Abstract
The mRNA is co-transcriptionally bound by a number of RNA-binding proteins (RBPs) that contribute to its processing and formation of an export-competent messenger ribonucleoprotein particle (mRNP). In the last few years, increasing evidence suggests that RBPs play a key role in preventing transcription-associated genome instability. Part of this instability is mediated by the accumulation of co-transcriptional R loops, which may impair replication fork (RF) progression due to collisions between transcription and replication machineries. In addition, some RBPs have been implicated in DNA repair and/or the DNA damage response (DDR). Recently, the Npl3 protein, one of the most abundant heterogeneous nuclear ribonucleoproteins (hnRNPs) in yeast, has been shown to prevent transcription-associated genome instability and accumulation of RF obstacles, partially associated with R-loop formation. Interestingly, Npl3 seems to have additional functions in DNA repair, and npl3∆ mutants are highly sensitive to genotoxic agents, such as the antitumor drug trabectedin. Here we discuss the role of Npl3 in particular, and RBPs in general, in the connection of transcription with replication and genome instability, and its effect on the DDR.
Keywords: DNA damage response, DNA repair, Npl3, R loops, RNA-binding proteins, transcription-associated genome instability, transcription-replication conflicts
Introduction
In eukaryotic cells, transcription and translation occur in different cellular compartments. Whereas DNA is transcribed to mRNA in the nucleus, protein synthesis takes place in the cytoplasm, implying that the mRNA has to be exported for translation. This export occurs through a macromolecular complex embedded in the nuclear membrane termed the nuclear pore complex (NPC).1 Transcription and export are coupled with the processing of pre-mRNAs into mRNAs, which includes 5′-end capping, splicing, and 3′-end cleavage and polyadenylation. During these processes, the mRNA is co-transcriptionally packaged by a number of RNA-binding proteins (RBPs) leading to the formation of an export-competent messenger ribonucleoprotein particle (mRNP).2 The most abundant classes of RBPs in eukaryotes are the heterogeneous nuclear ribonucleoproteins (hnRNPs) and the mammalian serine-arginine-rich (SR) proteins. Both protein families carry out different functions from pre-mRNA packaging to splicing, mRNA export and translation.3,4
One of the most abundant hnRNPs in Saccharomyces cerevisiae is Npl3, which is also an SR-like protein and shares structural homologies with both protein families.5,6 Npl3 is a multifunctional protein that participates in a variety of RNA-related processes, including transcription, splicing, mRNA export, and translation.7-10 Additionally, a recent report has provided evidence of an Npl3 role in preventing genome instability.11 The function of Npl3 at transcribed genes is necessary to avoid R-loop formation between the nascent mRNA and the transcribed DNA strand, thus protecting the genome against harmful transcription–replication collisions. The sensitivity of npl3∆ cells to double-strand break (DSB)-inducing genotoxic agents, including the antitumor drug trabectedin, suggests a role of Npl3 in the DNA damage response (DDR) and provides new evidence on the importance of RBPs in the maintenance of genome integrity and cancer prevention that we discuss here from a global perspective.
Npl3, a Multifunctional Protein Mediating RNA Export
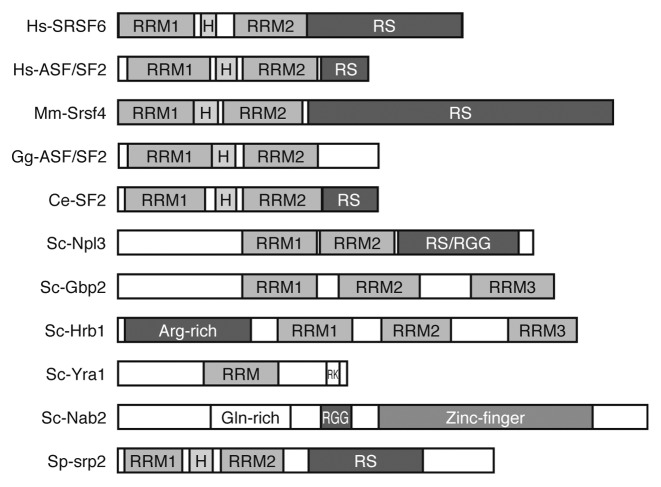
Npl3 was initially identified as a protein required for the localization of nuclear proteins (nuclear protein localization 3) and for pre-rRNA processing.12,13 However, it was soon described for its role as an mRNA export factor and its ability to shuttle between the nucleus and the cytoplasm together with the mRNAs.8,14 Npl3 shares structural homologies with hnRNPs and SR proteins. It has 2 RNA-recognition motifs (RRMs), a conserved RNA-binding domain shared by a number of RBPs from yeast to humans (Fig. 1), and an N-terminal domain composed of RGG tripeptides and RS dipeptides that confer to Npl3 a high RNA-binding capacity.5 There are 2 other SR-like proteins in Saccharomyces cerevisiae, Gbp2 and Hrb1, which also function in mRNA export. In contrast to Npl3, Gbp2 and Hrb1 physically associate with the THO complex, a key nuclear RNA binding factor connecting transcription, mRNP biogenesis, and genome instability.15 In addition, Npl3 promotes pre-mRNA splicing, while Gbp2 and Hrb1 are involved in quality control of spliced mRNAs.9,16
Figure 1. RNA-binding domains in different eukaryotic RBPs. RRM, RNA-recognition motif; H, hinge domain; RS, Arg-Ser-rich; RGG, Arg-Gly-Gly-rich; RK, Arg-Lys-rich; Hs, Homo sapiens; Mm, Mus musculus; Gg, Gallus gallus; Ce, Caenorhabditis elegans; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe.
Npl3 binds to genes in a transcription-dependent manner by its physical interaction with the C-terminal domain (CTD) of RNA polymerase II (RNAPII) at the phosphorylated Ser2,7,17 which is a mark of transcription elongation. Binding of Npl3 to RNAPII promotes transcription elongation and inhibits termination by competing with the CF1A cleavage and polyadenylation complex.18 This mechanism is believed to avoid premature termination at cryptic termination sites and serves as a self-regulation mechanism of Npl3 due to the existence of a weak alternative poly-A site downstream of the NPL3 gene.19 The anti-termination activity of Npl3 is progressively reduced during transcription elongation by casein-kinase Cka1-dependent phosphorylation, allowing the action of termination factors.7 Then, mRNA 3′-end processing stimulates Npl3 dephosphorylation by Glc7 phosphatase, which causes Npl3 binding to the nascent mRNA and promotes its export to the cytoplasm.20 Consistent with this model, Npl3 is distributed genome-wide throughout the length of transcribed ORFs in an increasing gradient toward the 3′ end and decreasing again around the poly-A site,11 not easily observed in another study.21 The increasing accumulation of Npl3 toward the 3′ end of genes correlates with that of Ser2P of the RNAPII CTD, with which Npl3 physically interacts.7,22,23 Indeed, the THO complex and several transcription elongation factors also bind to active genes in a 5′ to 3′ gradient.23-25 Therefore, it is possible that the RNAPII CTD participates in recruiting Npl3 during transcription elongation.
After export to the cytoplasm, Npl3 is phosphorylated by Sky1 and released from the mRNA.26 Indeed, purification of the mRNPs by pull down with different RNA-binding tagged-proteins has revealed that Npl3 is a component of the nuclear mRNPs, but not of the cytoplasmic mRNPs, such as the Cap-binding complex (CBC) proteins Cbp20 and Cbp80, the THO subunit Thp2, the RNA export factor Yra1, the export receptor Mex67, the NPC-associated THSC/TREX-2 subunit Sac3, and the nucleoporin Mlp1.27,28 These observations indicate that Npl3 is bound to the mRNA at early stages of mRNP biogenesis and remains associated until it reaches the cytoplasm, where it is dephosphorylated and reimported to the nucleus.
A Role of Npl3 in the Maintenance of Genome Integrity
Improper mRNP biogenesis and export leads to defects in transcription and mRNA export and transcription-associated genome instability.29 Part of this instability is mediated by the accumulation of R loops, consisting on a DNA–RNA hybrid and the displaced non-template DNA strand. R loops form naturally during specific cellular processes, such as Escherichia coli plasmid replication, mitochondrial DNA replication, and immunoglobulin class switching. However, they can also be generated as transcriptional by-products that compromise genome integrity.30 This has been shown for cells from yeast to humans depleted of a number of proteins involved in RNA metabolism, including the THO complex of yeast, Caenorhabditis elegans, and human cells,31-33 the ASF/SF2 splicing factor of chicken and human cells,34 the yeast and human topoisomerase I,35,36 the yeast DNA-RNA helicase Sen1/Senataxin,37 and the yeast non-canonical polyA-polymerase Trf4.38 In addition, several screenings in yeast and human cells have revealed a number of RNA-processing factors preventing R-loop-mediated genome instability.39-41 Interestingly, absence of Npl3 leads to genome instability, as npl3∆ cells accumulate DSBs and have high levels of recombination.11 This hyperrecombination is dependent on transcription and partially suppressed by overexpression of RNase H1. Therefore, these results strongly support a model suggesting that an improper mRNP assembly may lead to R-loop accumulation in cells lacking Npl3.
Interestingly, the hyperrecombination phenotype of npl3∆ cells, as well as their sensitivity to genotoxic agents and lethality at 37 °C, is suppressed by overexpression of several hnRNP genes.11 This includes the THO-associated protein Sub2 and the polyA-binding proteins Nab2 and Tho1. Complete suppression of hyperrecombination by SUB2 overexpression may indicate a function of Npl3 related to that of THO/TREX, at early steps of mRNP biogenesis. Despite their different structures (Fig. 1), both Nab2 and Npl3 share functional similarities. They bind poly-A RNA, function as Mex67 adaptors, shuttle between the nucleus and the cytoplasm, and participate in pre-ribosomal subunits export.8,20,42-44 Whereas Nab2 associates with mRNAs encoding transcription-related proteins, Npl3 preferentially binds to transcripts encoding ribosomal proteins and other highly transcribed mRNAs,45 suggesting specific functions for Nab2 and Npl3 in alternative mRNP biogenesis pathways. Conversely, overexpression of NPL3 reduces the hyperrecombination phenotype of the THO mutant hpr1∆, suggesting the existence of partially overlapping functions of Npl3 and THO. In this sense, it is worth noting that NPL3 overexpression per se is able to increase recombination in wild-type cells, so that Npl3 cellular levels has to be tightly regulated. However, a contribution to this phenomenon of late transcription termination caused by excess of Npl3 cannot be discarded.18
Coordination of Transcription, mRNA Export, and Replication to Prevent Genome Instability
Transcription may become an obstacle for replication progression, so that a tight coordination is required for the RF to traverse transcribed DNA sequences.46 In addition, transcription may occur in close contact to the NPC, according to the “gene gating” hypothesis, thanks to the participation of a number of mRNA export factors and components of the NPC, as shown in yeast, worms, flies, and mammals.47,48 In this sense, a mechanism has been proposed by which the DNA damage checkpoint releases transcribed genes from the NPCs to facilitate RF progression.49 A non-proficient transcription and/or mRNA export may lead to replication impairment, as shown by the genome-wide analysis of the replicative Rrm3 helicase distribution in yeast. As Rrm3 is required for the RF to pass through obstacles,50 its accumulation at specific genomic regions has been used to identify RF pauses or stalls. Indeed, Rrm3 binding sites highly correlate with transcribed areas.51 Deletion of NPL3 increases the genome-wide accumulation of Rrm3 to the highest transcribed genes.11 Interestingly, Rrm3 accumulation is suppressed by RNase H1 overexpression, suggesting that R loops constitute an obstacle to RF progression in npl3∆ cells. Mutants of the THO component Hpr1 also show R-loop-mediated Rrm3 accumulation at highly transcribed genes,24 suggesting that co-transcriptional R-loops may lead to genome instability by interfering with RF progression.
Taking into account the reported effects of Npl3 on transcription, replication, and genome integrity, our current view is that Npl3 is a key player preventing conflicts between these processes. Npl3 stimulates transcription elongation and is highly abundant at the nascent mRNPs, helping prevent transcription–replication conflicts and R-loop accumulation (Fig. 2). In the absence of Npl3, transcription elongation is slower and the mRNAs would not be properly assembled into export-competent mRNPs, enhancing the probability of R-loop accumulation and transcription-associated genome instability.
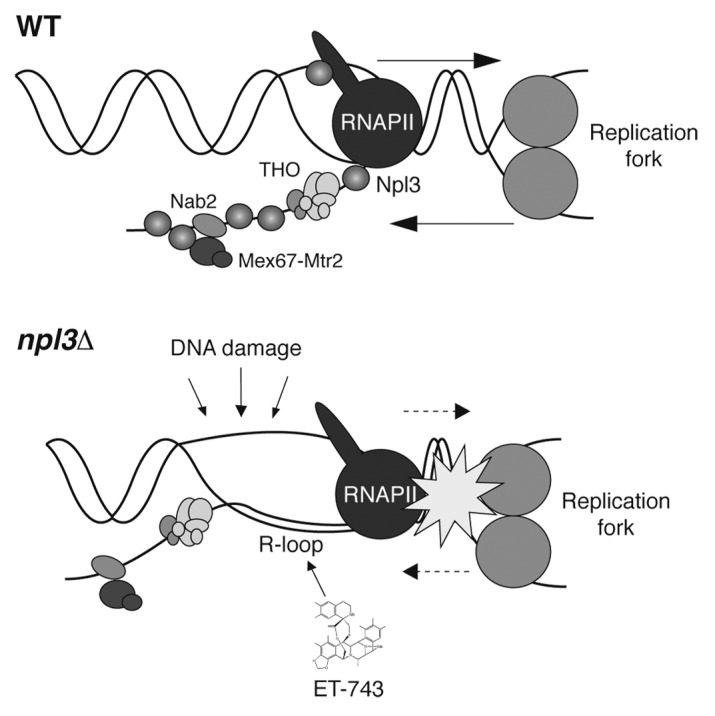
Figure 2. Model illustrating the role of Npl3 coupling mRNP biogenesis and export. In wild-type cells (top), the mRNA is properly assembled into an export-competent mRNP with the collaboration of RNA-binding factors such as Npl3, THO/TREX, Nab2, and Mex67-Mtr2. This facilitates RF progression through transcribed DNA regions. However, in npl3∆ cells (bottom) the mRNA packaging is defective, and the nascent mRNA may hybridize with the transcribed DNA strand, leading to R loops that favor the accessibility of DNA damaging agents to the non-transcribed single-stranded DNA and that constitute an obstacle for RF progression. Antitumor drug trabectedin (ET-743) might act similarly to an R loop.
A Possible Role of Npl3 in the DNA Damage Response
Although a direct role of RBPs in DNA repair and the DDR has not yet been established, several reports have revealed new functions of specific RBPs in DNA repair in higher eukaryotes.52 In yeast, different mRNA export mutants, including THO mutants hpr1∆ and tho2∆ and THSC/TREX-2 mutant thp1∆, show increased sensitivity to genotoxic agents, such as 4NQO, UV, or MMS.53 Interestingly, the npl3∆ mutant is sensitive to MMS, HU, phleomycin, and UV, agents causing DNA lesions that can result in stalled RFs and/or DSBs, indicating a role of Npl3 preventing DNA damage accumulation.11 Importantly, simultaneous deletion of NPL3 and RAD52 or YKU80, 2 key components of the homologous recombination (HR) and non-homologous end-joining (NHEJ) pathways of DSB repair, respectively, induces increased sensitivity to MMS, suggesting a connection between Npl3 and DSB repair. Although npl3∆ cells proficiently repair DSBs by sister-chromatid recombination, the main HR mechanism, they show a mild defect in the repair by NHEJ. This suggests the possibility that Npl3 had a role in DNA repair consistent with that reported for other RBPs, as in the case of the human p54nrb/NONO, which binds to DSBs sites and favors their repair by NHEJ over HR.54 On the other hand, sensitivity of npl3∆ cells to UV may imply a connection of Npl3 with the nucleotide excision repair (NER) pathway, which repairs bulky adducts in the DNA, such as those induced by UV. Consistently, several mRNA export mutants (hpr1∆, tho2∆, sub2∆, and thp1∆) have been involved in transcription-coupled NER (TC-NER), the RNAPII-dependent NER sub-pathway.55 However, this phenotype may also be the consequence of an increase in the collisions between transcription an replication promoted by UV lesions occurring during the S phase, which would lead to DNA breaks that would demand DSB repair functions for their repair and RF restart.
DNA repair genes are not downregulated in npl3∆ cells,11 so that it seems clear that Npl3 does not specifically control the expression of DNA repair genes that could explain their repair phenotypes. Interestingly, however, Npl3 was identified in a proteomic screening for Mec1/Tel1- and Rad53-dependent phosphorylation sites, together with components of the nuclear basket of the NPC.56 Therefore, it is possible that Npl3 also plays a role in the DDR. Extensive work will be required to understand the molecular mechanisms underlying the connection of yeast RBPs and DNA repair.
One important observation in our study is the hypersensitivity of npl3∆ cells to the antitumor drug trabectedin. The mechanism of action of this drug has been proposed to be dependent on the NER machinery both in fission yeast and humans.57,58 Binding of trabectedin to the DNA would form an adduct recognized by the NER machinery, leading to the stabilization of a complex with the fission yeast/human nuclease Rad13/XPG (Rad2 in budding yeast). The Rad13–DNA–trabectedin ternary complex would block the repair by NER, leading to DSBs and DNA damage checkpoint activation.57,59 A similar situation was reported for the rad3–102 mutant in budding yeast, which encodes a defective Rad3/XPD allele that blocks NER repair in a post-incision step and requires HR for RF restart.60 Consequently, npl3∆ cells treated with trabectedin could over-accumulate DSBs, mimicking the effect of MMS or phleomycin.
An additional, but a non-excluding possibility, to explain trabectedin hypersensitivity could be related to the fact that trabectedin binding to the DNA generates an R-loop-like structure.61 According to this, Npl3 could be required to prevent or solve this sort of structure, thus explaining the R-loop-mediated genome instability and replication impairment of npl3∆ cells. Given the importance of the Npl3 resistance to trabectedin anti-tumor drug, and that genome instability is a common hallmark of cancer cells,62,63 these observations open the possibility of exploring the use of specific RBPs as new targets for anti-cancer drug designs.
Concluding Remarks
Transcription-associated genome instability is a common hallmark of mRNA processing defects from yeast to human cells. Depletion of proteins involved in mRNP biogenesis and processing leads to an increase in DNA breaks in a transcription-dependent manner, in part due to R-loop formation, and as a consequence of replication impairment. Finding that the yeast SR-like protein Npl3, one of the most abundant RNA-binding proteins in yeast, prevents genome instability and R-loop formation as the major trigger of transcription-associated instability provides strong evidence of the key importance of proper mRNA biogenesis and assembly in preventing the nascent RNA from interacting with the template DNA and from compromising genome integrity. Thus, Npl3 is a multifunctional protein acting from transcription to mRNA processing and export with a function in preventing genome instability. Interestingly, it seems to have additional roles in DNA repair, as is the case of other RBPs from mammalian cells, although this is still poorly understood. Thus, RBPs constitute a conserved group of nuclear factors that safeguard genome integrity in many different ways. Defining the specific functions of Npl3 and other RBPs in genome integrity, as well as their involvement in DNA repair, the DDR, and cancer is important to understand the role of RNA and RNA-binding factors in genome dynamics and in the origin of cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank D. Haun for style supervision. Research in the labs of A.A. and S.M. are funded by grants from the Spanish Ministry of Economy and Competitiveness and the European Union (A.A. and S.M.) and the Junta de Andalucía (A.A.).
Glossary
Abbreviations:
- RBP
RNA-binding protein
- mRNP
messenger ribonucleoprotein particle
- RF
replication fork
- DDR
DNA damage response
- hnRNP
heterogeneous nuclear ribonucleoprotein
- NPC
nuclear pore complex
- SR
Ser-Arg-rich
- DSB
double-strand break
- RRM
RNA-recognition motif
- CTD
carboxy-terminal domain
- RNAPII
RNA polymerase II
- Ser2P
phosphorylation of Serine 2
- CBC
cap-binding complex
- 4NQO
4-nitroquinoline 1-oxide
- UV
ultraviolet light
- MMS
methyl-methane sulfonate
- HU
hydroxyurea
- HR
homologous recombination
- NHEJ
non-homologous end joining
- NER
nucleotide excision repair
- TC-NER
transcription-coupled nucleotide excision repair
References
- 1.Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8:761–73. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- 2.Müller-McNicoll M, Neugebauer KM. How cells get the message: dynamic assembly and function of mRNA-protein complexes. Nat Rev Genet. 2013;14:275–87. doi: 10.1038/nrg3434. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol. 2002;3:195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- 4.Shepard PJ, Hertel KJ. The SR protein family. Genome Biol. 2009;10:242. doi: 10.1186/gb-2009-10-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deka P, Bucheli ME, Moore C, Buratowski S, Varani G. Structure of the yeast SR protein Npl3 and Interaction with mRNA 3′-end processing signals. J Mol Biol. 2008;375:136–50. doi: 10.1016/j.jmb.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–41. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 7.Dermody JL, Dreyfuss JM, Villén J, Ogundipe B, Gygi SP, Park PJ, Ponticelli AS, Moore CL, Buratowski S, Bucheli ME. Unphosphorylated SR-like protein Npl3 stimulates RNA polymerase II elongation. PLoS One. 2008;3:e3273. doi: 10.1371/journal.pone.0003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MS, Henry M, Silver PA. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–46. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- 9.Kress TL, Krogan NJ, Guthrie C. A single SR-like protein, Npl3, promotes pre-mRNA splicing in budding yeast. Mol Cell. 2008;32:727–34. doi: 10.1016/j.molcel.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baierlein C, Hackmann A, Gross T, Henker L, Hinz F, Krebber H. Monosome formation during translation initiation requires the serine/arginine-rich protein Npl3. Mol Cell Biol. 2013;33:4811–23. doi: 10.1128/MCB.00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos-Pereira JM, Herrero AB, García-Rubio ML, Marín A, Moreno S, Aguilera A. The Npl3 hnRNP prevents R-loop-mediated transcription-replication conflicts and genome instability. Genes Dev. 2013;27:2445–58. doi: 10.1101/gad.229880.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossie MA, DeHoratius C, Barcelo G, Silver P. A mutant nuclear protein with similarity to RNA binding proteins interferes with nuclear import in yeast. Mol Biol Cell. 1992;3:875–93. doi: 10.1091/mbc.3.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell ID, Tollervey D. NOP3 is an essential yeast protein which is required for pre-rRNA processing. J Cell Biol. 1992;119:737–47. doi: 10.1083/jcb.119.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson MS. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol. 1994;127:1173–84. doi: 10.1083/jcb.127.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rondón AG, Jimeno S, Aguilera A. The interface between transcription and mRNP export: from THO to THSC/TREX-2. Biochim Biophys Acta. 2010;1799:533–8. doi: 10.1016/j.bbagrm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Hackmann A, Wu H, Schneider UM, Meyer K, Jung K, Krebber H. Quality control of spliced mRNAs requires the shuttling SR proteins Gbp2 and Hrb1. Nat Commun. 2014;5:3123. doi: 10.1038/ncomms4123. [DOI] [PubMed] [Google Scholar]
- 17.Lei EP, Krebber H, Silver PA. Messenger RNAs are recruited for nuclear export during transcription. Genes Dev. 2001;15:1771–82. doi: 10.1101/gad.892401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucheli ME, Buratowski S. Npl3 is an antagonist of mRNA 3′ end formation by RNA polymerase II. EMBO J. 2005;24:2150–60. doi: 10.1038/sj.emboj.7600687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund MK, Kress TL, Guthrie C. Autoregulation of Npl3, a yeast SR protein, requires a novel downstream region and serine phosphorylation. Mol Cell Biol. 2008;28:3873–81. doi: 10.1128/MCB.02153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell. 2004;13:201–12. doi: 10.1016/S1097-2765(04)00030-9. [DOI] [PubMed] [Google Scholar]
- 21.Meinel DM, Burkert-Kautzsch C, Kieser A, O’Duibhir E, Siebert M, Mayer A, Cramer P, Söding J, Holstege FC, Sträßer K. Recruitment of TREX to the transcription machinery by its direct binding to the phospho-CTD of RNA polymerase II. PLoS Genet. 2013;9:e1003914. doi: 10.1371/journal.pgen.1003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Erickson B, Luo W, Seward D, Graber JH, Pollock DD, Megee PC, Bentley DL. Gene-specific RNA polymerase II phosphorylation and the CTD code. Nat Struct Mol Biol. 2010;17:1279–86. doi: 10.1038/nsmb.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer A, Lidschreiber M, Siebert M, Leike K, Söding J, Cramer P. Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol. 2010;17:1272–8. doi: 10.1038/nsmb.1903. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-González B, García-Rubio M, Bermejo R, Gaillard H, Shirahige K, Marín A, Foiani M, Aguilera A. Genome-wide function of THO/TREX in active genes prevents R-loop-dependent replication obstacles. EMBO J. 2011;30:3106–19. doi: 10.1038/emboj.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson SA, Kim H, Erickson B, Bentley DL. The export factor Yra1 modulates mRNA 3′ end processing. Nat Struct Mol Biol. 2011;18:1164–71. doi: 10.1038/nsmb.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert W, Siebel CW, Guthrie C. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA. 2001;7:302–13. doi: 10.1017/S1355838201002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oeffinger M, Wei KE, Rogers R, DeGrasse JA, Chait BT, Aitchison JD, Rout MP. Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods. 2007;4:951–6. doi: 10.1038/nmeth1101. [DOI] [PubMed] [Google Scholar]
- 28.Bretes H, Rouviere JO, Leger T, Oeffinger M, Devaux F, Doye V, Palancade B. Sumoylation of the THO complex regulates the biogenesis of a subset of mRNPs. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaillard H, Herrera-Moyano E, Aguilera A. Transcription-associated genome instability. Chem Rev. 2013;113:8638–61. doi: 10.1021/cr400017y. [DOI] [PubMed] [Google Scholar]
- 30.Aguilera A, García-Muse T. R loops: from transcription byproducts to threats to genome stability. Mol Cell. 2012;46:115–24. doi: 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Castellano-Pozo M, García-Muse T, Aguilera A. R-loops cause replication impairment and genome instability during meiosis. EMBO Rep. 2012;13:923–9. doi: 10.1038/embor.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domínguez-Sánchez MS, Barroso S, Gómez-González B, Luna R, Aguilera A. Genome instability and transcription elongation impairment in human cells depleted of THO/TREX. PLoS Genet. 2011;7:e1002386. doi: 10.1371/journal.pgen.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. 2003;12:711–21. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Manley JL. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–78. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.El Hage A, French SL, Beyer AL, Tollervey D. Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev. 2010;24:1546–58. doi: 10.1101/gad.573310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuduri S, Crabbé L, Conti C, Tourrière H, Holtgreve-Grez H, Jauch A, Pantesco V, De Vos J, Thomas A, Theillet C, et al. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat Cell Biol. 2009;11:1315–24. doi: 10.1038/ncb1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mischo HE, Gómez-González B, Grzechnik P, Rondón AG, Wei W, Steinmetz L, Aguilera A, Proudfoot NJ. Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol Cell. 2011;41:21–32. doi: 10.1016/j.molcel.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavaldá S, Gallardo M, Luna R, Aguilera A. R-loop mediated transcription-associated recombination in trf4Δ mutants reveals new links between RNA surveillance and genome integrity. PLoS One. 2013;8:e65541. doi: 10.1371/journal.pone.0065541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, Hesley JA, Miller SC, Cromwell EF, Solow-Cordero DE, et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 2009;35:228–39. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stirling PC, Chan YA, Minaker SW, Aristizabal MJ, Barrett I, Sipahimalani P, Kobor MS, Hieter P. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 2012;26:163–75. doi: 10.1101/gad.179721.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahba L, Amon JD, Koshland D, Vuica-Ross M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol Cell. 2011;44:978–88. doi: 10.1016/j.molcel.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hackmann A, Gross T, Baierlein C, Krebber H. The mRNA export factor Npl3 mediates the nuclear export of large ribosomal subunits. EMBO Rep. 2011;12:1024–31. doi: 10.1038/embor.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iglesias N, Tutucci E, Gwizdek C, Vinciguerra P, Von Dach E, Corbett AH, Dargemont C, Stutz F. Ubiquitin-mediated mRNP dynamics and surveillance prior to budding yeast mRNA export. Genes Dev. 2010;24:1927–38. doi: 10.1101/gad.583310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green DM, Marfatia KA, Crafton EB, Zhang X, Cheng X, Corbett AH. Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J Biol Chem. 2002;277:7752–60. doi: 10.1074/jbc.M110053200. [DOI] [PubMed] [Google Scholar]
- 45.Kim Guisbert K, Duncan K, Li H, Guthrie C. Functional specificity of shuttling hnRNPs revealed by genome-wide analysis of their RNA binding profiles. RNA. 2005;11:383–93. doi: 10.1261/rna.7234205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bermejo R, Lai MS, Foiani M. Preventing replication stress to maintain genome stability: resolving conflicts between replication and transcription. Mol Cell. 2012;45:710–8. doi: 10.1016/j.molcel.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci U S A. 1985;82:8527–9. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–17. doi: 10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- 49.Bermejo R, Capra T, Jossen R, Colosio A, Frattini C, Carotenuto W, Cocito A, Doksani Y, Klein H, Gómez-González B, et al. The replication checkpoint protects fork stability by releasing transcribed genes from nuclear pores. Cell. 2011;146:233–46. doi: 10.1016/j.cell.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivessa AS, Lenzmeier BA, Bessler JB, Goudsouzian LK, Schnakenberg SL, Zakian VA. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol Cell. 2003;12:1525–36. doi: 10.1016/S1097-2765(03)00456-8. [DOI] [PubMed] [Google Scholar]
- 51.Azvolinsky A, Giresi PG, Lieb JD, Zakian VA. Highly transcribed RNA polymerase II genes are impediments to replication fork progression in Saccharomyces cerevisiae. Mol Cell. 2009;34:722–34. doi: 10.1016/j.molcel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dutertre M, Lambert S, Carreira A, Amor-Guéret M, Vagner S. DNA damage: RNA-binding proteins protect from near and far. Trends Biochem Sci. 2014;39:141–9. doi: 10.1016/j.tibs.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Gaillard H, Tous C, Botet J, González-Aguilera C, Quintero MJ, Viladevall L, García-Rubio ML, Rodríguez-Gil A, Marín A, Ariño J, et al. Genome-wide analysis of factors affecting transcription elongation and DNA repair: a new role for PAF and Ccr4-not in transcription-coupled repair. PLoS Genet. 2009;5:e1000364. doi: 10.1371/journal.pgen.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krietsch J, Caron MC, Gagné JP, Ethier C, Vignard J, Vincent M, Rouleau M, Hendzel MJ, Poirier GG, Masson JY. PARP activation regulates the RNA-binding protein NONO in the DNA damage response to DNA double-strand breaks. Nucleic Acids Res. 2012;40:10287–301. doi: 10.1093/nar/gks798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaillard H, Wellinger RE, Aguilera A. A new connection of mRNP biogenesis and export with transcription-coupled repair. Nucleic Acids Res. 2007;35:3893–906. doi: 10.1093/nar/gkm373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smolka MB, Albuquerque CP, Chen SH, Zhou H. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc Natl Acad Sci U S A. 2007;104:10364–9. doi: 10.1073/pnas.0701622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrero AB, Martín-Castellanos C, Marco E, Gago F, Moreno S. Cross-talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedin. Cancer Res. 2006;66:8155–62. doi: 10.1158/0008-5472.CAN-06-0179. [DOI] [PubMed] [Google Scholar]
- 58.Takebayashi Y, Pourquier P, Zimonjic DB, Nakayama K, Emmert S, Ueda T, Urasaki Y, Kanzaki A, Akiyama SI, Popescu N, et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat Med. 2001;7:961–6. doi: 10.1038/91008. [DOI] [PubMed] [Google Scholar]
- 59.Guirouilh-Barbat J, Redon C, Pommier Y. Transcription-coupled DNA double-strand breaks are mediated via the nucleotide excision repair and the Mre11-Rad50-Nbs1 complex. Mol Biol Cell. 2008;19:3969–81. doi: 10.1091/mbc.E08-02-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moriel-Carretero M, Aguilera A. A postincision-deficient TFIIH causes replication fork breakage and uncovers alternative Rad51- or Pol32-mediated restart mechanisms. Mol Cell. 2010;37:690–701. doi: 10.1016/j.molcel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Marco E, García-Nieto R, Mendieta J, Manzanares I, Cuevas C, Gago F. A 3.(ET743)-DNA complex that both resembles an RNA-DNA hybrid and mimicks zinc finger-induced DNA structural distortions. J Med Chem. 2002;45:871–80. doi: 10.1021/jm010370d. [DOI] [PubMed] [Google Scholar]
- 62.Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 63.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr., Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–13. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]