Abstract
Objectives
We analyzed the outcomes following clinical management of parotid masses that were determined to be malignant tumors after parotidectomy.
Methods
We evaluated data from 70 patients with parotid malignancies between November 1994 and December 2005.
Results
Among salivary histotypes (n=49), the most significant prognostic parameter was cT4 stage at diagnosis (P=0.0055, log-rank) both for clinical involvement of the facial nerve and for invasion of other structures. The main cause of cancer-related death was a distant metastasis.
Conclusion
The present series confirms that the main prognostic parameter in salivary parotid malignancies was cT4 classification at diagnosis, often due to clinical involvement of the facial nerve. The oncological outcome of salivary malignancies was influenced by distant metastasis more than most other head and neck sites. We recommend dissecting and preserving the functioning VIIth cranial nerve during surgery for parotid malignancies.
Keywords: Parotid malignancies, Parotidectomy, Facial nerve, VII cranial nerve dissection, Clinical prognostic factors
INTRODUCTION
Salivary histotypes make up approximately 3% of all head and neck malignancies diagnosed in North America annually, and about 60% are in the parotid glands [1]. Parotid gland tumors account for 80% of all salivary gland neoplasms, but 80% of parotid tumors are benign. In the presence of a parotid mass, a physical examination is the first diagnostic tool and, in most cases, it guides the clinician in the appropriate direction. A fine needle aspiration biopsy (FNAB) has been indicated by several authors for the diagnostic work-up [2,3]. Anatomic imaging, and in particular ultrasonography, computed tomography, and/or magnetic resonance imaging are useful complementary studies for proper surgical planning. Nevertheless, none of these tools can provide definitive information about the nature and precise histology of a parotid mass. Open biopsy of a parotid mass is not recommended because of the risk of seeding in cases of a solid malignancy. Therefore, most parotid masses are operated on to obtain the histological diagnosis. The aim of this study was to retrospectively analyze a series of malignant masses of the parotid gland, and to obtain useful information about facial nerve preservation from our survival and outcome data.
MATERIALS AND METHODS
We evaluated 70 consecutive patients who underwent parotidectomy between November 1994 and December 2005 at the Institute of Otorhinolaryngology of Università Cattolica del Sacro Cuore, Policlinico Agostino Gemelli, Rome, Italy and who were affected by a malignant tumor at the histopathological exam.
We obtained clinical data by revision of charts, direct clinical re-examination, imaging of surviving patients, and by phone calls to relatives of dead patients (Table 1). The median follow up was 53 months. No patient was affected by Sjogren syndrome or by any other inflammatory disease of the parotid. We also collected familial history with particular regard to malignant tumors (salivary and not) in relatives.
Table 1.
Characteristics of the patients, tumors, and the surgical resection (n=70)
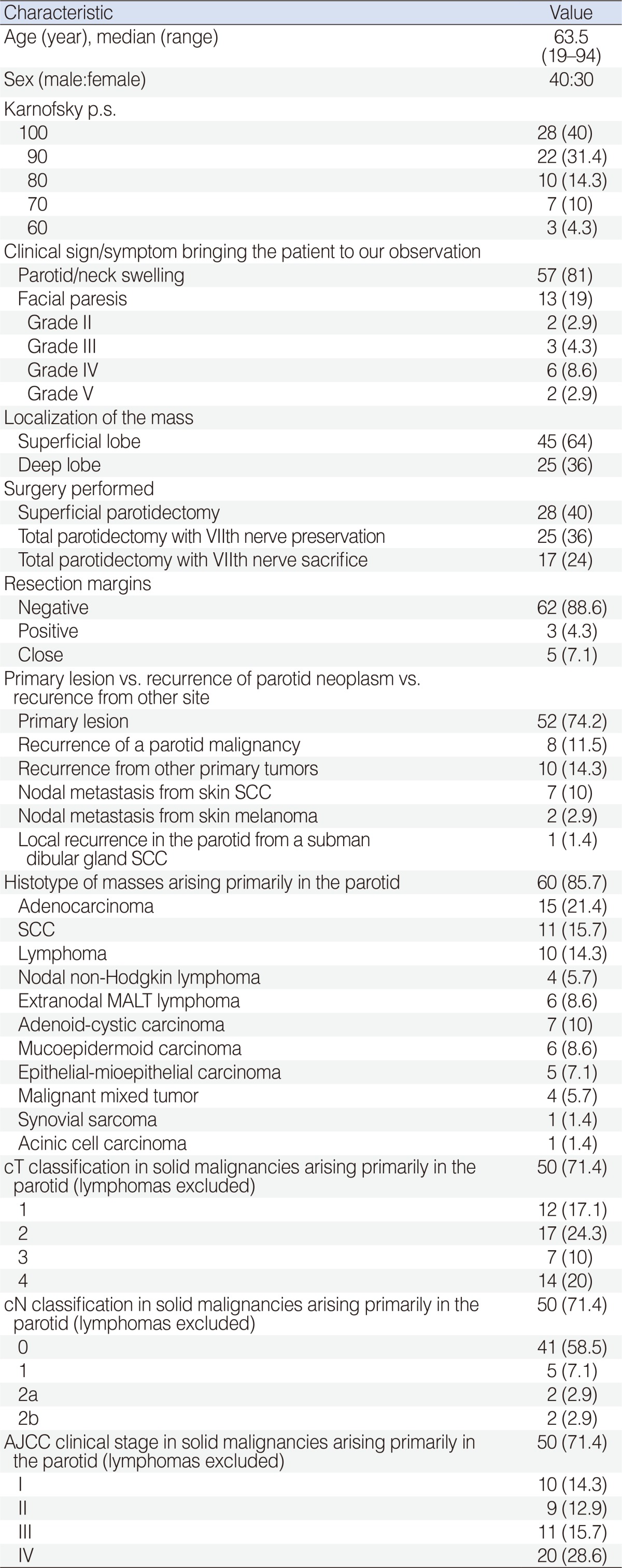
Values are presented as numbers (%).
SCC, squamous cell carcinoma; MALT, mucosa-associated lymphoid tissue; AJCC, American Joint Committee on Cancer.
Based on the site of origin, we classified the masses as primitive (when the parotid was the site of origin of the neoplasm) or metastatic and as primary or recurrent based on the clinical history (Table 2). We also evaluated the surgical parameters and incidental complications (Tables 1, 2). We did not use a transmandibular approach, as the deep lobe tumors were always resectable through a standard parotidectomy approach [4] by modifying the neck incision when needed. Resection was enlarged to other structures in 15 cases (external auditory canal, four cases; auricle, one; overlying skin, nine; mastoid, two; sternocleidomastoid muscle, seven; digastic muscle, seven; masseter, three; and submandibular gland, one). We reconstructed the defect with a free flap (one deep inferior epigastric perforator flap and one anterolateral thigh flap) or a regional pedicled flap (pectoralis major) in three cases, in which a wide area of skin had to be resected because of suspected or evident clinical involvement.
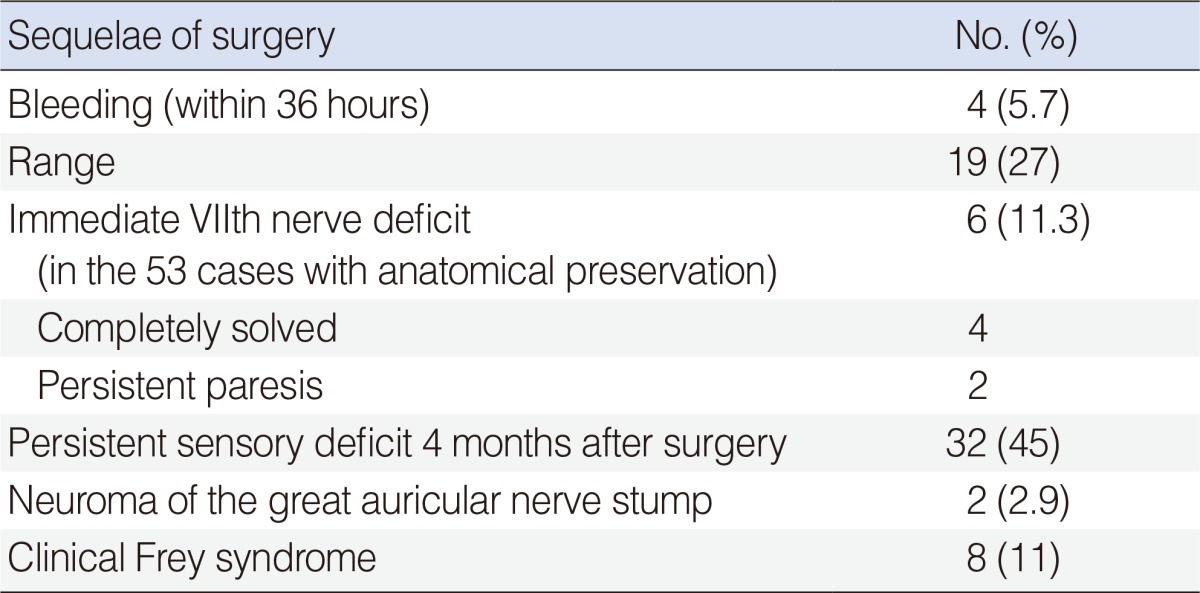
Table 2.
Complications and late sequelae of surgery in 70 patients undergoing parotidectomy for malignancies
We performed seven homolateral comprehensive neck dissections for metastases of skin neoplasms (two melanomas and five squamous cell carcinomas [SCCs]), and nine comprehensive (eight modified radical type III and one modified radical type I) in cN+primaries of the parotid (2pN0). We also performed 13 homolateral prophylactic neck dissections (selective of levels I, II, and III), in locally advanced primary tumors, with only one pN+(N2b).
Adjuvant treatment was recommended and performed in all patients with metastatic masses: radiochemotherapy in SCCs (seven cases, 60 Gy and three cycles of concurrent 100 mg/m3 cisplatin [CDDP] on days 1, 22, and 43), interferon-α for melanomas (two cases). Primitive neoplasms of epithelial origin underwent adjuvant radiotherapy (50-60 Gy) on the surgical bed and on the neck nodes when indicated by international guidelines [5], including intermediate or high grade or adenoid cystic tumors, close or positive margins, neural/perineural invasion, lymph node metastases, lymphatic/vascular invasion, stage IV disease, deep lobe salivary malignancies. When we found two or more of the above cited adverse characteristics, we added concurrent 100 mg/m3 CDDP on days 1, 22, and 43. In total, 16 patients (23%) with a parotid primary tumor underwent adjuvant radiochemotherapy and 14 (20%) underwent radiotherapy alone. Patients with lymphomas were referred to hematologists and underwent chemotherapy.
Survival curves were calculated since the day of the surgery by the Kaplan-Meier method using JMP ver. 5.1 (SAS Institute, Cary, NC, USA). We considered disease-specific, disease-free, and metastasis-(regional and distant) free survival as endpoints for the Kaplan-Meier analysis. We used both the log-rank and Wilcoxon tests to compare the survival curves. We also performed a multiviariate analysis by the Cox proportional hazards model. The significance level was fixed at 0.05 for all statistical tests.
RESULTS
The patient and tumor characteristics are shown in Table 1. No patient had distant metastases at diagnosis. Among the seven nodal metastases from skin SCC, three were thought to be primitive parotid neoplasms before resection, because the patients had not reported resection or they were considered negligible small skin tumors several months before.
Among the 40 cases that underwent FNAB before surgery, we had 25 true positive cases (with a cytological diagnosis of malignancy), 10 false negative cases, and five inadequate samples. Cytology of 10 operated lymphomas was unable to suggest the final histological diagnosis.
Eight of 70 patients (11%) had a second metachronous malignant tumor preceding or following the parotid malignancy (three adenocarcinomas of the prostate, two breast cancers, two kidney adenocarcinomas, and one Hodgkin lymphoma). Such metachronous malignancies were never the cause of death.
None of the patients reported a case of salivary malignant neoplasm among relatives. Twenty-six of 70 patients in the whole series and 23 of 60 with primary salivary malignant neoplasms reported one or more malignancies among first-degree relatives; the sites of such tumors were lung (four cases), colon or rectum (four cases), breast (four cases), bladder (one case), hematopoietic and lymphatic system (three cases), larynx (one case), oral cavity (one case), stomach (six cases), kidney (one case), liver (one case), and ovary (one case). Five of six gastric cancers occurred in relatives of patients with a primary parotid cancer arising from the glandular epithelium (two mucoepidermoid carcinomas, two adenocarcinomas, and one malignant mixed tumor).
The VIIth nerve was functioning normally at the time of surgery in four of 17 patients undergoing parotidectomy with VIIth nerve sacrifice (Table 1). It was possible to preserve the superior (orbitofrontal) branch in one of these cases and in one case we reconstructed the nerve with a sural graft. Among the salivary malignancies, we obtained close/positive resection margins in six of 17 operations (35%) with nerve sacrifice and in two of 32 operations (6%) with nerve preservation.
The most frequent early complication of surgery was sialocele (27%), which was always a self-limiting problem. The most frequent late complaint (4 months after surgery) was residual sensory deficit in the territory of the great auricular nerve (23/32 were irradiated patients) (Table 2). Frey syndrome was not as frequent as in other series probably because we diagnosed it clinically, did not perform routine objective tests, and because of the frequent recourse to irradiation, which inhibits both glandular secretion and nerve regeneration (none of the irradiated patients had Frey syndrome). We observed permanent paresis (grade III and grade IV, both of the inferior branches) in two cases with anatomical preservation of the facial nerve. The facial nerve reconstructed with a sural graft resulted in an unsatisfactory functional recovery; which may have been due to adjuvant radiochemotherapy.
We did not find differences when comparing survival among the different histological outcomes by the log-rank and Wilcoxon tests (P=0.4 and 0.53, respectively), recording the worst 5 year disease-specific survival among metastatic melanomas (both dead), skin SCC (74%) and primitive parotid SCC (68%) [6].
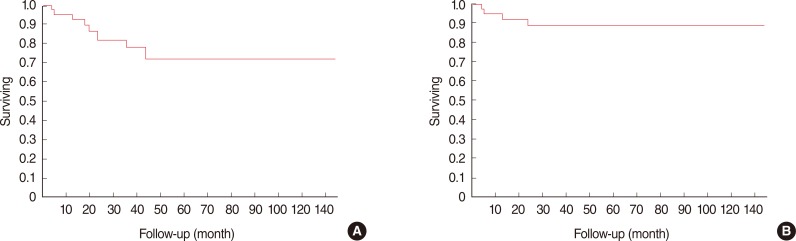
Overall survival in the group of 49 salivary tumors of the parotid (excluding tumors arising outside the parotid, lymphomas and the synovial sarcoma), was 85% at 2 years and 72% at 5 years (Fig. 1A); disease-specific survival was 93% at 2 years and 89% at 5 years (Fig. 1B). In the group of 49 salivary tumors of the parotid, no significant differences in survival were observed between lesions arising in the deep (5 year survival, 89%) or superficial lobes (5 year survival, 92%) or between resections with or without VII nerve preservation (92% and 79% 5 year survival respectively).
Fig. 1.
Overall survival in our series was 85% at 2 years and 72% at 5 years (A); disease-specific survival was 93% at 2 years and 89% at 5 years (B).
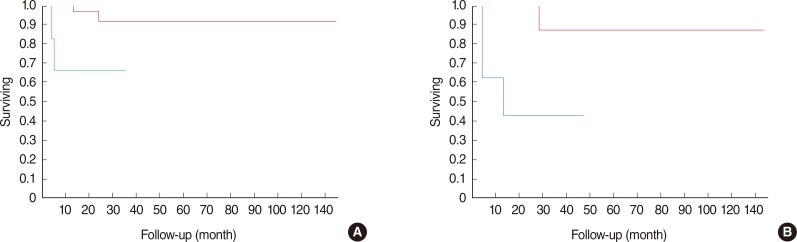
The most significant parameters for disease-specific survival were the presence of positive/close margins (P=0.01, log-rank) and cT4 (P=0.0055, log-rank) stage at diagnosis. Both parameters retained their prognostic value in the Cox regression model (Table 3). When considering the reasons why we classified the malignancies as cT4, both paresis of the facial nerve at diagnosis (P=0.006, log-rank) (Fig. 2A) and involvement of extra-parotid structures (skin, mandible, ear canal: T4a; or skull base: T4b; P<0.0001, log-rank) (Fig. 2B) were significant clinical prognostic markers.
Table 3.
Univariate and multivariate analysis of prognostic covariates for disease-specific survival among patients affected by primary malignant salivary histotypes of the parotid
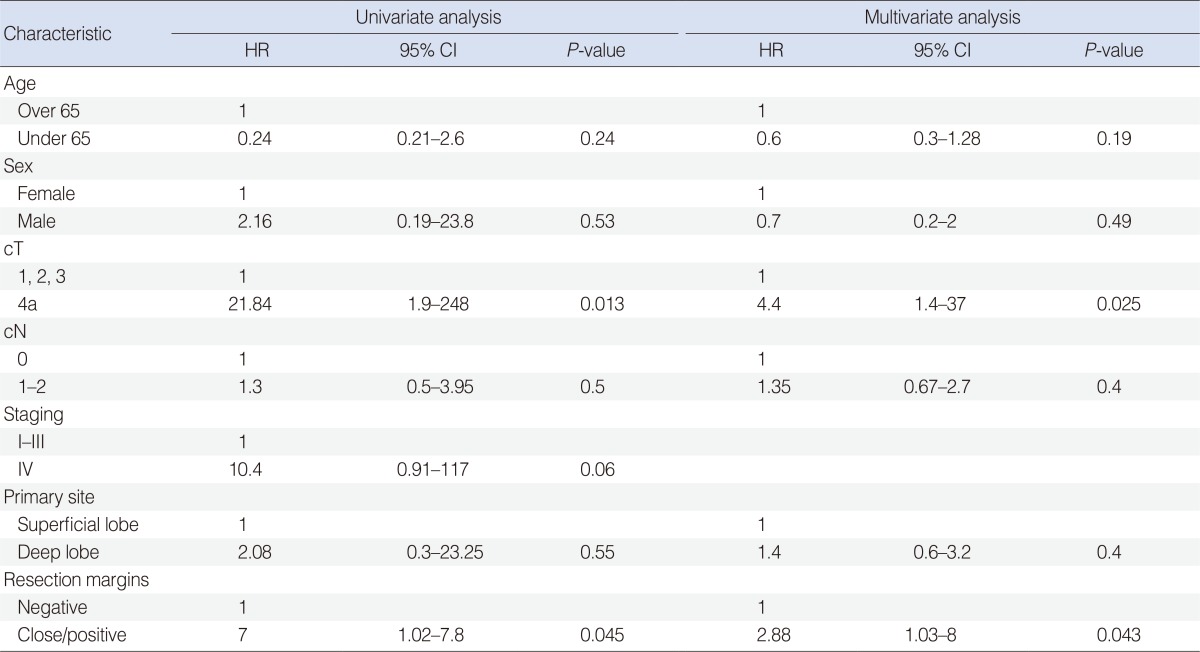
HR, hazard ratio; CI, confidence interval.
Fig. 2.
The most significant clinical parameter in our series was the cT4 at diagnosis, and in particular. (A) Paresis of the facial nerve (T4a; P=0.006, log-rank) was associated with a 66% 5-year disease-specific survival (DSS) (green line) vs. 91% 5-year DSS for patients with normal nerve function at diagnosis. (B) Involvement of other extra-parotid structures such as skin, ear canal, mandible (T4a) or skull base (T4b; P<0.0001, log-rank) was associated with a 44% 5-year DSS (azure line), vs. a 94% 5-year DSS for patients without involvement of the above cited structures at diagnosis. Notably, facial nerve function was always impaired when involvement of such structures was evident.
Distant metastases to the lung occurred in five patients with salivary neoplasms and in both patients with melanoma. Three of the patients with metastases from salivary cancers are currently alive with disease, two died because of the metastases. Therefore, distant metastases were the main cause of cancer related death; no patient died due to regional relapse.
DISCUSSION
The causes and risk factors for salivary gland malignancies have been evaluated only in a few reports [7,8], and they remain undetermined. In the present study, we describe an increased incidence of malignant second primary tumors arising from glandular epithelia, i.e., prostate, among patients with a primary salivary tumor and their relatives. Notably, our patients did not develop second primary metastases in the lungs, which is a typical occurrence in mucosal head and neck malignancies [9,10,11,12]. We recorded a positive familial history for gastric cancer in 5/49 of our patients (10.2%) with a primary parotid malignancy (excluding lymphomas), which usually accounts for 1.5% of all malignancies in the general population of Western countries [13]. These observations are interesting, even if they require further studies to be confirmed, as they may indicate common predisposing genetic factors for salivary and gastric cancer or for other glandular malignancies, as already postulated by some authors [14,15].
It is mandatory for the surgeon to assess the risk of malignancy before surgery, because it changes the prognosis and, the attitude toward facial nerve, whose sacrifice may be necessary in solid malignancies. Therefore, a suspicion of malignancy changes informed consent, avoiding the legal controversies related to the so-called "histological surprises". We believe that histological surprises are extremely rare; a malignancy can almost always be at least suspected, we only had four (about 6%) histological surprises, with no suspicion of malignancy before the operation. I two of these, cases the surprise was intraoperative, with difficulties dissecting the nerve (which was preserved). Avoidance of surprises is achieved by adequate anamnesis, physical examination, imaging, and by FNAB. The National Comprehensive Cancer Network (NCCN) guidelines for managing head and neck cancer suggest some suspicion criteria for a mass >4 cm, or one arising from the deep lobe [5]. The frequency (35%) of deep lobe masses in our series of malignancies was definitely higher than what has been reported in literature for benign tumors [15,16].
The nodal and extranodal lymphatic tissue within the parotid demonstrated a notable clinical relevance in our series. In fact, the parotid is the only salivary gland with intraparenchymal lymph nodes (5-7) that can collect metastatic cells; such nodes within the substance of the gland are not easily palpated and become noticeable only when they are enlarged. Distinguishing these nodes from primary parotid gland tumors by palpation or by imaging can be difficult. Masses with aggressive growth can therefore be secondary, most frequently nodal metastases from cutaneous SCC and melanomas [17], or ascribable to hematologic malignancies and in particular to lymphomas. Lymphomas of the parotid arising from diffuse lymphatic tissue within the gland (extranodal lymphomas) have been described as well, particularly in Sjogren disease [18], and they are often low grade maltomas. All of these non salivary malignancies may undergo surgical resection before obtaining a histopathological diagnosis, even if their treatment might be nonsurgical as with lymphomas.
Nodal metastases from salivary malignancies were less crucial in out series than those in previous studies [19,20]: we performed a prophylactic neck dissection in 13 cN0 cases with only 1 pN+ (7.7%) and we had no treatment failures for regional relapse. Neck irradiation in high risk cases (as defined above and by the NCCN guidelines) seems adequate for regional control in most salivary cancers and in high risk patients [21,22]. We performed prophylactic irradiation of the neck when we irradiated the surgical bed.
Disease-specific survival decreases for many years, particularly in patients with adenoid cystic carcinoma and malignant mixed tumor, because of distant metastases, which have been reported in approximately 20% of mainly high grade parotid malignancies and are predictive of a poor prognosis [19]. In particular, 40% of patients with adenoid cystic carcinoma and 26%-32% with malignant mixed tumors demonstrated this feature [23,24]. In all these lesions, the site of distant metastasis is most often the lung(s). Lung metastases occurred in 10% of all of out patients with malignancies arising from salivary tissue. Nevertheless distant metastasis may not always represent a terminal event and, therefore, does not necessarily preclude treatment of primary disease, particularly in patients with adenoid cystic carcinoma. We have three patients still alive with metastases. One with adenoid cystic carcinoma is alive 2 years after the diagnosis of the pulmonary relapse. Nevertheless, none of our patients had a diagnosed distant metastasis when we performed a parotidectomy.
Several previous studies showed that advanced stage, high grade, and submandibular location were prognostic for a poorer outcome, and differences in histological features were reported to affect natural history [8,23,25,26,27,28,29]. Positive/close margins on histopathology and cT4 stage for VII nerve involvement or for involvement of other structures were the only clinical parameters at diagnosis retaining their value also at multivariate analysis (Fig. 2).
The significance of positive/close margins might suggest extending the indications to nerve sacrifice, because the attempt to preserve the nerve sometimes leads the surgeon to leave microscopic (or even macroscopic) disease behind. Nevertheless such a "destructive" attitude with liberal resection of a facial nerve no longer dominates surgical philosophy. Instead the surgeon's reliance on postoperative radiation therapy to manage microscopic disease and the likelihood of a distant metastasis make many surgeons reluctant to sacrifice a functioning facial nerve or in the event of a hard-to-dissect clear malignancy. Our results also suggest this form of surgical minimalism which is gaining consent in the last few years. In fact, facial nerve sacrifice seems to be associated with worse survival. Furthermore, most of our patients with positive margins (R1) had undergone a total parotidectomy with nerve sacrifice, which is not a solution for the margin issue. In these cases, the positive margins are more often outside the parotid, and involve other structures; thus, a more aggressive attitude is warranted on extra-glandular structures, possibly by resorting reconstructive techniques. We believe that clinical nerve dysfunction and extra-parotid extension (that is T=4), which is often not associated with bulky tumors in our experience, are by themselves expression of intrinsically more aggressive tumors and can be read as independent prognostic factors.
Our approach is to sacrifice the nerve when it is clinically involved and/or when it is totally embedded in a clearly malignant neoplasm, but to at least attempt to dissect and preserve it in other cases. This attitude is strengthened by the consideration that the ultimate diagnosis of malignancy always relies on definitive histology of a surgical sample (often with the help of immunohistochemistry) and frozen sections or biopsies cannot provide sure and legally safe indications for VII nerve sacrifice.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Spiro R, Spiro J. Cancer of the salivary glands. In: Meyers E, Suen J, editors. Cancer of the head and neck. 2nd ed. New York: Churchill Livingstone; 1984. pp. 645–699. [Google Scholar]
- 2.Zbären P, Schar C, Hotz MA, Loosli H. Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope. 2001 Nov;111(11 Pt 1):1989–1992. doi: 10.1097/00005537-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Sergi B, Contucci AM, Corina L, Paludetti G. Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope. 2004 Apr;114(4):789. doi: 10.1097/00005537-200404000-00041. [DOI] [PubMed] [Google Scholar]
- 4.Shah JP, Patel KJ. Head and neck surgery and oncology. 3rd ed. Philadelpia, PA: Mosby; 2003. [Google Scholar]
- 5.Pfister DG, Ang KK, Brizel D, Burtness B, Cmelak AJ, Colevas AD, et al. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: head and neck cancers. ver. 2.2011. Fort Washington, PA: NCCN; 2011. [Google Scholar]
- 6.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986 Jan-Feb;8(3):177–184. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 7.Katz AD, Preston-Martin S. Salivary gland tumors and previous radiotherapy to the head or neck: report of a clinical series. Am J Surg. 1984 Mar;147(3):345–348. doi: 10.1016/0002-9610(84)90164-8. [DOI] [PubMed] [Google Scholar]
- 8.Hollander L, Cunningham MP. Management of cancer of the parotid gland. Surg Clin North Am. 1973 Feb;53(1):113–119. doi: 10.1016/s0039-6109(16)39937-6. [DOI] [PubMed] [Google Scholar]
- 9.Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, El Mofty S, et al. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope. 2001 Jun;111(6):1079–1087. doi: 10.1097/00005537-200106000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Narayana A, Vaughan AT, Fisher SG, Reddy SP. Second primary tumors in laryngeal cancer: results of long-term follow-up. Int J Radiat Oncol Biol Phys. 1998 Oct;42(3):557–562. doi: 10.1016/s0360-3016(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 11.Franchin G, Minatel E, Gobitti C, Talamini R, Vaccher E, Sartor G, et al. Radiotherapy for patients with early-stage glottic carcinoma: univariate and multivariate analyses in a group of consecutive, unselected patients. Cancer. 2003 Aug;98(4):765–772. doi: 10.1002/cncr.11575. [DOI] [PubMed] [Google Scholar]
- 12.Almadori G, Bussu F, Cadoni G, Galli J, Rigante M, Artuso A, et al. Multistep laryngeal carcinogenesis helps our understanding of the field cancerisation phenomenon: a review. Eur J Cancer. 2004 Nov;40(16):2383–2388. doi: 10.1016/j.ejca.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jul-Aug;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 14.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953 Apr;1(4814):799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CC, Tsai MH, Huang CC, Hua CH, Tseng HC, Huang ST. Parotid tumors: a 10-year experience. Am J Otolaryngol. 2008 Mar-Apr;29(2):94–100. doi: 10.1016/j.amjoto.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Bussu F, Parrilla C, Rizzo D, Almadori G, Paludetti G, Galli J. Clinical approach and treatment of benign and malignant parotid masses, personal experience. Acta Otorhinolaryngol Ital. 2011 Jun;31(3):135–143. [PMC free article] [PubMed] [Google Scholar]
- 17.Cassisi NJ, Dickerson DR, Million RR. Squamous cell carcinoma of the skin metastatic to parotid nodes. Arch Otolaryngol. 1978 Jun;104(6):336–339. doi: 10.1001/archotol.1978.00790060038010. [DOI] [PubMed] [Google Scholar]
- 18.Hyjek E, Smith WJ, Isaacson PG. Primary B-cell lymphoma of salivary glands and its relationship to myoepithelial sialadenitis. Hum Pathol. 1988 Jul;19(7):766–776. doi: 10.1016/s0046-8177(88)80259-4. [DOI] [PubMed] [Google Scholar]
- 19.Spiro RH, Huvos AG, Strong EW. Cancer of the parotid gland: a clinicopathologic study of 288 primary cases. Am J Surg. 1975 Oct;130(4):452–459. doi: 10.1016/0002-9610(75)90483-3. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong JG, Harrison LB, Thaler HT, Friedlander-Klar H, Fass DE, Zelefsky MJ, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992 Feb;69(3):615–619. doi: 10.1002/1097-0142(19920201)69:3<615::aid-cncr2820690303>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong JG, Harrison LB, Spiro RH, Fass DE, Strong EW, Fuks ZY. Malignant tumors of major salivary gland origin: a matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990 Mar;116(3):290–293. doi: 10.1001/archotol.1990.01870030054008. [DOI] [PubMed] [Google Scholar]
- 22.Malata CM, Camilleri IG, McLean NR, Piggot TA, Kelly CG, Chippindale AJ, et al. Malignant tumours of the parotid gland: a 12-year review. Br J Plast Surg. 1997 Dec;50(8):600–608. doi: 10.1016/s0007-1226(97)90505-1. [DOI] [PubMed] [Google Scholar]
- 23.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin: a clinicopathologic study of 242 cases. Am J Surg. 1974 Oct;128(4):512–520. doi: 10.1016/0002-9610(74)90265-7. [DOI] [PubMed] [Google Scholar]
- 24.Spiro RH, Huvos AG, Strong EW. Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer. 1977 Feb;39(2):388–396. doi: 10.1002/1097-0142(197702)39:2<388::aid-cncr2820390204>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Spiro RH, Huvos AG, Strong EW. Adenocarcinoma of salivary origin: clinicopathologic study of 204 patients. Am J Surg. 1982 Oct;144(4):423–431. doi: 10.1016/0002-9610(82)90416-0. [DOI] [PubMed] [Google Scholar]
- 26.Borthne A, Kjellevold K, Kaalhus O, Vermund H. Salivary gland malignant neoplasms: treatment and prognosis. Int J Radiat Oncol Biol Phys. 1986 May;12(5):747–754. doi: 10.1016/0360-3016(86)90032-5. [DOI] [PubMed] [Google Scholar]
- 27.Matsuba HM, Simpson JR, Mauney M, Thawley SE. Adenoid cystic salivary gland carcinoma: a clinicopathologic correlation. Head Neck Surg. 1986 Jan-Feb;8(3):200–204. doi: 10.1002/hed.2890080312. [DOI] [PubMed] [Google Scholar]
- 28.Spiro RH, Huvos AG, Berk R, Strong EW. Mucoepidermoid carcinoma of salivary gland origin: a clinicopathologic study of 367 cases. Am J Surg. 1978 Oct;136(4):461–468. doi: 10.1016/0002-9610(78)90262-3. [DOI] [PubMed] [Google Scholar]
- 29.Lima RA, Tavares MR, Dias FL, Kligerman J, Nascimento MF, Barbosa MM, et al. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol Head Neck Surg. 2005 Nov;133(5):702–708. doi: 10.1016/j.otohns.2005.08.001. [DOI] [PubMed] [Google Scholar]