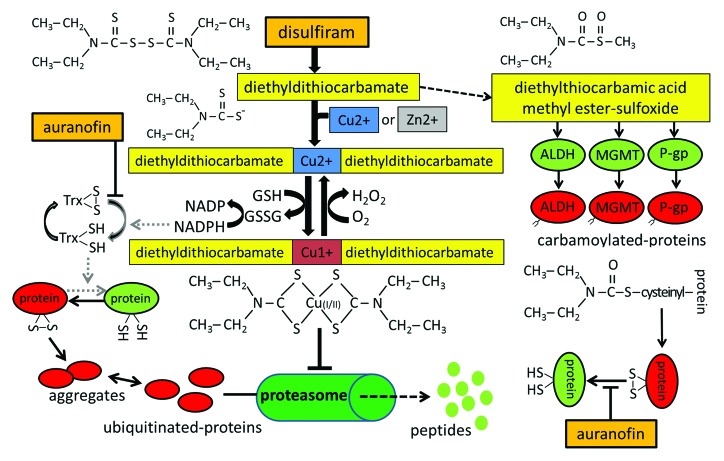
Disulfiram (tetraethylthiuram disulfide) is a highly versatile reagent and has been used for a variety of applications ranging from a vulcanization accelerator to an ointment against endo- and ectoparasites.1 It was noticed more than 70 years ago that rubber-processing workers and patients with scabies exposed to thiuram disulfides experienced strong adverse reactions after drinking alcoholic beverages. This led to identification of the alcohol-deterring quality of disulfiram.1 Since then, disulfiram, also known by its trade name Antabuse, has been approved and used clinically for over 60 years as an alcohol deterrent in the treatment of alcoholism.
Clinically, in the absence of alcohol consumption, disulfiram is quite well-tolerated. Orally administered disulfiram is rapidly converted by the gastrointestinal tract or within the blood stream into 2 molecules of its diethyldithiocarbamate salt (Fig. 1). Disulfiram-derived diethyldithiocarbamate can further be metabolized into diethylthiocarbamic acid methyl ester sulfoxide (Fig. 1). This metabolite of disulfiram has been identified as a highly active inhibitor of the catalytic sulfhydryl group of human aldehyde dehydrogenase (ALDH), which metabolizes ethanol-derived noxious acetaldehyde into nontoxic acetate. By the same cysteine-interacting mechanism, disulfiram also inhibits a variety of cancer-promoting proteins, including protein kinase C, P-glycoprotein (P-gp), and DNA methyltransferases (DNMT) (Fig. 1).
Figure 1. Metabolism and action of disulfiram. Disulfiram-derived metabolites can inactivate essential sulfhydryl groups either directly by protein carbamoylation or indirectly by complexing with divalent copper or zinc ions to form a redox-active metallo–organic complex. Oxidized proteins with non-physiological disulfide bonds can be repaired by the thioredoxin (Trx) system, whose inhibition by auranofin can lead to further accumulation of possibly cytotoxic proteins and aggregates.
Disulfiram metabolites have also been shown to complex with copper or zinc ions, resulting in partial depletion of the intracellular heavy metal ion pool, thereby inhibiting zinc- and copper-dependent enzymes such as superoxide dismutase (protective against oxidative stress), matrix metalloproteinases (promoting cancer cell invasion and metastasis), or dopamine β-monooxygenase (involved in cocaine addiction, for which disulfiram is also under investigation).
A strong anti-cancer effect of disulfiram was observed in combination with divalent heavy metal ions, often found enriched in cancer tissues, or orally supplied either as copper or zinc gluconate.2 The redox cycling between Cu(I) and Cu(II) within this complex has been proposed to be responsible for both glutathione oxidation and hydrogen peroxide generation, leading to a combined and perpetual oxidative stress mechanism (Fig. 1).
The combination of disulfiram with copper was also shown to have a strong inhibitory effect on the proteasomal protein degradation system.3,4 Since a functional proteasome system is essential for a coordinated expression of cell cycle-regulatory and apoptosis-controlling proteins, its inhibition is particularly fatal for cancer cells, in which proteasome inhibitors induce massive disturbances in protein homeostasis and accumulation of poly-ubiquitinated proteins and cytotoxic protein aggregates.3,4
Another mechanism by which disulfiram may specifically target cancer cells is by its preferential activity against various forms of aldehyde dehydrogenases. The ALDH genes, although primarily known as biotransformation genes, also belong to a small group of genes coding for proteins that, although called “stem cell markers,” are mediators of the range of attributes of what we call stem cells. Cancer stem cells are preferentially resistant to cytotoxic chemotherapies as currently constituted. Selective targeting of ALDH-positive cancer cell sub-populations by disulfiram was shown, as predicted, to preferentially inhibit cancer stem cells.4,5
Disulfiram, and in particular its metabolites, have repeatedly been demonstrated to cause a pro-oxidative environment in cancer cells.2 A recent study performed in our laboratory revealed a pronounced and irreversible induction of apoptosis in ovarian cancer cells by disulfiram in a copper-supported manner, associated with the occurrence of oxidative stress, formation of protein aggregates, and induction of heat shock-response genes.6
Cells protect against cytotoxic oxidative stress by the expression of endogenous antioxidants, which function either as radical scavengers or provide reducing equivalents to re-hydrogenate oxidized disulfide bonds (Fig. 1).2 Two systems provide the main contribution to cellular redox homeostasis: the glutathione and the thioredoxin system. These are both often upregulated in cancer cells to cope with increased intracellular production of oxidants resulting from characteristic metabolic patterns in cancer.7 We reasoned therefore that the combined action of disulfiram with other drugs that interfere with cellular antioxidative defense systems might enhance the anti-tumoral effects of disulfiram.2 Auranofin, an anti-rheumatic drug, is an effective inhibitor of thioredoxin reductase, and it has been proposed that the co-application of auranofin might enhance the anti-cancer efficacy of disulfiram.2 In fact, a proof-of-principle study on ovarian cancer cells revealed that auranofin indeed augmented the cytotoxic effects of disulfiram.6 By preventing thioredoxin-mediated reduction of oxidized proteins (Fig. 1), protein inactivation, misfolding, and aggregation is enhanced. Both auranofin and disulfiram are well-tolerated drugs and indicate that a combined application of repurposed drugs may have promising potential for anti-cancer therapy.
In vitro studies, xenograft models, and preliminary data from clinical trials and clinical observations indicate that disulfiram might have promising prospects in cancer therapy that should be further explored by ongoing and future clinical studies.2,8 Therefore, we have several clues which indicate that the repurposed “old drug” disulfiram, particularly when combined with its new partner auranofin, may find a further application as a prospective new medication in cancer.
References
- 1.Ellis PM, et al. Drug Alcohol Rev. 2013;32:342–4. doi: 10.1111/dar.12018. [DOI] [PubMed] [Google Scholar]
- 2.Kast RE, et al. Oncotarget. 2013;4:502–30. doi: 10.18632/oncotarget.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, et al. Cancer Res. 2006;66:10425–33. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 4.Hothi P, et al. Oncotarget. 2012;3:1124–36. doi: 10.18632/oncotarget.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SK, et al. PLoS One. 2013;8:e78130. doi: 10.1371/journal.pone.0078130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaioannou M, et al. Oncoscience. 2014;1:21–9. doi: 10.18632/oncoscience.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotblat B, et al. Oncotarget. 2013;4:2577–90. doi: 10.18632/oncotarget.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cvek B. Curr Cancer Drug Targets. 2011;11:332–7. doi: 10.2174/156800911794519806. [DOI] [PubMed] [Google Scholar]