Abstract
Pleural effusion is not a rare disease in Korea. The diagnosis of pleural effusion is very difficult, even though the patients often complain of typical symptoms indicating of pleural diseases. Pleural effusion is characterized by the pleural cavity filled with transudative or exudative pleural fluids, and it is developed by various etiologies. The presence of pleural effusion can be confirmed by radiological studies including simple chest radiography, ultrasonography, or computed tomography. Identifying the causes of pleural effusions by pleural fluid analysis is essential for proper treatments. This review article provides information on the diagnostic approaches of pleural effusions and further suggested ways to confirm their various etiologies, by using the most recent journals for references.
Keywords: Pleural Effusion, Pleurisy, Diagnosis
Introduction
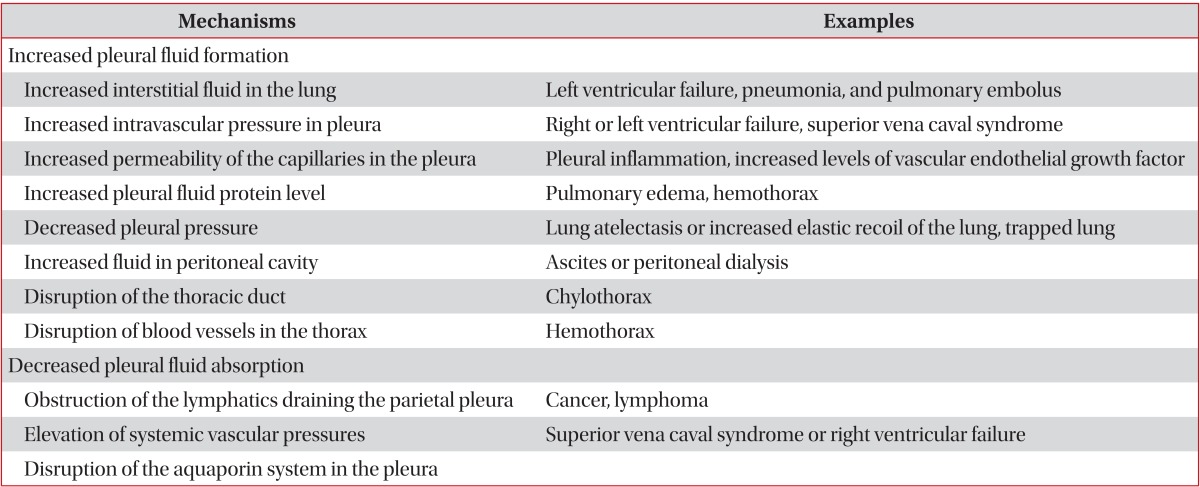
The mean amount of pleural fluid in the normal is as small as 8.4±4.3 mL. Fluid that enters the pleural space can originate in the pleural capillaries, the interstitial spaces of the lung, the intrathoracic lymphatics, the intrathoracic blood vessels, or the peritoneal cavity. Pleural fluid is usually absorbed through the lymphatic vessels in the parietal pleura by means of stomas in the parietal pleura, or through the alternative transcytosis1. But various pathogenic mechanisms increase the amount of pleural fluids by increasing the rates of pleural fluid formation exceeding the rate of pleural fluid absorption (Table 1)1.
Table 1.
General causes of pleural effusions
The first step of differential diagnosis or determination of pathogenesis for pleural fluid is to determine whether the patient has a transudative or exudative pleural effusion2. Transudates are caused by increased hydrostatic pressures (e.g., heart failure), decreased oncotic forces (e.g., hypoproteinemia), increased negative intrapleural pressure (e.g., atelectasis), or movement of ascitic fluid through the diaphragm (e.g., hepatic hydrothorax). In contrast, exudates are due to the increased capillary permeability and/or impaired lymphatic drainage which results from the proliferative (e.g., malignancy) or inflammatory (e.g., parapneumonic effusions) processes3.
This article aims to review the history taking and the physical examinations of patients who were suspected to have symptoms and signs of pleural effusions, to provide precise radiological approaches including thoracic ultrasonography for diagnosing the presence of pleural effusions, and to diagnose the different causes of effusions by using the thoracentesis and pleural biopsy.
History, Symptoms and Signs
The clinical history, symptoms and signs may be very helpful for evaluating many causes of the pleural effusions (Table 2)3. Especially, because the effusion by drugs is misdiagnosed, the clinical history which includes the medication history is important. If the causes of pleural effusions are suspected to by drugs, such drug list can be easily found in http://www.pneumothox.com.
Table 2.
Medical history and physical examinations of pleural effusions
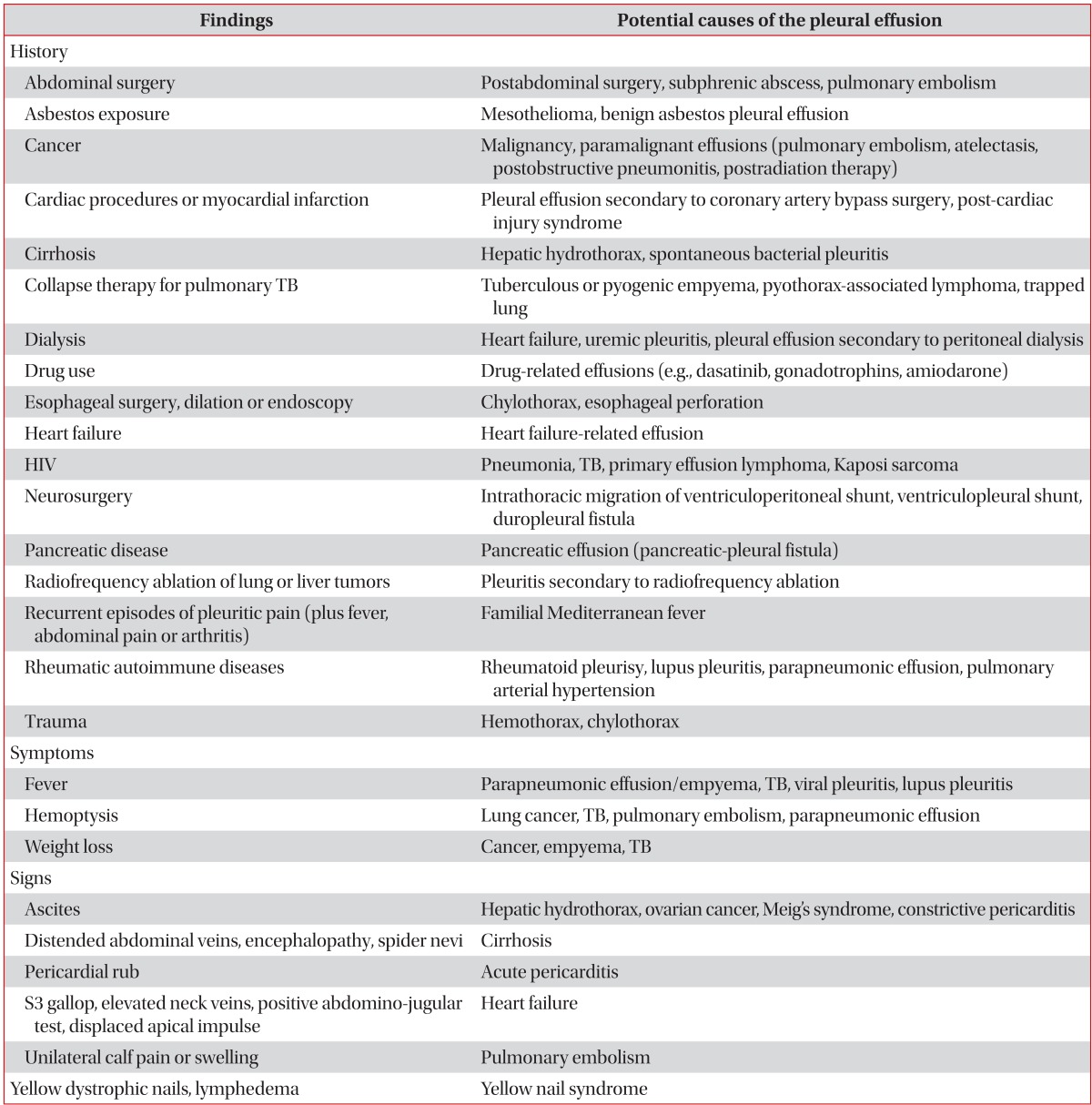
TB: tuberculosis; HIV: human immunodeficiency virus.
While small amounts of effusions are usually asymptomatic, the increasing effusion produces dyspnea, trepopnea, chest pain or cough. Dyspnea is the consequence of a combination of restrictive lung defects, a ventilation-perfusion mismatch, and a decrease in cardiac output4. Although large volumes of pleural effusions usually correlate with the degree of dyspnea, dyspnea is frequently not proportional to the size of the pleural effusions. Patients with underlying lung diseases (chronic obstructive pulmonary disease [COPD], carcinomatous lymphangitis, pulmonary emboli) may experience intense dyspnea with just small-to-moderate sized pleural effusions. Trepopnea is a form of positional dyspnea in which the patients experience less dyspnea when lying on the side of the pleural effusion3. Chest pain accompanied with a parietal pleural inflammation clinically is a sharp localized pain, worsening on deep inspiration or coughing, and occasionally twisting or bending movements. A malignancy involving the parietal pleura frequently produces a chronic dull ache localized to the relevant anatomic region5. Pleuritic chest pain may be referred to the abdomen, and to the ipsilateral shoulder when the diaphragmatic pleura is involved. Cough is dry and nonproductive, a consequence of pleural inflammation or compression of the bronchial trees, but they are rarely helpful for diagnosing pleural effusions. If hemoptysis is combined, it may suggest endobronchial cancer or pulmonary thromboembolism1.
Upon physical examination, tactile fremitus in palpation is either absent or attenuated, and the percussion note over a pleural effusion is dull or flat. In auscultation, the breathing sounds are decreased or absent, and auscultatory percussion (Guarino's second method) is abnormal. Pleural rubs often appear as pleural effusions which diminish in size, and will disappear once the effusion develops5.
Radiologic Diagnosis
1. Chest radiograph
If symptoms and signs are suspected to have pleural effusions, the chest radiograph is usually the diagnosing method.
1) Chest posteroanterior and lateral view
The chest posteroanterior (PA) view is the abnormal blunting of sharp lateral costophrenic angle when pleural fluid is over 200 mL. In addition, the lateral radiography may show blunting of the sharp posterior costophrenic angle when the fluid exceeds 50 mL6. Increasing amounts of the effusion form a meniscus, opacify the lung, and obscure the diaphragmatic margin. Radiological characteristics of subpulmonic effusions are elevations of either one or both diaphragms, the displacement of the apex of the apparent diaphragm more laterally, and separation between the lower border of the lung and gastric bubble greater than 2 cm in the left-sided effusion1.
2) Anteroposterior radiograph
The anteroposterior (AP) chest radiography is abnormal when pleural fluid is over 300 mL. The earliest sign is blunting of the costophrenic angle. Subsequently, it also causes increased density of the hemithorax, loss of the hemidiaphragm, and decreased visibility of the lower lobe vasculature1.
3) Lateral decubitus view
In the lateral decubitus view, pleural effusion is easily detected by free pleural fluids shifting between the dependent chest wall and the lower border of the lung. Diagnostic thoracentesis is safe when the distance of shifting is more than 10 mm. In general, bilateral decubitus chest radiographs should be ordered to assess the underlying lungs for infiltrates or atelectasis1.
4) Chest radiographic finding (Table 3)
Table 3.
Useful radiological signs in pleural effusions
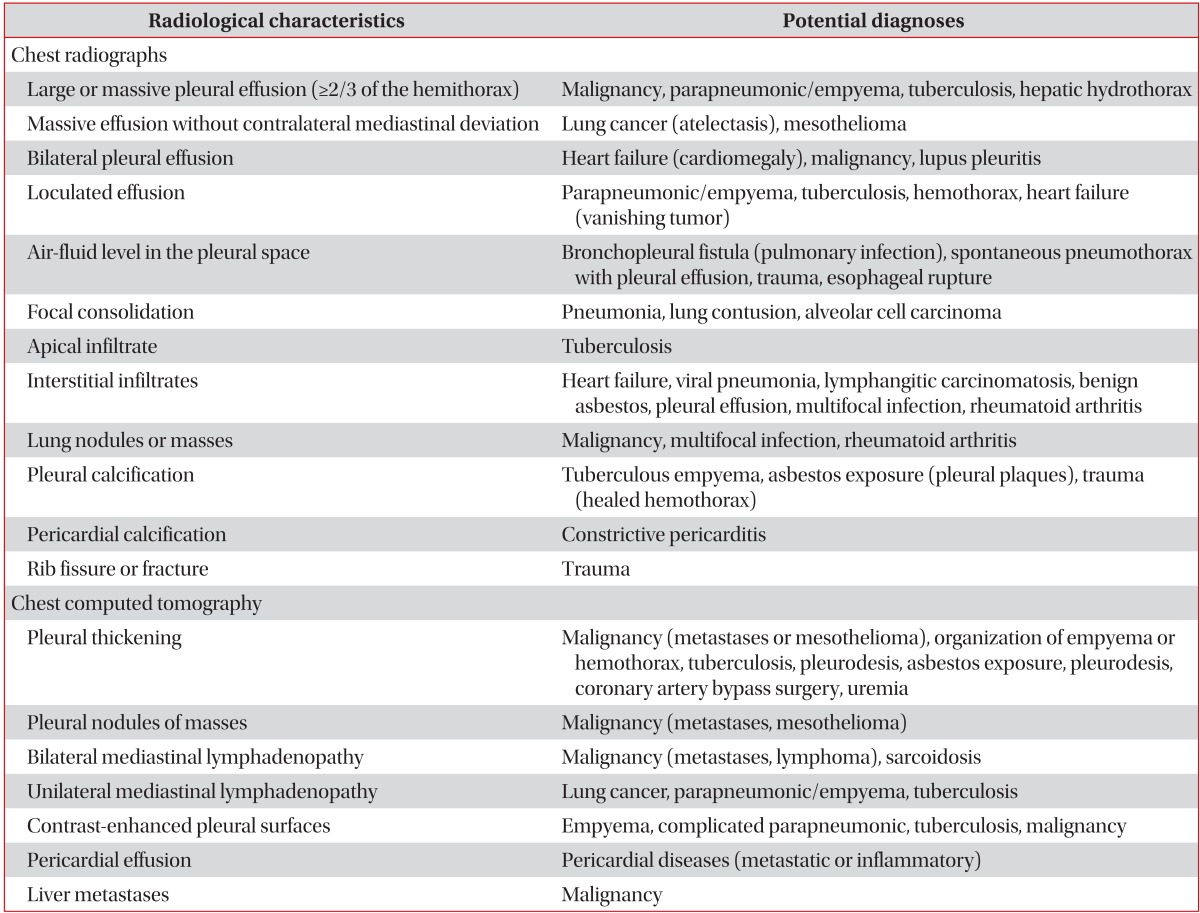
Useful radiological findings of pleural effusions are summarized in Table 33.
(1) Bilateral effusion
Bilateral pleural effusion is commonly seen in heart failure. For bilateral effusion with a normal heart size, the differential diagnosis should include malignancy and, less commonly, lupus pleuritis and constrictive pericarditis3.
(2) Massive effusion more than half of hemithorax
The most frequent cause of massive pleural effusions is malignancy (55%), followed by complicated parapneumonic or empyema (22%), and tuberculosis (TB) (12%). If massive effusions are without contralateral displacement of mediastinal structures, the endobronchial obstructions by lung cancer or mediastinum fixation by mesothelioma should be considered7.
(3) Loculated effusion
The loculation of pleural space is caused by adhesions between contiguous pleural surfaces. It occurs most frequently in conditions that cause intense pleural inflammations, such as empyema, hemothorax, or TB pleurisy. In patients with congestive heart failure after treatment, the loculated effusion in fissure may simulate a mass, termed as the vanishing tumor or pseudotumor in chest PA view1.
(4) Combined pneumonia in lower lobe
The AP, PA, and lateral chest radiographs are not sensitive methods to identify parapneumonic effusions in patients with pneumonia, because all views missed more than 10% of significant effusions. The existence of a lower lobe parenchymal consolidation concealed the identification of some pleural effusions. Therefore, such considerations should be used for obtaining additional imaging, such as thoracic ultrasonography in patients with lower lobe parenchymal consolidations on plain film radiographs8.
2. Thoracic ultrasonography
Thoracic ultrasonography (TUS) will detect the presence of as little as 5-50 mL of pleural fluids and is 100% sensitive for effuions9. Ultrasonography can be used under several different situations, including the following: 1) determining the presence of pleural fluid; 2) identification of the appropriate locations for an attempted thoracentesis, pleural biopsy, or chest tube placement; 3) identification of pleural fluid loculations; 4) distinction of pleural fluids from thickening; 5) semiquantitation on the amount of pleural fluids; 6) differentiation of a pyopneumothorax from a lung abscess; 7) assessment of whether a pleurodesis is present; and 8) evaluation of the trauma patient for the presence of a hemothorax or a pneumothorax1. TUS is also an useful instrument for the diagnosis and treatment of pleural diseases, especially in the intensive care units.
Pleural fluids on ultrasonograph can be characterized either as echo free (anechoic), complex septated (fibrin strands or septa), complex nonseptated (heterogeneous echogenic material), or homogenrously echogenic1. If echogenecity is extremely high, immediate thoracentesis is required for differentiation of empyema or hemothorax. The TUS findings which suggest malignancy include parietal pleural thickening of more than 1 cm, pleural nodularity and diaphragmatic thickness >7 mm (sensitivity 42% and specificity 95% for each criteria)10.
3. Computed tomography
Chest computed tomography (CT) in pleural effusion is available for differentiation of pleural collections or masses, detection of loculated fluid collections, demonstration of abnormalities in lung parenchyme, distinguishing empyema with air-fluid levels from lung abscess, identification of pleural thickening, evaluation of major and minor fissures, and distinguishing benign and malignant effusions1. CT findings which are suggestive of malignancy are as follows: pleural nodularity, pleural rind, mediastinal pleural involvement, and pleural thickening greater than 1 cm11.
4. Magnetic resonance imaging
Magnetic resonance imaging of chest is currently less satisfactory and higher cost than CT or TUS in pleural disease due to poor spatial resolutions and motion artifacts1.
5. Positron emission tomography scan
Because the 18-fluorodeoxyglucose (18FDG) concentrated in malignant cells are more avid than normal tissues, the FDG-positron emission tomography is useful for differentiating benign and malignant pleural effusions including mesothelioma. But, false positive scans may be due to infections (parapneumonics, empyema, TB) and talc pleurodesis3.
Thoracentesis
If the thickness of the pleural fluid on decubitus radiograph, TUS or the CT scan is greater than 10 mm, diagnostic thoracentesis should be performed. Although the effusion is due to obvious congestive heart failure, the thoracentesis should be concerned under the following conditions: 1) not bilateral and comparable sized effusions; 2) pleuritic chest pain; 3) febrile; and 4) no responses to diuretics. Using TUS is safe for small or loculated effusions1.
The main contraindication of thoracentesis is a hemorrhagic diathesis. Some guidelines recommend correcting an international normalized ratio to less than 2, transfusing platelets to more than 50,000/µL, or withholding certain medications (oral anticoagulants, heparin or clopidogrel) before performing minimally invasive procedures such as thoracentesis12. Thoracentesis should not be attempted in local cutaneous conditions such as pyoderma or herpes zoster infection1.
The most common complication of thoracentesis is pneumothorax. The incidence of pneumothorax after thoracentesis with conventional techniques was 18%, whereas it was only 3% with the TUS13. Other common complications of thoracentesis are cough, chest pain, vasovagal reflux characterized by bradycardia and a decreased blood pressure, infection of the pleural space, hemothorax due to laceration of intercostal artery, splenic or hepatic laceration, soft tissue infection and seeding of the needle tract with tumor cells. Postprocedure chest radiograph is only recommended in aspiration of air, developing of symptoms, and the loss of tactile fremitus1.
To relieve dyspnea in massive pleural effusion, therapeutic thoracentesis is helpful. The removal as little as 300-500 mL at once is generally sufficient to relieve dyspnea in patients with undiagnosed effusions3. Although most physicians tend to avoid performing thoracentesis with more than 1,500 mL at one time, the re-expansion pulmonary edema is uncommon (0.5%), even when >1,000 mL of pleural fluids are removed14. To prevent re-expansion pulmonary edema, therapeutic thoracentesis should be stopped in symptoms such as chest tightness, chest pain, dyspnea or more than minimal coughing1. If the dyspnea does not improve after therapeutic thoracentesis in malignant effusions, underlying diseases such as pulmonary carcinomatous lymphangitis, atelectasis, pulmonary or tumor embolism, or COPD should be considered3.
Analysis of Pleural Fluid
1. Preparation of pleural fluid sample for test
Approximately 20-40 mL of aspirated fluid should be immediately placed into appropriate anticoagulant (EDTA or heparin) coated tubes for biochemistry (5 mL), microbiology (5-10 mL), cytology (10-25 mL), and heparin coated syringe for the pH measurement. Pleural fluids should be analyzed within 4 hours of extraction. If pleural fluid contacts with air for a long time, the CO2 will escape and the pH level will increase3. Measurements of pH level should be accomplished more accurately with a blood gas machine either than the pH indicator paper or pH meter1. For aerobic and anaerobic bacterial cultures, the pleural fluids should be inoculated directly into the blood culture media by the bedside to improve positively cultured results15.
Routine and optional tests of pleural fluids are summarized in Tables 4 and 53.
Table 4.
Routine pleural fluid tests for pleural effusions
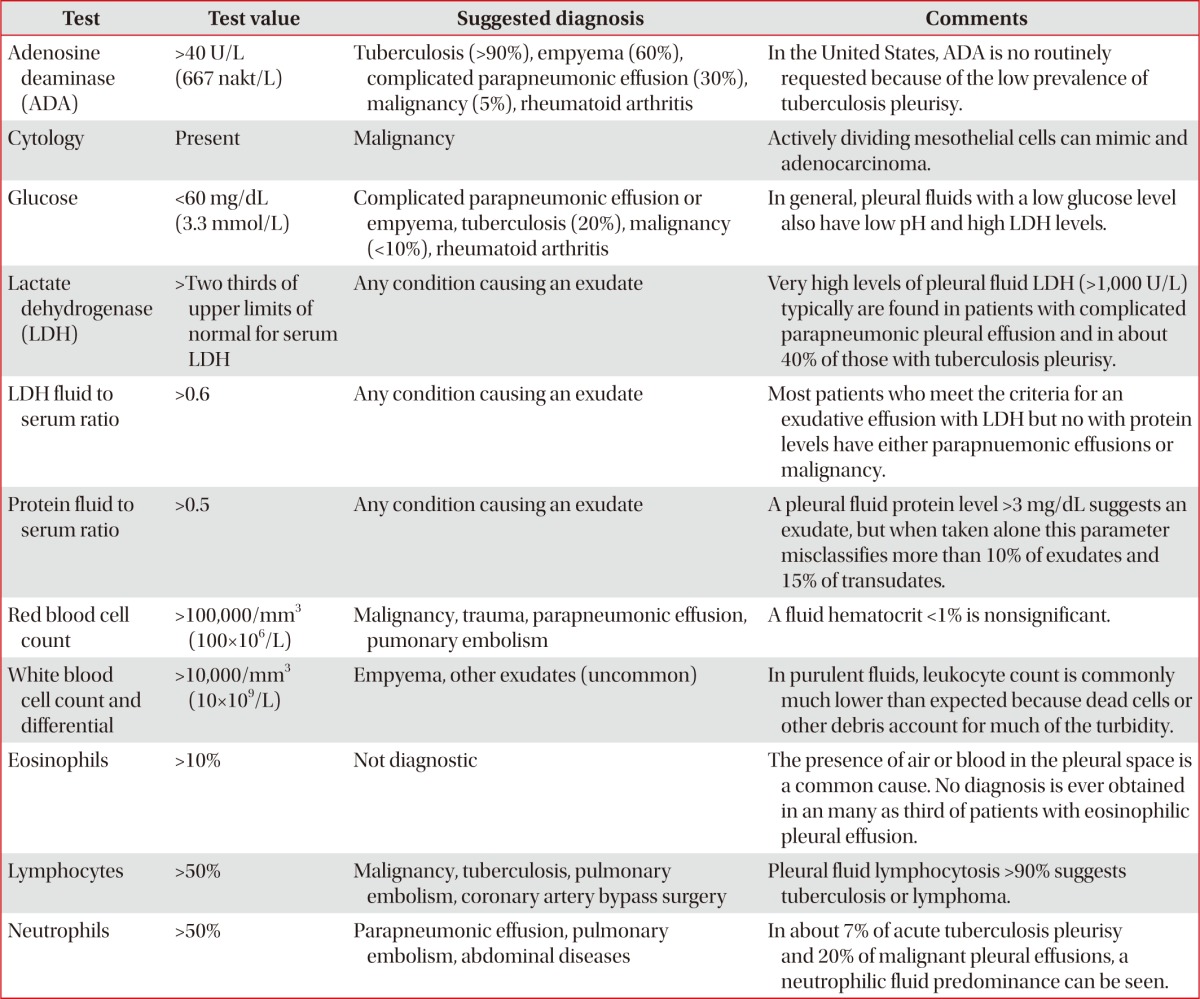
Table 5.
Optional pleural fluid tests for pleural effusions
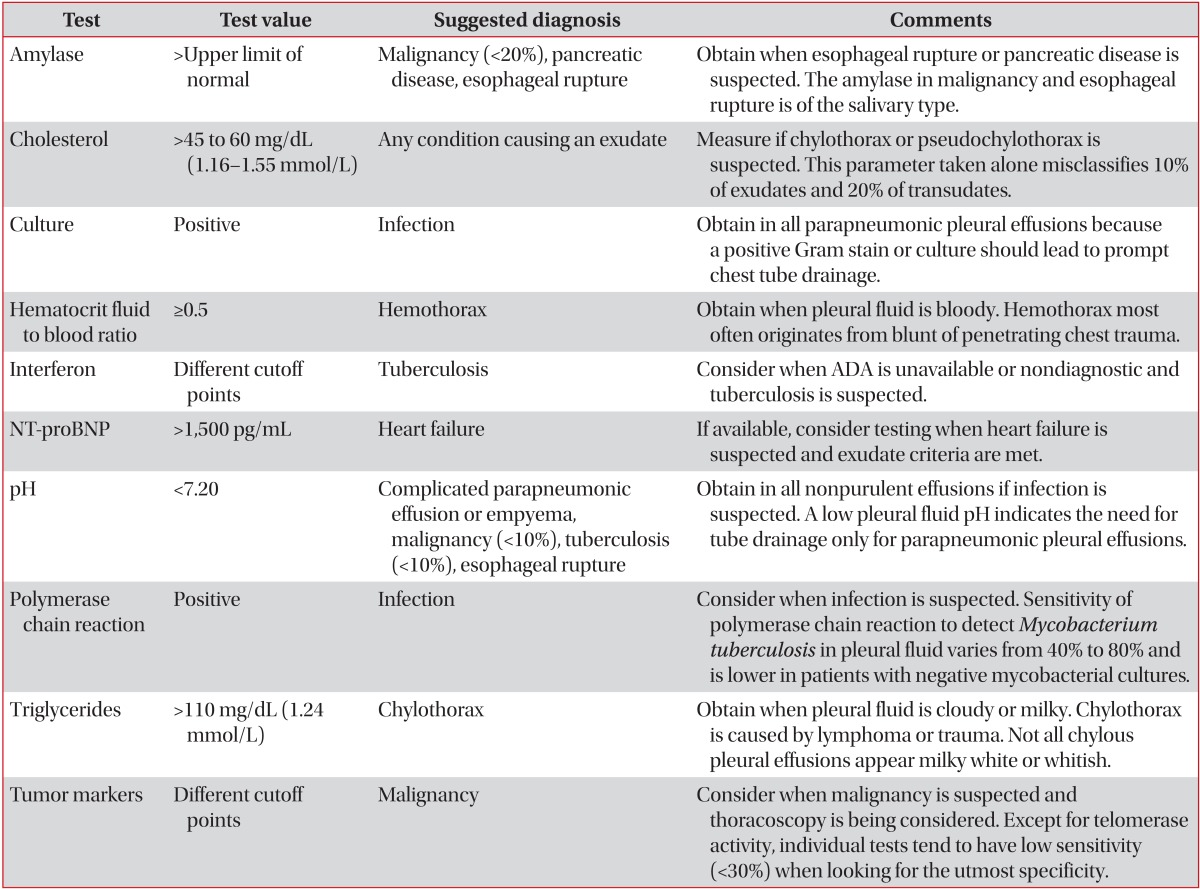
ADA: adenosine deaminase; NT-proBNP: N-terminal pro-b-type natriuretic peptide.
2. Differentiation of transudate and exudate
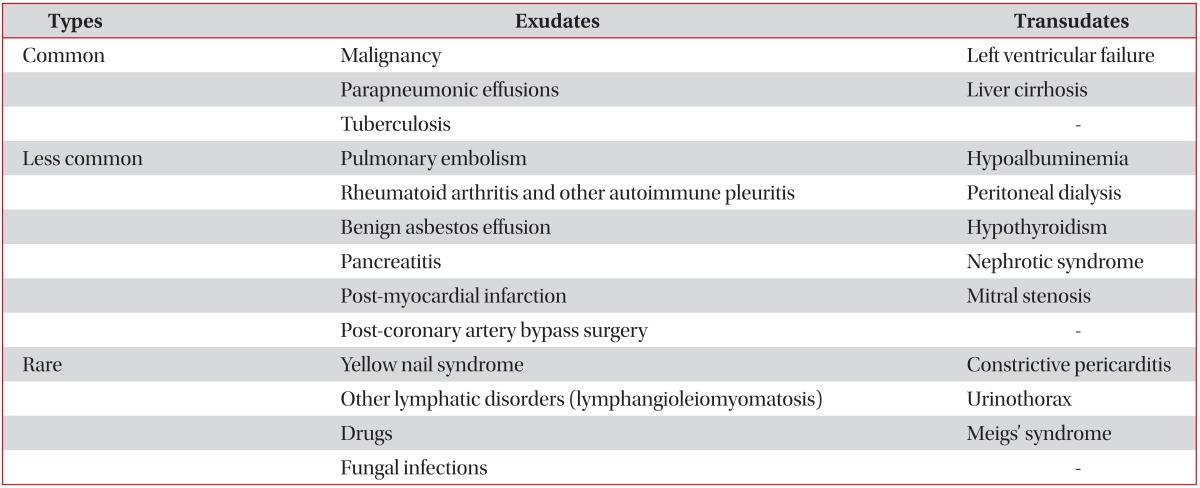
The first step in differential diagnosis of pleural effusion is to determine whether the effusion is transudative or exudative. Light's criteria (Table 6)1 should be used to differentiate transudates and exudates by analyzing the levels of protein and lactate dehydrogenase (LDH) in the pleural fluid and serum. Transudate is mostly due to systemic diseases, such as congestive heart failure, liver cirrhosis, or nephrosis that easily treated with diuretics. Exudate resulted from local diseases, where the fluid originated, and further investigations should be directed at the genesis of the local disease1. Common causes of exudative pleural effusion is malignancy, parapneumonic effusion and TB (Table 7)16.
Table 6.
Light's criteria for identifying transudates and exudates
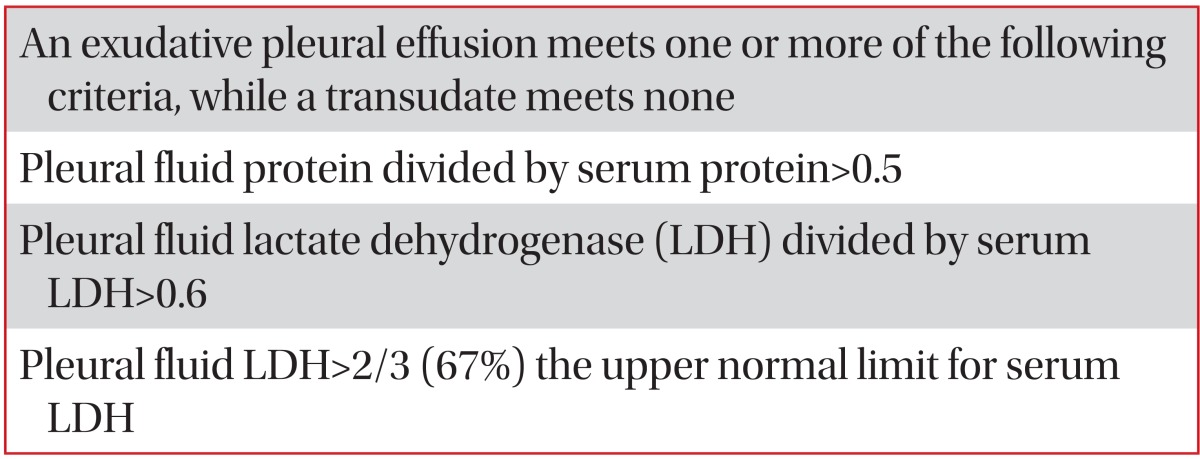
Table 7.
Causes of transudative and exudative pleural effusions
Light's criteria is the gold standard to differentiate the transudate and exudate over the last 30 years. However, the concentrations of the biochemical components (protein, LDH, and albumin) in pleural fluids increase progressively during the diuretic therapy of patients with congestive heart failure17. In patients with congestive heart failure receiving diuretic therapy, the misclassification of transudate to exudate (29%) in Light's criteria can be more accurately corrected in the albumin gradient (>1.2 g/dL) than the protein gradient (>3.1 g/dL) between the serum and the pleural fluid. In patients with liver cirrhosis misclassified as exudate (18%) in Light's criteria, their diagnostic accuracy increased more in albumin ratios (<0.6) than albumin or protein gradient18. Although the accuracy of the albumin gradient is higher than the protein gradient, it is recommend to use protein gradient easily obtained in Light's criteria. Increased levels of pleural fluid or serum N-terminal pro-brain natriuretic peptide (NT-proBNP) is more diagnostic the heart failure-associated transudative effusion which are misclassified as exudate by Light's criteria19.
3. Appearance of pleural fluid
The gross appearance of pleural fluid frequently serves as useful diagnostic information. A reddish pleural fluid indicates that the blood is present (malignant disease, trauma, or pulmonary embolization), and a brownish tinge indicates that the blood has been present for a prolonged period. Because the gross bloody pleural fluid indicates hemothorax, a hematocrit (>1/2 of blood hematocrit) should be obtained. Black pleural fluids are pleural infections with Aspergillus niger or Rhizopus oryzae, or following massive bleeding due to metastatic carcinoma and melanoma. Turbid pleural fluids can occur from either increased cellular contents (pyothorax) or increased lipid contents (chylothorax or pseudochylothorax). If the fluid is centrifuged, the supernatant is clear in pyothorax. The odor of pleural fluids is important for finding the etiology. A feculent odor indicates anaerobic bacterial infection of pleural space, and the urine-like smell is related to urinothorax1. Although a watery appearance is may suggest transudate, only 13% of transudates are classified to be watery20.
4. Biochemical analysis of pleural fluid
1) Pleural fluid white blood cell differential counts
In patients with exudative pleural effusions, the differential cell counts provide clues for the etiology of pleural effusions. Exudative pleural effusions with predominantly polymorphonuclear leukocytes (>50%) mean acute process, and such causes are parapneumonic effusion, pulmonary embolus, viral infection, gastrointestinal disease, asbestos pleural effusion, malignant pleural disease, or acute TB pleurisy. Exudative pleural effusions with predominantly mononuclear cells (>50%) indicate chronic processes, and the most common causes are malignant disease, pulmonary embolization, pleural effusion following coronary arterial bypass surgery and TB. Causes of eosinophilic pleural effusions (>10%) are air (most common) or blood in the pleural space, malignancy, parapneumonic, transudates, TB, pulmonary embolism, asbestos-related pleural effusion, drug reaction, parasitic disease and Churg-Strauss syndrome1,21.
2) Pleural fluid pH level
The sample of pleural fluid which measures the pH levels should be collected anaerobically in a heparinized syringe, placed on ice, with residual lidocaine removed, and then analyzed within 1 hour by the blood gas analyzer. The accurate method of measuring pleural fluid pH level is through the blood gas analyzer rather than the pH meter, pH indicator stick, and litmus paper. But, the gross pus should be avoided when using the blood gas analyzer22. Exposures to local anesthetic (lidocaine) or heparin in the syringe decreases pH levels of pleural fluid. Pleural fluid pH level is also increased for the exposure of the sample to air (escape of CO2) and the delay of test for over 4 hours23.
Pleural fluid pH level may decrease as a result of increased acid productions by pleural fluid cells and bacteria (e.g., complicated parapneumonic effusion and empyema, esophageal rupture) or by an abnormal pleural membrane that blocks hydrogen ion efflux from the pleural space into the circulatory system (e.g., malignancy, TB, and chronic rheumatoid pleurisy)3. Pleural pH levels of 7.2 or less in the parapneumonic effusion is related with a poor outcome, as well as pleural loculation and the needs of invasive procedure with thoracotomy tube drainage1. A low pleural fluid pH level (≤7.30) in patients with malignant pleural effusions showed greater cytology positivity, potentially worse outcomes, and poor responses to chemical pleurodesis as compared with patients of normal pleural fluid pH24.
3) Pleural fluid glucose
In general, the pleural fluid with low glucose (<60 mg/dL) also have a low pH (<7.20) and high LDH levels. A low pleural fluid glucose (<60 mg/dL) is mainly caused by complicated parapneumonic effusion, malignancy, TB and rheumatoid pleuritis, and caused rarely hemothorax, paragonimiasis, Churg-Strauss syndrome, and occasionally, lupus pleuritis1,2.
4) Pleural fluid adenosine deaminase
Adenosine deaminase (ADA) is the enzyme that catalyzes the conversions of adenosine to inosine. In general, elevated effusions higher than the cutoff level of 40-45 U/L means TB effusion, but it is also possible in rheumatoid pleuritis or empyema. The increase in ADA activity with TB pleurisy is mainly due to ADA2, but the additional ADA isoenzymes are not helpful to diagnose TB pleurisy1. Notably, when pleural fluid ADA activity is extremely high (>250 U/L), the empyema or lymphoma, rather than TB, should be the first consideration25.
5) Amylase
Elevation of pleural fluid amylase, defined levels greater than the upper limits of normal serum level (100-130 U/L) and a pleural fluid to serum ratio >1.0, developed in pancreatic disease, esophageal rupture or malignancy26.
6) Triglycerides
Chylothorax is a milky exudate with high pleural fluid triglycerides (TG) values more than 110 mg/dL. Pleural fluid TG less than 50 mg/dL can exclude chylothorax. In cases of pleural fluid TG 50-110 mg/dL, lipoprotein analysis is required for the demonstration of chylomicrons. For fasting or malnourished patients, lipoprotein analysis is necessary even with TG less than 50 mg/dL27.
7) C-reactive protein
A neutrophilic exudate with pleural fluid C-reactive protein (CRP) levels >45 mg/L will most likely be parapneumonic, and if pleural fluid CRP >100 mg/dL, it will be complicated parapneumonic effusion28. But, it is not clear whether the high CRP is superior to low pH levels (<7.2) and low glucose (<60 mg/dL) which diagnose the complicated parapneumonic effusions.
8) Procalcitonin
Procalcitonin (PCT) is a biomarker used to diagnose the systemic bacterial infection. Both the serum and pleural fluid PCT is higher in parapneumonic effusions than TB pleurisy or malignant effusion29. However, pleural fluid PCT lacks the ability of separating complicated parapneumonic effusions from uncomplicated ones30. Thus, the diagnostic value of PCT is limited.
9) NT-proBNP
NT-proBNP is neurohormones released by ventricular cardiomyocytes in response to increased pressure or volume. Pleural fluid NT-proBNP is a very useful biomarker with high diagnostic accuracy for distinguishing pleural effusions of cardiac origins with 94% of sensitivity and specificity31. Because of the high correlations between the pleural and serum level of NT-proBNP, diagnostic thoracentesis may not be necessary in high serum NT-proBNP levels in patients with pleural effusion32. The most widely used cut-off point is 1,500 pg/mL. Pleural NT-proBNP rather than BNP can discriminate between transudates of hepatic or cardiac origins, and can also correctly identify more than 80% of cardiac transudates mislabeled by Light's criteria33.
10) Tumor marker
Several tumor markers, such as carcinoembryonic antigen (CEA), cancer antigen (CA) 125, CA 15-3, CYFRA 21-1 have low sensitivity (<30%) at cutoff values with 100% specificity. But, if combined, the sensitivity is similar to that of pleural fluid cytology (approximately 50%)34. The pleural fluid mesothelin and fibulin-3 have been recently reported with good results for diagnosis of mesothelioma. But, pleural tumor marker measurements cannot replace the definitive cytohistological study3.
5. Cytological analysis of pleural fluid
The accuracy of cytologic examinations of malignant pleural effusion is around 60% (range of 40-87%). Several factors influence the diagnostic yield of cytology. 1) Presence of paramalignant pleural effusion: negative malignant cells in congestive heart failure, pulmonary emboli, pneumonia, lymphatic blockade, hypoproteinemia, atelectasis, or drugs and radiation; 2) tumor types: more frequent positive in adenocarcinoma than sarcoma; 3) the types of specimen examined: greater positivity in the combination of both cell blocks and smears; 4) repeated examinations up to 2 times: positivity of 65% in the first specimen, further 27% in second specimen, and further only 5% in third specimen; 5) skill of cytologist; 6) higher tumor burden in the pleural space1,35. Combination of immunocytochemical markers that use epithelial membrane antigen, CEA, calretinin, and thyroid transcription factor-1 is very helpful for distinguishing benign mesothelial cells, mesothelioma, and adnocarcinoma19.
6. Microbiologic analysis of pleural fluid
In pleural effusions due to bacterial infection, the gram stain and culture (both aerobic and anaerobic) of the pleural fluid should be obtained. Although the result of culture would be lower in patients with previous antibiotics treatment, the direct inoculation of pleural fluid into blood culture media at the bedside increased the identification rate of pathogen from 37.7% to 58.5% in one study15. Non-groupable streptococci (S. viridans, S. milleri) and pneumococcus are the most commonly isolated pathogens in community-acquired empyema, whereas the staphylococcal species (particularly methicillin-resistant Staphylococcus aureus), Enterococcus and Enterobacteriaceae lead in causing hospital-acquired infections3.
Pleural fluid cultures are positive for less than 40% of Mycobacterium tuberculosis and smears are virtually always negative. If TB pleurisy is suspected, the use of a BACTEC system with bedside inoculation provides higher yields and faster results than the conventional methods1,36.
Undiagnosed Pleural Effusions after Initial Thoracentesis
If pleural fluid analysis and chest CT (for pulmonary emboli and abnormality in the chest) are not helpful for identifying the cause of pleural effusions, other options should be followed.
1. Observation
Observation is the best action if the patient is improving, such as pleural effusion due to viral illness that is self-limited. If pleural fluids re-accumulate after therapeutic thoracentesis, the time of re-accumulation is important to differentiate the causes. When effusion re-accumulates rapidly within 24-72 hours, the clinician should consider transudative causes, such as trapped lung, peritoneal dialysis, hepatic hydrothorax and extravascular migration of a central venous catheter, with saline or glucose infusion. Exudates that recur rapidly following the thoracentesis can be produced in angiosarcoma, chylothorax, lung entrapment by malignancy and parapneumonic effusions, malignant ascites, Meigs syndrome and blood by iatrogenic haemothorax26. Effusions that typically persist for more than 6 months are limited to unexpandable lung, post-coronary artery bypass graft surgery, benign asbestos pleural effusion, rheumatoid pleurisy, lymphangioleiomyomatosis (chylothorax), cholesterol effusions, and yellow nail syndrome3.
2. Bronchoscopy
Bronchoscopy is useful in patients with pleural effusions for one or more of the following: 1) pulmonary infiltrates in chest radiograph or CT scan; 2) hemoptysis; 3) massive pleural effusion more than three fourths of the hemithorax; and 4) mediastinum shifted toward the side of the effusion1.
3. Pleural biopsy
Blind needle pleural biopsies were frequently performed primarily to establish the diagnosis of TB pleurisy or malignancy. However, it is rarely indicated because the TB pleurisy is easily diagnosed by pleural fluid ADA>40 U/L, and the blind biopsy of malignant effusion is diagnostic only about 20% of the patients with cytology negative malignant effusions2,37.
CT-guided cutting needle biopsy provides a significantly higher diagnostic yield than the blind needle biopsy in pleural mass or pleural thickening38.
Thoracoscopy allows direct visualization of the pleural surface, biopsy of areas which appear to be abnormal, and therapeutic maneuvers such as complete fluid drainage and talc pleurodesis during the same procedure3. Thoracoscopy should only be performed when less invasive procedures are non-diagnostic. Malignant pleural effusion is suspected in 1) a symptomatic period of more than a month; 2) absence of fever; 3) blood-tinged or bloody pleural fluid; and 4) CT findings suggestive of malignancy (pulmonary or pleural masses, pulmonary atelectasis, or lymphadenopathy)39. Despite the medical thoracoscopy in one series, 12% (5/142) of the patients initially diagnosed as nonspecific pleuritis/fiborsis were subsequently diagnosed with malignant mesothelioma after a mean interval of 9.8 months40.
Summary
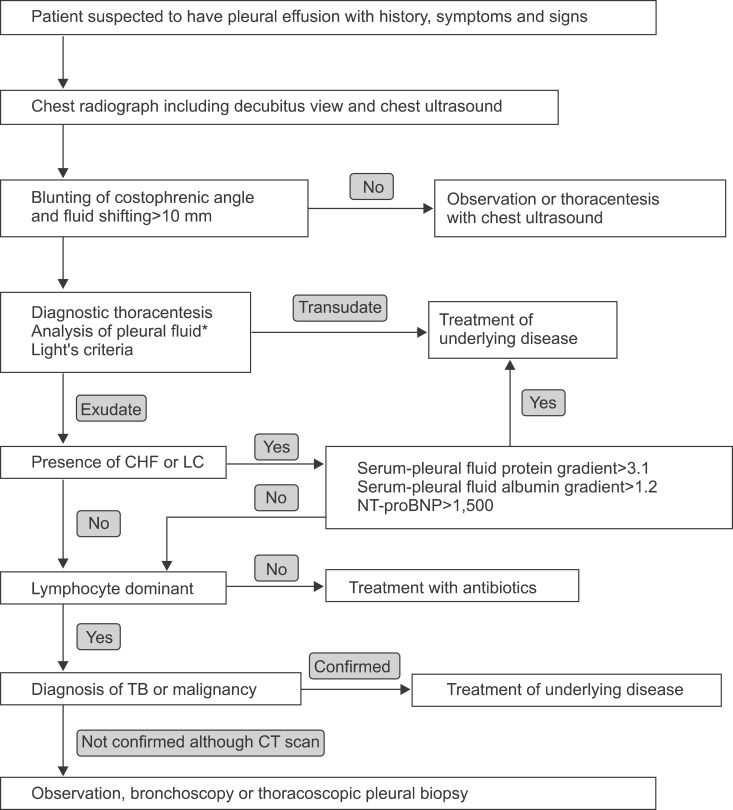
For patients with clinical history, symptoms and signs related to pleural diseases, physicians have to use diagnostic radiographical studies to diagnose pleural effusions, and the use of TUS is essential. Thoracentesis is necessary to identify the causes of pleural effusion, and it is a safe procedure without any complications when using the TUS. Through the analysis of pleural fluid, it is possible to differentiate between transudative or exudative pleural effusions, and to prove the causes of pleural effusions. Transudative pleural effusion is easily treated with corrections of underlying diseases, such as diuretics. If the causes of exudative pleural effusions are not proved by conventional diagnostic methods including CT scans, the next necessary steps are observations, bronchoscopy and/or pleural biopsy by using thoracoscopy. Diagnostic algorithm is summarized in Figure 1.
Figure 1.
Algorithm for diagnostic approaches for patients suspected of pleural effusions. *Analysis of pleural fluid includes protein and lactate dehydrogenase of pleural fluids and serum, gross appearance, red blood cell, white blood cell with differential count, pH levels, glucose, amylase, cholesterol, triglyceride, cytology, acid-fast bacilli stain, TB culture, TB-polymerase chain reaction, Gram stain, routine culture, carcinoembryonic antigen and adenosine deaminase of pleural fluids. CHF: congestive heart failure; LC: liver cirrhosis; NT-proBNP: N-terminal pro-b-type natriuretic peptide; TB: tuberculosis; CT: computed tomography.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Light RW. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 2.Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055–1070. doi: 10.1016/j.mcna.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Porcel JM, Light RW. Pleural effusions. Dis Mon. 2013;59:29–57. doi: 10.1016/j.disamonth.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75:297–303. doi: 10.3949/ccjm.75.4.297. [DOI] [PubMed] [Google Scholar]
- 5.Brims FJ, Davies HE, Lee YC. Respiratory chest pain: diagnosis and treatment. Med Clin North Am. 2010;94:217–232. doi: 10.1016/j.mcna.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Froudarakis ME. Diagnostic work-up of pleural effusions. Respiration. 2008;75:4–13. doi: 10.1159/000112221. [DOI] [PubMed] [Google Scholar]
- 7.Porcel JM, Vives M. Etiology and pleural fluid characteristics of large and massive effusions. Chest. 2003;124:978–983. doi: 10.1378/chest.124.3.978. [DOI] [PubMed] [Google Scholar]
- 8.Brixey AG, Luo Y, Skouras V, Awdankiewicz A, Light RW. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16:1000–1004. doi: 10.1111/j.1440-1843.2011.02006.x. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi NR, Gleeson FV. Imaging of pleural disease. Clin Chest Med. 2006;27:193–213. doi: 10.1016/j.ccm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64:139–143. doi: 10.1136/thx.2008.100545. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz U, Polat G, Sahin N, Soy O, Gulay U. CT in differential diagnosis of benign and malignant pleural disease. Monaldi Arch Chest Dis. 2005;63:17–22. doi: 10.4081/monaldi.2005.653. [DOI] [PubMed] [Google Scholar]
- 12.Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156:917–920. doi: 10.2214/ajr.156.5.2017951. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123:418–423. doi: 10.1378/chest.123.2.418. [DOI] [PubMed] [Google Scholar]
- 15.Menzies SM, Rahman NM, Wrightson JM, Davies HE, Shorten R, Gillespie SH, et al. Blood culture bottle culture of pleural fluid in pleural infection. Thorax. 2011;66:658–662. doi: 10.1136/thx.2010.157842. [DOI] [PubMed] [Google Scholar]
- 16.Hooper C, Lee YC, Maskell N BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii4–ii17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Candeira S, Fernandez C, Martin C, Sanchez-Paya J, Hernandez L. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med. 2001;110:681–686. doi: 10.1016/s0002-9343(01)00726-4. [DOI] [PubMed] [Google Scholar]
- 18.Bielsa S, Porcel JM, Castellote J, Mas E, Esquerda A, Light RW. Solving the Light's criteria misclassification rate of cardiac and hepatic transudates. Respirology. 2012;17:721–726. doi: 10.1111/j.1440-1843.2012.02155.x. [DOI] [PubMed] [Google Scholar]
- 19.Porcel JM. Pleural fluid biomarkers: beyond the Light criteria. Clin Chest Med. 2013;34:27–37. doi: 10.1016/j.ccm.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Villena V, Lopez-Encuentra A, Garcia-Lujan R, Echave-Sustaeta J, Martinez CJ. Clinical implications of appearance of pleural fluid at thoracentesis. Chest. 2004;125:156–159. doi: 10.1378/chest.125.1.156. [DOI] [PubMed] [Google Scholar]
- 21.Kalomenidis I, Light RW. Eosinophilic pleural effusions. Curr Opin Pulm Med. 2003;9:254–260. doi: 10.1097/00063198-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Putnam B, Elahi A, Bowling MR. Do we measure pleural fluid pH correctly? Curr Opin Pulm Med. 2013;19:357–361. doi: 10.1097/MCP.0b013e3283620844. [DOI] [PubMed] [Google Scholar]
- 23.Mishra EK, Rahman NM. Factors influencing the measurement of pleural fluid pH. Curr Opin Pulm Med. 2009;15:353–357. doi: 10.1097/MCP.0b013e32832b98d4. [DOI] [PubMed] [Google Scholar]
- 24.Heffner JE, Heffner JN, Brown LK. Multilevel and continuous pleural fluid pH likelihood ratios for evaluating malignant pleural effusions. Chest. 2003;123:1887–1894. doi: 10.1378/chest.123.6.1887. [DOI] [PubMed] [Google Scholar]
- 25.Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med. 2010;21:419–423. doi: 10.1016/j.ejim.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Sahn SA. Getting the most from pleural fluid analysis. Respirology. 2012;17:270–277. doi: 10.1111/j.1440-1843.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 27.Skouras V, Kalomenidis I. Chylothorax: diagnostic approach. Curr Opin Pulm Med. 2010;16:387–393. doi: 10.1097/MCP.0b013e328338dde2. [DOI] [PubMed] [Google Scholar]
- 28.Porcel JM, Bielsa S, Esquerda A, Ruiz-Gonzalez A, Falguera M. Pleural fluid C-reactive protein contributes to the diagnosis and assessment of severity of parapneumonic effusions. Eur J Intern Med. 2012;23:447–450. doi: 10.1016/j.ejim.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Lee EJ, Min KH, Hur GY, Lee SY, Kim JH, et al. Procalcitonin as a diagnostic marker in differentiating parapneumonic effusion from tuberculous pleurisy or malignant effusion. Clin Biochem. 2013;46:1484–1488. doi: 10.1016/j.clinbiochem.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Porcel JM, Vives M, Cao G, Bielsa S, Ruiz-Gonzalez A, Martinez-Iribarren A, et al. Biomarkers of infection for the differential diagnosis of pleural effusions. Eur Respir J. 2009;34:1383–1389. doi: 10.1183/09031936.00197208. [DOI] [PubMed] [Google Scholar]
- 31.Janda S, Swiston J. Diagnostic accuracy of pleural fluid NT-pro-BNP for pleural effusions of cardiac origin: a systematic review and meta-analysis. BMC Pulm Med. 2010;10:58. doi: 10.1186/1471-2466-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han CH, Choi JE, Chung JH. Clinical utility of pleural fluid NT-pro brain natriuretic peptide (NT-proBNP) in patients with pleural effusions. Intern Med. 2008;47:1669–1674. doi: 10.2169/internalmedicine.47.1276. [DOI] [PubMed] [Google Scholar]
- 33.Porcel JM. Utilization of B-type natriuretic peptide and NT-proBNP in the diagnosis of pleural effusions due to heart failure. Curr Opin Pulm Med. 2011;17:215–219. doi: 10.1097/MCP.0b013e3283455cda. [DOI] [PubMed] [Google Scholar]
- 34.Porcel JM, Vives M, Esquerda A, Salud A, Perez B, Rodriguez-Panadero F. Use of a panel of tumor markers (carcinoembryonic antigen, cancer antigen 125, carbohydrate antigen 15-3, and cytokeratin 19 fragments) in pleural fluid for the differential diagnosis of benign and malignant effusions. Chest. 2004;126:1757–1763. doi: 10.1378/chest.126.6.1757. [DOI] [PubMed] [Google Scholar]
- 35.Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665–668. [PubMed] [Google Scholar]
- 36.Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15:451–458. doi: 10.1111/j.1440-1843.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 37.Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc. 1985;60:158–164. doi: 10.1016/s0025-6196(12)60212-2. [DOI] [PubMed] [Google Scholar]
- 38.Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326–1330. doi: 10.1016/s0140-6736(03)13079-6. [DOI] [PubMed] [Google Scholar]
- 39.Ferrer J, Roldan J, Teixidor J, Pallisa E, Gich I, Morell F. Predictors of pleural malignancy in patients with pleural effusion undergoing thoracoscopy. Chest. 2005;127:1017–1022. doi: 10.1378/chest.127.3.1017. [DOI] [PubMed] [Google Scholar]
- 40.Davies HE, Nicholson JE, Rahman NM, Wilkinson EM, Davies RJ, Lee YC. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg. 2010;38:472–477. doi: 10.1016/j.ejcts.2010.01.057. [DOI] [PubMed] [Google Scholar]