Abstract
Disgust reactions can be elicited using stimuli that engender orogastric rejection (e.g., pus and vomit; Core Disgust stimuli), but also using images of bloody injuries or medical procedures (e.g., surgeries; Blood-[Body] Boundary Violation [B-BV] Disgust stimuli). These two types of disgust reaction are believed to be connected by a common evolutionary function of avoiding either food- or blood-borne contaminants. However, reactions to the category of bloody injuries are typically conflated with reactions to the potential pain being experienced by the victim. This may explain why the two forms of ‘disgust,’ though similarly communicated (through self-report and facial expressions) evince different patterns of physiological reactivity. We therefore tested whether the communicative similarities and physiological dissimilarities would hold when markers of potential contamination in the latter category are removed, leaving only painful injuries that lack blood or explicit body-envelope violations. Participants viewed films that depicted imagery associated with (1) core disgust, (2) painful injuries, or (3) neutral scenes while we measured facial, cardiovascular, and gastric reactivity, respectively. Whereas communicative measures (self-report and facial muscles) suggested that participants experienced increased disgust for both core disgust and painful injuries, peripheral physiology dissociated the two: core disgust decreased normal gastric activity and painful-injury disgust decelerated heart rate and increased heart rate variability. These findings suggest that expressions of disgust toward bodily injuries may reflect a fundamentally different affective response than those evoked by core disgust, and that this (cardiovascularly-mediated) response may in fact be more closely tied to pain-perceptions (or empathy) rather than contaminant-laden stimuli.
The term ‘disgusting’ is applied to more stimuli in our environment than just those we want to avoid ingesting orally, including ones as distantly related to oral rejection as filthy environments, unwashed animals and people, and morally reprehensible acts (up to and including violations of basic social norms; for a review, see Chapman & Anderson, 2012). The disgust umbrella has grown so large, in fact, that many have suggested differentiating cases in which this term reflects an evolutionarily conserved feeling of disgust versus cases where disgust is potentially used as a metaphor or proxy for another underlying feeling (Nabi, 2002; Royzman & Kurzban, 2011; Rozin, Haidt, & Fincher, 2009). While this question has been raised primarily in the context of norm violations (e.g., fair vs. unfair offers in an ultimatum game; Chapman, Kim, Susskind, & Anderson, 2009), the same might be asked of one of the most commonly studied form of disgust: reactions elicited by images involving blood or (body) boundary violations (BBV injury), such as surgery videos (Rottenberg, Ray, & Gross, 2007).
Reactions to B-BV injury have two main features that encourage researchers to compare them directly to more basic forms of disgust reactions elicited by stimuli that are orogastrically objectionable (e.g., rotting foods, feces; stimuli that are said to elicit core disgust). First, participants generate similar verbal reports and facial expressions – including contractions of the levator labii muscles at the sides of their nose (Olatunji, Haidt, McKay, & David, 2008; Stark, Walter, Schienle, & Vaitl, 2005; Vrana, 1993) – for both core disgust and B-BV injury stimuli. Second, both reactions are believed to share analogous evolutionary functions. Just as core disgust is believed to protect individuals from potential orally-consumed (e.g., food-borne) contaminants, B-BV injury disgust is believed to signal potential infectious contaminants carried in the bloodstream, now exposed by a violation of the body envelope (Curtis & Biran, 2001; Oaten, Stevenson, & Case, 2009; Tybur, Lieberman, Kurzban, & DeScioli, 2013).
The comparisons between these two domains of disgust reaction therefore rely on disgust reactions being elicited by the bloody or boundary-violation aspects of the imagery rather than, for instance, reactions to the painful aspects of the injury or procedure itself. This concern is particularly salient given that facial expressions of disgust share commonalities with facial expressions associated with pain (Chapman & Anderson, 2012). More generally, it has been argued that self-report and facial expressions of disgust can have less to do with a disgust feeling than with a communicative signal that the stimulus in question is objectionable, disliked, and/or worth avoiding (Gilbert, Fridlund, & Sabini, 1987; Jäncke & Kaufmann, 1994; Royzman & Kurzban, 2011; Rozin et al., 2009; Tybur et al., 2013). When researchers have instead explored peripheral physiological signals associated with core-disgust versus B-BV injury disgust, they tend to find more differences than commonalities. Specifically, reactions to B-BV injury are more closely associated with cardiac changes (in particular, decelerated heart rate; Gross, 1998; Gross & Levenson, 1993; Rohrmann & Hopp, 2008; reviewed in Kreibig, 2010), whereas core disgust has been more consistently associated with gastric reactivity (in particular, slowing or irregularity of stomach contraction; Harrison, Gray, Gianaros, & Critchley, 2010; Peyrot des Gachons, Beauchamp, Stern, Koch, & Breslin, 2011; Stern, Jokerst, Levine, & Koch, 2001). The latter association with gastric reactivity might be expected given the presumed evolutionary origins of disgust (Angyal, 1941; Curtis & Biran, 2001; Rozin & Fallon, 1987; Vrana, 2009). The question then becomes whether the analogy between these two underlying physiological responses still holds at the teleological level (i.e., whether these two mechanisms are directed at contaminant avoidance), or whether the dissociated physiological mechanisms betray a fundamental distinction between what participants are reacting to within the different sets of stimuli (i.e., potential contaminants on the one hand and simulated pain on the other). In other words, would the same dissociation be observed if participants viewed instances of painful injury that lacked blood or explicit violations of the body envelope? Our study addresses this question.
We tested whether reactions while viewing instances of painful injury, in the absence of blood and body-envelope violations, produce the communicative hallmarks of disgust while at the same time producing physiological patterns distinct from those associated with core disgust. Participants viewed videos of core disgust imagery (e.g., vomiting), painful injuries (e.g., sports injuries), or affectively neutral imagery (documentary clips). We used a variety of measures of disgust – affective and somatic self-reports, facial electromyography (EMG), cardiac measures of sympathetic and parasympathetic changes (inter-beat interval [IBI] and respiratory sinus arrhythmia [RSA]), and a measure of enteric reactivity (electrogastrography [EGG]) – in order to test whether painful injuries and core disgust stimuli are similarly communicated as disgusting, but dissociate in their peripheral physiological responses. Consistent with the literature described above differentiating B-BV injury from core disgust, we predicted that “painful-injury disgust” and core disgust would be differentially associated with cardiac versus gastric reactivity, respectively. Moreover, we predicted that these different physiological indicators would be related to individual differences in the degree to which a given participant would rate their experience as “disgusting.”
Method
Participants
We recruited 95 participants (47 [49%] female) from the study pool at a New England university and the surrounding community. We excluded participants who had cardiovascular disease, were taking medications that could affect cardiovascular functioning and those with Body Mass Indices (BMIs) over 33. Upon arrival at the laboratory four participants did not comply with pre-session instructions regarding fasting prior to the session1 and six exceeded our BMI criteria. One participant did not consent to watch the films, and four additional participants were excluded for other reasons: two due to incomplete sessions, one due to technical difficulties, and one (in the control condition) who did not watch any of the films. After these a priori exclusions, 80 participants remained (39 [49%] female; Mage = 27.1, SD = 9.0).
Procedure
After participants provided initial consent, which only described the sensors that would be attached, we confirmed compliance with study day instructions. Participants were then asked to consume a high-protein snack (Cliff® Bar) in order to help generate normal gastric activity (Stern, personal communication, 2009). Physiological sensors were then applied, and participants completed baseline self-report measures of affective states, followed by a five-minute period of resting/baseline physiological recording.
Participants were then randomly assigned to one of three affect induction conditions: Neutral/Control (N=27), Core Disgust (N=28), or Painful Injury (N=25). They provided a second consent and proceeded to watch one of three film montages we created (see below) in three separate viewings of unique content, with tasks inserted after each viewing. After the third film and subsequent tasks, participants provided ratings of their affective and somatic reactions to the films they watched. Between each set of films, participants in all conditions performed the same set of distracter tasks, including solving word problems and providing judgments in various scenarios, which lasted approximately 5-6 minutes total between each set of films. No significant between-group differences were found for any of these secondary tasks, so they are not discussed further.
Stimuli
We culled short film clips from movies, television shows, and the internet, and compiled them into montages centered on: a) neutral scenes, b) elicitors of core disgust, or c) painful injuries. Neutral clips were derived primarily from documentary film segments, and included a variety of scenes of animals in nature, people in rural and urban settings, landscapes, and landmarks. Core Disgust clips focused on the production of and interactions with bodily products such as pus, feces, and vomit and were sourced from films used in previous induction studies – including scenes from Pink Flamingos (Gross & Levenson, 1995; Rottenberg et al., 2007; Vianna & Tranel, 2006), Trainspotting (Lerner, Small, & Loewenstein, 2004; Schnall, Haidt, Clore, & Jordan, 2008), and an episode of the television show Jackass during which an omelet containing vomit is made and consumed (Rohrmann & Hopp, 2008) – as well as internet content. Painful Injury clips focused on images of bodily injuries primarily caused through accident/misstep, including visible leg fractures in football and kickboxing and falls in skateboarding and gymnastics. Injuries caused by external agents were excluded to minimize induction of additional emotions such as anger or fear. In order to avoid contamination between the Core Disgust and Painful Injury conditions, Core Disgust film clips excluded imagery of painful acts/procedures, and the Painful Injury clips excluded images of bodily byproducts of injury (e.g., blood). Moreover Painful Injury clips focused on external injuries, avoiding images of explicit body envelope violations (e.g., bodily impalement).
A total of approximately seven minutes of film clips were collected for each condition, and divided into three montages of approximately 2min 20s each. None of the films included an audio track. Film clips are available from the authors.
Measures
Electrogastrography (EGG)
EGG recordings were taken to measure the frequency and amplitude of gastric muscle contractions. Two disposable Ag Ag-Cl electrodes were placed on the lower abdomen, one approximately 4cm directly above the umbilicus and the other 6-8cm from the first, above and to the participant’s left, so that the electrodes formed a 45° angle approximating the stomach’s antral axis (Chang, 2005; Stern et al., 2001; Stern, Koch, Stewart, & Vasey, 1987). A ground electrode was placed below the participant’s right rib. When necessary, a hair trimmer was used to remove excess hair from regions of the abdomen where these sensors needed to be applied (participants were notified of this possibility in advance of the session).
EGG data were analyzed with purpose-written routines in Matlab. Following a preprocessing stream similar to Harrison et al. (2010), participants’ data were re-sampled at 10Hz and analyzed in 4-minute segments. Each segment was detrended, mean-centered, a Hamming window was applied, the data were forward-reverse Butterworth filtered (third order, window: 0.5-9.75cpm), and then submitted to a Fast Fourier Transform. In order to perform statistical analysis between conditions, the data were reduced to average power within each of the frequency bands of interest (relative to total power across the 0.5-9.75cpm window) for 4-minute segments beginning at the start of each film clip and for two 75% overlapping 4-minute segments during the 5min baseline period. In order to minimize generic effects of novelty and expectancy violation when examining physiological reactivity to the different film types (cf. Vrana, 2009), analyses for this and all other physiological sensors focused on the final two sets of film clips for each condition, compared to baseline. The frequency band of interest was the well-characterized normogastric range (2.5-3.75cpm), which is associated with normal digestion and where power has been shown to decrease when asked to ingest undesirable foods (Stern et al., 2001). Analyses were also performed over the tachygastric (3.75-9.75cpm) range, where power has been shown to increase in the context of motion-induced nausea (Gianaros, Quigley, Mordkoff, & Stern, 2001). Given the short length of our film clips we did not have enough data to reliably calculate power in the bradygastric (0.5-2.5cpm) range.
Electromyography (EMG)
In order to evaluate the magnitude of facial muscle contraction in response to each of the film types, facial EMG recording was performed with standard 4mm Ag Ag-Cl electrodes placed at three facial muscles: the orbicularis oris, levator labii, and corrugator supercilli. All electrodes were placed on the right side of the participant’s face, and placement followed the muscle-specific recommendations of Fridlund & Cacciopo (1986). Prior to application of electrodes, experimenters cleaned and abraded the skin at each site to reduce resistance across each electrode pair. Experimenters trained on pilot subjects on preparation and application at all of the facial recording sites used in this study until they reliably achieved proficiency at reaching resistance below 15 kΩ for the initial attempt at each site without needing to re-abrade the skin. The participants were not given any explicit cover story regarding what EMG or any of the other physiological sensors were measuring.
EMG data were analyzed as the mean of the EMG response in non-overlapping 20-second intervals across the baseline period and for 2min 20s prior to the end of each film clip. Averages of mean EMG signal across film segments were compared to the minimum EMG signal at baseline (i.e., the 20-second interval with the lowest average response). As recommended, we log-transformed the difference scores, which were significantly positively skewed (Blascovich, Mendes, Vanman, & Dickerson, 2011).
Cardiovascular measures
In order to obtain measures of heart rate variability (HRV), electrocardiographic (ECG) recording was performed with disposable electrodes attached in a Modified Lead II configuration (right upper chest, left lower rib). ECG data were scored in 60-second intervals at baseline and during film viewing using Mindware software (HRV 2.6). We focus here on the high frequency band of heart rate variability (RSA) given its link to parasympathetic activity, as well as the inter-beat interval (IBI) of the cardiac signal. Each measure was averaged over film segments and compared to the final minute of baseline to form a change score.
Subjective Experience
Participants completed the Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988), modified to include ratings for ‘disgust’ and ‘pain.’ They rated their affect on a computerized analog scale (anchored at ‘not at all’ and ‘extremely’) before the baseline recordings (with respect to their present state) and at the end of the session (with respect to their state during film presentation). Alphas for positive and negative affect were acceptable and ranged from .70 to .90.
As a subjective measure of somatic reactivity, participants completed the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) after the second PANAS, providing retrospective ratings of their bodily states while watching the films. Ratings were given on a 4-point Likert scale to indicate how much the participant currently felt, for example, ‘shaky,’ ‘faint,’ and ‘indigestion or discomfort in abdomen’ (α = .90). Analyses of subjective reactivity to the films examined absolute scores on the somatic reactivity scale, as well as difference scores comparing a participant’s ratings of a given affective state on the PANAS during the film to their baseline ratings of those same items. Analyses of these difference scores utilize ANCOVAs that control for baseline ratings.
Results
Self-reported affective responses
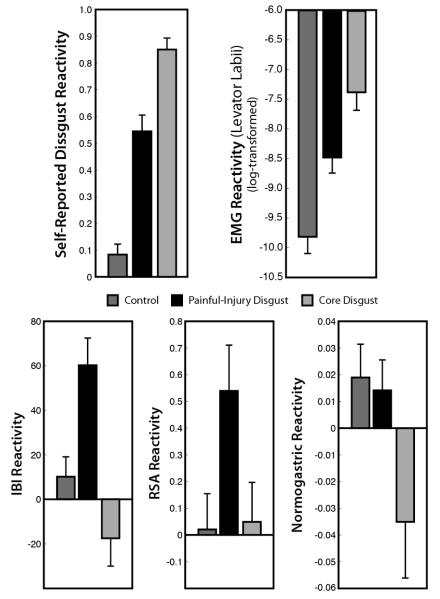
We first examined overall changes in positive and negative affect. Relative to baseline ratings, participants in both Core Disgust and Painful Injury groups reported significantly higher ratings of negative affect, F (2, 76) = 8.58, p < .0005, η2= 0.18, lower ratings of positive affect, F (2, 76) = 10.49, p < .0001, η2 = 0.20, and higher ratings of somatic reactivity, F (2, 77) = 11.09, p < .0001, η2 = 0.23, than those in the Control group. However, the two affect groups did not differ from each other either along these composite ratings of affect or in ratings of somatic reactivity (all F’s < 1.65).
We then examined individual items from the PANAS. Relative to other affective states, “disgust” showed the greatest change of any other affective item for participants in both the Core Disgust and Painful Injury conditions. We then compared these ratings to participants in the Control (neutral film) condition. The simple comparison between Core Disgust and Control conditions was significant (F (1, 76) = 140.60, p < .0001, d = 3.63), as was Painful Injury compared to Control film, (F (1, 76) = 49.94, p < .0001, d = 1.85). Disgust ratings were also higher for Core Disgust versus Painful Injury conditions, (F (1, 76) = 19.76, p < .0001, d = 1.19). That is, the two affect imagery conditions clearly increased “disgust” self-reports relative to control imagery, although core-disgust stimuli produced a larger effect than stimuli that elicited ‘painful-injury disgust.’
Facial EMG
We observed a significant main effect of film condition on EMG activity at the levator labii muscle, F(2,73) = 18.93, p<0.0001, η2 = 0.34, with participants in the Core (M [log-transformed] = −7.38) and Painful-Injury Disgust (M = −8.48) conditions displaying significantly greater increases relative to Control participants (M = −9.82) (F(1,73) = 37.79, p<.0001, d = 1.64; F(1,73) = 10.48, p<.005, d = 1.01). Core Disgust also elicited significantly greater levator activity than Painful-Injury Disgust, F(1,73) = 7.22, p<.01, d = 0.79,. Activity at the corrugator muscles exhibited a similar but weaker pattern to that of the levator muscle (Core Disgust vs. Control: F(1,75) = 9.01, p<.005, d = 0.75; Painful-Injury Disgust vs. Control: F(1,75) = 2.67, p=.11; Core vs. Painful-Injury Disgust: F(1,75) = 1.52, ns), and orbicularis oris activity did not differ significantly across the film conditions, F(2,70) = 1.61.
Cardiovascular reactivity
We observed main effects of film condition on cardiac changes, IBI: F(2,77) = 11.85, p<0.0001, η2 = 0.24; RSA: F(2,77) = 3.61, p < .04, η2 = 0.09, both driven primarily by reactivity to Painful-Injury Disgust relative to the other two film types (Figure 1). Participants in the Painful-Injury Disgust condition showed significantly lower HR (longer IBI; i.e., greater deceleration) and higher RSA while watching the films than the other two conditions: IBI: Painful Injury vs. Core Disgust F (1,77) = 23.27, p<.0001, d = 1.24; Painful-Injury Disgust vs. Control F (1,77) = 9.50, p<.005, d = 0.95; RSA: Painful-Injury vs. Core Disgust F (1, 77) = 5.23, p=.02, d = 0.61; Painful-Injury Disgust vs. Control F(1, 77) = 5.75, p=.02, d = 0.68. The differences between Core Disgust and Control were not significant for RSA (F < 0.10) and showed a marginally significant increase in HR (shorter IBI) for Core Disgust, F (1, 77) = 3.06, p=.08, d = 0.49.
Figure 1.
Average affective, facial, and physiological reactivity to film viewing, relative to baseline, for the three conditions. Top: Relative to Control, both Core Disgust and Painful-Injury Disgust engendered increased self-reported disgust and contraction of the levator labii, albeit to varying degrees. Bottom: Painful-Injury but not Core Disgust let to increased heart rate variability (greater RSA) and heart rate deceleration (greater IBI). Conversely, Core Disgust, but not Painful-Injury Disgust, resulted in a decrease of stomach muscle contractions within the normal range (normogastry). Error bars represent s.e.m.
Another way to examine these responses is to test changes from a resting state. Only the Painful-Injury Disgust condition showed a significant change from baseline such that IBI and RSA reactivity significantly increased from resting states, t (24) = 4.9, p < .0001, d = 0.99; t (24) = 3.1, p < .005, d = 0.63, respectively. Core Disgust and Control films did not engender significant cardiac changes (all p-values > .17).
Gastric reactivity
We then examined changes in the normogastric range (2.5-3.75cpm) by emotion induction and observed a significant main effect, F(2, 74) = 3.73, p < .05, η2 = 0.09. In contrast to measures of cardiac reactivity, this main effect was driven by reactivity to the Core Disgust films relative to the other two film types (Figure 1). Consistent with the prediction that core disgust would reduce amplitude in the normogastric range, participants in the Core Disgust condition had significantly lower normogastric activity while watching the films, relative to the other two conditions: Core Disgust vs. Control: F(1, 74) = 5.99, p < .05, d = −0.64; Core vs. Painful-Injury Disgust: F(1, 74) = 4.97, p < .05, d = −0.59) (Figure 1). Control and Painful-Injury Disgust did not differ from each other, F(1, 74) = 0.05, ns. These effects appeared to be specific to normogastry, as we did not find a main effect of group on percent EGG power within the tachygastric range (3.75-9.75cpm), F(2,74) = 0.46, ns.
In summary, we observed several key patterns of physiological reactivity as a function of our emotion induction films that were consistent with our predictions. While both disgust conditions induced higher ratings of disgust and greater activation of the levator labii muscles relative to neutral films (albeit to different degrees), the two conditions differed in their influence on cardiac versus gastric reactivity. Cardiac measures (IBI and heart rate variability) were specifically influenced (sympathetic activation2 decreased, whereas parasympathetic activation increased) when watching the Painful-Injury films, whereas electrogastric activity was influenced (decreased) when watching Core Disgust films compared to the other conditions. This final dissociation between cardiac and gastric reactivity was confirmed by a significant interaction of measurement type (normogastry, RSA, IBI) by condition (Control, Core Disgust, Painful-Injury Disgust) on physiological reactivity, mixed-effect ANOVA F(4, 151.3) = 2.61, p < .05.
Correlations between subjective ratings and physiological reactivity to disgust
If disgust is differently expressed in the context of core disgust versus painful-injury stimuli according to levels of gastric versus cardiac reactivity, then we might expect self-reported ratings of disgust within the two conditions to correlate with the physiological measure most closely aligned with that condition. Specifically, we expected disgust ratings in the Core Disgust condition to correlate with gastric reactivity, and we expected disgust ratings in the Painful-Injury Disgust condition to correlate with cardiac reactivity. Because the ratings for the single-item disgust response were clustered near ceiling, we created a more conservative and normally distributed disgust index by averaging ratings of disgust with ratings of abdominal discomfort on the somatic reactivity questionnaire (these two items were significantly correlated: Spearman’s rho (80) = 0.56, p < .0001). Moreover, we controlled for effects of age, gender, BMI, and baseline physiological response, factors that can affect individual differences in our physiological measures of interest (Blascovich et al., 2011; Chang, 2005). We use robust regression to obtain significance values and partial correlations to obtain effect sizes (Pearson’s r).
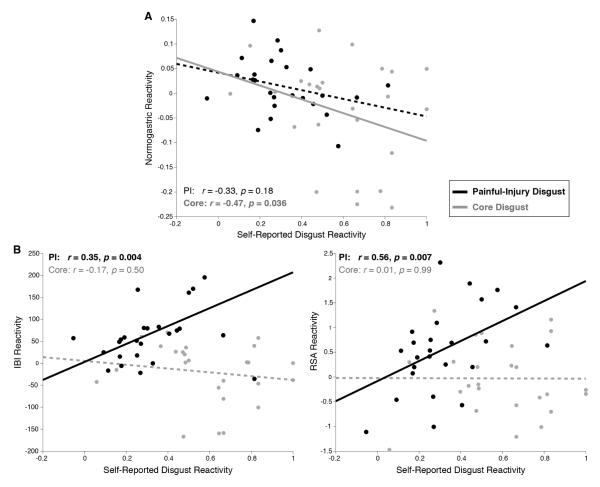
For participants in the Core Disgust condition, we observed a significant relationship between subjective experiences of disgust and lower gastric activity during film viewing (p < .04), whereas the relationship between the disgust index and gastric activity was not significant in the Painful-Injury disgust condition (p = .18) (Figure 2A). In contrast, participants viewing the Painful-Injury imagery showed significant relationships between their subjective experiences of disgust and cardiac reactions (greater IBI and RSA reactivity) (ps < .003), while participants viewing core-disgust imagery did not yield correlated responses between subjective experiences of disgust and cardiac measures (ps > .50) (Figure 2B)3. We then examined if film condition significantly moderated relationships between subjective experiences and physiological responses. The interaction was significant for cardiac measures (IBI: b = 238.7, p < .02; RSA: b = 2.06, p < .06), but not gastric reactivity (b=.03, p = .74).
Figure 2.
Individual differences in disgust reactivity are predicted by individual differences in physiological reactivity. (A) The magnitude of Painful-Injury Disgust reactions correlated with the magnitude of cardiovascular reactivity during film viewing, with greater increases in RSA and IBI predicting greater self-reported disgust. No such correlations were observed for the Core Disgust condition. All correlations control for effects of age, gender, BMI, and baseline measures associated with a given physiological response. Partial Pearson’s r-values are supplemented with p-values from a robust regression. (B) The magnitude of Core Disgust reactions correlated with the magnitude of normogastric reactivity during film viewing, with greater decreases in normogastry predicting greater self-reported disgust. A non-significant trend was observed in the Painful-Injury Disgust condition.
Finally, we tested whether correlations between cardiac reactivity and disgust ratings in the Painful-Injury condition were, in part, due to the subjective empathy for the injury victims. First, we noted that both self-reports of pain and sadness were significantly elevated in the Painful-Injury condition relative to Controls, Fpain(1, 77) = 12.7, p < 0.001, d = 1.00, Fsadness(1, 77) = 26.4, p < 0.001, d = 1.50. We then examined whether either cardiac measure (IBI or RSA) was correlated with reports of pain or sadness during film viewing. We found that neither measure of cardiac reactivity was correlated with pain, sadness, or a composite of the two ratings (ps > .40) in the Painful-Injury group. Furthermore, we found that the correlations between Painful-Injury disgust ratings and cardiac reactivity were robust even when controlling for ratings of pain and sadness (IBI: r = 0.33, p < .03; RSA: r = 0.56, p < .02).
Discussion
In writing The Jungle (Sinclair, 1906), Upton Sinclair’s original aim was to incite a call to action against the poor working conditions for the individuals in American meat factories. When he found that attention had instead focused on the grotesque practices of meat preparation his book had revealed, he famously lamented, “I aimed at the public’s heart, and by accident I hit it in the stomach” (Arthur, 2007, p. 83). Our study suggests that Sinclair’s metaphorical assessment may have been more prescient than previously thought. We found that reactions to painful injuries and to orogastrically objectionable products both elicited self-reported and facial signatures of disgust, albeit to different degrees. However, they clearly engendered different responses in the sympathetic/parasympathetic versus enteric systems. Whereas core disgust evoked decreases in normal gastric contraction, painful-injury disgust brought about decelerated heart rate and increased parasympathetic response. These physiological signatures separately predicted the level of disgust reported in their respective domain: gastric reactivity correlated with self-reported Core Disgust, and cardiac reactivity correlated with self-reported Painful-Injury Disgust.
As recent reviews of the disgust literature have pointed out (Chapman & Anderson, 2012; Tybur et al., 2013), emotion theorists have struggled to identify the common threads that motivate both our verbal and facial expressions of disgust across a widely varied set of stimuli. One approach to resolving this has been to posit an evolutionary basis for all expressions of disgust that typically involves an early-evolved system for detecting contaminants/pathogens (Tybur, Lieberman, & Griskevicius, 2009; Tybur et al., 2013), with an associated disgust feeling, that is later co-opted to serve other adaptive means, such as rejecting unfit partners (Sexual Disgust) or unfit group members (Moral Disgust). Under Tybur et al.’s (2009; 2013) model, the evolutionarily early category of ‘pathogen disgust’ includes both food- and blood-borne contaminants (see also Rozin, Haidt, & McCauley, 2008) and encompasses the cases we have referred to as Core and B-BV Disgust. An alternative account concurs that feelings of disgust may have evolved for more basic purposes (e.g., to avoid orogastrically objectionable items) but that its use in common vernacular and in communicative facial expressions has, in certain instances, been co-opted on a metaphorical basis to express a more general feeling (Royzman & Kurzban, 2011; Royzman & Sabini, 2001; Rozin et al., 2009) – such as the desire to distance oneself from the cue in question (cf. Royzman & Sabini, 2001) – or a different feeling altogether (e.g., anger; Nabi, 2002), rather than to reference the actual feeling of disgust. The metaphorical account has been offered particularly to explain disgust reactions that appear less directly linked to contaminant avoidance, such as disgust at norm violations. It is supported by evidence that facial expressions associated with disgust – including contraction of the levator – are non-specific to disgust (Haidt & Keltner, 1999; Russell, 1994; Russell, Bachorowski, & Fernández-Dols, 2003; Wolf et al., 2005), and their use in expressing disgust is under conscious control and influenced by social context (e.g., whether they are aware of being observed; Fridlund, 1992; Gilbert et al., 1987; Jäncke & Kaufmann, 1994).
Consistent with this latter account, we found that ‘disgust’ is reliably communicated in response to images of acute external injury in the absence of blood or body envelope violation, On its face this result fits less parsimoniously with a pathogen avoidance account than a general motivation to withdraw (and/or offer help; see below). Furthermore, we found that this form of disgust was associated with a physiological signature dissociable from that of Core Disgust, which by contrast involved stimuli that would intuitively engender pathogen avoidance and was directly associated with the orogastric channel, as predicted by most models of disgust (Angyal, 1941; Curtis & Biran, 2001; Rozin & Fallon, 1987; Rozin et al., 2008; Tybur et al., 2013). While by no means definitive on the matter, our results therefore at least call into question whether the ‘disgust’ expressed in our Painful-Injury condition shares a biological origin with this more basic form of disgust, and therefore whether it is undergirded by a disgust feeling. By association, the fact that similar physiological dissociations have been observed when previous researchers contrasted Core Disgust with B-BV Disgust – i.e., when stimuli may have forewarned blood-borne contamination but typically also conveyed painful injury (Chapman & Anderson, 2012; Harrison et al., 2010) – raises concerns regarding the degree to which a disgust feeling is being induced by the B-BV stimuli in these studies.
One response to this metaphorical account might simply be to suggest that the Painful-Injury disgust we observed is still a form of Pathogen Disgust but that the pathogen avoidance role has been made so general as to extend to cases where any injury is observed, regardless of whether contamination to the observer is hypothetically possible (even when imagining the event occurring nearby). If this were the case, the physiological dissociation we observe might simply represent similar heterogeneity of mechanisms within Pathogen Disgust as has been proposed between this class of disgust and others (e.g., Moral Disgust; Schaich Borg, Lieberman, & Kiehl, 2008). While difficult to argue against this perspective with the current data, the adaptive value of such a broad filter for pathogen avoidance – relative to the potential costs of overestimating disease risk at the sight of any external injury – would need to be addressed. It would be similarly difficult to argue categorically that the variety of injuries our participants observed did not in some way serve as reminders of their “animal natures” (Rozin & Fallon, 1987; Rozin et al., 2008; Rozin, Lowery, & Ebert, 1994; but see Tybur et al., 2013, for a discussion of the limitations of this explanatory account).
It also might still be the case that elements of B-BV injury stimuli (particularly, blood or viscera) elicit a disgust response for their orogastrically objectionable properties or even for their direct association with disease-related contaminants. However, to the extent that researchers have and continue to find different substrates for reactions to these compared to core disgust stimuli, it is worth considering whether it may in part be due to their painful rather than simply contamination-related properties. Our current study is limited in addressing this in the case of BBV injury disgust, but follow-up experiments will seek to probe this question by comparing these stimuli directly to the painful-injury stimuli used in this experiment.
Whatever the appropriate label for these experiences, our findings may offer insight into why some “disgust-like” reactions only engender withdrawal whereas others can also ultimately lead to helping behavior. The paradoxical possibility that short-term indicators of withdrawal can also elicit empathic concern towards others is borne out by a recent set of studies by Tullett and colleagues (Tullett, 2012; Tullett, Harmon-Jones, & Inzlicht, 2012). They show, for instance, that verbal and facial expressions of disgust towards graphic images of the suffering predict levels of empathic concern towards those same individuals. As discussed above, self-report and facial expressions of disgust in our study are consistent with the expression of such withdrawal motivations during Painful-Injury films. Interestingly, we further show that ratings of disgust account for individual differences in cardiac reactivity to these films better than ratings of pain or sadness, suggesting that empathic pain was either not reflected in these physiological responses or that it was present but participants are more accustomed to expressing such experiences through withdrawal (or they are more introspectively aware of the associated withdrawal motivations).
Relative to Core Disgust reactions, the expressions and underlying physiology associated with pain-associated disgust reactions may therefore have different time courses and be differently prioritized when it comes to motivating action. Exploring how these affective and physiological reactions evolve and interact in real time, particularly when an individual perceives stimuli that elicit both reactions, represents an important avenue for future research. Indeed, building on the current findings in such a way may help to answer whether Sinclair’s goals would have been best served by focusing his prose more squarely on the worker’s pain rather than what was going into their product.
Acknowledgments
We would like to thank our undergraduate research assistants and summer interns from the Health and Psychophysiology Lab. This research was supported by an NHLBI grant to W.B.M. and an NSF Graduate Research Fellowship to A.S.
Footnotes
Based on recommendations (Stern, personal communication, 2009), participants were asked to refrain from eating four hours prior to their arrival for the study. Time to last meal and last meal contents were probed at the start of each session. Participants who reported eating any meals in the previous two hours at the start of the session were excluded from participation. Subsequent analyses confirmed that the EGG results reported were not influenced by accounting for time to last meal.
IBI increases (like heart rate) are influenced by both sympathetic deactivation and parasympathetic activation so it is by no means a pure measure of sympathetic nervous system responses. We did not measure responses that would allow us to estimate a pure measure of SNS activation (like pre-ejection period) so we note that IBI in this case is a substandard measure of SNS.
Similar correlations between subjective ratings and physiological reactivity obtain when only using the single disgust item rather than the (more normally-distributed) composite reported in Figure 2: Painful-Injury Disgust - rIBI = 0.52, p < .02; rRSA = 0.38, p < .03; rEGG = −0.31, p = .14; Core Disgust - rIBI = 0.11, p = .54; rRSA = −0.19, p = .13; rEGG = −0.54, p < .03.
References
- Angyal A. Disgust and related aversions. The Journal of Abnormal and Social Psychology. 1941;36(3):393–412. [Google Scholar]
- Arthur A. Radical Innocent: Upton Sinclair. Random House; 2007. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of consulting and clinical psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Mendes WB, Vanman E, Dickerson S. Social Psychophysiology for Social and Personality Psychology. Sage Publications; 2011. [Google Scholar]
- Chang F-Y. Electrogastrography: basic knowledge, recording, processing and its clinical applications. J Gastroenterol Hepatol. 2005;20(4):502–516. doi: 10.1111/j.1440-1746.2004.03751.x. doi: 10.1111/j.1440-1746.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Anderson AK. Understanding disgust. Annals of the New York Academy of Sciences. 2012;1251:62–76. doi: 10.1111/j.1749-6632.2011.06369.x. doi: 10.1111/j.1749-6632.2011.06369.x. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Kim DA, Susskind JM, Anderson AK. In Bad Taste: Evidence for the Oral Origins of Moral Disgust. Science. 2009;323(5918):1222–1226. doi: 10.1126/science.1165565. doi: 10.1126/science.1165565. [DOI] [PubMed] [Google Scholar]
- Curtis V, Biran A. Dirt, disgust, and disease. Is hygiene in our genes?. Perspectives in biology and medicine. 2001;44(1):17–31. doi: 10.1353/pbm.2001.0001. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ. Darwin’s anti-Darwinism in the expression of the emotions in man and animals. In: Strongman KT, editor. Intemationai review of studies on emotion. Vol. 2. Wiley; Oxford, England: 1992. pp. 117–137. [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23(5):567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Quigley K, Mordkoff J, Stern RM. Gastric myoelectrical and autonomic cardiac reactivity to laboratory stressors. Psychophysiology. 2001;38(4):642–652. [PMC free article] [PubMed] [Google Scholar]
- Gilbert AN, Fridlund AJ, Sabini J. Hedonic and social determinants of facial displays to odors. Chemical Senses. 1987;12(2):355–363. doi: 10.1093/chemse/12.2.355. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion Elicitation Using Films. Cognition and Emotion. 1995;9(1):87–108. [Google Scholar]
- Haidt J, Keltner D. Culture and Facial Expression: Open-ended Methods Find More Expressions and a Gradient of Recognition. Cognition & Emotion. 1999;13(3):225–266. [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The Embodiment of Emotional Feelings in the Brain. Journal of Neuroscience. 2010;30(38):12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Kaufmann N. Facial EMG responses to odors in solitude and with an audience. Chemical Senses. 1994;19(2):99–111. doi: 10.1093/chemse/19.2.99. doi: 10.1093/chemse/19.2.99. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Small DA, Loewenstein G. Heart strings and purse strings: Carryover effects of emotions on economic decisions. Psychological Science. 2004;15(5):337–341. doi: 10.1111/j.0956-7976.2004.00679.x. doi: 10.1111/j.0956-7976.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Nabi RL. The theoretical versus the lay meaning of disgust: Implications for emotion research. Cognition & Emotion. 2002;16(5):695–703. [Google Scholar]
- Oaten M, Stevenson RJ, Case TI. Disgust as a disease-avoidance mechanism. Psychological Bulletin. 2009;135(2):303–321. doi: 10.1037/a0014823. [DOI] [PubMed] [Google Scholar]
- Olatunji B, Haidt J, McKay D, David B. Core, animal reminder, and contamination disgust: Three kinds of disgust with distinct personality, behavioral, physiological, and clinical correlates. Journal of Research in Personality. 2008;42(5):1243–1259. [Google Scholar]
- Peyrot des Gachons C, Beauchamp GK, Stern RM, Koch KL, Breslin PAS. Bitter taste induces nausea. Current Biology. 2011;21(7):R247–248. doi: 10.1016/j.cub.2011.02.028. doi: 10.1016/j.cub.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Hopp H. Cardiovascular indicators of disgust. International Journal of Psychophysiology. 2008;68(3):201–208. doi: 10.1016/j.ijpsycho.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Ray RR, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJB, editors. The handbook of emotion elicitation and assessment. Oxford University Press; New York: 2007. pp. 9–28. [Google Scholar]
- Royzman EB, Kurzban R. Minding the Metaphor: The Elusive Character of Moral Disgust. Emotion Review. 2011;3(3):269–271. doi: 10.1177/1754073911402371. [Google Scholar]
- Royzman EB, Sabini J. Something it Takes to be an Emotion: The Interesting Case of Disgust. Journal for the Theory of Social Behaviour. 2001;31(1):29–59. doi: 10.1111/1468-5914.00145. [Google Scholar]
- Rozin P, Fallon A. A perspective on disgust. Psychological Review. 1987;94(1):23. [PubMed] [Google Scholar]
- Rozin P, Haidt J, Fincher K. Psychology. From oral to moral. Science. 2009;323(5918):1179–1180. doi: 10.1126/science.1170492. doi: 10.1126/science.1170492. [DOI] [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley CR. Disgust. In: Lewis M, Havilland-Jones JM, Barrett LF, editors. Handbook of emotions. 3rd ed Guilford Press; New York, NY: 2008. pp. 757–776. [Google Scholar]
- Rozin P, Lowery L, Ebert R. Varieties of disgust faces and the structure of disgust. Journal of Personality and Social Psychology. 1994;66(5):870. doi: 10.1037//0022-3514.66.5.870. [DOI] [PubMed] [Google Scholar]
- Russell JA. Is there universal recognition of emotion from facial expressions? A review of the cross-cultural studies. Psychol Bull. 1994;115(1):102–141. doi: 10.1037/0033-2909.115.1.102. [DOI] [PubMed] [Google Scholar]
- Russell JA, Bachorowski J, Fernández-Dols J. Facial and vocal expressions of emotion. Annual Review of Psychology. 2003;54(1):329–349. doi: 10.1146/annurev.psych.54.101601.145102. [DOI] [PubMed] [Google Scholar]
- Schaich Borg J, Lieberman D, Kiehl KA. Infection, incest, and iniquity: investigating the neural correlates of disgust and morality. Journal of Cognitive Neuroscience. 2008;20(9):1529–1546. doi: 10.1162/jocn.2008.20109. doi: 10.1162/jocn.2008.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnall S, Haidt J, Clore GL, Jordan AH. Disgust as Embodied Moral Judgment. Personality and Social Psychology Bulletin. 2008;34(8):1096–1109. doi: 10.1177/0146167208317771. doi: 10.1177/0146167208317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair U. The Jungle. Doubleday, Jabber, & Company; 1906. [Google Scholar]
- Stark R, Walter B, Schienle A, Vaitl D. Psychophysiological Correlates of Disgust and Disgust Sensitivity. Journal of Psychophysiology. 2005;19(1):50–60. doi: 10.1027/0269-8803.19.1.50. [Google Scholar]
- Stern RM, Jokerst M, Levine M, Koch K. The stomach’s response to unappetizing food: cephalic–vagal effects on gastric myoelectric activity. Neurogastroenterology & Motility. 2001;13(2):151–154. doi: 10.1046/j.1365-2982.2001.00250.x. [DOI] [PubMed] [Google Scholar]
- Stern RM, Koch KL, Stewart WR, Vasey MW. Electrogastrography: current issues in validation and methodology. Psychophysiology. 1987;24(1):55–64. doi: 10.1111/j.1469-8986.1987.tb01862.x. [DOI] [PubMed] [Google Scholar]
- Tullett AM. Withdrawal Motivation and Empathy:Do Empathic Reactions Reflect the Motivation to “Reach Out” or the Motivation to “Get Out”? (PhD Dissertation) University of Toronto; Ontario, Canada: 2012. [Google Scholar]
- Tullett AM, Harmon-Jones E, Inzlicht M. Right frontal cortical asymmetry predicts empathic reactions: Support for a link between withdrawal motivation and empathy. Psychophysiology. 2012;49:1145–1153. doi: 10.1111/j.1469-8986.2012.01395.x. [DOI] [PubMed] [Google Scholar]
- Tybur JM, Lieberman D, Griskevicius V. Microbes, mating, and morality: individual differences in three functional domains of disgust. Journal of Personality and Social Psychology. 2009;97(1):103–122. doi: 10.1037/a0015474. [DOI] [PubMed] [Google Scholar]
- Tybur JM, Lieberman D, Kurzban R, DeScioli P. Disgust: Evolved Function and Structure. Psychological Review. 2013;120(1):65–84. doi: 10.1037/a0030778. [DOI] [PubMed] [Google Scholar]
- Vianna E, Tranel D. Gastric myoelectrical activity as an index of emotional arousal. International Journal of Psychophysiology. 2006;61(1):70–76. doi: 10.1016/j.ijpsycho.2005.10.019. doi: 10.1016/j.ijpsycho.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Vrana SR. The psychophysiology of disgust: Differentiating negative emotional contexts with facial EMG. Psychophysiology. 1993;30(3):279–286. doi: 10.1111/j.1469-8986.1993.tb03354.x. doi: 10.1111/j.1469-8986.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR. The psychophysiology of disgust: Motivation, action, and autonomic support. In: Olatunji BO, McKay D, editors. Disgust and its disorders: Theory, assessment, and treatment. American Psychological Association; Washington, DC: 2009. pp. 123–143. [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988 doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wolf K, Mass R, Ingelbleek T, Kiefer F, Naber D, Weidemann K. The facial pattern of disgust, appetence, excited joy and relaxed joy: An improved facial EMG study. Scandinavian Journal of Psychology. 2005;46(5):403–409. doi: 10.1111/j.1467-9450.2005.00471.x. doi: 10.1111/j.1467-9450.2005.00471.x. [DOI] [PubMed] [Google Scholar]