]
- 150.Sawai SK, Williams MC, O’Brien WF, et al. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstet Gynecol 1991; 78:19–23 [PubMed] [Google Scholar]
- 151.Sawai SK, O’Brien WF, Mastrogiannis DS, et al. Patient-administered outpatient intravaginal prostaglandin E2 suppositories in post-date pregnancies: a double-blind, randomized, placebo-controlled study. Obstet Gynecol 1994;84:807–10 [PubMed] [Google Scholar]
- 152.Shoaib F. Management of premature rupture of memebranes with unfavourbale cervix at term, by prostaglandins. Pak J Med Sci 1994;10:227–32 [Google Scholar]
- 153.Spallicci MDB, Bittar RE. Randomized double blind study of ripening the cervix with hyaluronidase in term gestations [article in Spanish]. Rev Bras Ginecol Obstet 2003;25:67 [Google Scholar]
- 154.Sperling LS, Schantz AL, Wahlin A, et al. Management of prelabor rupture of membranes at term. A randomized study. Acta Obstet Gynecol Scand 1993;72:627–32 [DOI] [PubMed] [Google Scholar]
- 155.Srisomboon J, Tongsong T, Tosiri V. Preinduction cervical ripening with intravaginal prostaglandin E1 methyl analogue misoprostol: a randomized controlled trial. J Obstet Gynaecol Res 1996;22:119–24 [DOI] [PubMed] [Google Scholar]
- 156.Stenlund PM, Ekman G, Aedo AR, et al. Induction of labor with mifepristone — a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand 1999;78:793–8 [PubMed] [Google Scholar]
- 157.Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol 2000; 96:684–8 [DOI] [PubMed] [Google Scholar]
- 158.Tamsen L, Lyrenas S, Cnattingius S. Premature rupture of the membranes — intervention or not. Gynecol Obstet Invest 1990; 29:128–31 [DOI] [PubMed] [Google Scholar]
- 159.Tan PC, Yow CM, Omar SZ. Effect of coital activity on onset of labor in women scheduled for labor induction: a randomized controlled trial. Obstet Gynecol 2007;110:820–6 [DOI] [PubMed] [Google Scholar]
- 160.Tannirandorn Y, Jumrustanasan T. A comparative study of membrane stripping and nonstripping for induction of labor in uncomplicated term pregnancy. J Med Assoc Thai 1999;82:229–33 [PubMed] [Google Scholar]
- 161.Tey A, Eriksen NL, Blanco JD. A prospective randomized trial of induction versus expectant management in nondiabetic pregnancies with fetal macrosomia [abstract]. Am J Obstet Gynecol 1995;172:293 [Google Scholar]
- 162.Thiery M, Decoster JM, Parewijck W, et al. Endocervical prostaglandin E2 gel for preinduction cervical softening. Prostaglandins 1984;27:429–39 [DOI] [PubMed] [Google Scholar]
- 163.Thomas N, Longo SA, Rumney PJ, et al. Intravaginal misoprostol in prelabor rupture of membranes at term. Am J Obstet Gynecol 2000;182:S136 [Google Scholar]
- 164.Trofatter KF, Jr, Bowers D, Gall SA, et al. Preinduction cervical ripening with prostaglandin E2 (Prepidil) gel. Am J Obstet Gynecol 1985;153:268–71 [DOI] [PubMed] [Google Scholar]
- 165.Ulmsten U, Wingerup L, Belfrage P, et al. Intracervical application of prostaglandin gel for induction of term labor. Obstet Gynecol 1982;59:336–9 [PubMed] [Google Scholar]
- 166.Ulmsten U, Ekman G, Belfrage P, et al. Intracervical versus intravaginal PGE2 for induction of labor at term in patients with an unfavorable cervix. Arch Gynecol 1985;236:243–8 [DOI] [PubMed] [Google Scholar]
- 167.Valentine BH. Intravenous oxytocin and oral prostaglandin E2 for ripening of the unfavourable cervix. Br J Obstet Gynaecol 1977;84:846–54 [DOI] [PubMed] [Google Scholar]
- 168.van den Hove MM, Willekes C, Roumen FJ, et al. Intrauterine growth restriction at term: Induction or spontaneous labour? Disproportionate intrauterine growth intervention trial at term (DIGITAT): a pilot study. Eur J Obstet Gynecol Reprod Biol 2006;25:54–8 [DOI] [PubMed] [Google Scholar]
- 169.Wingerup L, Andersson KE, Ulmsten U. Ripening of the uterine cervix and induction of labour at term with prostaglandin E2 in viscous gel. Acta Obstet Gynecol Scand 1978;57:403–6 [DOI] [PubMed] [Google Scholar]
- 170.Wiqvist I, Norstrom A, Wiqvist N. Induction of labor by intracervical PGE2 in viscous gel. Mechanism of action and clinical treatment routines. Acta Obstet Gynecol Scand 1986;65:485–92 [DOI] [PubMed] [Google Scholar]
- 171.Wiriyasirivaj B, Vutyavanich T, Ruangsri RA. A randomized controlled trial of membrane stripping at term to promote labor. Obstet Gynecol 1996;87:767–70 [DOI] [PubMed] [Google Scholar]
- 172.Witter FR, Weitz CM. A randomized trial of induction at 42 weeks gestation versus expectant management for postdates pregnancies. Am J Perinatol 1987;4:206–11 [DOI] [PubMed] [Google Scholar]
- 173.Witter FR, Rocco LE, Johnson TR. A randomized trial of prostaglandin E2 in a controlled-release vaginal pessary for cervical ripening at term. Am J Obstet Gynecol 1992;166:830–4 [DOI] [PubMed] [Google Scholar]
- 174.Wong SF, Hui SK, Choi H, et al. Does sweeping of membranes beyond 40 weeks reduce the need for formal induction of labour? BJOG 2002;109:632–6 [DOI] [PubMed] [Google Scholar]
- 175.Yonekura ML, Songster G, Smith-Wallace T. Preinduction cervical priming with PGE2 intracervical gel. Am J Perinatol 1985;2: 305–10 [DOI] [PubMed] [Google Scholar]
- 176.Ziaei S, Rosebehani N, Kazeminejad A, et al. The effects of intramuscular administration of corticosteroids on the induction of parturition. J Perinat Med 2003;31:134–9 [DOI] [PubMed] [Google Scholar]
- 177.van der Ham DP, Vijgen SM, Nijhuis JG, et al. PPROMEXIL trial group. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med 2012;9:e1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bezircioglu I, Baloglu A, Bicer M. The efficacy of dinoprostone vaginal insert for active management of premature rupture of membranes at term: a randomized controlled trial. Clin Exp Obstet Gynecol 2012;39:356–8 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.