Abstract
Leukotrienes generated by 5-lipoxygenase (5-LOX)–catalyzed reaction are key regulators of inflammation. In ionophore-stimulated (A23187; 1–2.5 μM) human blood neutrophils or differentiated HL-60 cells, vitamin E forms differentially inhibited leukotriene B4 (LTB4) with an IC50 of 5–20 μM for γ-tocopherol, δ-tocopherol (δT), and γ-tocotrienol, but a much higher IC50 for α-tocopherol. 13′-Carboxychromanol, a long-chain metabolite of δT, suppressed neutrophil- and HL-60 cell-generated LTB4 with an IC50 of 4–7 μM and potently inhibited human recombinant 5-LOX activity with an IC50 of 0.5–1 μM. In contrast, vitamin E forms had no effect on human 5-LOX activity but impaired ionophore-induced intracellular calcium increase and calcium influx as well as the subsequent signaling including ERK1/2 phosphorylation and 5-LOX translocation from cytosol to the nucleus, a key event for 5-LOX activation. Further investigation showed that δT suppressed cytosolic Ca2+ increase and/or LTB4 formation triggered by ionophores, sphingosine 1-phosphate, and lysophosphatidic acid but not by fMLP or thapsigargin, whereas 13′-carboxychromanol decreased cellular production of LTB4 regardless of different stimuli, consistent with its strong inhibition of the 5-LOX activity. These observations suggest that δT does not likely affect fMLP receptor-mediated signaling or store depletion-induced calcium entry. Instead, we found that δT prevented ionophore-caused cytoplasmic membrane disruption, which may account for its blocking of calcium influx. These activities by vitamin E forms and long-chain carboxychromanol provide potential molecular bases for the differential anti-inflammatory effects of vitamin E forms in vivo.
Leukotrienes are generated by activated leukocytes via 5-lipoxygenase (5-LOX)–catalyzed oxidation of arachidonic acid (AA). Leukotriene B4 (LTB4) and leukotriene C4 (LTC4), produced by neutrophils and eosinophils, respectively, are important lipid regulators of inflammation (1, 2). When leukocytes are stimulated, 5-LOX, which is primarily located in the cytosol under resting condition, translocates to the membrane of the nucleus where it interacts with 5-LOX activating protein to form the functionally active enzyme (3). The translocation of 5-LOX is triggered by intracellular calcium increase, which subsequently activates downstream signaling including protein kinase C (PKC) and ERK (4, 5). Ca2+ release can be stimulated by calcium ionophores such as A23187, thapsigargin (THAP), which induces depletion of endoplasmic reticulum (ER) Ca2+ storage (6), or fMLP, which triggers receptor-mediated calcium release from ER storage via a phospholipase C-mediated mechanism (7). In addition, it has been shown that sphingosine-1 phosphate (S1P) and lysophosphatidic acid (LPA), two important lipid mediators, activate intracellular calcium increase in neutrophils (8, 9). Given the regulatory role of leukotrienes, modulation of their production via potentiating 5-LOX activity or Ca2+-related signaling may have profound effects on inflammation and inflammation-associated diseases.
Previous studies suggest that some vitamin E isoforms may be capable of modulating leukotriene formation. Natural forms of vitamin E comprise eight lipophilic antioxidants; that is, α-, β-, γ-, and δ-tocopherol (αT, βT, γT, and δT) and α-, β-, γ-, and δ-tocotrienol (αTE, βTE, γTE, and δTE) (Fig. 1). In A23187-stimulated neutrophils, αT, the predominant form of vitamin E in tissues, has been reported to enhance 5-LOX–catalyzed LTB4 at low micromolar concentrations but to suppress LTB4 at higher concentrations (10). αT is shown to decrease LPS-stimulated LTB4 from human monocytes (11). Supplementation of αT appears to reverse αT deficiency-caused enhancement of LTB4 production in neutrophils (12, 13) but inconsistently modulates LTB4 release under αT-sufficient conditions (12, 14). Besides αT, we have demonstrated that γT, the major form of vitamin E in the United States diet, suppressed inflammation-enhanced leukotriene generation in several inflammation models in rodents (14–16). Despite these observations, the mechanisms underlying these modulatory effects are not completely understood. It is also not clear whether different forms of vitamin E show differential effects on 5-LOX–mediated reactions. In this study, we investigated the effect and mechanism of different forms of vitamin E on leukotriene formation from human neutrophils or neutrophil-like differentiated HL-60 cells and eosinophil-like differentiated clone 15 HL-60 cells. In addition, in light of our recent studies that 13′-carboxychromanol (13′-COOH) (Fig. 1), a long-chain metabolite of δT, competitively inhibited cyclooxygenases (cyclooxygenase [COX]-1 and COX-2) (17), we also examined whether this vitamin E metabolite has any effect on 5-LOX–catalyzed reactions.
FIGURE 1.

The structures of natural forms of vitamin E and 13′-carboxychromanol (13′-COOH), a metabolite of δT.
Materials and Methods
Chemicals and Abs
Vitamin E forms, A23187, ionomycin, S1P, fMLP, DMSO, and protease inhibitor mixture were purchased from Sigma (St. Louis, MO). LPA was obtained from Enzo Life Sciences (Farmingdale, NY). Zileuton was purchased from Tocris Cookson (St. Louis, MO). Fluo-4 AM, RPMI 1640 medium, DMEM, and HBSS buffer were purchased from Invitrogen Life Technologies (Carlsbad, CA). AA and human recombinant 5-LOX were from Cayman Chemicals (Ann Arbor, MI). The Abs for 5-LOX, ERK1/2 (pT202/pY204), and ERK (pan ERK) were purchased from BD Biosciences Pharmingen (San Diego, CA). The secondary Abs were from Santa Cruz Biotechnology (Santa Cruz, CA).
Isolation of human neutrophils
Neutrophils were isolated from fresh blood by density gradient centrifugation using Histopaque-1077 (Sigma). Blood was obtained from healthy donors following a protocol approved by human research protection program institutional review boards at Purdue University.
Cell differentiation
HL-60 cells and clone 15 HL-60 cells were purchased from American Type Culture Collection (Manassas, VA). These cells were routinely maintained in RPMI 1640 medium supplemented with 10% FBS under 5% CO2. For induction of neutrophil differentiation, 2.2 × 105 to 2.4 × 105 cells/ml were incubated in RPMI 1640 containing 10% FBS plus 1.25% DMSO for 5 d (18–20). For induction of eosinophil differentiation, clone 15 HL-60 cells were incubated in RPMI 1640 containing 10% FBS plus 0.5 mM butyric acid for 5 d (21–23).
Isolation of 13′-COOH from cell culture media
A549 cells were incubated with 50 μM δT for 72 h, and the media were collected for isolation of 13′-COOH using a Supelcosil LC-18-DB Semiprep column (Supelco, Bellefonte, PA) by HPLC as previously described (17). The isolated fractions were freeze-dried, quantified using HPLC, and stored at −80°C until use.
Neutrophil and eosinophil activation
Neutrophils and differentiated HL-60 or clone 15 HL-60 cells (1.6 × 106) were preincubated with DMSO (0.05%), vitamin E forms, or 13′-COOH in DMEM-1% FBS or HBSS (for experiments with S1P or LPA) at 37°C for 10 min. Cells were then stimulated with A23187 or ionomycin (final 0.1% DMSO) or other stimuli including S1P or LPA (final methanol at 1.9%) for another 15 min. After brief centrifugation, the medium was collected, and LTB4 or LTC4 was measured by ELISA (Cayman Chemicals, Ann Arbor, MI).
Western blotting
To study 5-LOX translocation, differentiated cells were stimulated by A23187 for 15 min, and the nuclear and cytosol fractions were isolated by a commercial kit, NE-PER nuclear and cytoplasmic extraction reagent (Thermo Scientific, Waltham, MA). For ERK1/2 phosphorylation, differentiated cells were stimulated with A23187 for 2 min and were frozen immediately to stop cellular reactions. Collected cells were lysed in Tris-EDTA containing 1% SDS, 2 mM sodium vanadate, and protease inhibitor cocktails. The resulting solution was heated at 95°C for 5 min. Proteins (25–50 μg) were resolved on 10% precast SDS-PAGE gels, transferred onto a polyvinylidene floride membrane (Millipore, Billerica, MA), and probed by Abs. Membranes were exposed to chemiluminescent reagent (PerkinElmer, Waltham, WA) and visualized on a Kodak film.
Assessment of 5-LOX activity
Potential effects on the activity of 5-LOX were evaluated using the ferrous oxidation–xylenol orange assay (FOX assay) as previously described (24). Briefly, human recombinant 5-LOX (9 U) was preincubated with vitamin E forms, 13′-COOH, or zileuton (a specific 5-LOX inhibitor) for 4 min at room temperature in 50 mM Tris-HCl buffer (pH 7.4) containing 0.4 mM CaCl2. Reactions were initiated by the addition of AA (final 75 μM). Four minutes later, the reaction was terminated by addition of FOX reagent containing 25 μM sulfuric acid, 100 μM xylenol orange, and 100 μM ferrous sulfate dissolved in methanol/water (9:1). After color development, the absorbance was measured at 560 and 575 nm using a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA).
Intracellular calcium measurement
Differentiated HL-60 cells were incubated with 2 μM fluo-4 AM in Ca2+-free HBSS in the dark at room temperature for 30 min (25). Cells were then washed twice and resuspended in HBSS buffer. In some studies, 3 × 106 cells were incubated with vitamin E at 37°C for 10 min in HBSS with Ca2+ and then stimulated. To study Ca2+ influx, cells were incubated with vitamin E for 10 min in Ca2+-free HBSS, followed by stimulation with A23187 or S1P for 1.3 min and Ca2+ added (final 1.25 mM). Intracellular calcium was monitored at emission of 526 nm with excitation of 496 nm using SpectraMax Gemini XS dual-scanning microplate spectrofluorometer (Molecular Devices).
Cytoplasm membrane permeability
Differentiated HL-60 cells were stimulated with A23187 in the presence or absence of δT (50 μM). Cells were then washed with cold PBS buffer and stained with a kit containing annexin V–fluorescein and propidium iodide (PI) (Roche Diagnostics, Indianapolis, IN). The change of cytoplasmic membrane permeability was monitored by Cell Lab Quanta SC–MPL flow cytometer (Beckman Coulter), with excitation at 488 nm and emission at 670 nm for PI.
Results
Vitamin E forms and 13′-COOH inhibited A23187-stimulated LTB4 in neutrophil-like differentiated HL-60 cells or human neutrophils isolated from fresh blood of healthy subjects
Human promyeloblast HL-60 cells have been shown to be differentiated into neutrophils by 1.2–1.5% DMSO (18–20), and lymphoblast clone 15 HL-60 cells are differentiated into eosinophils by butyric acid (21–23). To monitor the differentiation, we measured the expression of 5-LOX, which only exists in mature granulocytes and is the key enzyme for leukotriene formation (26). Under the culture conditions specified in Materials and Methods, 5-LOX expression reached maximum on the fourth to fifth day after the differentiation was initiated, at which time the differentiated cells can generate substantial amounts of leukotrienes; for example, when stimulated by A23187 (1 μM), differentiated HL-60 cells released 9.03–54.7 ng/ml LTB4 within 15 min compared with the baseline of 0.1–0.2 ng/ml.
We found that vitamin E forms (Fig. 1) differentially inhibited A23187-stimulated LTB4 and LTC4 formation from differentiated HL-60 cells and clone 15 HL-60 cells, respectively, and their inhibitory potency depended upon the concentrations of A23187 (Fig. 2). When 1 μM A23187 was used for cell stimulation, γT, δT, and γTE showed similar inhibitory potency with an IC50 of ~5 μM (Fig. 2A, 2B), whereas αT suppressed LTB4 and LTC4 at IC50 of 60 and 40 μM, respectively. Similar effects were seen with ionomycin-stimulated leukotriene formation (data not shown). When cells were stimulated by 2.5 μM A23187, the IC50 for γT and δT increased to 20 μM (Fig. 2C). With A23187 at 5 μM or higher, none of the vitamin E forms showed consistent inhibition even at 50 μM (data not shown). In contrast, 13′-COOH, a long-chain metabolite from δT, dose-dependently inhibited LTB4 when cells were stimulated by 1 or 5 μM A23187 with IC50 of 4 or 7 μM (Fig. 2D), respectively. In addition, similar to the effects in the differentiated cell lines, δT and 13′-COOH potently suppressed LTB4 generation from ionophore-stimulated neutrophils isolated from fresh blood of healthy subjects (Fig. 2E).
FIGURE 2.
Vitamin E forms differentially inhibited A23187-induced LTB4 (A, C) or LTC4 (B) from neutrophil-like HL-60 cells or eosinophil-like clone 15 HL-60 cells, respectively. 13′-COOH dose-dependently suppressed LTB4 production from A23187-stimulated (5 μM) HL-60 cells (D). δT and 13′-COOH inhibited LTB4 in stimulated human neutrophils isolated from fresh blood (E). Differentiated HL-60 and clone 15 HL-60 cells or freshly isolated neutrophils were preincubated with different vitamin E forms or 13′-COOH for 10 min and then activated by A23187 for 15 min. LTB4 and LTC4 were measured by ELISA assays. The relative LTB4 (or LTC4) is the ratio of LTB4 (or LTC4) formed in the presence of tested compounds in relation to that with solvent control. The results are reported as mean ± SD or SEM of two or more independent experiments. *p < 0.05; **p < 0.01 (difference between the treatment and solvent controls).
13′-COOH, but not vitamin E forms, potently inhibited human 5-LOX enzymatic activity
To understand the observed inhibition of leukotrienes by activated leukocytes, we investigated the effect of 13′-COOH and vitamin E on human recombinant 5-LOX activity in a cell-free FOX assay (24). In these assays, αT, γT, and δT at 50 μM did not exhibit any effect on 5-LOX activity (Fig. 3A), whereas γT at 200 μM showed 40% inhibition. In contrast, 13′-COOH potently inhibited the enzyme activity with an IC50 of 0.5–1 μM (Fig. 3B), which is comparable with that of the specific 5-LOX inhibitor zileuton, which showed an IC50 of 3–5 μM. These results indicated that the reduction of LTB4 in activated neutrophils by 13′-COOH, but not the unmetabolized vitamins, likely stems from its direct inhibition of the 5-LOX activity.
FIGURE 3.

The effect of vitamin E forms (A) and 13′-COOH (B) on human recombinant 5-LOX activity. Human recombinant 5-LOX was preincubated with vitamin E forms and 13′-COOH (at indicated concentrations, micromolar) or zileuton (10 μM) for 4 min at room temperature. Reactions were started by the addition of AA (final 75 μM) and terminated by addition of FOX reagent. After color development, the absorbance was measured at 560 and 575 nm. The 5-LOX activity (%) is the ratio of the absorbance in the presence of tested compounds in relation to that of solvent control. *p < 0.05; **p < 0.01 (difference between the treatment and solvent controls).
δT and γT inhibited A23187-stimulated 5-LOX translocation and ERK1/2 phosphorylation
It is well established that in vivo activation of 5-LOX requires translocation of this enzyme from the cytosol to nuclear membrane, where it binds to 5-LOX activating protein and catalyzes leukotriene formation (19, 27–29). In the current study, we observed translocation of 5-LOX from cytosol to the nucleus in response to the stimulation of A23187 in the differentiated HL-60 cells. Consistent with the inhibition of LTB4, γT, δT, and γTE, but not αT, almost completely abolished A23187-induced 5-LOX translocation (Fig. 4A).
FIGURE 4.
A, Effects of vitamin E forms on A23187-induced translocation of 5-LOX. Differentiated HL-60 cells were treated with 50 μM of different forms of vitamin E or solvent controls for 10 min and then stimulated by A23187 (2.5 μM) for 15 min. 5-LOX in the cytosolic and nuclear fractions were probed by Western blotting. B, The effect of specific inhibitors for various signaling pathways on LTB4 formation. Differentiated HL-60 cells were preincubated with inhibitors of MEK/ERK (U0126 at 20 μM, bar 3), PKC (Go-6983 at 2 or 10 μM, bars 4 and 5), or MAPK p38 (SB202190 at 5 or 10 μM, bars 6 and 7) for 10 min and then stimulated by A23187 (5 μM) for 15 min. Bars 1 and 2 are vehicle controls without or with A23187, respectively. *p < 0.05; **p < 0.01 (difference between cells treated with tested compounds and solvent control [bar 2]). C, γT and δT inhibited A23187-induced phosphorylation of ERK1/2 in neutrophils. Differentiated HL-60 cells were treated with or without δT or γT (50 μM) for 10 min and then stimulated by 1 μM A23187 for 2 min. Phosphorylated ERK1/2 and whole ERK1/2 were probed by Western blotting.
Calcium-regulated signaling such as PKC and MEK-ERK has been shown to be important to the activation of 5-LOX translocation in granulocytes (4, 5, 30). Consistently, the specific inhibitor for PKC (Go6389) and MEK (U0126), but not that for p38 MAPK, potently inhibited A23187-induced LTB4 (Fig. 4B) and LTC4 (data not shown). In agreement with the effect on 5-LOX translocation, γT and δT impaired ionophore-triggered ERK1/2 phosphorylation (Fig. 4C), suggesting that the upstream signaling such as intracellular Ca2+ increase may be modulated by vitamin E forms.
δT inhibited intracellular calcium increase stimulated by A23187
Cytosolic calcium increase is the pivotal upstream event triggering the activation of downstream signaling including PKC and ERK1/2 phosphorylation that leads to 5-LOX translocation. Because tocopherols inhibited 5-LOX translocation and ERK1/2 phosphorylation, we reason that they may modulate intracellular calcium increase. As expected, we found that δT greatly suppressed A23187-stimulated cytosolic calcium increase (Fig. 5A). In addition, δT appeared to block calcium influx when extracellular calcium was added (Fig. 5B).
FIGURE 5.

A, The effect of δT on intracellular calcium increase induced by A23187. Differentiated HL-60 cells were incubated with 2 μM fluo-4 AM in HBSS in the dark for 30 min. After a brief washing, cells were resuspended in HBSS buffer with Ca2+ and δT (50 μM) or 0.05% DMSO (solvent control) at 37°C for 10 min and then stimulated with 0.5 or 1 μM A23187. Symbols: triangles are calcium increase with A23187 at 1 μM (open, solvent; solid, δT), and circles are that with A23187 at 0.5 μM (open, solvent; solid, δT). B, The effect of δT on A23187-mediated Ca2+ influx. After preloading with fluo-4 AM, cells were incubated with δT (50 μM) for 10 min in HBSS without Ca2+. Cells were stimulated by A23187 (0.5 μM) for 1 min 20 s, and then Ca2+ added (final 1.25 μM). Symbols: open and solid circles represent calcium increase in vehicle controls and δT-treated cells, respectively. In all these studies, intracellular calcium was monitored by measuring fluo-4 AM at emission of 526 nm with excitation of 494 nm. Calcium increase is the subtraction of fluorescence intensity in the presence of A23187 minus fluorescence intensity without the stimulus.
δT inhibited LTB4 generation stimulated by S1P or LPA, but not by THAP or fMLP, and blocked S1P-stimulated intracellular calcium increase, whereas the inhibitory effects by 13′-COOH were independent of stimulus types
To understand the mechanisms underlying the inhibition of calcium increase, we tested the potential effect of δT on LTB4 generation induced by several stimuli that are known to increase cytosolic calcium via distinct mechanisms (Fig. 6A). δT showed no effects on LTB4 formation induced by fMLP, which causes calcium release from intracellular ER storage via a receptor-mediated, G protein-coupled, and phospholipase C-involved mechanism (7). δT treatment did not affect LTB4 induced by THAP (data not shown), which depletes ER Ca2+ storage by noncompetitively inhibiting ER Ca2+ ATPase (31). In contrast, δT potently suppressed LTB4 stimulated by S1P and LPA (Fig. 6A), which have been shown to stimulate calcium influx via a receptor-independent mechanism (8, 9). Consistent with the effect on S1P-mediated LTB4, δT also inhibited S1P-triggered calcium increase and calcium influx (Fig. 6B, 6C). Unlike δT, its metabolite 13′-COOH at low micromolar concentration strongly inhibited fMLP- and THAP-stimulated LTB4 (data not shown).
FIGURE 6.

A, Effects of δT on LTB4 production stimulated by fMLP, S1P, and LPA from differentiated HL-60 cells. Cells were preincubated with δT (50 μM) for 10 min and stimulated by fMLP (1 μM), S1P (50 μM), or LPA (100 μM) for 10 min. LTB4 was measured by ELISA. *p < 0.05; **p < 0.01 (difference between δT treatment and solvent controls for each indicated activator). B and C, Effects of δT on (B) S1P-triggered intracellular calcium increase and (C) calcium influx. The experimental procedure is the same as that described for Fig. 5; concentration of S1P was 50 μM.
δT counteracted ionophore-enhanced membrane permeability
Because δT suppressed LTB4 production stimulated by S1P, LPA, or ionophores that are known to increase intracellular Ca2+ by distinct mechanisms (8, 9), we reason that δT may modulate membrane perturbation induced by these stimuli. Therefore, we used PI to monitor potential cytoplasmic membrane disruption in response to the stimulation. Two minutes after cells were treated with A23187, the cytoplasmic membrane integrity was not affected (Fig. 7). In contrast, membrane permeability appeared to be enhanced during the extended 4-min incubation. δT completely reversed the enhanced PI staining when A23187 was at 1 μM, and only slightly attenuated 10 μM ionophore caused calcium release (Fig. 7). These data were consistent with the observation that when relatively high concentrations (>5 μM) of A23187 were used for cell stimulation, vitamin E forms failed to reduce LTB4 production. These results suggest that δT may block calcium entry via preventing membrane changes induced by the stimuli.
FIGURE 7.
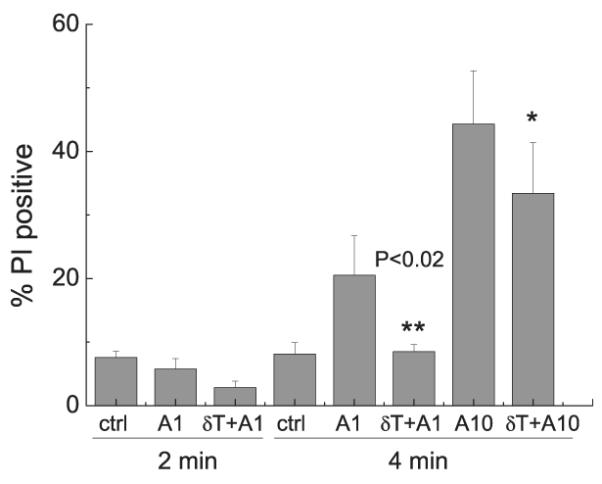
Effects of δT on A23187-enhanced membrane permeability. Differentiated HL-60 cells were preincubated with δT (50 μM) for 10 min and then stimulated with A23187 (1 or 10 μM) for 2 or 4 min. The membrane permeability was measured by PI staining using flow cytometry. *p < 0.02 (difference between A1 and δT + A1); *p < 0.05 (difference between A10 and δT + A10). A1, A23187 at 1 μM; A10, A23187 at 10 μM; Ctrl, no stimulus.
Discussion
LTB4 produced by 5-LOX–catalyzed reaction is one of the most potent chemotactic agents for leukocytes (1, 32) and thus plays a significant role in regulation of inflammatory response. Cysteinyl leukotrienes such as LTC4 that are generated by eosinophils or mast cells are known to contribute to the development of asthma and allergic inflammation (1, 33, 34). Leukotriene antagonists and 5-LOX inhibitors have been used to treat asthma and inflammatory diseases (2). Here we have demonstrated that γT, δT, and γTE, at physiologically relevant concentrations, suppressed neutrophil-mediated LTB4 production much more potently than αT by blocking intracellular calcium increase, which consequently led to inhibition of ERK phosphorylation and 5-LOX translocation, a key step for activation of 5-LOX. Unlike the unmetabolized vitamins, 13′-COOH, a long-chain metabolite of vitamin E, decreased leukotriene formation by direct inhibition of the 5-LOX activity.
We have previously demonstrated that 13′-COOH derived from δT strongly inhibits COX-1 and COX-2 by competing with AA for the binding to the substrate site of COXs (17). In this study, we found that 13′-COOH inhibits 5-LOX activity at a potency comparable with that of zileuton, a competitive inhibitor of 5-LOX. Consistent with its strong inhibition of 5-LOX activity, 13′-COOH suppressed cellular production of LTB4 regardless of the stimuli. Given that both 13′-COOH and AA share an end carboxylate group and both bind to the substrate site of COXs, we postulate that 13′-COOH may also competitively inhibit human 5-LOX, which warrants further investigation. Regardless of the mechanisms, the unique dual inhibition of COXs and 5-LOX by 13′-COOH makes it an interesting and novel anti-inflammatory agent. This is because compared with specific COX or 5-LOX inhibitors, 13′-COOH may exhibit more potent anti-inflammatory effects by suppressing multiple proinflammatory pathways and may have reduced adverse effects caused by a shunt of AA metabolism to either the COX- or 5-LOX–mediated pathway (35, 36).
In contrast with their long-chain metabolite, vitamin E forms failed to affect human recombinant 5-LOX activity at up to 50 μM, although αT has previously been reported to noncompetitively inhibit potato 5-LOX at much lowered concentrations (37). This discrepancy of vitamin E toward human and potato 5-LOX is likely due to the large sequence difference between the two enzymes (based on Blast protein sequence comparison). However, the lack of inhibition of human 5-LOX activity is in agreement with the fact that the inhibitory effect of vitamin E forms on cellular LTB4 formation varies with the specific stimuli, that is, showing inhibition to A23187, S1P, and LPA but not fMLP or THAP stimulation, even when comparable amounts of LTB4 were generated (Fig. 6A). Our results are also consistent with the previous study by Goetzel (10) showing that αT at <30 μM enhances 5-LOX–catalyzed products by A23187-stimulated human neutrophils, whereas a suppressive effect is observed at >120 μM (10). Notably, we also observed a slight enhancement of leukotriene by low micromolar concentration of αT (Fig. 2), and αT supplementation at 33 mg/kg body weight tends to increase inflammation-induced LTB4 in a rat inflammation model (14).
It is intriguing that vitamin E forms suppress LTB4 by modulation of calcium influx and that their effect is dependent upon the types of activator. Because δT failed to affect fMLP- or THAP-stimulated leukotriene, vitamin E forms do not likely have impact on fMLP receptor-mediated, phospholipase C-involved signaling, or Ca2+ store depletion. The lack of influence on the receptor-mediated pathway is also supported by the strong inhibition of LTB4 stimulated by S1P and LPA, which increase intracellular calcium via receptor-independent mechanisms in human neutrophils and HL-60 cells (8, 9). Although S1P and LPA appear to trigger store-operated and non-store-operated calcium entry, respectively (8, 9), the exact mechanisms leading to calcium influx is unknown. In contrast, because the ionophore appears to enhance membrane permeability as indicated by the increase of intracellular PI staining, we propose that δT blocks calcium influx by counteracting stimuli-caused disruption of cytoplasmic membrane integrity. However, when higher concentrations of ionophore (>5 μM) were applied, δT failed to extensively reverse the further increased membrane leak and was therefore ineffective in inhibition of LTB4 production. Although the exact mechanism remains to be further elucidated, antagonizing S1P- and LPA-mediated effects may have important physiological implications, as both have been recognized as important lipid regulators for growth, cancer, and inflammation (38–40). Notably, LPA has been shown to act as an ovarian cancer-activating factor and potently increase intracellular Ca2+ in ovarian cancer cells (41). Whether specific vitamin E forms are capable of modulating S1P- or LPA-mediated inflammation in vivo should be further tested. In addition, because the effect of vitamin E forms on Ca2+ influx is sensitive to the activators, their in vivo anti-inflammatory actions may vary with specific stimuli and depend upon whether disruption of membrane permeability contributes significantly to cell activation.
Although previous studies have investigated the effect of αT supplementation on 5-LOX product formation, the results are highly inconsistent and are likely influenced by oxidative stress and the basal/supplement levels of αT. 5-LOX activity is known to be enhanced by lipid hydroperoxide (42), and reactive oxygen species can also be produced by lipoxygenase-dependent mechanisms (43–45). It is conceivable that as the most important lipophilic antioxidant, αT may suppress oxidative stress-promoted leukotriene formation caused by vitamin E deficiency (12, 13) or under certain pathological conditions such as kidney disease (46). In contrast, due to the lack of potent inhibition of human 5-LOX activity or intracellular calcium at up to 50 μM (current study), αT at low or medium supplement doses shows variable effects on 5-LOX product formation in neutrophils or animals with sufficient vitamin E levels (10, 12, 14), whereas high αT (>100 μM) appears to be effective to suppress 5-LOX products (current study and Refs. 10, 47).
Unlike αT, the strong inhibition of leukotrienes by other vitamin E forms such as γT and their long-chain metabolites likely, at least in part, account for the in vivo anti-inflammatory activities that have been reported by us and others. This is because plasma and liver concentrations of γT have been reported to reach up to 20 or 60 μM (48, 49), respectively, and 13′-COOH is detected in tissues in response to vitamin E supplementation (48, 50). Thus, we have shown that γT but not αT potently inhibits carrageenan-induced LTB4 at the inflammation site in the rat air-pouch model (14), which is consistent with the current observation that γT more potently suppressed cellular leukotriene formation than that by αT. γT supplementation also alleviates OVA-sensitized airway inflammation and suppresses leukotriene increase in the broncho-alveolar lavage fluid in Brown Norway rats (15, 16). In addition, γT-rich mixed tocopherols lower LTB4 in an inflammation-promoted colon carcinogenesis model (51). Our current findings also suggest that δT and γTE, as well as long-chain carboxychromanols, appear to have equal or stronger anti-inflammatory effects compared with those of γT. Future studies should be carried out in animals and humans to further determine the role of supplementation of these vitamin E forms and their long-chain metabolites in inflammation-related diseases.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01AT001821, P01AT002620, and R21CA133651.
Abbreviations used in this article
- AA
arachidonic acid
- 13′-COOH
13′-carboxychromanol
- COX
cyclooxygenase
- FOX
ferrous oxidation–xylenol orange
- 5-LOX
5-lipoxygenase
- LPA
lysophosphatidic acid
- LTB4
leukotriene B4
- LTC4
leukotriene C4
- PI, propidium iodide
PKC, protein kinase C
- S1P
sphingosine 1-phosphate
- αT, βT, γT, or δT
α-, β-, γ-, or δ-tocopherol
- αTE, βTE, γTE, or δTE
α-, β-, γ-, or δ-tocotrienol
- THAP
thapsigargin
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science (New York, N. Y.) 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Peters-Golden M, Henderson WR., Jr. Leukotrienes. N. Engl. J. Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 3.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre TM, Reinhold SL, Prescott SM, Zimmerman GA. Protein kinase C activity appears to be required for the synthesis of platelet-activating factor and leukotriene B4 by human neutrophils. J. Biol. Chem. 1987;262:15370–15376. [PubMed] [Google Scholar]
- 5.Werz O, Bürkert E, Fischer L, Szellas D, Dishart D, Samuelsson B, Rådmark O, Steinhilber D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- 6.Islam MS, Berggren PO. Mobilization of Ca2+ by thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone in permeabilized insulin-secreting RINm5F cells: evidence for separate uptake and release compartments in inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. Biochem. J. 1993;293:423–429. doi: 10.1042/bj2930423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 2006;534:1–11. doi: 10.1016/j.ejphar.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J. Biol. Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itagaki K, Kannan KB, Hauser CJ. Lysophosphatidic acid triggers calcium entry through a non-store-operated pathway in human neutrophils. J. Leukoc. Biol. 2005;77:181–189. doi: 10.1189/jlb.0704390. [DOI] [PubMed] [Google Scholar]
- 10.Goetzl EJ. Vitamin E modulates the lipoxygenation of arachidonic acid in leukocytes. Nature. 1980;288:183–185. doi: 10.1038/288183a0. [DOI] [PubMed] [Google Scholar]
- 11.Devaraj S, Jialal I. Alpha-tocopherol decreases interleukin-1 beta release from activated human monocytes by inhibition of 5-lipoxygenase. Arterioscler. Thromb. Vasc. Biol. 1999;19:1125–1133. doi: 10.1161/01.atv.19.4.1125. [DOI] [PubMed] [Google Scholar]
- 12.Chan AC, Tran K, Pyke DD, Powell WS. Effects of dietary vitamin E on the biosynthesis of 5-lipoxygenase products by rat polymorphonuclear leukocytes (PMNL) Biochim. Biophys. Acta. 1989;1005:265–269. doi: 10.1016/0005-2760(89)90047-7. [DOI] [PubMed] [Google Scholar]
- 13.Reddanna P, Whelan J, Burgess JR, Eskew ML, Hildenbrandt G, Zarkower A, Scholz RW, Reddy CC. The role of vitamin E and selenium on arachidonic acid oxidation by way of the 5-lipoxygenase pathway. Ann. N. Y. Acad. Sci. 1989;570:136–145. doi: 10.1111/j.1749-6632.1989.tb14914.x. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin. Exp. Allergy. 2008;38:501–511. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 16.Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic. Biol. Med. 2007;43:1176–1188. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl ***sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kargman S, Rouzer CA. Studies on the regulation, biosynthesis, and activation of 5-lipoxygenase in differentiated HL60 cells. J. Biol. Chem. 1989;264:13313–13320. [PubMed] [Google Scholar]
- 20.Scoggan KA, Nicholson DW, Ford-Hutchinson AW. Regulation of leukotriene-biosynthetic enzymes during differentiation of myelocytic HL-60 cells to eosinophilic or neutrophilic cells. Eur. J. Biochem. 1996;239:572–578. doi: 10.1111/j.1432-1033.1996.0572u.x. [DOI] [PubMed] [Google Scholar]
- 21.Fischkoff SA, Brown GE, Pollak A. Synthesis of eosinophil-associated enzymes in HL-60 promyelocytic leukemia cells. Blood. 1986;68:185–192. [PubMed] [Google Scholar]
- 22.Fischkoff SA, Condon ME. Switch in differentiative response to maturation inducers of human promyelocytic leukemia cells by prior exposure to alkaline conditions. Cancer Res. 1985;45:2065–2069. [PubMed] [Google Scholar]
- 23.Ishihara K, Hong J, Zee O, Ohuchi K. Possible mechanism of action of the histone deacetylase inhibitors for the induction of differentiation of HL-60 clone 15 cells into eosinophils. Br. J. Pharmacol. 2004;142:1020–1030. doi: 10.1038/sj.bjp.0705869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YS, Kim HS, Kim CH, Cheon HG. Application of the ferrous oxidation-xylenol orange assay for the screening of 5-lipoxygenase inhibitors. Anal. Biochem. 2006;351:62–68. doi: 10.1016/j.ab.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 26.Reid GK, Kargman S, Vickers PJ, Mancini JA, Léveillé C, Ethier D, Miller DK, Gillard JW, Dixon RA, Evans JF. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J. Biol. Chem. 1990;265:19818–19823. [PubMed] [Google Scholar]
- 27.Brock TG, McNish RW, Bailie MB, Peters-Golden M. Rapid import of cytosolic 5-lipoxygenase into the nucleus of neutrophils after in vivo recruitment and in vitro adherence. J. Biol. Chem. 1997;272:8276–8280. doi: 10.1074/jbc.272.13.8276. [DOI] [PubMed] [Google Scholar]
- 28.Kargman S, Prasit P, Evans JF. Translocation of HL-60 cell 5-lipoxygenase. Inhibition of A23187- or N-formyl-methionyl-leucylphenylalanine-induced translocation by indole and quinoline leukotriene synthesis inhibitors. J. Biol. Chem. 1991;266:23745–23752. [PubMed] [Google Scholar]
- 29.Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc. Natl. Acad. Sci. USA. 2003;100:12165–12170. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werz O, Klemm J, Samuelsson B, Rådmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. USA. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ely JA, Ambroz C, Baukal AJ, Christensen SB, Balla T, Catt KJ. Relationship between agonist- and thapsigargin-sensitive calcium pools in adrenal glomerulosa cells. Thapsigargin-induced Ca2+ mobilization and entry. J. Biol. Chem. 1991;266:18635–18641. [PubMed] [Google Scholar]
- 32.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 33.Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med. Res. Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- 34.Soberman RJ, Christmas P. The organization and consequences of eicosanoid signaling. J. Clin. Invest. 2003;111:1107–1113. doi: 10.1172/JCI18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rainsford KD. Inhibition by leukotriene inhibitors, and calcium and platelet-activating factor antagonists, of acute gastric and intestinal damage in arthritic rats and in cholinomimetic-treated mice. J. Pharm. Pharmacol. 1999;51:331–339. doi: 10.1211/0022357991772330. [DOI] [PubMed] [Google Scholar]
- 36.Rainsford KD. The ever-emerging anti-inflammatories. Have there been any real advances? J. Physiol. Paris. 2001;95:11–19. doi: 10.1016/s0928-4257(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 37.Reddanna P, Rao MK, Reddy CC. Inhibition of 5-lipoxygenase by vitamin E. FEBS Lett. 1985;193:39–43. doi: 10.1016/0014-5793(85)80075-2. [DOI] [PubMed] [Google Scholar]
- 38.Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 2009;48:62–72. doi: 10.1016/j.plipres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Georas SN. Lysophosphatidic acid and autotaxin: emerging roles in innate and adaptive immunity. Immunol. Res. 2009;45:229–238. doi: 10.1007/s12026-009-8104-y. [DOI] [PubMed] [Google Scholar]
- 40.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin. Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 42.Rådmark O, Samuelsson B. Regulation of 5-lipoxygenase enzyme activity. Biochem. Biophys. Res. Commun. 2005;338:102–110. doi: 10.1016/j.bbrc.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 44.Swindle EJ, Coleman JW, DeLeo FR, Metcalfe DD. FcepsilonRI- and Fcgamma receptor-mediated production of reactive oxygen species by mast cells is lipoxygenase- and cyclooxygenase-dependent and NADPH oxidase-independent. J. Immunol. 2007;179:7059–7071. doi: 10.4049/jimmunol.179.10.7059. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi Y, Zhu H, Yoshimoto T. Essential roles of lipoxygenases in LDL oxidation and development of atherosclerosis. Antioxid. Redox Signal. 2005;7:425–431. doi: 10.1089/ars.2005.7.425. [DOI] [PubMed] [Google Scholar]
- 46.Maccarrone M, Taccone-Gallucci M, Meloni C, Cococcetta N, Di Villahermosa SM, Casciani CU, Finazzi-Agrò A. Activation of 5-lipoxygenase and related cell membrane lipoperoxidation in hemodialysis patients. J. Am. Soc. Nephrol. 1999;10:1991–1996. doi: 10.1681/ASN.V1091991. [DOI] [PubMed] [Google Scholar]
- 47.Devaraj S, Jialal I. Alpha-tocopherol decreases tumor necrosis factor-alpha mRNA and protein from activated human monocytes by inhibition of 5-lipoxygenase. Free Radic. Biol. Med. 2005;38:1212–1220. doi: 10.1016/j.freeradbiomed.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J. Lipid Res. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008;45:40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J. Nutr. 2009;139:884–889. doi: 10.3945/jn.108.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev. Res. (Phila.) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]




