Abstract
Objective
To determine the functionality of a wireless controlled implantable stimulator designed for stimulation and block of the pudendal nerve.
Materials and Methods
In 5 cats under α-chloralose anesthesia, the stimulator was implanted underneath the skin on the left side in the lower back along the sacral spine. Two tripolar cuff electrodes were implanted bilaterally on the pudendal nerves in addition to one bipolar cuff electrode that was implanted on the left side central to the tripolar cuff electrode. The stimulator provided high frequency (5-20 kHz) biphasic stimulation waveforms to the two tripolar electrodes and low frequency (1-100 Hz) rectangular pulses to the bipolar electrode. Bladder and urethral pressures were measured to determine the effects of pudendal nerve stimulation (PNS) or block.
Results
The maximal (70-100 cmH2O) urethral pressure generated by 20 Hz PNS applied via the bipolar electrode was completely eliminated by the pudendal nerve block induced by the high frequency stimulation (6-15 kHz, 6-10 V) applied via the two tripolar electrodes. In a partially filled bladder 20-30 Hz PNS (2-8 V, 0.2 ms) but not 5 Hz stimulation applied via the bipolar electrode elicited a large sustained bladder contraction (45.9±13.4 to 52.0±22 cmH2O). During cystometry, the 5 Hz PNS significantly (P<0.05) increased bladder capacity to 176.5±27.1% of control capacity.
Conclusions
The wireless controlled implantable stimulator successfully generated the required waveforms for stimulation and block of pudendal nerve, which will be useful for restoring bladder functions after spinal cord injury (SCI).
Keywords: pudendal nerve, stimulation, block, spinal cord injury, cat
INTRODUCTION
After SCI above the lumbosacral level, the coordinated action between bladder and external urethral sphincter (EUS) disappears (1). Instead, the bladder and EUS contract simultaneously (termed detrusor sphincter dyssynergia or DSD), which generates high bladder pressure, prevents complete elimination of urine, and requires daily urethral catheterization (1, 2). High bladder pressure can cause vesicoureteral reflux and renal failure in the long-term. Frequent urethral catheterization can cause low urinary tract infection (2). In addition, detrusor overactivity (DO) induces poor bladder storage function and frequent incontinence (1, 2). Currently, no medication can treat both DSD and DO. However, in the 1970s Brindley and his team developed an implantable sacral anterior root stimulator to restore bladder function after SCI. This system is now commercially available (Finetech Medical Limited, UK) and has been implanted in over 2000 SCI persons around the world (3). It requires sacral posterior root rhizotomy to prevent DO and DSD, which is destructive and irreversible and results in the loss of reflex sexual and defecation functions (3).
Our previous studies (4-6) in chronic SCI cats under anesthesia have revealed a frequency-dependent pudendal-to-bladder spinal reflex. PNS at 5 Hz can inhibit reflex bladder activity and increase bladder capacity, but at 20 Hz it can excite the bladder and induce a large bladder contraction (4-6). In addition, our previous studies (7-9) in cats have shown that high frequency (>6 kHz) biphasic stimulation can block the pudendal nerve and completely relax the EUS. When the 20 Hz PNS was combined with high frequency pudendal nerve block in anesthetized chronic SCI cats, a large bladder contraction was induced simultaneously with an EUS relaxation, resulting in efficient voiding (10). These results indicate that both micturition and continence functions could be restored after SCI by stimulation and block of the pudendal nerve, which will not require a dorsal root rhizotomy and thereby preserving the residual reflexes for bowel and sexual functions after SCI.
However, to test the stimulation/block strategy in a future clinical trial to restore bladder functions after SCI, an implantable stimulator is needed to generate the required 5 Hz and 20 Hz PNS as well as the high frequency (>6 kHz) biphasic waveform for pudendal nerve block. Currently, such an implantable stimulator is not commercially available. Therefore, in this study we designed and constructed a small, wireless controlled, battery powered, implantable stimulator, that we showed in anesthetized cats can generate the required stimulation waveforms to reflexively excite or inhibit the bladder, and block axonal conduction in the pudendal nerve. This study represents the first step in developing a device for future testing in awake chronic SCI animals, and eventually in SCI human subjects.
MATERIALS AND METHODS
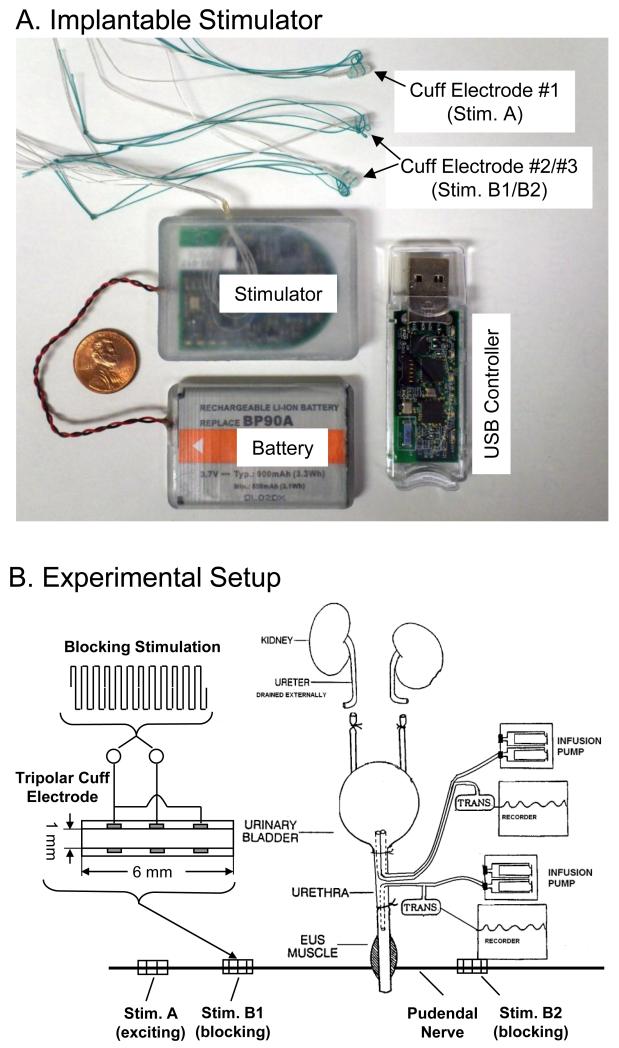
The Animal Care and Use Committee at the University of Pittsburgh approved all protocols involving the use of animals in this study. Fig.1A shows a picture of the implantable stimulator (dimensions: 5.6×4.0×0.8 cm) connected to a rechargeable battery (3.7 V and 900 mAh; dimensions: 5.4×3.6×0.6 cm). The stimulator has 2 output channels providing charge-balanced rectangular pulses (1-100 Hz, 0-11 V, 0.05-0.2 ms) to the bipolar cuff electrode #1 (i.e., Stim. A in Fig.1B) and a high frequency (5-20 kHz, 0-11 V) biphasic square waveform (see Fig.1B) to the tripolar cuff electrodes #2 and #3 (i.e., Stim. B1 and B2 in Fig.1B). The stimulator can be wireless controlled within a 2-meter distance by a USB controller that is connected to a USB port of a portable computer (the computer is not shown). The computer software can set stimulation parameters for each output channel and turn on/off each channel by wireless communication between the implanted stimulator and the USB controller. The stimulator and battery are implanted underneath the skin on the left side of the lower back of the cat along the sacral spine. The battery can be charged by the charging circuitry in the stimulator that can be powered wirelessly across the skin by an external charging coil (not shown). At the beginning of each experiment, the battery was fully charged from 2.8 V to 3.7 V in about 15-20 minutes and it could be run for the entire experiment (5-6 hours) with repeated stimulation. At the end of the experiment, the battery, stimulator, and electrodes were removed from the animal.
Fig.1.
The implantable stimulator (A) and the locations of cuff electrodes implanted on the pudendal nerves in the cat (B). EUS – External Urethral Sphincter.
A total of 5 cats (3 female and 2 male, 2.9 to 3.3 kg) were used in this study. Isoflurane (2-5% in O2) was used to anesthetize the animals during surgery and then replaced by α-chloralose anesthesia (65 mg/kg i.v. and supplemented as needed) during data collection. The temperature of the animal was maintained at 35 °C to 37 °C using a heating pad. The ureters were cut and drained externally in order to accurately measure the bladder capacity when bladder was infused through a catheter in the urethra. A double lumen catheter (5F) was inserted into the bladder through a small incision in the proximal urethra and secured by a ligature (Fig.1B). One lumen of the catheter was attached to a pump to infuse the bladder with saline, and the other lumen was connected to a pressure transducer to monitor the bladder activity. Another catheter (5F) was also inserted at the same site in the proximal urethra but directed toward the distal urethra and secured by a ligature (Fig.1B). This catheter was attached to an infusion pump and to a pressure transducer via a T connector. The pudendal nerves were accessed posteriorly in the sciatic notch lateral to the tail. The three cuff electrodes (NCE112/113, MicroProbes Inc., Gaithersburg, MD) as shown in Fig.1A were tunneled underneath the skin from the stimulator to the exposed pudendal nerves and were placed around the left and right pudendal nerves as shown in Fig.1B. After implanting the stimulator, the battery, and the cuff electrodes, the muscle and skin were closed by sutures.
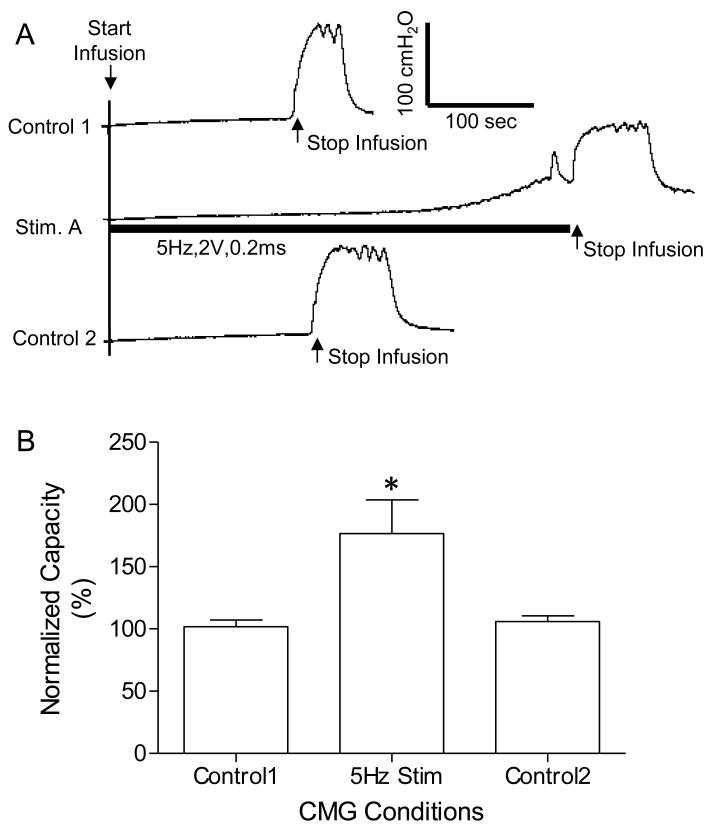
Three tests were performed in this study to determine the ability for the implanted stimulator to: (1) block the pudendal nerve, (2) induce large bladder contraction, or (3) inhibit the micturition reflex. In the first test (N=5 cats), the urethra was infused continuously with saline at a rate of 1-2 ml/min. Back pressure in the urethral perfusion system caused by EUS contraction was recorded via the pressure transducer (Fig.1B). First, the high frequency biphasic pudendal nerve stimulation (PNS) at 10 kHz was applied to both Stim. A and Stim. B sites (see Fig.1B) at different intensities (0.5-10V) to determine the effective intensities for pudendal nerve block. Then, using an intensity effective in blocking the nerve at 10 kHz, different frequencies (5-20 kHz) were tested to determine the threshold and optimal frequencies for pudendal nerve block. Finally, the high frequency biphasic stimulation determined to be effective in blocking the pudendal nerve conduction was applied to block the maximal EUS contraction induced by the 20 Hz PNS applied at Stim. A (Fig.1B). In the second test (N=5 cats) a cystometrogram (CMG) was performed to determine the bladder capacity (i.e., the volume necessary to induce a large micturition contraction, >30 cm H2O). Then the bladder was infused to about 90% of its capacity and PNS (2-8 V, 0.2 ms) of different frequencies (5-40 Hz) was applied at Stim. A (Fig.1B) to determine the optimal frequency for inducing a large (>30 cmH2O) bladder contraction. In the third test (N=4 cats), repeated CMGs were performed to determine the bladder capacity. Each CMG consisted of a slow infusion of saline (1-2 ml/min) starting with an empty bladder until the first micturition contraction occurred. Initially, two or three control CMGs were performed without PNS to determine the control bladder capacity and evaluate reproducibility. Then, PNS (5 Hz, 2-5 V, 0.2 ms) was applied at Stim. A (Fig.1B) during the CMG to increase bladder capacity. A control CMG without stimulation was also performed after the PNS CMG.
To quantify the PNS inhibitory effect, the bladder capacity measured during each CMG was normalized to the measurement during the first control CMG. Bladder contraction amplitude and area under bladder contraction curve were measured to determine the excitatory PNS effect. Urethral pressure induced by 20 Hz PNS was measured to determine the effect of high frequency block. The data from different animals are presented as mean ± standard error. One-way ANOVA followed by Dunnett multiple comparison was used to detect statistical significance (P<0.05).
RESULTS
Pudendal nerve block by high frequency biphasic stimulation
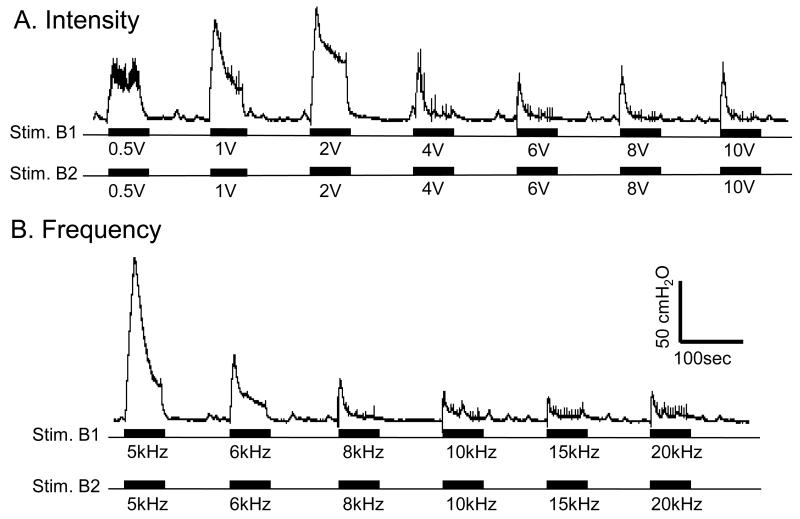
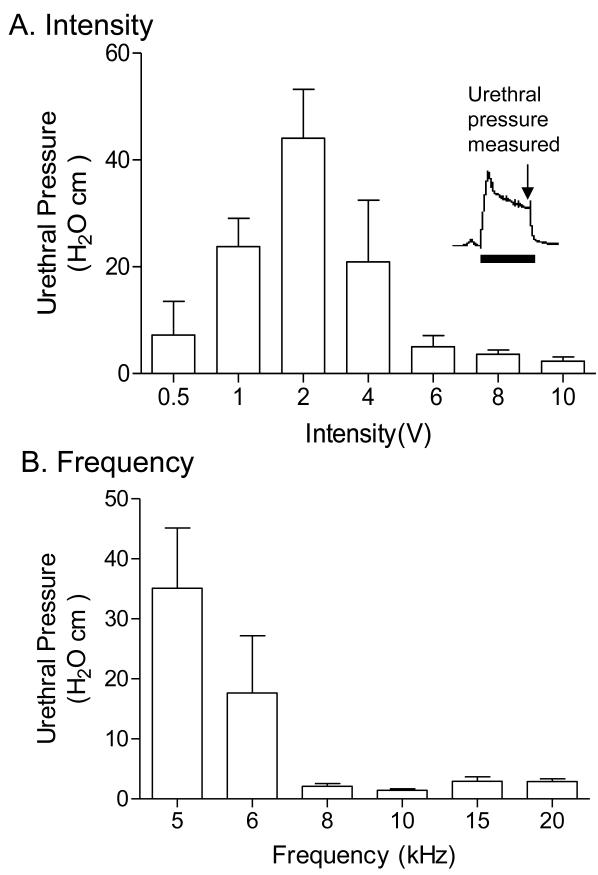
The high frequency biphasic PNS waveform generated by the implanted stimulator successfully blocked pudendal nerve conduction and eliminated the PNS evoked EUS contraction when applied bilaterally on the pudendal nerves (Figs.2-5). The high frequency biphasic PNS applied bilaterally at 10 kHz for 60 sec at locations Stim. B1 and Stim. B2 (Fig.1B) activated the pudendal nerves, induced a strong EUS contraction, and caused large increases in urethral pressure at intensities of 0.5-2 V (Fig.2A and Fig.3A). However, as the intensity was increased the urethral pressure response gradually decreased in amplitude and duration, and became minimal (<5 cmH2O) at the end of the 60-second high frequency stimulation when the intensity was 6-10 V (Fig.2A and Fig.3A). Within this range of intensities (6-10 V), the high frequency biphasic PNS also generated large urethral pressure responses at frequencies below 6 kHz, but the response was minimal (<5 cmH2O) at the end of the 60 second stimulation once the frequency was increased above 6 kHz (Fig.2B and Fig.3B).
Fig.2.
Urethral pressure induced by the high frequency biphasic electrical stimulation applied bilaterally on the pudendal nerves. A. Different intensities at 10 kHz frequency. B. Different frequencies at 6 V intensity. The urethral infusion rate is 1 ml/min. The black bars under the pressure traces indicate the stimulation duration. The calibration bars are for both A and B. The Y-calibration bar indicates the urethral pressure in 50 cmH2O and the X-calibration bar indicates the time in 100 seconds.
Fig.5.
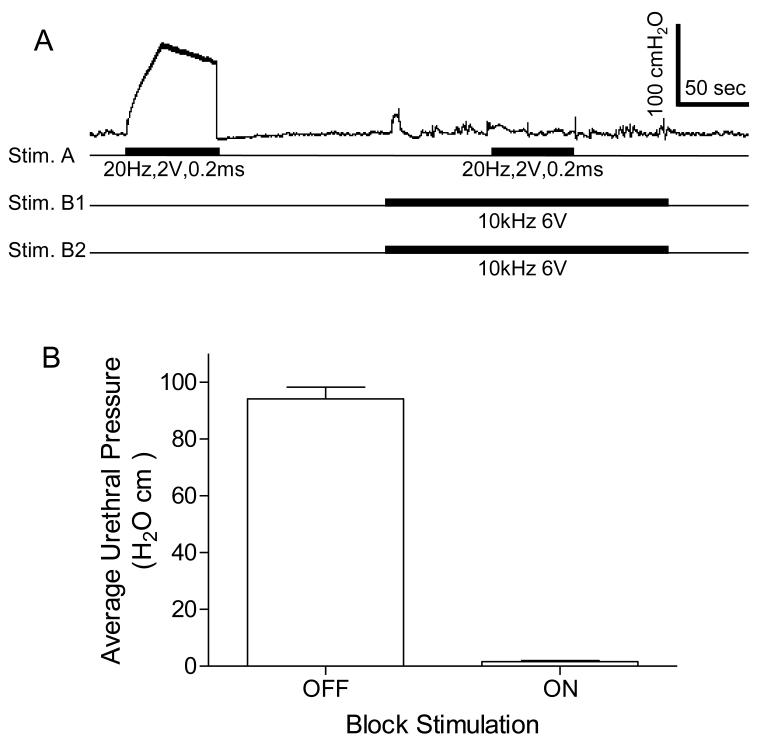
High frequency biphasic stimulation of the pudendal nerves bilaterally completely blocked the increase in urethral pressure induced by unilateral pudendal nerve stimulation. A. Records from one female cat showing the representative block of the response elicited by Stim. A during bilateral pudendal nerve stimulation (Stim. B1 and Stim. B2). High frequency stimulation delivered to Stim. B1 and Stim. B2 (see Fig.1B) completely reduced the urethral pressure induced by Stim. A. The black bars under pressure trace indicate the stimulation duration. The Y-calibration bar indicates the urethral pressure in 100 cmH2O and the X-calibration bar indicates the time in 50 seconds. B. Average urethral pressure induced by Stim. A when the high frequency stimulation was either on or off. Stim. A: frequency 20 Hz, pulse width 0.2 ms, intensity 2-5 V. Stim. B1/B2: frequency 6-15 kHz, intensity 6-10 V. N = 4 cats. A total of 8 tests were performed in 4 cats with 1-3 tests in each cat.
Fig.3.
Urethral pressure measured at the end of high frequency biphasic electrical stimulation. A. Different intensities at 10 kHz frequency. B. Different frequencies at 6-10 V intensity. N = 5 cats. The inserted figure in A shows the time point for urethral pressure measurement.
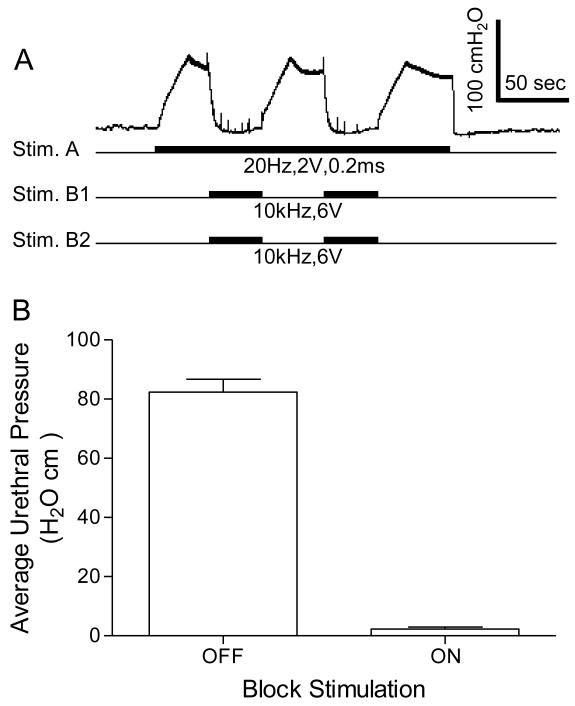
In order to show that the high frequency biphasic PNS blocked pudendal nerve conduction when the urethral pressure response was diminished at the end of the 60-second stimulation, a 20 Hz PNS (2-5 V, 0.2 ms) was applied at Stim. A (Fig.1B) central to the high frequency stimulation site to induce a maximal (70-100 cmH2O) urethral pressure response (Fig.4 and Fig.5). This urethral response was completely blocked by the high frequency biphasic PNS (6-15 kHz, 6-10 V) (Fig.4 and Fig.5). The pudendal nerve conduction recovered quickly from the block once the high frequency PNS was terminated (Fig.5A).
Fig.4.
High frequency, biphasic electrical stimulation of the pudendal nerves bilaterally blocked the urethral pressure increase induced by 20 Hz unilateral pudendal nerve stimulation. A. A typical representation of the block effect. High frequency stimulation delivered to Stim. B1 and Stim. B2 (see Fig.1B) completely blocked the urethral pressure increase induced by Stim. A. The black bars under pressure trace indicate the stimulation duration. The Y-calibration bar indicates uretheral pressure in 100 cmH2O and the X-calibration bar indicates the time in 50 seconds. B. Average urethral pressure measured during Stim. A when the high frequency stimulation was either on or off. Stim. A: frequency 20 Hz, pulse width 0.2 ms, intensity 2-5 V. Stim. B1/B2: frequency 6-15 kHz, intensity 6-10 V. N = 5 cats. A total of 17 tests were performed in 5 cats with 2-5 tests in each cat.
Bladder excitation or inhibition by PNS
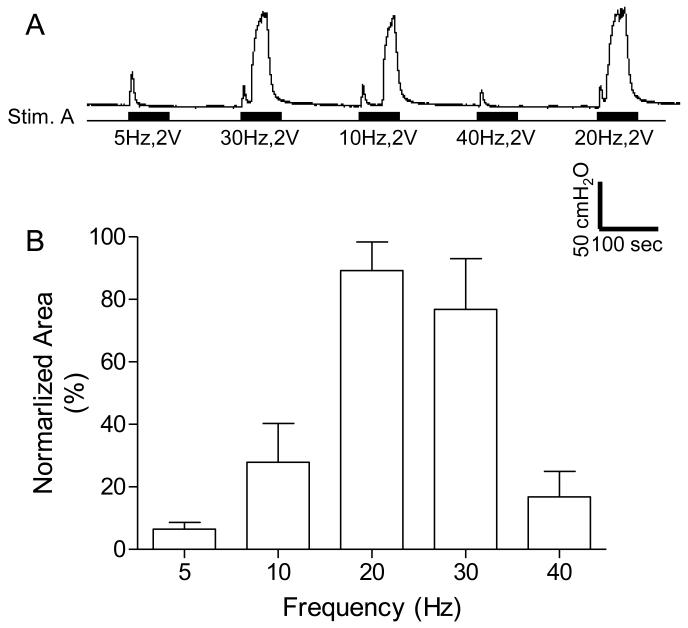
The implanted stimulator also generated rectangular pulses at the cuff electrode #1 (at Stim. A in Fig.1 A and B) that reflexively excited or inhibited the bladder depending on the frequency of stimulation (Fig.6 and Fig.7). Several CMGs were performed in each animal to determine the bladder capacity. In one group of experiments, 20-30 Hz PNS (2-8 V, 0.2 ms) induced a large amplitude (45.9±13.4 to 52.0±22 cmH2O) sustained bladder contraction (Fig.6) when the bladder was filled to 90% of capacity. When the frequency was changed to 5 Hz, PNS (2-5 V, 0.2 ms) applied during CMG inhibited reflex micturition and significantly (P<0.05) increased bladder capacity to 176.5 ± 27.1% of the control capacity (8.2±1.9 ml) (Fig.7).
Fig.6.
Bladder contractions induced by pudendal nerve stimulation at different frequencies. A. Increase in bladder pressure elicited by unilateral pudendal nerve stimulation (Stim. A). The black bars under pressure trace indicate the stimulation duration. The Y-calibration bar indicates bladder pressure in 50 cmH2O and the X-calibration bar indicates the time in 100 seconds. B. Normalized area under bladder pressure curve during stimulation. The area under curve was normalized to the maximal response in each animal. Stim. A: frequency 20 Hz, pulse width 0.2 ms, intensity 2-8 V. N = 5 cats.
Fig.7.
Increase in bladder capacity induced by 5 Hz unilateral pudendal nerve stimulation. A. Repeated CMG recordings (infusion rate 2 ml/min in a female cat) showing in the middle trace the reversible increase in bladder capacity during stimulation. The black bar under the CMG trace indicates the stimulation duration. The Y-calibration bar indicates the bladder pressure in 100 cmH2O and the X-calibration bar indicates the time in 100 seconds. B. Normalized bladder capacity measured during different CMGs. The capacity was normalized to the measurement from control 1 CMG. * indicates significantly different from the control 1. Stimulation: frequency 5 Hz, pulse width 0.2 ms, intensity 2-5 V. N = 4 cats.
DISCUSSION
In this study we constructed and successfully tested an implantable, wireless controlled, rechargeable battery powered stimulator that can provide the required stimulation waveforms to: 1) block pudendal nerve conduction and relax the EUS (Figs.2-5), 2) induce a large sustained reflex bladder contraction (Fig.6), or 3) inhibit reflex bladder activity (Fig.7). These acute in vivo experiments in anesthetized normal cats establish the effectiveness of the device as well as stimulation parameters that will be used in future long term experiments to modulate abnormal lower urinary tract function in awake chronic SCI cats. In the chronic experiments, the 5 Hz PNS will be used to inhibit bladder overactivity and to promote urine storage. However to elicit voiding, the 5 Hz PNS will be switched to 20 Hz to induce a sustained bladder contraction and at the same time a high frequency (6-10 kHz) pudendal nerve block will be used to relax the EUS and prevent DSD, so that efficient, low pressure voiding can be induced.
The elimination of EUS contraction by the high frequency stimulation as shown in Figs.4-5 is due to the block of pudendal nerve conduction. It is not due to EUS fatigue caused by the stimulation, because a previous study (9) showed that the EUS could still contract if the Stim. A (Fig.1B) was moved to a location distal to the high frequency blocking stimulation. Our previous studies (7,8) have shown that the EUS contraction was gradually reduced as the stimulation frequency increased from 1 kHz to 10 kHz. A complete block of EUS contraction could only be achieved at 6 kHz and above. Therefore, we designed the stimulator to provide 5-20 kHz for pudendal nerve block (Fig.2B). In the present study, the high frequency blocking stimulation was applied bilaterally on the pudendal nerves (Fig.1B) to block an excitatory pudendal-to-pudendal reflex (10, 11) that can be triggered by afferent axonal volleys elicited by Stim. A that in turn can elicit efferent activity on the contralateral as well as the ipsilateral pudendal nerves to cause an EUS contraction. In addition, bilateral block of the pudendal nerves would be needed to control DSD after chronic SCI because afferent activity elicited by bladder contractions can generate pudendal efferent activity and EUS contractions bilaterally. Therefore, in this study we implanted the blocking electrodes bilaterally on the pudendal nerves (Stim. B1 and B2 in Fig.1B) to mimic the conditions when the implantable stimulator is used to restore bladder functions after chronic SCI. Our approach combining pudendal nerve stimulation and block is different from the approach used in other studies (12,13) that attempted to induce a reflex bladder contraction without an EUS contraction by selectively stimulating pudendal afferent nerves without stimulating efferent nerves. Activation of pudendal afferent nerves will reflexively activate pudendal efferent nerves through an excitatory pudendal-to-pudendal reflex (10, 11). In addition, the bladder contraction induced by pudendal afferent stimulation will produce pudendal efferent activity and EUS contraction due to DSD after SCI. This is why we positioned the electrodes bilaterally on the whole pudendal nerve to completely block pudendal efferent activity and relax the EUS.
The high frequency blocking stimulation generated by the implantable stimulator produced a reversible block of the pudendal nerve. Pudendal nerve conduction recovered quickly once the high frequency blocking stimulation was off (Fig.5), indicating that the stimulation was safe and no nerve damage was induced. It is worth noting that the quick recovery from pudendal nerve block as shown in Fig.5 was achieved even after repeatedly (>14 times, see Fig.2 and Fig.4) applying the high frequency blocking stimulation of 1-3 minute duration for a total duration greater than 15 minutes. In clinical applications to restore micturition function after SCI, the high frequency blocking stimulation will be applied about 3-5 times per day for only 1-2 minutes each time during voiding. Therefore, the possibility of pudendal nerve damage caused by the high frequency blocking stimulation should be minimal. In addition, the safety of high frequency blocking stimulation has also been verified by recent clinical studies to block the vagus nerve for diabetes treatment (14, 15). The reversibility from pudendal nerve block is not only important for safety reasons but also necessary for maintaining continence function during urine storage.
About 15-20 minutes were needed to fully charge the battery wirelessly across the skin. After each charging, the battery successfully powered the stimulator in the animal for the entire experimental period (5-6 hours). After removing the stimulator and battery from the animal at the end of experiment, the battery power lasted for about 2-3 days before the stimulator stopped functioning and lost communication with the USB controller due to a low battery level (<2.8 V). Although the stimulator was not used to stimulate/block the pudendal nerve once it was removed from the animal, it is estimated that the battery should still be able to power the stimulator for the same amount of time (2-3 days) if it were used for PNS/block. This is because the electrical power used by PNS/block is only a very small portion of the total power consumption by the electrical circuits including wireless communication, microprocessor, and the output amplifiers. Therefore, it is expected that the implantable stimulator will continuously function after chronic implantation in SCI cats as long as we continue to charge it for 15-20 minutes every day.
Currently there is a commercially available, implantable stimulator (InterStim™, Medtronic Inc.) that stimulates the sacral S3 root to treat overactive bladder (OAB) (16). It has also been used to stimulate the pudendal nerve for the treatment of OAB or interstitial cystitis (IC) (17,18). However, the InterStim stimulator does not generate kHz frequencies and cannot be used to block the pudendal nerve and prevent DSD. Our stimulator is designed to treat both DO (5 Hz) and DSD (20 Hz + kHz) after SCI by stimulating and/or blocking the pudendal nerves. Although our stimulator could potentially be used for OAB or IC treatment, the kHz stimulation generated by our stimulator would be unnecessary for this purpose. Since pudendal nerve also innervates the penis and anal sphincter in addition to the EUS, our pudendal nerve stimulator might have other potential applications to induce defecation, ejaculation, or penile erection etc. However, before these applications become possible, the responses from these visceral organs to PNS will have to be determined.
This study only tested the functionality of the implantable stimulator. The durability can only be tested when the stimulator is implanted in awake chronic SCI cats in our future experiments. Although many improvements on this prototype stimulator may have to be implemented as our study progresses, this study is the first step of our long-term effort in designing an implantable pudendal nerve stimulator for restoring bladder functions after SCI.
Acknowledgments
Sources of financial support: the DOD Spinal Cord Injury Research Program (SCIRP) under contract number W81XWH-11-1-0819, and the NIH under grants DK-094905, DK-068566, DK-090006, and DK-091253.
Footnotes
Conflict of interest: Dr. Tai is the inventor of a pending patent application related to this study.
Authorship statement: All authors designed and conducted the study. All authors approved the final manuscript. DOD and NIH provided the funding for the study. Dr. Tai had complete access to the study data.
REFERENCES
- 1.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system, nervous control of the urogenital system. Harwood Academic Publishers; London: 1993. pp. 227–289. [Google Scholar]
- 2.Burns AS, Rivas DA, Ditunno JF. The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine. 2001;26:s129–s136. doi: 10.1097/00007632-200112151-00022. [DOI] [PubMed] [Google Scholar]
- 3.van Kerrebroeck PEV, Koldewijn EL, Rosier PFWM, Wijkstra H, Debruyne FMJ. Results of the treatment of neurogenic bladder dysfunction in spinal cord injury by sacral posterior root rhizotomy and anterior sacral root stimulation. J Urol. 1996;155:1378–1381. doi: 10.1097/00005392-199604000-00069. [DOI] [PubMed] [Google Scholar]
- 4.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition and voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 6.Tai C, Chen M, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cat. Exp Neurol. 2011;228:109–117. doi: 10.1016/j.expneurol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol. 2004;172:2069–2072. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- 8.Tai C, Roppolo JR, de Groat WC. Response of external urethral sphincter to high frequency biphasic electrical stimulation of pudendal nerve. J Urol. 2005;174:782–786. doi: 10.1097/01.ju.0000164728.25074.36. [DOI] [PubMed] [Google Scholar]
- 9.Tai C, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Influence of temperature on pudendal nerve block induced by high frequency biphasic electrical current. J Urol. 2008;180:1173–1178. doi: 10.1016/j.juro.2008.04.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn. 2007;26:879–886. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thor KB, Hisamitsu T, Roppolo JR. Selective inhibitory effects of ethylketocyclazocine on reflex pathways to the external urethral sphincter of the cat. J Pharm Exp Therap. 1989;248:1010–1025. [PubMed] [Google Scholar]
- 12.Woock JP, Yoo PB, Grill WM. Finite element modeling and in vivo analysis of electrode configurations for selective stimulation of pudendal afferent fibers. BMC Urol. 2010;10:11. doi: 10.1186/1471-2490-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in cats. Exp Neurol. 2008;212:218–225. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarr MG, Billington CJ, Brancatisamo R, Brancatisano J, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikore S, Reeds DN, Eagon JC, Wolfe BM, O’Rourke RW, Fujioka K, Takata M, Swain JM, Morton JM, Ikramuddin S, Schweitzer M, Chand B, Rosenthal R. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22:1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 15.Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng. 2011;8:056013. doi: 10.1088/1741-2560/8/5/056013. [DOI] [PubMed] [Google Scholar]
- 16.van Kerrebroeck PEV, van Voskuilen AC, Heesakkers JPFA, Nijholt AABL, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunctions: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2020–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 18.Peters KM, Feber KM, Bennett RC. A prospective, single-blinded, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100:835–839. doi: 10.1111/j.1464-410X.2007.07082.x. [DOI] [PubMed] [Google Scholar]