Abstract
INTRODUCTION
The needle biopsy technique for the soleus muscle is of particular interest because of its unique fiber type distribution, contractile properties, and sensitivity to unloading. Unlike other commonly biopsied muscles, the soleus is not fully superficial and is in close proximity to neurovascular structures resulting in a more challenging biopsy. Because of this, a standardized protocol for performing needle biopsies on the human soleus muscle that is safe, reliable, and repeatable is presented.
METHODS
Ultrasonography was used on an initial set of 12 subjects to determine the optimal biopsy zone thereby guiding the location of the incision site. Forty-five subjects were recruited and attended two separate biopsy sessions. Each biopsy session incorporated 3 passes of the biopsy needle proximal, posterior, and distal using suction from a portable vacuum source producing 3 separate muscle specimens.
RESULTS
Eighty-four soleus muscle biopsy procedures were successfully conducted yielding 252 total samples without complication. Ultrasonography was used to confirm biopsy needle infiltration of the soleus muscle. Average sample weight obtained per pass was 61.5 ± 15.7 mg. Histochemistry and molecular analyses demonstrated a considerably higher amount of slow type I MHC in comparison to the vastus lateralis providing verification for the successful sampling of the soleus muscle.
DISCUSSION
The procedure presented consists of a detailed protocol to accurately and consistently obtain muscle biopsy samples from the human soleus muscle. We have demonstrated that the human soleus biopsy is a safe, reliable and repeatable procedure providing ample tissue for multiple types of analyses.
Keywords: human soleus, muscle biopsy, suction
INTRODUCTION
The needle biopsy technique for human skeletal muscle is a proven and convenient method for retrieving muscle samples that can be used for histological, biochemical and molecular analyses [8, 11]. Needle biopsies can be done in a clinical setting under local anesthesia, provide only minor discomfort and scarring, and permit immediate resumption to normal daily activities. Needle biopsies are therefore an effective tool for the study of skeletal muscle in both research and clinical settings.
The vastus lateralis is one of the most commonly biopsied muscles [7, 13, 17] as it is large, subcutaneous and distant from major neurovascular structures [16]. Unlike the vastus lateralis and other commonly biopsied muscles, the soleus is in close proximity to major neurovascular structures and is deep to other musculature, the gastrocnemius, thereby making for a more challenging biopsy. The soleus muscle, in the superficial posterior compartment of the lower leg, is of specific research concern and perhaps clinical interest because of its unique characteristics including fiber type distribution and contractile properties [5, 10], role in ambulatory activities [9, 21], and resistance to hypertrophy [19]. The soleus is particularly sensitive to the adverse effects of unloading such as unilateral lower limb suspension, bed rest, or the microgravity spaceflight environment.
A properly designed and well-described protocol is necessary for accurate muscle sample retrieval and avoidance of direct neurovascular injury that may lead to subsequent hemorrhage or neural compromise [2]. Although results obtained from human soleus biopsies have been reported, we were unable to locate a published protocol of sufficient detail to safely learn and replicate the technique. The procedure detailed here provides a thorough and repeatable protocol for soleus muscle retrieval verified through cadaver dissection and ultrasonography. Tissue amounts are sufficient for performing a wide variety of analyses of which several examples are presented here. It is assumed that the reader has basic familiarity and experience with the needle biopsy technique and is encouraged to read the classic paper by Bergström [3].
METHODS
Subjects
Soleus samples were taken from 45 subjects (27 men; 18 women; 21.4 ± 2.9 yrs; 67.24 ± 12.3 kg; 167.5 ± 9.1 cm) participating in an integrated endurance and resistance exercise study using a gravity independent training device. All subjects were healthy, reported no cardiovascular or neuromuscular disease, and were considered sedentary at the beginning of the study. Subjects attended 2 separate sessions with 1 incision being made during each session (Figure 1). Muscle biopsies were performed at the Institute for Clinical and Translational Science at the University of California, Irvine where subjects were monitored at least 2 weeks following the procedure for bleeding, swelling, infection, skin irritation, discomfort, symptoms of compartment syndrome and healing progress. All biopsies were performed by a Board Certified Orthopedic Surgeon. Pre- and post-procedure use of platelet inhibiting preparations, such as aspirin and non-steroidal anti-inflammatory medications were discouraged for 1 week prior to and following the procedure. If it is noted that platelet inhibiting preparations may have been used, the investigative team should analyze the individual circumstances for the perceived level of risk before a decision to proceed or postpone the procedure is made. Depending upon the purpose of the biopsy and the research protocol; activities, exercise, and pharmaceuticals should be specified. The study protocol was approved in advance by the Institutional Review Board at the University of California, Irvine. Each subject provided written informed consent before participating.
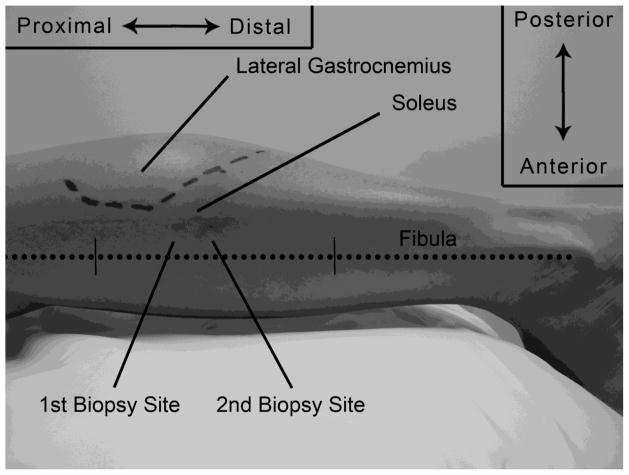
FIGURE 1. Lateral left leg detailing the biopsy sites in relation to anatomical structures.
Two biopsy sites from the same subject are shown in relation to the lateral gastrocnemius and fibula (dotted line). The fibula is marked in its middle third. The dashed line represents the junction of the lateral gastrocnemius and its aponeurisis.
Prior to undertaking human soleus muscle samples, prudence required a thorough understanding of the three dimensional anatomy utilizing cadaveric dissections in the University of California, Irvine, Human Anatomy Laboratory. During the examination by dissection, the optimal zone for the soleus biopsy was determined to be located where there is large muscle girth that is centrally located within the muscle. Surface anatomy was additionally examined to then determine a proposed needle entry site, trajectory, and depth. For technical reasons, we avoided using a biopsy needle on formalin persevered specimens. Rather, a 3 cc syringe filled with 1cc of printer ink attached to a 25 gauge 1½ in needle was used. Three undissected specimens were used to insert the needle by means of the proposed entry site, trajectory, and depth. Once the specified orientation of the needle was obtained, the ink was injected. A dissection of the calf was undertaken to confirm infiltration of the soleus muscle without disturbing the neurovascular bundle.
Ultrasonography was used to confirm the proposed needle entry site, trajectory, and depth on living subjects as determined by cadaver examination. The soleus muscle was located by the use of ultrasound guidance on an initial set of 12 subjects. The subject laid prone, knees extended, with a pillow supporting the lower legs (Figure 1). A Sonosite S-Fast ultrasound (Bothel, WA) was used with an L38 linear probe (frequency 5.0–10.0 MHz). The probe was oriented in the transverse plane at the lateral aspect of the subject’s mid-calf commencing distally near the ankle. The probe was slowly advanced superiorly towards the proposed biopsy zone until the soleus could be clearly distinguished from the superficial gastrocnemius. Once the soleus was identified in reference to the proposed incision site, the skin on the lateral edge of the soleus was marked indicating the incision site. The proposed incision site as determined by cadaver examination correlated well with obtaining the optimal biopsy zone by ultrasonography on living subjects.
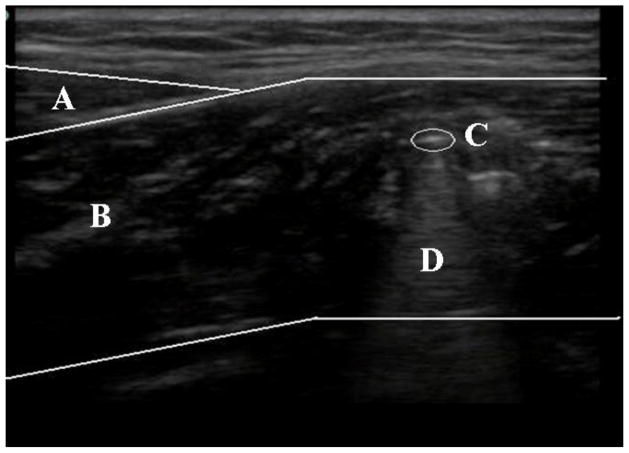
Ultrasonography was additionally utilized during the biopsy procedure to confirm infiltration of the soleus muscle. After insertion of the biopsy needle, the probe was placed longitudinally on the lower leg directly posterior to the position of the needle. This orientation allows for the gastrocnemius, soleus, and needle infiltration to be viewed (Figure 2)
FIGURE 2. Ultrasound confirmation in the transverse view of the lower leg.
The biopsy needle is shown in short axis detailing its position within the soleus muscle. (A) Gastrocnemius. (B) Soleus. (C) Biopsy Needle. (D) Artifact.
Equipment
For these biopsies, the University College Hospital (UCH) skeletal muscle biopsy needle (6G × 4 3/4″, Cadence Science, Lake Success, NY) was used (Figure 3A–C). The needle was cleaned and autoclave sterilized before each use. A surgical pack was put together prior to the biopsy. The pack contains pre-packaged sterile materials for each subject (Table I; Figure 3D).
FIGURE 3. U.C.H. skeletal muscle biopsy needle and surgical pack setup.
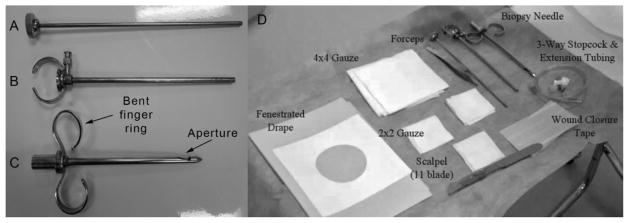
(A) Clearing rod, which expels the sample when inserted into the cylindrical cutting cannula. (B) Cyclindrical cutting cannula. (C) Outer needle with bent finger ring indicating direction of aperture. (D) Surgical pack setup in a sterile environment.
TABLE I.
Surgical pack.
| Units per Pack | Disposable Materials for Surgical Pack |
|---|---|
| 1 | Disposable razor |
| 1 | Povidone Iodine Swabstick |
| 1 | Syringe, 3cc |
| 1 | Needle, 18g |
| 1 | Needle, 30g 1in |
| 1 | Utility Drape** |
| 1 | Minor Procedure Fenestrated Adhering Drape** |
| 1 | Scalpel, disposable #11** |
| 2 | Gauze, Sterile 4×4in** |
| 2 | Gauze Sterile 2×2in** |
| 2 | 3-way stopcock** |
| 1 | Tubing 20in (50.8cm) Extension Set** |
| 1 | Bandaid** |
| 1 | Strip type wound closure tape** |
| 1 | Surgical Gloves Sterile** |
To be opened and used with sterile technique
In addition to the pre-packed materials, some materials were needed for multiuse such as an indelible marking pen, autoclave sterilized fine forceps (without teeth), ethyl chloride topical anesthetic spray (Gebauer Company, Cleveland, OH), and 2% lidocaine without epinephrine (Hospira, Inc., Lake Forest, IL). Furthermore, a vacuum pump was utilized (model: DOA-P707-AA; Gast Manufacturing, Benton Harbor, MI) with 500 mmHg suction controlled by an on/off foot controlled power switch. Suction pressure was measured after a stabilization period by a pressure gauge at the end of the attached tubing that was connected to the vacuum pump. This allowed for consistent suction with each pass that was easily engaged by pressing the foot pedal. Automated suction utilizing a portable vacuum pump or with wall suction may provide more consistent sampling than other methods such as applying suction with a syringe. The vacuum pump utilized here does not permit alterations in the level of suction and although higher than 200 mm Hg reported for wall suction [14], we have found no associated issues with the level of suction.
Procedures
Prior to the procedure, the subject is instructed to notify the team immediately should any pain, tingling, numbness, burning or other sensation be felt down the leg, distal to the needle insertion site during the procedure. This could indicate the needle is too close to the neurovascular bundle to proceed with the biopsy. The subject is reassured that there may be pressure, but not sharp pain, experienced with needle insertion. If pain is felt, additional local anesthetic is administered with the procedure immediately terminating if the discomfort is intolerable. This has not occurred in our series.
The subject is positioned prone on a standard examination table (Figure 1). A pillow supports the lower legs with the toes and ankle suspended. Shoe-wear is optional, but should preferentially be removed if obstructing lower leg anatomy. Pants may be rolled above the knee providing that there is no constriction. This positioning relaxes the posterior compartments of the leg and maximizes local soleus girth.
The subject is instructed that there may be some local soreness at the biopsy site post procedure. The subject is provided a means of contacting the research team should any symptoms of compartment syndrome or other potential complications arise in the first 72 hours after the procedure. Early indicators include severe pain, difficulty walking and calf swelling or tenseness. Subjects are instructed to not wait for numbness or color changes in the limb as these are more advanced signs that could indicate irreversible damage. The subject is directed to the emergency room for evaluation if there is any doubt.
Based upon cadaveric and ultrasound observations, the optimal zone for the soleus biopsy was determined by locating the area of greatest muscle girth within the central portion of the muscle. The superior-inferior borders of the optimal zone are within 2 cm proximal and 2 cm distal at the junction of the lateral head of the gastrocnemius and its aponeurosis (Figure 1). To determine this junction in those subjects where ultrasound was not used, the subject is asked to plantar flex the ankle against manual resistance at the metatarsal head plantar surface by asking the subject to “step on the gas” or “point your toes.” In lean subjects with prominent muscle definition, the two heads of the gastrocnemius clearly come into view. Otherwise, the lateral head of the gastrocnemius is easily palpated. The distal end of the muscle belly, at the junction of the aponeurosis, is marked with indelible ink (Figure 1, dashed lines). The medial-lateral borders of the optimal zone are within 1 cm of the midline of the calf. The distance between the tip of the needle and the aperture should be considered when advancing the biopsy needle to the optimal zone.
Additional biopsy site planning is required if subsequent biopsy sites are anticipated following the initial procedure. It is recommended to allow a distance of 1–2 cm proximal or distal between sampling sites to minimize specimen artifact caused by trauma, inflammation, and/or scarring (Figure 1). Biopsy sites are on the same longitudinal line within the optimal zone with the initial biopsy site being chosen in anticipation of any succeeding biopsies that will occur. It is our practice to choose a site distal to that of the initial site and have found no histological evidence of tissue damage or inflammation. The entry site for the biopsy needle is marked on the surface of the skin using an indelible marker on the lateral side of the leg, 1–2 cm posterior to the fibular shaft in its middle third (Figure 1, dotted line). The most lateral projection of the fibular head and lateral malleolus are subcutaneous and easily palpated. The middle third of the fibula may be visually estimated or measured. In those few subjects where the fibular diaphysis cannot be palpated, a straight line between the proximal and distal projections can be used to approximate the fibular path.
On a technical note, quantification of the optimal biopsy site as a ratio of the planned biopsy site from ultrasound imaging to the measured length of the fibula obtained from magnetic resonance imaging was attempted. Significant variability was found with multiple outliers and therefore this effort was abandoned. However, the reader is encouraged to become familiar with the anatomy by way of cadaver dissection or medical imaging of the biopsy site.
The area surrounding the biopsy site is shaved if required. A 10 cm diameter area surrounding the site is prepped with the 10% povidone-iodine solution swabstick. Local anesthesia is obtained with approximately 3 cc of 2% lidocaine utilizing a 30 gauge 1 in needle. Ethyl chloride spray is used as a topical anesthetic prior to needle insertion. The skin and subcutaneous tissue and fat are infiltrated. The fascia and gastrocnemius aponeurosis may also be anesthetized anticipating the needle trajectory described below.
Allowing the lidocaine time to achieve effect, the material and equipment are prepared. Sterile technique is used throughout. The sterile utility drape is opened onto a horizontal surface near the subject. The fenestrated adhering drape, scalpel, gauze, 3-way stopcock, extension tubing, wound closure tape and band-aid are opened onto the sterile utility drape (Figure 3D). Sterile gloves are donned.
The 3-way stopcock is attached to the biopsy needle hub. The 20 in extension tubing is attached to the other port of the 3-way stopcock. The opposite end of the extension tubing is handed off to the gloved (non-sterile) assistant for the application of suction. Although suction may be applied by pulling on a large bore syringe, we prefer an electric foot-switch controlled vacuum pump as it provides continuous and reproducible suction. Additionally, this also allows for convenient sampling in rooms that may not have the availability of wall suction.
The fenestrated adhering drape is applied to the leg with the biopsy site centered in the fenestration. The scalpel is used to make a longitudinal stab incision fully through the skin and subcutaneous tissue approximately 5–8 mm deep and 5–6 mm long. Any bleeding is dabbed with 4×4 in gauze.
Before proceeding with the needle insertion, several precautionary steps are advised. The biopsy needle is checked for proper suction at the aperture with the stopcock opened, the pump foot-switch activated and a thumb over the aperture at the needle base. If little to no suction is experienced, further examination of the stopcock and vacuum settings should be investigated. The fit of the outer needle and inner cutting cannula should be snug and not loose fitting. The cutting surface should be sharp and free of defects.
The biopsy needle is introduced into the incision. The preferred trajectory is:
Perpendicular to the long axis of the tibia,
Approximately 10 degrees upward from horizontal (Figure 5), and
With biopsy needle aperture directed posteriorly (toward the ceiling) and opposite the neurovascular structures.
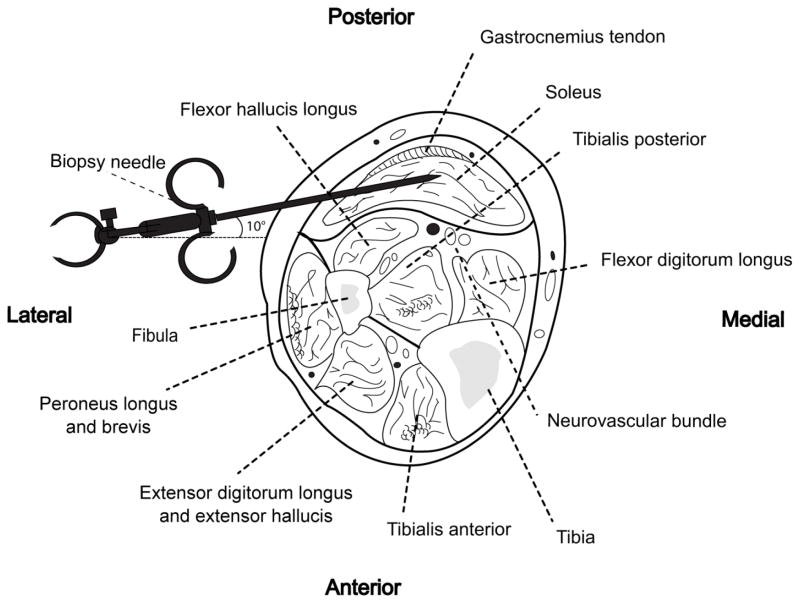
FIGURE 5. Cross sectional illustration of biopsy site and surrounding structures of the soleus.
(Redrawn from Hollinshead W.H.: Anatomy for Surgeons: Volume 3, New York, Harper, 1982) [12]
To monitor the orientation of the aperture, we have found it beneficial to slightly bend the metal finger ring on the side of the aperture (Figure 3C). This allows for easy identification of where the aperture is located even when the needle has been inserted into tissue. Once the orientation has been determined, the needle is advanced, with suction off, until resistance from the fascia and gastrocnemius aponeurosis is felt. The needle is then further advanced through the resistance into the belly of the soleus. Advancement is stopped when the aperture is at about the midline of the calf. It may be helpful to lay the needle across the calf prior to insertion to estimate the optimal depth. The vacuum pump foot-switch is depressed, activating suction and the biopsy cut is progressed in the usual fashion [3]. The operator’s other hand may gently squeeze the calf medially and laterally to “thicken” the soleus. Once the tissue has been cut, suction is shut off via the 3-way stopcock while the needle remains in the leg. The vacuum pump foot-switch is then released and the needle removed.
Depending on a variety of factors, such as desired fiber orientation, volume of required tissue, etc., multiple passes of the biopsy needle may be obtained through the same incision site. It is our practice to remove the needle after each biopsy and reintroduce it through the same trajectory and fascial hole although the needle may be left in the leg with multiple samples being taken before needle withdrawal. We chose to remove the needle with each pass due to the requirement for rapid freezing of the sampled tissue. There may be an advantage for subject comfort with taking multiple samples with a single pass due to reduced needle insertions although this will elongate the amount of time it takes for tissue to be frozen. Desired outcomes will dictate which method investigators decide to undertake. The orientation of the needle’s aperture is rotated proximally, posteriorly, and distally providing three muscle samples (Figure 1). We avoid taking biopsies with the biopsy needle aperture oriented anteriorly so as to minimize the risk of injury to the neurovascular structures located deep to the muscle (Figures 1 & 5). When adequate sample has been obtained, blood is removed from the skin with 4×4 in gauze, the wound is closed with the wound closure tape, and a band-aid is placed. Manual pressure may be required for a few minutes to aid hemostasis. The wound is then wrapped with moderate pressure using an elastic wrap such as Coban™ Self-Adhering Wrap (3M, St. Paul, MN). Subjects were monitored for at least 30 minutes in a relaxed, seated position following the procedure to ensure that bleeding at the incision site has stopped and that no other immediate complications have occurred. Subjects were then given a discharge kit containing a cold pack, gauze tape, wound closure tape and plastic bandages accompanied with instructions covering standard post-biopsy care. Subjects were instructed to:
Keep the incision site clean
Apply pressure/bandage if oozing occurs
For discomfort, apply ice to the incision site and take acetaminophen but avoid aspirin and ibuprofen for 1 week following the procedure
Resume normal activities but to refrain from intense exercise such as running as it may induce bleeding at the incision site
Go to the emergency room for persistent pain, swelling, or bleeding
Subjects were monitored for at least 2 weeks following the procedure with no complications being experienced other than reported feelings of minor soreness and bleeding at the incision site. A list of the rationale for the applied procedures for the soleus biopsy technique is provided with the manuscript (Table II).
TABLE II.
Rationale for the applied procedures of the soleus biopsy.
| Applied Procedures | Rationale |
|---|---|
| 1. Subject should be prone | Neurovascular bundle drops away from biopsy site |
| 2. Ankle in relaxed plantar flexion | Neurovascular bundle is not under tension |
| 3. Lateral head of muscular/aponeurosis junction defines optimal biopsy region | In this area, the soleus is thick and the gastrocnemius is thin |
| 4. Incision 1–2 cm posterior (above) to fibula | Avoids painful contact with fibula |
| 5. Biopsy needle trajectory is directly medial and angled posteriorly (upward) approximately 10 degrees (Figure 5) | Avoids the neurovascular bundle lying anterior to the soleus muscle belly |
| 6. Biopsy needle advanced until the aperture is at the midline of the calf | Biopsy needle aperture is at thickest part of soleus. |
| 7. Biopsies needle is rotated so aperture will obtain samples in the posterior (upper) one-half of soleus | Avoids potential neurovascular injury. |
| 8. Subject asked to immediately report any symptoms of distal pain, tingling, etc. toward the foot | Could indicate biopsy needle too close to neurovascular structure. Abort and reposition needle |
| 9. Subject counseled regarding procedure for post-biopsy increasing pain, swelling, etc. | Early recognition and treatment of potential compartment syndrome is critical to avoid complications |
The ideal specimen is a plug approximately 3 mm in diameter and approximately 5–6 mm in length obtained from each insertion of the biopsy needle. Fragmentation can occur where the plug does not come out as one single unit but in many smaller units. This fragmentation often occurs due to the usage of dull needles. Fragmented specimens are not optimal for histochemical analysis but are adequate for molecular or biochemical analyses. If tissue is badly fragmented, the sample is immediately placed in an aluminum foil pouch and frozen for biochemical/molecular analyses. If continual fragmentation occurs with multiple passes, a new biopsy needle with a sharp cutting end should be employed. With the limited amount of passes that may be performed per incision site, the technician will have to make an informed decision on which sample(s) to dedicate to mounting versus biochemical/molecular analyses. Once the needle has been removed from the tissue, the specimen usually remains at the needle’s aperture.
The plunger may need to be used to move the tissue sample into view at the needle’s aperture. The forceps are used to place the specimen on 2×2 in gauze by gently scooping or grabbing at the corners of the sample and handed off to a technician, using sterile technique, for processing and storage as required. Removal and transfer of the tissue should be done with extreme caution as to not crush or damage the muscle fibers, especially in preparation for such analyses as histochemistry and electron microscopy.
Tissue to be used for molecular/biochemical analyses is placed in an aluminum foil pouch which is immediately frozen in liquid nitrogen. As an example, muscle total RNA extraction and myosin heavy chain (MHC) mRNA was determined by competitive reverse transcription – polymerase chain reaction (RT-PCR) and gel electrophoresis as described previously for rat samples [1, 6]. A small amount of muscle biopsy (~5 mg) was homogenized in 30 volume PBS and the homogenate was used for MHC protein isoform analyses using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described previously for rat samples [1, 6]. These methods were used to identify differences in MHC makeup between the soleus and vastus lateralis to confirm soleus muscle retrieval.
For histochemistry, tissue samples are placed under a dissection microscope at 5x magnification in order to observe muscle fiber orientation. Any fragmented tissue at the ends of the sample are trimmed using a sharp blade and placed into an aluminum foil pouch and immediately frozen as described above for molecular/biochemical analysis. Using fine Dumont forceps, the sample is then oriented and mounted onto a circular piece of cork using HistoPrep (Fisher Scientific, Fair Lawn, NJ) to ensure that the muscle sample will be cut in cross-section. The mounted sample is rapidly frozen into a cup of 2-methylbutane (isopentane) cooled by liquid nitrogen and is then placed in a snap cap vial. The vial should have a vented cap to protect against internal pressure changes that may cause the cap to dislodge upon submission in liquid nitrogen. The sample is stored in liquid nitrogen until transferred to a −80°C freezer. Cross-sections of the muscle tissue were cut in a cryostat at −20°C at a thickness of 10 μm. To confirm muscle fiber type composition, ATPase staining of the section was performed after pre-incubation at 4.2 pH according to the technique described by Brooke and Kaiser [4].
RESULTS
Soleus muscle samples were obtained from 45 subjects with the described technique. Three samples were collected from succeeding needle insertions for each session. Ultrasonography was used on 12 subjects after needle insertion to demonstrate the biopsy needle directly in the belly of the soleus (Figure 2). A total of 84 soleus muscle biopsies were completed yielding 252 individual muscle specimens. Six subjects completed only the pre-biopsy session due to withdrawal from the study. Of the 252 specimens, 84 were mounted for histochemical analysis and the remaining 168 were frozen for biochemical/molecular analyses. Specimens obtained for biochemical/molecular analyses (2 out of the 3 passes) had an average weight of 61.5 ± 15.7 mg. There was no difference between sample weights obtained from men (61.5 ± 19.8 mg) and women (61.4 ± 23.0 mg). Average difference between the two sample specimens within a given biopsy session was 21.3 ± 18.5 mg. The minimum amount of tissue obtained from a single pass was 13 mg with 3 out of the 168 specimens having weights less than 20 mg.
Complications experienced during and after the procedure were minimal. Most subjects communicated a sense of pressure that was experienced as the needle was inserted into the muscle belly. Additionally, a dull ache at the biopsy site starting the day of or the following day was commonly reported. One subject experienced skin irritation due to the adhesives in the wound closure tape.
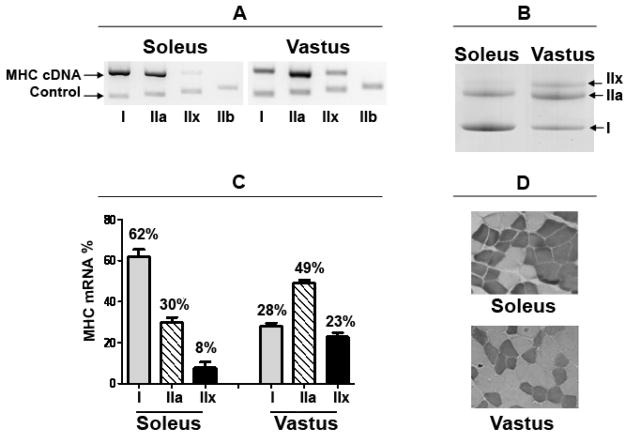
The MHC mRNA composition was 62% type I, 30% IIa, and 8% IIx for the soleus (N=12) whereas the vastus lateralis was found to be 28% type I, 49% IIa, and 23% IIx (N=37) (Clay Pandorf; written communication 09-03-2012; Figure 4A, C). These MHC distributions were also in the same range at the protein level based on MHC isoform separation by SDS-PAGE (Figure 4B). Myosin ATPase staining demonstrated that the cross sectional area of the soleus was 73 ± 15% slow-twitch type I (N=3) whereas the vastus lateralis in comparison was 31 ± 11% (N=3) (Figure 4D). Histological analysis of tissue in cross-section showed no instances of large vessels, nerves or fat.
FIGURE 4. MHC isoform composition for the soleus and vastus lateralis muscles.
(A) Representative gels for MHC mRNA PCR products showing control fragment along with co-amplified cDNA signals. (B) Representative gels for MHC protein isoforms as separated by SDS-PAGE. (C) Bar graph for MHC mRNA expression as % of the total MHC mRNA pool for both the soleus and vastus lateralis. Data are presented as means±SEM; soleus (N=12), vastus lateralis (N=37). (D) Muscle biopsy specimens were stained for ATPase at pH 4.2. Dark fibers are type I; light fibers are type II.
DISCUSSION
The results of the present study demonstrate that the human soleus muscle biopsy is a safe, reliable and repeatable procedure with techniques similar to that of the more commonly biopsied vastus lateralis. Tissue obtained from this procedure can be used for a wide variety of analyses such as RT-PCR, gel electrophoresis and histochemistry as was shown here. Additional methods for analyzing rates of protein synthesis and degradation [5, 19] and single fiber muscle mechanics have also been performed [18]. We have reported a predominance of type I fibers that is in agreement with previous studies [10, 15, 20]. This demonstrates that in addition to the ultrasound confirmation, the procedure was successful in obtaining samples from the soleus muscle.
Yield for each pass of the biopsy needle resulted in an average of 61.5 mg of muscle tissue. This is considerably less than the reported yield for tissue obtained from using wall suction despite the usage of 60% less suction (200 mm Hg versus 500 mm Hg) [14]. Using wall suction, investigators were able to retrieve an average of 233 mg of muscle tissue. This discrepancy may be due to the materials used (i.e. different biopsy needle) or the employed protocol. We were unable to determine if multiple applications of suction were used before removal of the needle or if the reported yield is from a single pass with one instance of suction. If multiple samples were taken before needle removal within a single pass, this would explain the nearly 4 times greater yield that was obtained despite a lower magnitude of suction. Additionally, weight of the biopsy sample may be dependent upon the internal diameter of the blade portion of the needle as well as the aperture dimensions. We used a needle with an internal cutting diameter of 3.5 mm and an aperture size of 11.5 mm × 5 mm. Regardless, the quantity of tissue obtained from the described protocol here provides ample tissue for a multitude of analyses.
At the mid-calf level, where the preferred biopsy site is located, the medial and lateral gastrocnemius bellies terminate and give rise to a broad thickened tendon, known as an aponeurosis, that envelopes the posterior surface of the soleus muscle. Immediately anterior to the soleus muscle lays a thin intramuscular septum separating the superficial and deep posterior compartments. This thin fascia separates the soleus muscle belly from important neurovascular structures including the tibial artery, vein and nerve. The very thin flexor hallucis longus muscle belly lies between the soleus and the peroneal artery and veins. A misdirected biopsy needle has potential for permanently injuring these critical structures warranting a detailed protocol for obtaining soleus muscle samples in a safe and effective manner (Figure 5). With the aid of cadaver dissection and ultrasonography, the authors are confident in the retrieval of human soleus muscle samples and comfortable that no major neurovasculature will be affected with this technique.
The presented manuscript is intended to establish a safe, reliable and repeatable protocol for the human soleus muscle biopsy. Sample tissue is accurately obtained in adequate amounts to perform a large amount of histochemical, biochemical, and molecular analyses. We feel that this procedure will permit further analysis of the soleus muscle and given its unique characteristics, will add to musculoskeletal and neuromuscular research as well as to clinical diagnosis and management.
Acknowledgments
The authors thank the following: Per Tesch, Department of Health Science, Mid Sweden University, Östersund for his guidance and encouragement in this manuscript. Marc Hamilton of the Pennington Biomedical Research Institute for the proofreads, suggestions and providing the first technique trainee. Anqi Qin, Ming Zeng, and Connie H. Chan for their technical support. Barbara Bodenhoefer, RN, and the nursing staff at the Institution for Clinical and Translational Science of UC Irvine for their exceptional care of our subjects. Marinelle Camilon and Theresa Hoang for their untiring work as organizers, technical support, and subject care. And finally, Tomasz Owerkowicz for his guidance and support. This work was supported by NSBRI-NASA NCC 9-58 and by the UCI Institute for Clinical and Translational Science NIH-UL1 TR000153.
Footnotes
Authors’ contributions
JC* recruited subjects, assisted with the biopsies, cadaver analysis, and ultrasound procedures, supervised the overall project and contributed to writing the manuscript. AY* assisted with the biopsies, cadaver analysis, and ultrasound procedures, and contributed to writing the manuscript. AK is a Board Certified Orthopaedic Surgeon that assisted with the cadaver analysis and ultrasound procedures, performed the soleus biopsies and contributed to writing the manuscript. FH conducted the mRNA and protein analyses and contributed to writing the manuscript. MB assisted with the biopsy and ATPase staining procedures and contributed to the manuscript. JF is Board Certified in Emergency Medicine and conducted the ultrasound procedures and contributed to writing the manuscript. GA provided financial support, supervised the overall project and contributed to writing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Joshua A. Cotter, Email: jcotter@uci.edu.
Alvin Yu, Email: yuam@uci.edu.
Arthur Kreitenberg, Email: akreit@msn.com.
Fadia H. Haddad, Email: fhaddad@uci.edu.
Michael J. Baker, Email: m2baker@uci.edu.
John C. Fox, Email: jfox@uci.edu.
Gregory R. Adams, Email: gradams@uci.edu.
References
- 1.Adams GR, Haddad F, McCue SA, Bodell PW, Zeng M, Qin L, et al. Effects of spaceflight and thyroid deficiency on rat hindlimb development. II. Expression of MHC isoforms. J Appl Physiol. 2000;88:904–16. doi: 10.1152/jappl.2000.88.3.904. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft LW, Peterson JJ, Kransdorf MJ, Berquist TH, O’Connor MI. Compartmental anatomy relevant to biopsy planning. Semin Musculoskelet Radiol. 2007;11:16–27. doi: 10.1055/s-2007-984410. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J. Muscle electrolytes in man. Scand J Clin Lab Med. 1962;14:511. [Google Scholar]
- 4.Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–2. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- 5.Carroll CC, Fluckey JD, Williams RH, Sullivan DH, Trappe TA. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Metab. 2005;288:E479–85. doi: 10.1152/ajpendo.00393.2004. [DOI] [PubMed] [Google Scholar]
- 6.di Maso NA, Haddad F, Zeng M, McCue SA, Baldwin KM. Role of denervation in modulating IIb MHC gene expression in response to T(3) plus unloading state. J Appl Physiol. 2000;88:682–9. doi: 10.1152/jappl.2000.88.2.682. [DOI] [PubMed] [Google Scholar]
- 7.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199:71–81. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards RH, Round JM, Jones DA. Needle biopsy of skeletal muscle: a review of 10 years experience. Muscle Nerve. 1983;6:676–83. doi: 10.1002/mus.880060910. [DOI] [PubMed] [Google Scholar]
- 9.Ericson M, Nisell R, Ekholm J. Quantified electromyography of lower-limb muscles during level walking. Scand J Rehabil Med. 1986;18:159–63. [PubMed] [Google Scholar]
- 10.Gollnick PD, Sjodin B, Karlsson J, Jansson E, Saltin B. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflugers Arch. 1974;348(3):247–55. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- 11.Greig PD, Askanazi J, Kinney JM. Needle biopsy of skeletal muscle using suction. Surg Gynecol Obstet. 1985;160:466–8. [PubMed] [Google Scholar]
- 12.Hollinshead WH. Anatomy for Surgeons. Vol. 3. New York: Harper & Row; 1969. [Google Scholar]
- 13.Lexell J, Taylor CC. A morphometrical comparison of right and left whole human vastus lateralis muscle: how to reduce sampling errors in biopsy techniques. Clin Physiol. 1991;11:271–6. doi: 10.1111/j.1475-097x.1991.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 14.Melendez MM, Vosswinkel JA, Shapiro MJ, Gelato MC, Mynarcik D, Gavi S, et al. Wall suction applied to needle muscle biopsy—A novel technique for increasing sample size. J Surg Res. 2007;142:301–3. doi: 10.1016/j.jss.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Ohira Y, Yoshinaga T, Nonaka I, Ohara M, Yoshioka T, Yamashita-Goto K, et al. Histochemical responses of human soleus muscle fibers to long-term bedrest with or without countermeasures. Jpn J Physiol. 2000;50:41–7. doi: 10.2170/jjphysiol.50.41. [DOI] [PubMed] [Google Scholar]
- 16.Paoli A, Pacelli QF, Toniolo L, Miotti D, Reggiani C. Latissimus dorsi fine needle muscle biopsy: A novel and efficient approach to study proximal muscles of upper limbs. J Surg Res. 2010;164:e257–e63. doi: 10.1016/j.jss.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma K, Watanabe K, Hotta N, Koike T, Ishida K, Katayama K, et al. The adaptive responses in several mediators linked with hypertrophy and atrophy of skeletal muscle after lower limb unloading in humans. Acta Physiol (Oxf) 2009;197:151–9. doi: 10.1111/j.1748-1716.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 18.Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N, et al. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol. 2008;294:R939–47. doi: 10.1152/ajpregu.00761.2007. [DOI] [PubMed] [Google Scholar]
- 19.Trappe TA, Raue U, Tesch PA. Human soleus muscle protein synthesis following resistance exercise. Acta Physiol Scand. 2004;182:189–96. doi: 10.1111/j.1365-201X.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- 20.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, et al. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915–30. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winter D, Yack H. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol. 1987;67:402–11. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]