Abstract
Fibroblast growth factor receptor 3 (FGFR3) activating mutations are drivers of malignancy in several human tissues, including bladder, lung, cervix, and blood. However, in skin, these mutations are associated predominantly with benign, common epidermal growths called seborrheic keratoses (SKs). How epidermis resists FGFR3 mediated transformation is unclear, but previous studies have suggested that FGFR3 activation in skin keratinocytes may serve a tumor-suppressive role by driving differentiation and antagonizing Ras signaling. To define the role of FGFR3 in human normal and neoplastic epidermis, and to directly test the hypothesis that FGFR3 antagonizes Ras, we engineered human skin grafts in vivo with mutant active FGFR3 or shRNA FGFR3 knockdown. We show that FGFR3 active mutants drive mild hyperproliferation, but are insufficient to support benign or malignant tumorigenesis, either alone, or in combination with G1–S checkpoint release. This suggests that additional cell-intrinsic or stromal cues are required for formation of benign SKs with FGFR3 mutations. Further, FGFR3 activation does not alter the growth kinetics or differentiation status of engineered human epidermal SCCs driven by Ras, and FGFR3 protein itself is dispensable for Ras-driven SCC. To extend these findings to patients, we examined a uniquely informative human tumor in which SCC developed in continuity with a SK, raising the hypothesis that one of the tumors evolved from the other. However, mutational analysis from each tumor indicates that the overlapping SK and SCC evolved independently and supports our conclusion that FGFR3 activation is insufficient to drive SCC.
Keywords: extracellular signal-regulated kinase, fibroblast growth factor receptor 3, seborrheic keratosis, skin, squamous cell carcinoma
Introduction
Seborrheic keratoses (SKs) are the most common benign tumor of elderly humans, present in 88% of people over age 64.1 Although the tumors are hyperproliferative, they respect the underlying basement membrane, are well-differentiated, rarely exhibit cytological abnormalities that are common to squamous cell carcinomas (SCCs), and are thought to rarely progress to malignancy.2,3 39–85% of clinical SK tumors harbor a mutation in the fibroblast growth factor receptor 3 (FGFR3) gene, depending on the histological sub-type and patient demographic.2,4-7 Most FGFR3 mutations in SKs, as well as malignant tumors in other organs, are localized to positions 248 or 249, where cysteine substitutions lead to increased FGFR3 dimer stability and constitutive receptor activation in the absence of ligand.8 This results in increased downstream activation of Shp2, PLCγ, ERK1/2, STAT3, and PI3K.8 FGFR3 R248C and S249C mutations have been reported with a frequency ranging from 8–37% and 7–15% in seborrheic keratoses, respectively.2,4-7
An additional less common FGFR3 mutation (G697C) was found in a large group (62%) of keratinocyte-derived oral SCC tumors from Japan. This FGFR3 mutant was determined to cause increased FGFR3 auto-phosphorylation, and presumed to serve as a driver oncogene in this setting.9 While these 3 FGFR3 mutations are associated with both benign and malignant human tumors, it has not been established whether FGFR3 activation is sufficient to drive SK formation, or has the capacity to serve as a bona fide oncogene in human skin. Whether FGFR3 is required for epidermal SCC has also not been explored previously.
The identification of activating FGFR3 mutations only in benign epidermal lesions is surprising, given that the same mutations have been identified in subsets of malignant tumors in many other tissues.10-14 Small-molecule tyrosine kinase inhibitors targeting FGFR3 or shRNA knockdown of mutant FGFR3 have been shown to be effective in reducing tumor burden in mouse models of multiple myeloma and bladder carcinoma, indicating that mutant FGFR3 likely serves as an oncogene in these tumor types.15-19 Despite an oncogenic role in some malignancies, recent data suggests that FGFR3 activation may prevent SCC development in the skin through upregulation of FOXN1, a transcription factor that may antagonize Ras activity by promoting differentiation.20 However, this study did not directly link mutant FGFR3 expression to inhibition of SCC formation.
Here we present data defining the role of FGFR3 in normal and neoplastic human epidermis by determining: (1) the role of disease-associated FGFR3 mutations (R248C, S249C, G697C) in human skin; (2) the ability of FGFR3 mutants to serve as oncodrivers of epidermal squamous cell carcinoma (SCC) either alone or in combination with other common tumor-associated genetic changes; (3) whether FGFR3 activation antagonizes oncogenic Ras activity; (4) whether FGFR3 is necessary for Ras-driven epidermal SCC; and (5) whether FGFR3 activation plays an oncogenic role in the occasionally encountered clinical specimen in which SCC appears to develop with a previously existing benign SK.
Results
FGFR3 mutations R248C and S249C, but not G697C, activate MAPK and drive mild epidermal hyperplasia
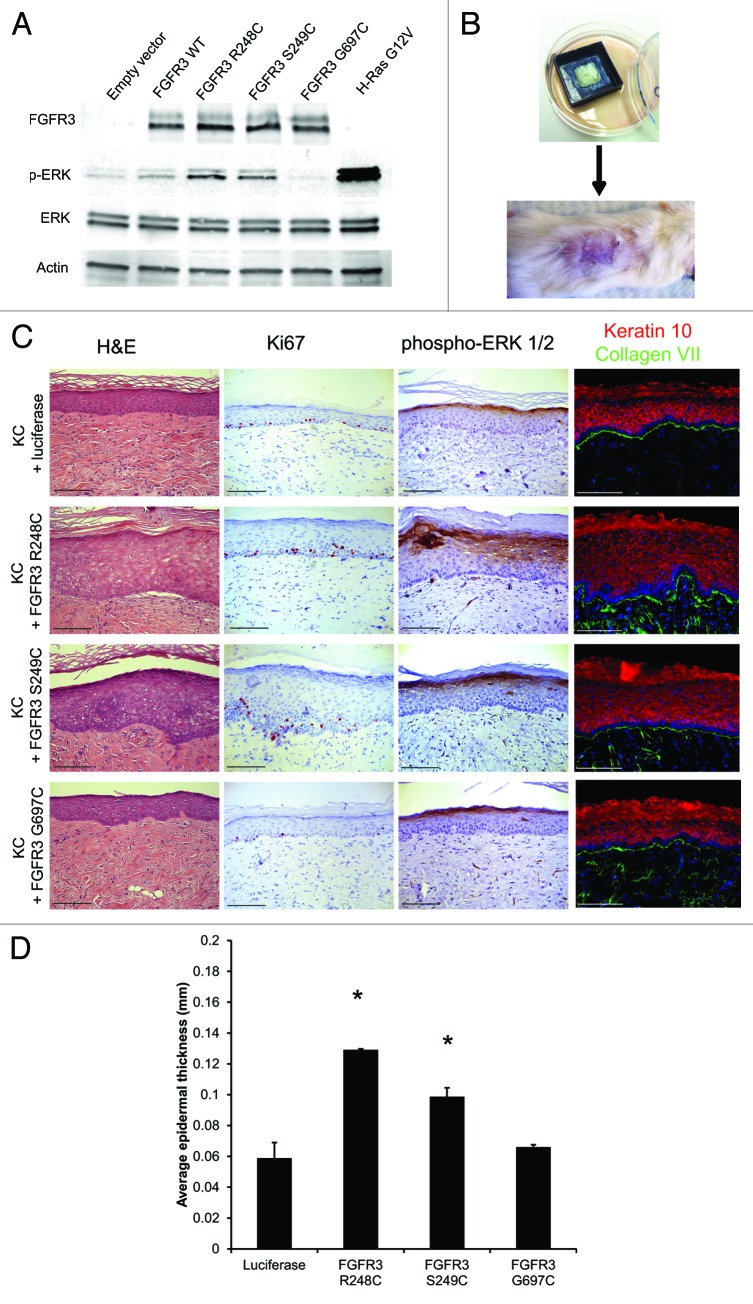
The relative oncogenic activities of the 3 mutant tumor-associated FGFR3 proteins (R248C, S249C, G697C) were determined by measuring downstream activation of the mitogen-activated protein kinase (MAPK) pathway (Fig. 1A). While the R248C and S249C FGFR3 mutants stimulated ERK phosphorylation, intermediate between that of control and oncogenic H-RasG12V, the G697C mutant did not differ from WT control (Fig. 1A). As increased MEK-ERK signaling is a near ubiquitous feature of epidermal SCC, these results suggested that the R248C/S249C mutants may drive a subset of the Ras-associated tumor-oncogenic changes, while the G697C mutant is likely not an oncodriver. To determine the effects of these FGFR3 proteins in an architecturally faithful 3-dimensional human skin model, we used retroviral transduction to stably introduce each mutant FGFR3 protein into primary human keratinocytes, and then used the transduced keratinocytes to generate organotypic human skin xenografts on the backs of SCID mice (Fig. 1B).21,22 On histologic examination, epidermis expressing FGFR3 R248C or S249C had approximately 2-fold increased tissue thickness, contained suprabasal Ki67+ keratinocytes, and displayed increased MAPK activation (Fig. 1C and D). Epidermis expressing FGFR3, R248C, or S249C also had expansion of the Keratin 10-positive tissue compartment, consistent with the increased tissue thickness in these grafts (Fig. 1C). These tissues did not display premature expression of this differentiation marker, since all basal keratinocytes in contact with the basement membrane remained Keratin 10-negative. Despite this hyperplasia, the tissues lacked many of the hallmark features of typical SKs, including prominent epidermal hyperplasia and pseudohorn cysts (keratin containing cystic structures within the expanded epidermis). This suggests that human SKs require additional genetic changes or stromal cues lacking in our system that cooperate with FGFR3 activation to promote formation of the epidermal cancer. In contrast to the mild hyperproliferation seen in the R248C or S249C xenografts, the G697C-expressing tissue appeared unremarkable and did not manifest epidermal hyperplasia (Fig. 1C and D). These results suggest that the G697C mutation, despite its presence in a large fraction of Japanese oral SCCs, does not contribute to hyperproliferation in the human epidermis.9 Subsequent follow-up studies on oral SCC from other countries failed to find the G697C mutation, indicating that this mutation might be a geographically restricted variant not widely associated with the pathogenesis of this tumor.23 Given the high mutation rate in head and neck squamous cell carcinoma, it is likely that this particular FGFR3 mutation is simply a passenger mutation, or perhaps can only act in the context of additional driver mutations.24
Figure 1. FGFR3, R248C, and S249C, but not G697C, induce MAPK activation and hyperproliferation in human skin grafts, but are not sufficient to drive SKs. (A) Immunoblot of HEK293T cells transfected with WT, R248C, S249C or G697C FGFR3 or H-Ras G12V. (B) Engineered human tissue xenograft model. Transduced human keratinocytes are seeded onto devitalized human dermis and grafted onto SCID mice. (C) H&E, Ki67, and phospho-ERK 1/2 IHC, and Keratin 10 and collagen VII immunofluorescence from in vivo human tissue expressing the indicated FGFR3 mutants. (D) Epidermal thickness of tissues displayed in panel (C). Error bars indicate ± SD *P < 0.05. Scale bar = 100 μm.
FGFR3 activation does not cooperate with G1–S checkpoint release to drive neoplasia
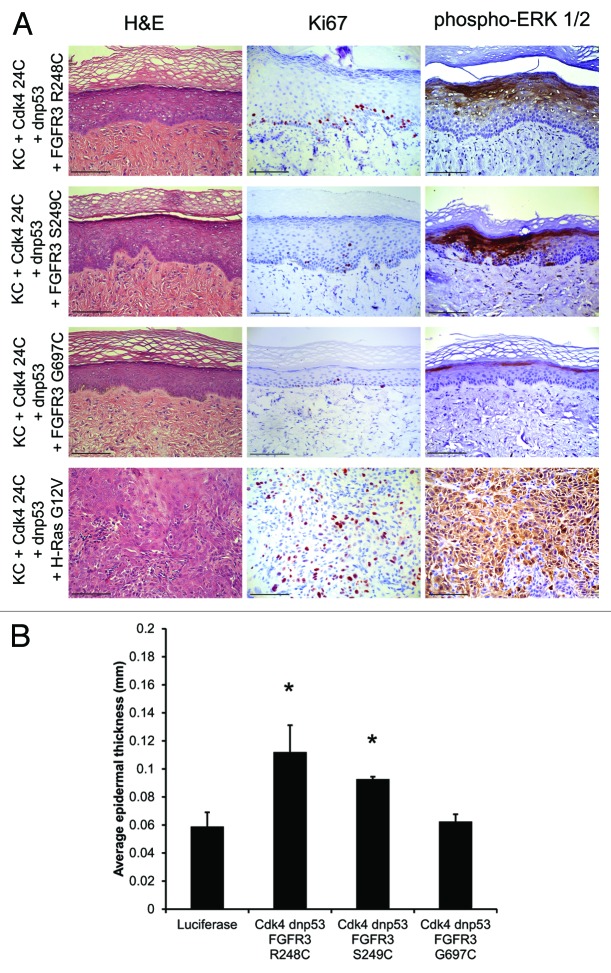
While the FGFR3 mutants alone are not sufficient to drive epidermal cancer, we determined whether they could cooperate with other common SCC-associated genetic changes to drive tumorigenesis. Each FGFR3 mutant was expressed in epidermis with activated Cdk4 (R24C) and dominant-negative p53 (dnp53, R248W).21,22 The Cdk4R24C and p53 R248W promote release of the Rb-mediated G1–S cell cycle checkpoint control and inhibit anti-proliferative signals preventing malignant transformation. When Cdk4R24C and dnp53 are expressed with oncogenic H-RasG12V in the human xenograft model, invasive neoplasia recapitulating human SCC develops (Fig. 2A, bottom panels). However, none of the FGFR3 mutants was able to drive SK or SCC formation in the permissive tissue environment created by Cdk4R24C and dnp53 (Fig. 2A). Somewhat surprisingly, addition of Cdk4R24C and dnp53 did not increase epidermal thickness beyond that observed with the FGFR3 mutants alone (Figs. 1D and 2B). Therefore, while the FGFR3 R248C/S249C mutants activate the MEK-ERK pathway, the level of activation falls short of the threshold necessary to support benign or malignant tumor growth. These mutant FGFR3 proteins are thus unlikely to function as primary oncogenes in epidermis, but may cooperate with stromal cues, or additional somatic mutations, that potentiate these otherwise relatively weak mitogenic drivers to support the benign SK tumor growth seen clinically.
Figure 2. FGFR3 R248C and S249C in combination with G1–S checkpoint release are insufficient to drive SK formation. (A) H&E, Ki67, and phospho-ERK ½ IHC from in vivo human tissue expressing the indicated FGFR3 mutants, Cdk4R24C and dnp53. (B) Epidermal thickness of tissues displayed in (A). Error bars indicate ± SD *P < 0.05. Scale bar = 100 μm.
FGFR3 activation does not inhibit SCC tumorigenesis
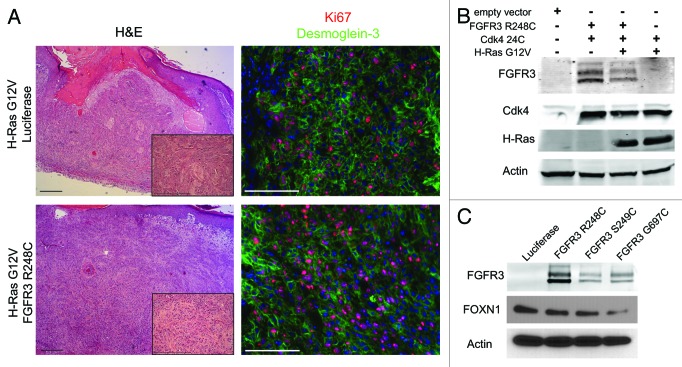
While the level of MAPK activity may not be sufficient to drive tumor formation, we thought it formally possible that additional FGFR3-activated signaling pathways operating downstream serve to inhibit oncogenesis. This possibility is suggested by 2 previous experimental observations from other laboratories. First, while activating mutations in FGFR3 and Ras are both seen in bladder carcinoma, they are mutually exclusive.13 This suggests that there is either no selective advantage to cells harboring both mutations, or that there is active antagonism between the two. Second, upregulation of FOXN1 was shown to be associated with seborrheic keratoses, and overexpression of FOXN1 in oncogenic Ras-expressing SCC cells slowed tumor growth.20 Whether FGFR3 activation antagonizes oncogenic Ras was not directly examined. Intrigued by the possibility that FGFR3 could possibly inhibit Ras-mediated transformation, we established human skin xenografts expressing active H-RasG12V and mutant Cdk4R24C, with and without active FGFR3 (Fig. 3B). In these grafts, FGFR3 did not affect tumor growth, degree of invasiveness, mitotic index, or differentiation status (Fig. 3A). Additionally, we found that expression of each FGFR3 mutant in human primary keratinocytes did not result in an increase in expression of FOXN1 at the protein level (Fig. 3C). This suggests that FGFR3 activation may not be sufficient for the increase in FOXN1 protein expression seen in human SKs, and that additional genetic or microenvironmental changes are responsible for its upregulation.20
Figure 3. FGFR3 activation does not antagonize H-Ras G12V-mediated tumorigenesis. (A) H&E (left panels) and Ki67/Desmoglein-3 immunofluorescence (right panels) of skin grafts expressing Cdk4R24C, H-RasG12V, and luciferase or FGFR3 R248C. (B) Western blot showing efficient transduction of FGFR3R248C, Cdk4R24C, and H-RasG12V in human keratinocytes. (C) Western blot showing FOXN1 expression in keratinocytes transduced with various FGFR3 mutations. Scale bar = 100 μm.
FGFR3 is not necessary for SCC progression
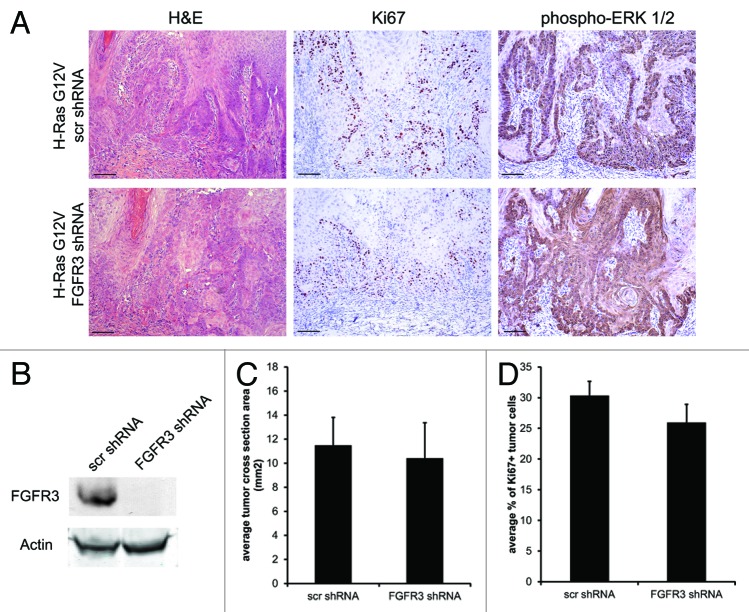
While FGFR3 activity was not sufficient to drive or inhibit epidermal tumorigenesis in our human system, we next conducted experiments to determine whether FGFR3 is necessary for maintenance of malignant human skin tissue. This is particularly germane, as FGFR3 inhibitors are currently under development as potential anti-cancer therapeutics. Constitutive FGFR3 knockdown in keratinocytes was achieved using lentiviral-delivered shRNA (Fig. 4B). FGFR3 proved to be dispensable for normal keratinocyte growth in vitro and in vivo. Keratinocytes transduced with non-targeting shRNA or FGFR3 shRNA were then used to establish tumors expressing constitutively active Cdk4R24C and oncogenic H-RasG12V (Fig. 4B). Tumors lacking FGFR3 expression showed no difference in size, proliferative index, invasion, or MAPK activation (Fig. 4A, quantified in 4C and D), indicating that FGFR3 is not necessary for human SCC. This result also indicates that, despite their potential utility in myeloma, FGFR3 small-molecule inhibitors and blocking antibodies are not likely to be useful therapeutics for Ras-driven epidermal cancers.17,18
Figure 4. FGFR3 is dispensable for SCC tumor formation. (A) H&E, Ki67, and phospho-ERK IHC of skin grafts expressing Cdk4R24C, H-RasG12V, and a scrambled shRNA or FGFR3 shRNA. (B) Western blot showing efficient knock down of FGFR3 expression in keratinocytes by lentiviral transduction of shRNAs. (C) Average cross-sectional area of tumors depicted in (A). (D) Ki67 quantification of tumors depicted in (A). Error bars indicate ± SD *P < 0.05. Scale bar = 100 μm.
Clinical case of contiguous SK-SCC contains distinct tumors with mutually exclusive genetic aberrations
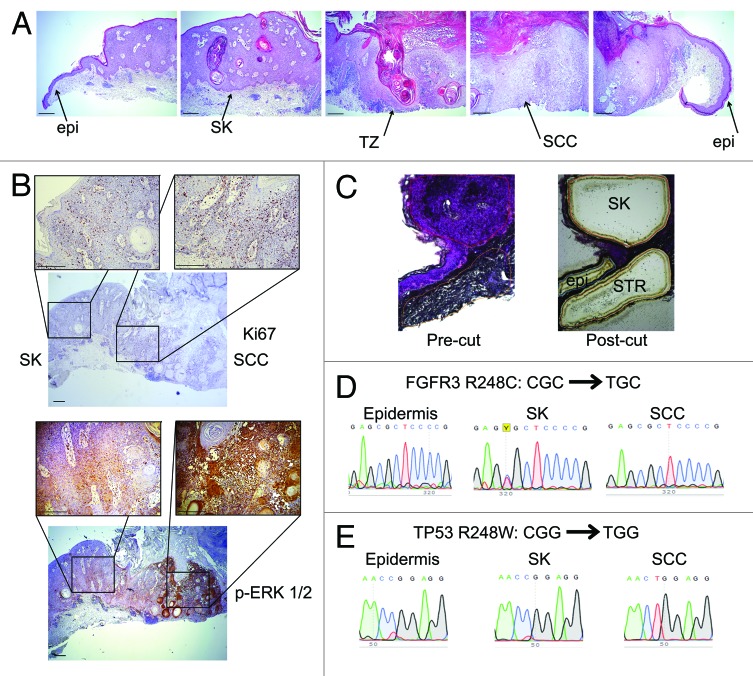
In order to directly extend our findings to human clinical specimens, we obtained formalin-fixed, paraffin-embedded tissue from a uniquely informative case in which a patient harbored a histologically classic SCC that developed in continuity with a pre-existing SK, suggesting that the overlapping tumors were genetically related (Fig. 5A). In this specimen, proliferation index and MAPK activation are elevated in the benign tumor, and to a higher degree in the invasive squamous cell carcinoma (Fig. 5B). We performed laser capture microdissection (LCM) to separate the SK, SCC, adjacent histologically normal epidermis, and obtained DNA and RNA from each region (Fig. 5C). PCR amplification from genomic DNA, followed by Sanger sequencing showed the typical FGFR3 R248C mutation (CGC→TGC) in the SK, but not in the adjacent epidermis or SCC (Fig. 5D). This indicates that, despite the histological appearance, the SCC did not arise directly from the SK. We also identified the common dominant-negative TP53 R248W (CGG→TGG) mutation present in the SCC, but not in the adjacent epidermis or SK (Fig. 5E), indicating that the SK did not arise directly from the SCC. This clinical example suggests that the occasionally encountered case in which an SK appears to progress into an SCC are in fact collisional lesions of 2 common genetically distinct tumors of sun-damaged, aged skin. To date, FGFR3 mutations have not been observed in SCC. This is perhaps a bit unexpected, given that SK lesions are hyperproliferative, with elevated MAPK activity, which would seem to make them more prone to malignant transformation. While we have demonstrated that the FGFR3 mutation itself does not prevent progression to SCC, it is possible that other mechanisms work to minimize the development of additional mutations.
Figure 5. Contiguous SK-SCC specimen consists of genetically distinct tumors. (A) H&E images from patient sample in which SCC appears to arise from a pre-existing SK, presented as contiguous frames. Epi, normal epidermis; SK, seborrheic keratosis; TZ, transition zone; SCC, squamous cell carcinoma. (B) Ki67 and phospho-ERK 1/2 IHC in SK-SCC sample. (C) Hematoxylin-stained tissue pre- and post- laser capture microdissection. (D) Genomic DNA sequencing trace for FGFR3 in epidermis, SK, and SCC. (E) cDNA sequencing of TP53 in epidermis, SK, and SCC. Scale bar = 200 μm.
Discussion
FGFR3 is not a primary driver of epidermal tumors
While FGFR3 mutations have been identified in benign human epidermal tumors, they have not previously been functionally characterized in human tissue. In this report, we use an in vivo human skin model to demonstrate that the most common activating FGFR3 mutations, R248C and S249C, drive mild epidermal hyperplasia, but are insufficient alone or in combination with G1–S checkpoint release to drive benign or malignant epidermal tumors. These findings are consistent with a murine study in which FGFR3 (R249C) was targeted to epidermis using the K5 promoter.7 In this model, all epidermal tissue expresses active FGFR3 from E13.5, and mice are born with normal skin.7 Over several months these animals develop a limited number of small hyperproliferative epidermal papules in specific areas subject to mechanical trauma and scratching, including nose and eyelids, suggesting that FGFR3 activation in this murine context is also not sufficient to support SK formation.
In addition to SKs, FGFR3-activating mutations are also seen in epidermal nevi—benign hyperproliferative epidermal lesions resulting from a developmentally acquired mosaic FGFR3 mutation in epidermal keratinocytes. Our results and conclusions are consistent with the clinical observation that EN are often not clinically visible until late childhood or adolescence, despite the fact that keratinocytes in the epidermis contain the mutation at birth.4 This indicates that, while FGFR3 certainly plays a role in benign tumorigenesis, additional genetic, hormonal, or microenvironmental changes are required for epidermis containing mutant FGFR3 to progress to neoplasia. Consistent with this notion is the fact that SKs are typically associated with an underlying inflammatory reaction, and the lesions are often itchy for patients. In our case, the inflammatory cells were more prominent under the SK than in the surrounding normal epidermis (Fig. 5B).
It is currently unknown what additional oncogenic or epigenetic alterations occur alongside FGFR3 mutation to drive benign epidermal tumorigenesis. It has been shown that loss of one allele of the tumor suppressor PTEN in combination with UVA irradiation leads to both SK and SCC tumor formation in mice.25 It is therefore possible that additional Akt signaling is required in combination with FGFR3-induced phosphorylation of ERK 1/2 for full formation of SKs. Mutations in the PI3K–Akt pathway have been found in SKs, but at a very low frequency.26,27
It is also not well understood why FGFR3 activation may play an oncogenic role in certain cell types—as is the case in multiple myeloma or urothelial carcinoma—but has restricted oncogenic capacity in keratinocytes. This is likely due to differential activation of the different pathways downstream of FGFR3, including Shp2, PLCγ, ERK1/2, STAT3, or PI3K. Alternatively, additional mutations in other oncodrivers or signals from tissue stress, perhaps due to injury or inflammation, may be required to potentiate FGFR3 signaling capacity and drive malignant tumorigenesis. Interestingly, a patient who exhibited mosaicism for an activating HRAS mutation presented with multiple urothelial cell carcinomas and an epidermal nevus, but no malignant skin tumors.28 This indicates that signaling through other MAPK activators may also display restricted oncogenic capacity in keratinocytes, and that this is not a unique feature of FGFR3.
Role of FGFR3 in established tumors
FGFR3 inhibitors have shown preclinical success in various mouse models, and one inhibitor, Dovitinib, is currently being used in clinical trials for breast, prostate, gastric, urogenital, and lung carcinomas.17,18 In addition, previous literature suggested that FGFR3 activation antagonizes Ras and is therefore actually a tumor suppressor in the skin.20 This would suggest that patients treated with Dovitinib may be predicted to develop increased numbers of skin cancers, as the supposed tumor-suppressor function of FGFR3 might be inhibited by this drug. In contrast to this literature, we have shown in human skin tissue that FGFR3 activation does not block Ras-driven tumorigenesis, nor does it upregulate FOXN1 protein expression. We have also demonstrated that FGFR3 protein is not necessary for squamous cell carcinoma formation, which suggests that inhibitors such as Dovitinib may have limited clinical use for epidermal tumors.
While we have demonstrated that FGFR3 is not required for SCC formation, we have not shown whether FGFR3 activity is required for SK formation. Direct testing of this hypothesis would require development of a tissue model for SKs, based ideally on SK-derived keratinocytes harboring FGFR3 mutations. Even these cells, however, may not recapitulate the lesions in xenografts, if critical stromal elements are missing in the model system. We have attempted to establish primary keratinocyte cultures from 10 fresh human SK lesions, but were unsuccessful. While we occasionally obtain viable keratinocyte cultures from these lesions, genetic analysis has so far failed to show FGFR3 mutation, indicating that the cultures are likely derived from normal contaminating keratinocytes obtained in the biopsies. SK keratinocytes may have specific requirements for growth in vivo that are lacking in vitro. Interestingly, the same technical challenge is observed with primary SCC cells, as it is extremely difficult to establish SCC cell lines from cutaneous SCC.
Why don’t SKs progress to malignancy?
Increased MAPK signaling downstream of activated FGFR3 would seemingly render keratinocytes more susceptible to tumor formation; however, this seems not to be the case, as SKs very rarely, if ever, progress to cancer. This clinical observation raised the possibility that FGFR3 serves as a tumor suppressor in skin despite its role as an oncodriver in other tissues. We tested this hypothesis directly and determined that constitutive FGFR3 activation itself does not prevent Ras-driven epidermal SCC. This experimental finding is consistent with our clinical overlapping SK-SCC case demonstrating that the 2 tumors are genetically distinct, and most likely arose independently. It is possible that the increased tissue thickness, hyperpigmentation, and hyperkeratosis associated with typical SK lesions prevent additional UV-related mutations in the proliferative basilar keratinocytes and, in so doing, minimize the risk of malignant transformation. Alternatively, FGFR3-mediated MAPK activation may alter the KC response to UV radiation, promoting either apoptosis or DNA damage repair pathways, making progression to SCC an unlikely event.
Materials and Methods
Cell culture
Human keratinocytes were isolated from neonatal foreskins obtained from the Hospital of the University of Pennsylvania as described in detail previously.22 Briefly, foreskins were incubated in 50:50 dispase:DMEM mixture overnight at 4 °C. The epidermis was peeled from the underlying stroma and incubated in trypsin at 37 °C for 10 min. The trypsin was quenched with DMEM containing FBS and spun at 300 g for 5 min. The supernatant was removed, and the pellet was plated in keratinocyte media. Keratinocytes were cultured in 50% Gibco Keratinocyte-SFM + L-glutamine + EGF and BPE, 50% Gibco Cascade Biologics 154 medium for keratinocytes and 1% penicillin/streptomycin. 293T cells and Phoenix cells were purchased from ATCC and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and 1% antibiotic/antimycotic.
Plasmids
FGFR3 WT, FGFR3 R248C, FGFR3 S249C, and FGFR3 G697C genes were generated using PCR overlap extension and cloned into the LZRS retroviral vector. All constructs were fully sequenced.
Retrovirus production and transduction
Phoenix cells were used to produce retrovirus for infection of primary keratinocytes as described previously.22 VSVG pseudotyped shRNA lentivirus targeting human FGFR3 (pGIPZ vector, OpenBiosystems) was produced in 293T cells. Primary keratinocytes were transduced at 10–40% confluence. Lentivirus was filtered through a 45-µm filter and supplemented with 5 µg/mL polybrene. Virus was added to primary keratinocytes, and cells were spun at 300 g for 1 h at room temperature. Keratinocyte media was replaced after 15 min incubation at 37 °C.
Human skin xenografts
Human skin was obtained from the New York Firefighters Skin Bank at Weill Cornell. Skin was incubated at 37 °C in sterile PBS containing penicillin/streptomycin for approximately 1 wk. Epidermis was then carefully peeled from the underlying dermis. Underlying dermis was washed thoroughly in PBS. One square centimeter pieces of dermis were cut, and the dermis was elevated onto a sterilized annular dermal support (ADS) with the basement membrane side up.22 Several drops of BD matrigel were placed on the bottom of the dermis to create a seal. One million primary keratinocytes were seeded onto the dermis in KGM. KGM contains a 3:1 mixture of DMEM:Ham F12, 10% FBS, 1.8 × 10–4M adenine, 0.4 µg/mL hydrocortisone, 5 µg/mL insulin, 10–10 M cholera toxin, 10 ng/mL EGF, 5 µg/mL transferrin, and 1.36 ng/mL triiodo-L-thyronine. ADS were incubated at 37 °C for 3–4 d before being grafted onto SCID mice. For skin grafting, SCID mice were anesthetized in an isoflurane chamber, and 1 cm2 of epidermis was removed on the dorsal region of the mouse, down to fascia. Reconstituted human skin was sown onto the mouse dorsal region with individual interrupted stitches using 6–0 nylon sutures. Mice were dressed with Bactroban ointment, Adaptic, Telfa pad, and Coban wrap. Mice were unwrapped 2 weeks after grafting. For all luciferase and FGFR3 R248C grafts, 4–10 biological replicates were performed. S249C and G697C grafts were repeated in duplicate. FGFR3 knockdown experiments were also repeated in duplicate.
Immunofluorescence
Tissue was frozen using OTC. Tissue was sectioned at 8 µm thickness using a cryostat. Tissue was fixed using ice-cold methanol for 2 min. Five% horse serum in PBS was used for blocking (30 min), and 1% horse serum in PBS was used for primary and secondary antibody incubations (30 min each). Slides were mounted using Prolong Gold Antifade plus DAPI. The following antibodies and concentrations were used: collagen VII (Calbiochem, 1:200), Cytokeratin10 (Covance, 1:100), Ki67 (Thermo Scientific, 1:200), Desmoglein-3 (Invitrogen, 1:200). All immunofluorescence images were taken using an Olympus BX61 inverted microscope.
Immunohistochemistry
Tissue was fixed using 10% neutral-buffered formalin. Immunostaining was performed on 5 µm formalin fixed paraffin embedded (FFPE) skin sections. Briefly, tissue sections were deparaffinized in xylene and rehydrated in alcohol. For antigen retrieval, tissues were immersed in 10 mM citrate buffer pH 6.0 and heated at 95 °C for 10 min, cooled at RT, and washed 2 times for 3 min. Endogenous peroxidase was quenched with 3% H2O2 and subsequently washed 2–3 times with PBS. Tissue sections were incubated with blocking buffer (1% BSA and 10% normal goat serum in PBS) for 30 min and primary antibody at 4 °C overnight. Following multiple washes, goat anti-rabbit HRP conjugated secondary antibodies were incubated for 20 min at RT. The signal was further amplified with DAB mix solution (Abcam). Slides were counterstained, dehydrated, and mounted with a coverslip. The following antibodies were used for IHC: phospho-ERK1/2 (Cell Signaling).
Cell lysis and western blotting
For the blots shown in Figure 1, cells were lysed in 50 mM TRIS, 150 mM NaCl, 1 mM EDTA, 1% NP-40, and immunoblotted using Li-Cor Odyssey blocking reagents and equipment. To detect endogenous FGFR3 protein in human keratinocytes, cells were lysed directly in 2× SDS loading buffer (3.33 mM TRIPZ pH 7.5, 50 mM NaCl, 0.33 mM EDTA, 0.33 % NP-40) and blotted using 5% milk and HRP detection reagents. The following antibodies were used for immunoblotting: β-actin (Santa Cruz Biotechnology, 1:1000), β-actin (Cell Signaling, 1:10 000), Cdk4 (Santa Cruz Biotechnology, 1:500), FGFR3 (Santa Cruz Biotechnology, 1:1000), FOXN1 (Abcam, 1:500), phospho-p44/p42 MAPK (Millipore, 1:1000), MAP kinase 1/2 (Millipore, 1:1000), and H-Ras (Santa Cruz Biotechnology, 1:500).
Laser capture microscopy, genomic DNA, and RNA isolation and sequencing
Human tissue was formalin-fixed and paraffin-embedded (FFPE) according to standard protocols. Twenty-eight PEN (polyethylene napthalate) membrane slides (Leica Microsystems) were cut at 10 μm thickness each and stored at −20 °C. Slides were thawed for 15 min at 4 °C, 15 min at RT and stained for 10 s with hematoxylin and eosin. After laser microdissection (using the Leica LMD7000 Laser Microdissection microscope) of 21 slides, material was processed using the Qiagen All Prep DNA/RNA FFPE kit. Samples were quantitated with the Qubit fluorometer, and then 100 ng of RNA was converted to cDNA using the Applied Biosystems RNA to cDNA kit. FGFR3 sequences were amplified from the genomic DNA using the following PCR primers: FGFR3 F- 5′-TGAAGAACGG CAGGGAGTTC-3′, FGFR3 R- 5′-ATTCACCTCC ACGTGCCTTG A-3′. TP53 was amplified from the cDNA using the following PCR primers: TP53 F 5′-GTGTGGTGGT GCCCTATGAG-3′, TP53 R 5′-TCAAAGCTGT TCCGTCCCAG-3′. PCR products were sequenced using Sanger sequencing with the forward PCR primer.
Statistics
All error bars represent one standard deviation from the mean. Two-tailed Student t test was used to determine significance. A P value of less than 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
T.W.R. is supported by a grant from the NIH/NCI (RO1 CA163566). E.K.D. is supported by an NIH/NIAMS training grant (T32 AR0007465-30). S.J.O. was supported by the Penn Skin Disease Research Center (SDRC) SDRC 5-P30-AR-057217. We thank the SDRC, Tzvete Dentchev, Vincent Anagnos, and Michelle Oros for tissue sections, tissue processing, histology, immunohistochemistry and assistance with L.C.M. We thank John Seykora and John Stanley for critical pre-submission review.
Glossary
Abbreviations:
- EN
epidermal nevi
- ERK
extracellular signal-regulated kinase
- FGFR3
fibroblast growth factor receptor 3
- PI3K
phosphatidylinositide 3-kinase
- PLCγ
phospholipase C γ
- SCID
severe combined immunodeficiency
- SK
seborrheic keratosis
- SCC
squamous cell carcinoma
- STAT
signal transducer and activator of transcription
References
- 1.Tindall JP, Smith JG., Jr. Skin Lesions of the Aged and Their Association With Internal Changes. JAMA. 1963;186:1039–42. doi: 10.1001/jama.1963.03710120021004. [DOI] [PubMed] [Google Scholar]
- 2.Hafner C, Toll A, Fernández-Casado A, Earl J, Marqués M, Acquadro F, Méndez-Pertuz M, Urioste M, Malats N, Burns JE, et al. Multiple oncogenic mutations and clonal relationship in spatially distinct benign human epidermal tumors. Proc Natl Acad Sci U S A. 2010;107:20780–5. doi: 10.1073/pnas.1008365107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hida Y, Kubo Y, Arase S. Activation of fibroblast growth factor receptor 3 and oncogene-induced senescence in skin tumours. Br J Dermatol. 2009;160:1258–63. doi: 10.1111/j.1365-2133.2009.09068.x. [DOI] [PubMed] [Google Scholar]
- 4.Hafner C, van Oers JM, Vogt T, Landthaler M, Stoehr R, Blaszyk H, Hofstaedter F, Zwarthoff EC, Hartmann A. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–7. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafner C, van Oers JM, Hartmann A, Landthaler M, Stoehr R, Blaszyk H, Hofstaedter F, Zwarthoff EC, Vogt T. High frequency of FGFR3 mutations in adenoid seborrheic keratoses. J Invest Dermatol. 2006;126:2404–7. doi: 10.1038/sj.jid.5700422. [DOI] [PubMed] [Google Scholar]
- 6.Hafner C, Hartmann A, Real FX, Hofstaedter F, Landthaler M, Vogt T. Spectrum of FGFR3 mutations in multiple intraindividual seborrheic keratoses. J Invest Dermatol. 2007;127:1883–5. doi: 10.1038/sj.jid.5700804. [DOI] [PubMed] [Google Scholar]
- 7.Logié A, Dunois-Lardé C, Rosty C, Levrel O, Blanche M, Ribeiro A, Gasc JM, Jorcano J, Werner S, Sastre-Garau X, et al. Activating mutations of the tyrosine kinase receptor FGFR3 are associated with benign skin tumors in mice and humans. Hum Mol Genet. 2005;14:1153–60. doi: 10.1093/hmg/ddi127. [DOI] [PubMed] [Google Scholar]
- 8.Hafner C, Vogt T, Hartmann A. FGFR3 mutations in benign skin tumors. Cell Cycle. 2006;5:2723–8. doi: 10.4161/cc.5.23.3509. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Hiraishi Y, Wang H, Sumi KS, Hayashido Y, Toratani S, Kan M, Sato JD, Okamoto T. Constitutive activating mutation of the FGFR3b in oral squamous cell carcinomas. Int J Cancer. 2005;117:166–8. doi: 10.1002/ijc.21145. [DOI] [PubMed] [Google Scholar]
- 10.Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 11.Chesi M, Nardini E, Brents LA, Schröck E, Ried T, Kuehl WM, Bergsagel PL. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet. 1997;16:260–4. doi: 10.1038/ng0797-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández S, Toll A, Baselga E, Ribé A, Azua-Romeo J, Pujol RM, Real FX. Fibroblast growth factor receptor 3 mutations in epidermal nevi and associated low grade bladder tumors. J Invest Dermatol. 2007;127:1664–6. doi: 10.1038/sj.jid.5700705. [DOI] [PubMed] [Google Scholar]
- 13.Jebar AH, Hurst CD, Tomlinson DC, Johnston C, Taylor CF, Knowles MA. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–25. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 14.Sibley K, Cuthbert-Heavens D, Knowles MA. Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma. Oncogene. 2001;20:686–91. doi: 10.1038/sj.onc.1204110. [DOI] [PubMed] [Google Scholar]
- 15.Grand EK, Chase AJ, Heath C, Rahemtulla A, Cross NC. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18:962–6. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- 16.Paterson JL, Li Z, Wen XY, Masih-Khan E, Chang H, Pollett JB, Trudel S, Stewart AK. Preclinical studies of fibroblast growth factor receptor 3 as a therapeutic target in multiple myeloma. Br J Haematol. 2004;124:595–603. doi: 10.1111/j.1365-2141.2004.04814.x. [DOI] [PubMed] [Google Scholar]
- 17.Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K, et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest. 2009;119:1216–29. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scuto A, Krejci P, Popplewell L, Wu J, Wang Y, Kujawski M, Kowolik C, Xin H, Chen L, Wang Y, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2011;25:538–50. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trudel S, Ely S, Farooqi Y, Affer M, Robbiani DF, Chesi M, Bergsagel PL. Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4;14) myeloma. Blood. 2004;103:3521–8. doi: 10.1182/blood-2003-10-3650. [DOI] [PubMed] [Google Scholar]
- 20.Mandinova A, Kolev V, Neel V, Hu B, Stonely W, Lieb J, Wu X, Colli C, Han R, Pazin MJ, et al. A positive FGFR3/FOXN1 feedback loop underlies benign skin keratosis versus squamous cell carcinoma formation in humans. J Clin Invest. 2009;119:3127–37. doi: 10.1172/JCI38543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarov M, Kubo Y, Cai T, Dajee M, Tarutani M, Lin Q, Fang M, Tao S, Green CL, Khavari PA. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat Med. 2002;8:1105–14. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- 22.Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med. 2010;16:1450–5. doi: 10.1038/nm.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aubertin J, Tourpin S, Janot F, Ahomadegbe JC, Radvanyi F. Analysis of fibroblast growth factor receptor 3 G697C mutation in oral squamous cell carcinomas. Int J Cancer. 2007;120:2058–9, author reply 2060. doi: 10.1002/ijc.22285. [DOI] [PubMed] [Google Scholar]
- 24.Lei Y, Lui VWY, Grandis JR, Egloff AM. Identification of Mutations in the PYRIN-Containing NLR Genes (NLRP) in Head and Neck Squamous Cell Carcinoma. PLoS One. 2014;9:e85619. doi: 10.1371/journal.pone.0085619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ming M, Shea CR, Feng L, Soltani K, He YY. UVA induces lesions resembling seborrheic keratoses in mice with keratinocyte-specific PTEN downregulation. J Invest Dermatol. 2011;131:1583–6. doi: 10.1038/jid.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafner C, López-Knowles E, Luis NM, Toll A, Baselga E, Fernández-Casado A, Hernández S, Ribé A, Mentzel T, Stoehr R, et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc Natl Acad Sci U S A. 2007;104:13450–4. doi: 10.1073/pnas.0705218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafner C, Vogt T, Landthaler M, Müsebeck J. Somatic FGFR3 and PIK3CA mutations are present in familial seborrhoeic keratoses. Br J Dermatol. 2008;159:214–7. doi: 10.1111/j.1365-2133.2008.08626.x. [DOI] [PubMed] [Google Scholar]
- 28.Hafner C, Toll A, Real FX. HRAS mutation mosaicism causing urothelial cancer and epidermal nevus. N Engl J Med. 2011;365:1940–2. doi: 10.1056/NEJMc1109381. [DOI] [PubMed] [Google Scholar]