Abstract
Purpose
To determine if older patients with breast cancer have cognitive impairment before systemic therapy.
Patients and Methods
Participants were patients with newly diagnosed nonmetastatic breast cancer and matched friend or community controls age > 60 years without prior systemic treatment, dementia, or neurologic disease. Participants completed surveys and a 55-minute battery of 17 neuropsychological tests. Biospecimens were obtained for APOE genotyping, and clinical data were abstracted. Neuropsychological test scores were standardized using control means and standard deviations (SDs) and grouped into five domain z scores. Cognitive impairment was defined as any domain z score two SDs below or ≥ two z scores 1.5 SDs below the control mean. Multivariable analyses evaluated pretreatment differences considering age, race, education, and site; comparisons between patient cases also controlled for surgery.
Results
The 164 patient cases and 182 controls had similar neuropsychological domain scores. However, among patient cases, those with stage II to III cancers had lower executive function compared with those with stage 0 to I disease, after adjustment (P = .05). The odds of impairment were significantly higher among older, nonwhite, less educated women and those with greater comorbidity, after adjustment. Patient case or control status, anxiety, depression, fatigue, and surgery were not associated with impairment. However, there was an interaction between comorbidity and patient case or control status; comorbidity was strongly associated with impairment among patient cases (adjusted odds ratio, 8.77; 95% CI, 2.06 to 37.4; P = .003) but not among controls (P = .97). Only diabetes and cardiovascular disease were associated with impairment among patient cases.
Conclusion
There were no overall differences between patients with breast cancer and controls before systemic treatment, but there may be pretreatment cognitive impairment within subgroups of patient cases with greater tumor or comorbidity burden.
INTRODUCTION
Many of the 290,000 women diagnosed each year in the United States with breast cancer1 will be candidates for systemic treatment.2,3 Despite survival benefits,4 short-5–7 and long-term8–10 cognitive deficits have been reported after these systemic therapies.11–19 However, the effects are not universal20–22 and may only affect certain subgroups of patients.12,23 There are emerging neuropsychological24 and neuroimaging25–27 data suggesting that some patients with cancer may have cognitive impairment before systemic treatment, independent of anxiety, depression, or fatigue,8,28 type of anesthesia or surgery,29,30 or nutritional status.31 There are several hypothesized biologic mechanisms whereby cancer might exert an influence on cognition function before systemic therapy,32 including inflammation,6,33 oxidative stress,34 DNA damage and compromised DNA repair,35 genetic susceptibility,36,37 decreased telomere length,38,39 and/or cell senescence.40,41
One common feature of these putative mechanisms is the aging process, leading to the speculation that older patients may be vulnerable to pre- and post-treatment cancer-related cognitive deficits.42,43 Unfortunately, there have been few studies examining outcomes for older patients,11,44–46 and the mean age in the most recent meta-analysis of cognition in patients with cancer was 53 years.13
The TLC (Thinking and Living With Cancer) study was a national, multisite prospective study designed to identify the impact of cancer and systemic cancer treatments on cognition among older women with breast cancer. We used presystemic therapy data to test the hypothesis that compared with older controls, older patients have lower baseline cognition as measured by neuropsychological testing. We also tested whether cognitive reserve, physiological reserve (defined by comorbidity level), age, or presence of APOE e4 alleles affects baseline pretreatment cognitive differences between patient cases and controls. Finally, we explored whether disease stage distinguishes patient cases with more versus less cognitive impairment.
PATIENTS AND METHODS
This study was conducted at Georgetown University (Washington, DC), Memorial Sloan-Kettering Cancer Center (New York, NY), Moffitt Cancer Center (Tampa, FL), and City of Hope Comprehensive Cancer Center (Los Angeles, CA). Assessment training and certification were conducted at Boston University School of Medicine (Boston, MA).47 The research protocol met Health Insurance Portability and Accountability Act standards and was approved by all institutional review boards.
Setting and Population
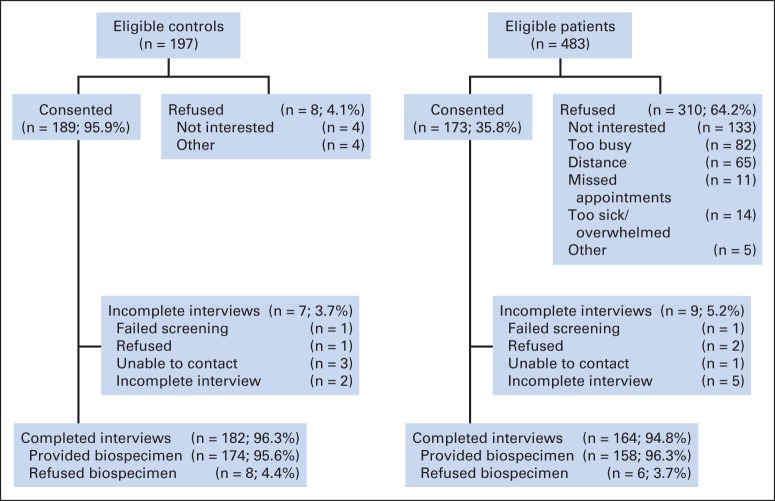
Women were recruited between August 1, 2010, and June 30, 2013. Eligible patient cases were age ≥ 60 years, newly diagnosed with primary nonmetastatic breast cancer (American Joint Committee on Cancer [AJCC] stages 0 to III), and English speaking; those with stroke, head injury, major Axis I psychiatric disorders, or neurodegenerative disorders were ineligible. Women with a history of other cancers were excluded if active treatment occurred < 5 years before or if they had ever received chemotherapy or hormonal therapy. Of eligible patient cases, 173 consented (median consent rate, 48.2%; range across sites, 24.7% to 67.4%; Fig 1). We recruited controls who were friends of participating patient cases; if no friend was available, we recruited an age-, race-, education-, and frequency-matched control from the same region as the patient case. Controls met the same eligibility criteria as patient cases. Consenting participants were screened for final eligibility using the Mini-Mental State Examination and the Wide Range Achievement Test (fourth edition; WRAT-4) to ensure ability to complete the study; those with a score < 24 or < third-grade level on either test were ineligible (patient case, n = 1; control, n = 1). The final sample included 164 patient cases and 182 controls.
Fig 1.
Sample for evaluation of cognition in older patients with breast cancer and noncancer controls.
Data Collection
Study staff identified potentially eligible patients from physician referrals and/or appointment schedules, obtained permission to contact patients, consented patients, and conducted testing. All data were collected after postoperative visits but before administration of radiation or systemic therapy. Assessment included neuropsychological testing (55 minutes), a structured survey, and a blood or saliva biospecimen (Oragene kit; DNA Genotek, Kanata, Ontario, Canada) for DNA testing to determine APOE genotype. All data were collected simultaneously or within 1 week of each other. Biospecimens were processed centrally and tested in batches; 100% of the specimens had sufficient DNA for analysis. We abstracted data on AJCC stage, estrogen receptor status, human epidermal growth factor receptor 2 status, and surgery for patient cases.
Measures
Outcomes.
The primary outcome variable was baseline presystemic therapy cognition based on neuropsychological testing. We selected domains most likely to be affected by cancer,48 relevant to older patients,49,50 and related to cognitive impairment and aging (eg, prefrontal or hippocampal involvement on functional magnetic resonance imaging),51,52 including attention, working memory, and psychomotor speed; executive functioning and language; learning and memory; and visual-spatial ability. The secondary outcome was impairment based on domain score.
Tests with established reliability and validity in diverse older populations were used to measure the domains, including some of those recommended by the International Task Force on Cancer and Cognition53 and/or part of the Unified Data Set of the National Alzheimer's Coordinating Center.54,55 The administration length (55 minutes) was deemed feasible for older patients.56 The test battery is summarized in Table 1, and raw scores are included on Appendix Table A1 (online only); Appendix Table A2 (online only) includes scores stratified by stage for patient cases.
Table 1.
Neuropsychological Tests and Domains Used to Assess Cognition in Older Patients With Breast Cancer and Noncancer Controls
| Test | Approximate Time to Administer (minutes) | Source |
|---|---|---|
| Attention, working memory, and processing speed domain | ||
| NAB digits forward | 4:00 | Stern and White55 |
| NAB digits backward | 4:00 | Stern and White55 |
| Trail making A | 2:00 | Reitan and Wolfson57 |
| Digit symbol test | 2:00 | Wechsler58 |
| NAB driving scenes | 12:00 | Stern and White55 |
| Language domain | ||
| Boston naming test | 4:00 | Kaplan et al59 |
| Category fluency | 1:30 | Morris et al60 |
| Executive Functioning | ||
| Trail making B | 3:00 | Reitan and Wolfson57 |
| Controlled oral word association test | 4:00 | Benton61 |
| NAB figure drawing | 3:00 | Stern and White55 |
| Learning and memory domain | ||
| Logical memory I | 3:00 | Wechsler58 |
| Logical memory II | 2:00 | Wechsler58 |
| NAB list A immediate recall | 4:00 | Stern and White55 |
| NAB list B immediate recall | 1:30 | Stern and White55 |
| NAB list A short delay recall | 1:00 | Stern and White55 |
| NAB long delay | 4:00 | Stern and White55 |
| Visual-spatial domain | ||
| NAB figure drawing copy | Variable | Stern and White55 |
Abbreviation: NAB, Neuropychological Assessment Battery.
Variables.
The primary variable related to cognition was patient case versus control status. We also present self-reported cognition based on the Functional Assessment of Cancer Therapy (FACT) –Cognition62 subscale for perceived cognitive impairment. This scale assesses difficulty in memory, concentration, and intellectual activities (α = 0.93). On the basis of prior research in cancer42,63 and other diseases,64 we expected that the effects of cancer on cognition might be moderated by stage, reserve, age, or presence of APOE e4 allele (ie, e2/e4, e3/e4, or e4/4). Stage was grouped as early (AJCC 0 to I) versus more advanced (AJCC II to III) based on tumor burden. Cognitive reserve was defined as high versus low based on median WRAT-4 score in controls and by education. Physiological reserve was estimated based on self-reported number of comorbid illnesses; we also dichotomized the number of illnesses at the median for ease of describing interactions. We further examined specific comorbidities, such as diabetes and cardiovascular disease. Age was considered as a continuous variable; we also dichotomized to examine subgroups (age 60 to 74 and ≥ 75 years).
Sociodemographic measures included age, race (white v nonwhite), family history of dementia (first- or second-degree relatives; yes v no), marital status, education (total years), usual occupation (clerical, administrative, management, and so on), insurance, and setting of care (health maintenance organization v non–health maintenance organization). Health habits included prior years of use of hormonal replacement therapy, age at menopause, self-reported cigarette (ever or current v never) and alcohol use (< two v ≥ two drinks per day), and level of physical activity.65
The Center for Epidemiologic Studies Depression Scale (20 items) measured depressive symptoms, with a cut point of 16 defining clinical depression.66,67 To measure anxiety, the state version of the 20-item State Trait Anxiety Inventory was used.68 Fatigue was assessed using the 13-item FACT-Fatigue subscale.69 The Medical Outcomes Study Short Form 12 measured physical and mental function in the 2 months before diagnosis or interview,70 and the FACT-General (for patient case–control comparisons) and FACT–Breast Cancer (for comparisons between patient cases) subscales captured current quality of life, including physical and emotional function.71,72 Current function was also adjusted for prior function. Clinical variables considered in case-case comparisons included surgery (mastectomy v lumpectomy, as proxy for anesthesia exposure, postoperative pain, and effects on function), estrogen receptor status, and human epidermal growth factor receptor 2 status.
Statistical Analysis
We used t and χ2 tests to compare characteristics of patient cases and controls. Raw neuropsychological test scores were calculated and converted to z scores by standardizing to control means and standard deviations (SDs). Standardized z scores were then averaged across each predefined domain. t tests compared patient case and control raw and standardized scores; two-way analysis of variance was employed to compare patient case and control domain z scores, adjusting for age, race, education, and site. Other factors were not related to patient case or control status or cognitive outcomes and so were not used in adjustment. Comparisons between patient cases included additional adjustment for surgery type to capture possible unmeasured postoperative effects. Differences between patient case and control groups were also examined within subgroups based on age (60 to 74 v ≥ 75 years), WRAT score (higher or lower than control median), comorbidity (< two v ≥ two illnesses based on overall median), APOE status (e4 allele v other), and stage (0 to I v II to III).
Because there is no universally accepted definition of lower-than-expected cognitive performance (ie, cognitive impairment),73 we used a common convention whereby relative cognitive impairment is defined as having any one domain z score that is two SDs below or two domain z scores that are 1.5 SDs below the control mean.42,53 In addition, we conducted sensitivity analyses using an alternative definition based on having ≥ two domains with z scores below one SD of the control mean. We did not consider definitions based on individual test scores, because these may overestimate impairment, given correlations of responses to tests within domains.74
We examined bivariate associations between cognitive impairment and covariates, overall and among the subgroups. We included any variable in logistic regression models that was related to impairment at a 0.05 level in bivariate analyses; site was retained to capture any unmeasured factors in comparisons between patient cases and controls and between patient cases; surgery type was also retained in comparisons between patient cases. There was a significant interaction between comorbidity and patient case or control status; thus, we also present separate models by patient case or control group.
We had 80% power (α = 0.05) to detect an effect size of 0.3 SDs (small to medium effect size) between groups for any cognitive domain score. We also had 80% power (α = 0.05) to detect an odds ratio of 2.1 for the association between patient case or control group and impairment, assuming 15% impairment among controls. All analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
Among eligible patient cases, those consenting were similar in age to nonparticipants. Participants were well educated and ranged in age from 60 to 98 years. There were no sociodemographic differences between patient cases and controls, except that controls had a minimally higher average education level than patient cases (15.7 [SD, 2.2] v 15.1 years [SD, 2.2]; P = .012; Table 2). Approximately one third of patient cases and controls reported a family history of dementia. Among patient cases, 65% had early-stage disease (stages 0 to I), and 88% were estrogen receptor positive. Patient cases reported significantly higher levels of anxiety, depression, and fatigue and quality of life than controls (P ≤ .005), but there were no self-reported differences in cognition between patient cases and controls.
Table 2.
Demographic and Clinical Characteristics of Older Participants
| Characteristic | Controls (n = 182)a |
Patient Cases |
P |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 164)a |
Stages 0 to I (n = 106) |
Stages II to III (n = 58) |
Patient Case v Control | Patient Case Stages 0 to I v II to III | Patient Case Stages 0 to I v Control | Patient Case Stages II to III v Control | ||||||
| No. | % | No. | % | No. | % | No. | % | |||||
| Age, years | .27 | .75 | .41 | .30 | ||||||||
| Mean | 67.3 | 68.1 | 68.0 | 68.4 | ||||||||
| SD | 6.5 | 6.7 | 6.7 | 6.8 | ||||||||
| Range | 60-90 | 60-98 | 60-98 | 60-91 | ||||||||
| Race | .92 | .85 | .86 | .95 | ||||||||
| White, non-Hispanic | 146 | 80.7 | 130 | 80.2 | 83 | 79.8 | 47 | 81.0 | ||||
| Nonwhite (black, Hispanic, AAPI) | 35 | 19.3 | 32 | 19.8 | 21 | 20.2 | 11 | 19.0 | ||||
| Marital status | .08 | .31 | .04 | .58 | ||||||||
| Married | 96 | 52.7 | 102 | 62.2 | 69 | 65.1 | 33 | 56.9 | ||||
| Widowed, divorced, or single | 86 | 47.3 | 62 | 37.8 | 37 | 34.9 | 25 | 43.1 | ||||
| Education, years | .01 | .15 | .12 | .006 | ||||||||
| Mean | 15.7 | 15.1 | 15.3 | 14.8 | ||||||||
| SD | 2.2 | 2.2 | 2.1 | 2.2 | ||||||||
| WRAT-4 score | .62 | .93 | .64 | .77 | ||||||||
| Mean | 111.2 | 112.0 | 112.1 | 111.9 | ||||||||
| SD | 16.0 | 15.4 | 16.0 | 14.5 | ||||||||
| Insurance coverage | .27 | .32 | .09 | .99 | ||||||||
| Private | 147 | 80.8 | 144 | 87.8 | 96 | 90.6 | 48 | 82.8 | ||||
| Medicaid | 4 | 2.2 | 4 | 2.4 | 3 | 2.8 | 1 | 1.7 | ||||
| 14 | 7.7 | 7 | 4.3 | 3 | 2.8 | 4 | 6.9 | |||||
| None | 17 | 9.3 | 9 | 5.5 | 4 | 3.8 | 5 | 8.6 | ||||
| Usual adult occupation | .65 | .95 | .72 | .71 | ||||||||
| Managerial or professional | 124 | 68.1 | 108 | 65.9 | 70 | 66.0 | 38 | 65.5 | ||||
| Other | 58 | 31.9 | 56 | 34.1 | 36 | 34.0 | 20 | 34.5 | ||||
| Smoking status | .35 | .72 | .33 | .68 | ||||||||
| Current or former | 96 | 53.0 | 94 | 58.0 | 62 | 59.0 | 32 | 56.1 | ||||
| Never | 85 | 47.0 | 68 | 42.0 | 43 | 41.0 | 25 | 43.9 | ||||
| Alcohol use, drinks per day | .56 | .92 | .64 | .64 | ||||||||
| < 2 | 173 | 95.1 | 158 | 96.3 | 102 | 96.2 | 56 | 96.6 | ||||
| ≥ 2 | 9 | 4.9 | 6 | 3.7 | 4 | 3.8 | 2 | 3.4 | ||||
| Age at menopause, years | .97 | .21 | .50 | .35 | ||||||||
| Mean | 49.5 | 49.4 | 48.9 | 50.4 | ||||||||
| SD | 6.1 | 6.7 | 7.1 | 6.0 | ||||||||
| Hormone replacement therapy duration, years | .37 | .21 | .77 | .09 | ||||||||
| < 1 | 6 | 5.9 | 8 | 8.9 | 3 | 5.4 | 5 | 14.7 | ||||
| 1 to < 5 | 39 | 38.6 | 25 | 27.8 | 19 | 33.9 | 6 | 17.6 | ||||
| 5 to < 10 | 23 | 22.8 | 20 | 22.2 | 11 | 19.6 | 9 | 26.5 | ||||
| ≥ 10 | 33 | 32.7 | 37 | 41.1 | 23 | 41.1 | 14 | 41.2 | ||||
| Family history of dementia | .57 | .10 | .80 | .12 | ||||||||
| Yes | 62 | 37.3 | 50 | 34.2 | 37 | 38.9 | 13 | 25.5 | ||||
| No | 104 | 62.7 | 96 | 65.8 | 58 | 61.1 | 38 | 74.5 | ||||
| No. of comorbidities | .34 | .62 | .53 | .31 | ||||||||
| Mean | 2.4 | 2.5 | 2.5 | 2.7 | ||||||||
| SD | 1.8 | 2.0 | 1.8 | 2.4 | ||||||||
| Physical activity | .15 | .92 | .21 | .41 | ||||||||
| Low or none | 42 | 23.3 | 41 | 27.7 | 26 | 26.8 | 15 | 29.4 | ||||
| Moderate | 75 | 41.7 | 70 | 47.3 | 47 | 48.5 | 23 | 45.1 | ||||
| High | 63 | 35.0 | 37 | 25.0 | 24 | 24.7 | 13 | 25.5 | ||||
| APOE genotypeb | .21 | .85 | .31 | .32 | ||||||||
| e4 negative | 131 | 75.3 | 128 | 81.0 | 83 | 80.6 | 45 | 81.8 | ||||
| e4 positive | 43 | 24.7 | 30 | 19.0 | 20 | 19.4 | 10 | 18.2 | ||||
| AJCC stage | — | — | — | — | ||||||||
| 0 | — | 3 | 1.8 | 3 | 2.8 | — | ||||||
| I | — | 103 | 62.8 | 103 | 97.2 | — | ||||||
| II | — | 51 | 31.1 | — | 51 | 87.9 | ||||||
| III | — | 7 | 4.3 | — | 7 | 12.1 | ||||||
| Surgery type | — | .001 | — | — | ||||||||
| Lumpectomy | — | 93 | 57.8 | 71 | 67.6 | 22 | 39.3 | |||||
| Mastectomy | — | 68 | 42.2 | 34 | 32.4 | 34 | 60.7 | |||||
| Time from surgery, daysc | — | .640 | — | — | ||||||||
| Mean | — | 50.6 | 51.1 | 49.5 | ||||||||
| SD | — | 20.4 | 19.9 | 21.5 | ||||||||
| ER status | — | .53 | — | — | ||||||||
| Positive | — | 144 | 88.3 | 94 | 89.5 | 50 | 86.2 | |||||
| Negative | — | 19 | 11.7 | 11 | 10.5 | 8 | 13.8 | |||||
| HER2 status | — | .18 | — | — | ||||||||
| Positive | — | 18 | 12.4 | 9 | 9.7 | 9 | 17.3 | |||||
| Negative | — | 127 | 87.6 | 84 | 90.3 | 43 | 82.7 | |||||
| Past physical functiond | .18 | .36 | .45 | .10 | ||||||||
| Mean | 52.0 | 50.8 | 51.3 | 50.0 | ||||||||
| SD | 7.5 | 8.7 | 8.0 | 9.9 | ||||||||
| Past emotional functiond | .55 | .35 | .31 | .77 | ||||||||
| Mean | 47.7 | 48.1 | 48.4 | 47.5 | ||||||||
| SD | 4.8 | 5.6 | 5.6 | 5.8 | ||||||||
| Depressione | .004 | .17 | .05 | < .001 | ||||||||
| > 16 on CES-D | 8 | 4.5 | 21 | 13.1 | 11 | 10.5 | 10 | 18.2 | ||||
| Anxietyf | < .001 | .16 | .004 | < .001 | ||||||||
| Mean | 26.7 | 29.7 | 29.0 | 31.0 | ||||||||
| SD | 5.5 | 8.5 | 8.1 | 9.0 | ||||||||
| Fatigueg | < .001 | .67 | < .001 | < .001 | ||||||||
| Mean | 46.9 | 43.8 | 44.0 | 43.4 | ||||||||
| SD | 5.7 | 8.7 | 8.7 | 8.7 | ||||||||
| Current physical functionh | < .001 | .24 | < .001 | < .001 | ||||||||
| Mean | 22.0 | 20.3 | 20.6 | 19.8 | ||||||||
| SD | 2.5 | 3.8 | 3.5 | 4.3 | ||||||||
| Current emotional functioni | < .001 | .13 | < .001 | < .001 | ||||||||
| Mean | 14.7 | 12.9 | 13.1 | 12.4 | ||||||||
| SD | 1.5 | 2.9 | 2.9 | 3.0 | ||||||||
| Overall QOLj | < .001 | .15 | .004 | < .001 | ||||||||
| Mean | 72.4 | 68.3 | 69.2 | 66.7 | ||||||||
| SD | 8.2 | 10.7 | 10.3 | 11.2 | ||||||||
| Self-reported cognitionk | .38 | .67 | .32 | .75 | ||||||||
| Mean | 62.1 | 61.1 | 60.9 | 61.6 | ||||||||
| SD | 8.6 | 10.8 | 11.4 | 9.6 | ||||||||
Abbreviations: AAPI, Asian American or Pacific Islander; AJCC, American Joint Committee on Cancer; CES-D, Center for Epidemiologic Studies Depression Scale; ER, estrogen receptor; FACT, Functional Assessment of Cancer Therapy; HER2, human epidermal growth factor receptor 2; QOL, quality of life; SD, standard deviation; WRAT-4, Wide Range Achievement Test (fourth edition).
Some numbers may not add to 100% because of missing data; three patients refused surgery.
Biospecimens provided by 95% of patient cases and 96.5% of controls; thus, results do not total 100%.
Time since surgery was not calculated for participants undergoing neoadjuvant therapy (lumpectomy, n = 6; mastectomy, n = 2).
Based on Short Form 12 assessed for period 2 months before cancer diagnosis; higher scores indicate better function.
Depression defined by score above cut point of 16 on CES-D.
Based on state version of State Trait Anxiety Inventory A scale; scores range from 20 to 80, with higher scores reflecting more anxiety.
Based on Functional Assessment of Chronic Illness Therapy–Fatigue subscale; scores range from 0 to 52, with higher scores reflecting less fatigue.
Based on FACT–General Population Physical Well-Being subscale; scores range from 0 to 24, with higher scores reflecting better functioning.
Based in FACT–General Population Emotional Well-Being subscale; scores range from 0 to 16, with higher scores reflecting better functioning.
Based on FACT-General total score; scores range from 0 to 84, with higher scores reflecting better functioning.
Based on FACT–Cognitive Function Perceived Cognitive Impairment score; scores range from 0 to 72, with higher scores reflecting better perceived cognition.
There were no differences between patient cases and controls with respect to unadjusted and adjusted means of standardized neuropsychological test domain scores (Table 3). There were also no differences between patient cases and controls in scores when stratified by age group, WRAT score, or APOE genotype results (data not shown). There was a trend among patient cases with more advanced disease stage toward lower executive function than controls after adjustment (P = .13; data not shown). There were differences among patient cases by stage group; those with more advanced disease stage had lower executive function than patient cases with earlier disease stage after adjustment (P = .05; Appendix Table A2, online only).
Table 3.
Baseline Pretreatment Neuropsychological Test Results for Older Patients With Breast Cancer Versus Noncancer Controls
| Test | Unadjusted Standardized z Score* |
Adjusted Standardized z Score† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls |
Patient Cases |
P | Controls |
Patient Cases |
P | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Attention, working memory, and processing speed domain | 0.00 | 0.05 | −0.03 | 0.05 | .699 | −0.17 | 0.05 | −0.12 | 0.05 | .464 |
| NAB digits forward | 0.00 | 0.07 | −0.04 | 0.08 | .682 | −0.19 | 0.08 | −0.20 | 0.09 | .913 |
| NAB digits backward | 0.00 | 0.07 | −0.01 | 0.08 | .890 | −0.19 | 0.08 | −0.16 | 0.08 | .838 |
| Trail making A | 0.00 | 0.07 | −0.04 | 0.08 | .738 | −0.18 | 0.08 | −0.13 | 0.08 | .620 |
| Digit symbol test | 0.00 | 0.08 | −0.05 | 0.08 | .640 | −0.16 | 0.08 | −0.12 | 0.08 | .679 |
| NAB driving scenes | 0.00 | 0.07 | 0.02 | 0.08 | .888 | −0.12 | 0.08 | −0.00 | 0.08 | .228 |
| Language domain | 0.00 | 0.06 | −0.07 | 0.07 | .410 | −0.24 | 0.06 | −0.23 | 0.06 | .840 |
| Boston naming test | 0.00 | 0.07 | −0.07 | 0.07 | .528 | −0.32 | 0.07 | −0.29 | 0.07 | .747 |
| Category fluency | 0.00 | 0.07 | −0.08 | 0.08 | .438 | −0.17 | 0.08 | −0.16 | 0.08 | .982 |
| Executive function domain | 0.00 | 0.05 | −0.12 | 0.05 | .091 | −0.12 | 0.05 | −0.17 | 0.05 | .498 |
| Trail making B | 0.00 | 0.08 | −0.13 | 0.08 | .229 | −0.22 | 0.08 | −0.23 | 0.08 | .883 |
| Controlled oral word association test | 0.00 | 0.07 | −0.26 | 0.07 | .013 | −0.10 | 0.08 | −0.30 | 0.08 | .042 |
| NAB figure drawing | 0.00 | 0.07 | 0.03 | 0.08 | .796 | −0.05 | 0.08 | 0.03 | 0.09 | .441 |
| Learning and memory domain | 0.00 | 0.06 | −0.04 | 0.06 | .620 | −0.13 | 0.06 | −0.08 | 0.06 | .480 |
| Logical memory I | 0.00 | 0.08 | −0.04 | 0.08 | .743 | −0.09 | 0.08 | −0.06 | 0.09 | .757 |
| Logical memory II | 0.00 | 0.08 | 0.04 | 0.08 | .686 | −0.09 | 0.08 | 0.04 | 0.09 | .219 |
| NAB list A immediate recall | 0.00 | 0.08 | −0.11 | 0.08 | .305 | −0.19 | 0.07 | −0.20 | 0.08 | .915 |
| NAB list B immediate recall | 0.00 | 0.07 | −0.01 | 0.07 | .912 | −0.15 | 0.07 | −0.08 | 0.07 | .421 |
| NAB list A short delay recall | 0.00 | 0.08 | −0.05 | 0.08 | .650 | −0.14 | 0.08 | −0.08 | 0.08 | .540 |
| NAB long delay | 0.00 | 0.07 | −0.09 | 0.08 | .425 | −0.14 | 0.08 | −0.12 | 0.08 | .877 |
| Visual-spatial domain | 0.00 | 0.07 | 0.09 | 0.08 | .419 | −0.06 | 0.08 | 0.08 | 0.09 | .173 |
| NAB figure drawing copy | 0.00 | 0.07 | 0.09 | 0.08 | .419 | −0.06 | 0.08 | 0.08 | 0.09 | .173 |
Abbreviation: NAB, Neuropychological Assessment Battery.
Patient case scores standardized using control mean and standard deviation as referent standard; therefore, by definition, unadjusted control mean scores are zero.
Adjusted for age, race (white v nonwhite), education (in years), and recruitment site.
There were no differences in unadjusted rates of cognitive impairment between patient cases and controls (14% v 15%; P = .81). However, as shown in Figure 2, patient cases with high comorbidity levels had higher rates of impairment than patient cases with low comorbidity levels (25.7% v 4.4%); this relationship was also seen for patient cases by surgery type, but controls had similar rates of impairment in the high versus low comorbidity groups (17.5% v 12.8%; P < .001 for overall comparison). Surgery, anxiety, depression, fatigue, health habits and physical activity, and current physical or emotional function were not associated with impairment in any group (Table 4).
Fig 2.
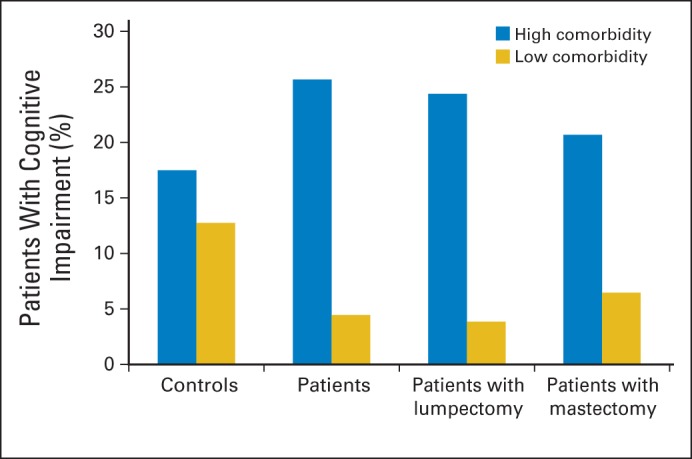
Percent with cognitive impairment among older patients with breast cancer and noncancer controls by comorbidity level. Comorbidity categorized based on overall median. High comorbidity defined as ≥ two comorbid illnesses; low defined as < two illnesses. Impairment rates based on ≥ one domain score two standard deviations (SDs) below control mean or two domain scores 1.5 SDs below control mean. Figure shows unadjusted rates of impairment. Overall comparison between all patient cases and controls significant at P < .001. Among all patient cases, association between impairment and comorbidity significant at P < .001, whereas control difference not significant (P = .98).
Table 4.
ORs for Associations With Pre–Systemic Treatment Cognitive Impairment* in Older Patients With Breast Cancer and Noncancer Controls
| Variable | All Participants |
Patient Cases (n = 159)† |
Controls (n = 181)† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted (N = 346) |
Adjusted (n = 343)ठ|
|||||||||||
| OR | 95% CI | P | OR | 95% CI | P | Adjusted OR | 95% CI | P | Adjusted OR | 95% CI | P | |
| Patient case (v control) | 0.93 | 0.51 to 1.71 | .83 | 0.74 | 0.37 to 1.47 | .39 | — | — | ||||
| Age, per 1-year increase | 1.12 | 1.07 to 1.17 | < .001 | 1.10 | 1.05 to 1.15 | < .001 | 1.09 | 1.02 to 1.18 | .02 | 1.10 | 1.03 to 1.18 | .003 |
| Education, per 1-year increase | 0.77 | 0.67 to 0.88 | < .001 | 0.81 | 0.69 to 0.95 | .008 | 0.83 | 0.64 to 1.08 | .17 | 0.80 | 0.65 to 1.00 | .050 |
| Nonwhite race (v white, non-Hispanic)‖ | 2.90 | 1.51 to 5.60 | .002 | 3.38 | 1.57 to 7.28 | .002 | 5.45 | 1.57 to 18.9 | .008 | 2.91 | 0.98 to 8.63 | .054 |
| ≥ 2 comorbidities (v < 2) | 2.81 | 1.50 to 5.27 | .001 | 2.31 | 1.14 to 4.69 | .020 | 8.77 | 2.06 to 37.4 | .003 | 0.98 | 0.39 to 2.49 | .97 |
| Mastectomy (v lumpectomy)¶ | — | — | 0.59 | 0.16 to 2.25 | .44 | — | ||||||
NOTE. ORs are from logistic regression models. Variables not considered in models because they were not significantly related to impairment: current physical (P = .22) or emotional function (P = .31), depression (P = .99), anxiety (P = .20), fatigue (P = .34), smoking (P = .93), physical activity (P = .33), and presence of APOE e4 allele (P = .21).
Abbreviation: OR, odds ratio.
Cognitive impairment defined as any one domain z score two SDs or two domain z scores 1.5 SDs below control mean.
Models stratified by patient case or control status included because of interaction between patient case or control status and comorbidity.
All adjusted ORs adjusted for covariates shown plus recruitment site.
Three patient cases were missing surgery or refused surgery and are not included.
Nonwhite race includes black, Hispanic, Asian American, and Pacific Islander.
Results for unadjusted association between surgery and impairment in patient case group: OR, 1.03; 95% CI, 0.41 to 2.60; P = .951.
The adjusted odds of having impairment were not related to patient case or control status, but they were significantly higher in women who were older, of nonwhite race, and with lower educational levels (P ≤ .001; Table 4). There was also an interaction between comorbidity and patient case or control status; comorbidity was associated with impairment among patient cases (adjusted odds ratio, 8.77; 95% CI, 2.06 to 37.4; P = .003) but not among controls (P = .97). The result was similar using the alternative definition of impairment (data not shown).
In exploratory analyses, there were no differences between patient cases and controls in rate of diabetes (10.4% v 7.7%; P = .39) or cardiovascular disease (52.4% v 47.3%; P = .34). However, the impairment rate was significantly higher in patient cases with diabetes (v no diabetes; 41% v 11%; P = .003) or cardiovascular disease (22% v 5%; P = .002), whereas impairment was not associated with these conditions in controls (data not shown).
DISCUSSION
This is one of the largest controlled studies of cognitive impairment before systemic treatment in an older population with breast cancer. Rates of impairment were low, and we did not find any overall presystemic treatment differences between patient cases and their frequency-matched noncancer controls. However, patient cases with more advanced disease stage tended to have lower executive function than patient cases with early-stage disease. Additionally, after adjustment, the odds of presystemic treatment impairment were higher in patient cases with greater (v less) comorbidity, whereas impairment was not associated with comorbidity among controls. Last, demographic factors, such as age, race, or education, were associated with impairment, whereas psychological variables like anxiety, depression, fatigue, lifestyle factors, APOE genotype, and surgery were not.
It is difficult to compare our rates of presystemic treatment cognitive impairment with those from other studies, because most prior research has been conducted among young patients,75,76 been based on self-report,77,78 or used different definitions of impairment.73 In one study that examined postmenopausal patients with breast cancer, Ahles et al42 reported a 22% rate of impairment among patient cases with stage I to III disease, similar but somewhat higher than our rate of 14% for those with stages 0 to III disease.
There have been inconsistent findings regarding cognitive differences between patient cases with breast13 or other cancers before systemic therapy53 and controls.7,42,79 For instance, Wefel et al24 reported cognitive deficits before systemic treatment, whereas we and others42,80,81 have not found these effects. There are several explanations for the conflicting results, including variability in definitions of impairment and referent standards.73,74 Alternatively, the effects may only be experienced by subgroups that have not been clearly identified. Our observation that patient cases with more advanced disease stage had lower executive function than controls suggests one such potential subgroup. This finding is consistent with the finding in a study that noted slower reaction times among patient cases with later-stage disease compared with controls before systemic treatment. Similar to our results, this was not attributable to differences in surgery, function, anxiety, depression, or fatigue.42 If confirmed, it would be important to understand if any executive dysfunction affects treatment decision making. A stage effect could also have biologic plausibility, with greater tumor burden exerting paraneoplastic effects or reflecting shared risk factors for cancer development and progression and cognitive impairment.42 Another explanation for the inconsistent results for pretreatment differences is that effects are subtle. This possibility is suggested by findings showing significant differences between patient cases with breast cancer before systemic therapy and healthy controls on functional magnetic resonance imaging tasks related to visual-spatial working memory25 or attention and working memory26 in the absence of differences on neuropsychological tests.
The finding that independent of age, surgery, and other factors, comorbidity was associated with pretreatment cognitive impairment in patient cases but not controls is intriguing. There are several potential explanations for this result. First, comorbidity may be a marker for chronic inflammation. There have been some reports of associations between proinflammatory cytokine signaling biomarkers and cognitive impairment after systemic cancer therapy.6,82,83 Another explanation is that comorbid illnesses such as diabetes or cardiovascular disease directly increase the risk of both cancer and cognitive impairment.81 Comorbidity may also be a marker of common underlying processes related to aging, cancer development, or frailty or of specific diseases that may accelerate aging.84 One such indicator may be insulin resistance, which has been suggested as a risk factor for dementia85 and cancer.86,87 Our study was not designed to elucidate or test these mechanisms. However, this will be an important area for further research, because older women generally have several comorbid illnesses.88–91
The result that older age, nonwhite race, and lower education increased the odds of having cognitive impairment was not unexpected. These demographic variables may help to identify individual older women at risk of cognitive problems, especially if considered within the context of a geriatric assessment.92 We were surprised that presence of any APOE e4 allele was not associated with impairment, because these genotypes have been previously related to cancer,93,94 cognitive impairment, and dementia.95,96 One explanation for our null result was the exclusion of those with pre-existing cognitive impairment. It is still possible that APOE e4 represents a marker for vulnerability to adverse cognitive effects of cancer systemic therapies among those meeting study entrance criteria. We will test this in longitudinal follow-up.
Despite the rigor of our design, there are several limitations that should be considered in interpreting the findings. First, patient cases were recruited primarily from academic centers and are not representative of all patients. However, the comparison of patient cases to well-matched noncancer controls and standardization of neuropsychological test scores to the control group enhance the internal validity of comparisons between patient cases and controls. Next, we cannot draw causal inferences about the association between comorbidity and impairment in patient cases, given the cross-sectional analysis. Also, despite the large sample, we were only powered to detect ≥ two-fold increase in the odds of impairment associated with patient case versus control status, so we cannot rule out smaller associations. However, we did have sufficient power to detect small to moderate effects of patient case versus control status for individual neuropsychological domains. Given the number of tests performed, some significant observations may have resulted from chance. The low overall impairment rate observed may reflect eligibility screening and the high education level in our sample. To the extent that education protects against cognitive decline, our estimates of impairment are likely to be conservative and lower than in the general population of older women. It would have also been interesting to look at our results by stage 0 versus invasive cancer, but the small number of patient cases with stage 0 disease precluded this analysis.
In summary, our results do not support an effect of cancer on pretreatment cognition among older patients with breast cancer. However, the findings do suggest that subgroups of older patients with breast cancer defined by greater tumor or comorbidity burden may be more susceptible to impairment before systemic therapy. It will be important to confirm these results, examine the biologic underpinnings of these observations, and observe the cohort longitudinally to fully understand how aging, cancer, and cancer treatments affect cognitive outcomes. Understanding at-risk groups could ultimately suggest avenues for intervention research to avoid adverse cognitive effects of breast cancer systemic treatments.
Acknowledgment
We thank the participants in the TLC (Thinking and Living With Cancer) study for sharing their time and experiences; without their generosity, this study would not have been possible. We also thank the following TLC study staff who contributed by ascertaining, enrolling, and interviewing participants: Trina McClendon, Elana Cooke, Julia Fallon, Maria Farberov, Alyssa Hoekstra, Mallory Cases, Maria Gomez, Olivia O'Brian, Renee Ornduff, Chie Akiba, Rupal Ramani, and Laura Zavala. We acknowledge the support of Irene Simpkin, PhD, and the staff at the Genetics Core Laboratory at Boston University as well as the support of Linda Abularch and Meenakshi Chivukula in processing samples. We also acknowledge the assistance of Adrienne Ryans in manuscript preparation.
Appendix
Table A1.
Raw Baseline Pretreatment Cognitive Performance z Scores for Older Patients With Breast Cancer and Noncancer Controls Based on Neuropsychological Testing
| Test | Controls (n = 182) |
Patient Cases |
P |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 164) |
Stages 0 to I (n = 106) |
Stages II to III (n = 58) |
Control v Patient Case | Control v Patient Case Stages 0 to I | Control v Patient Case Stages II to III | Patient Case Stages 0 to I v II to III | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Attention, working memory, and processing speed domain | 0.00 | 0.66 | −0.03 | 0.65 | 0.02 | 0.61 | −0.11 | 0.72 | .699 | .815 | .276 | .227 |
| NAB digits forwarda | 8.34 | 2.34 | 8.23 | 2.34 | 8.41 | 2.33 | 7.91 | 2.35 | .682 | .805 | .235 | .199 |
| NAB digits backwarda | 4.49 | 2.17 | 4.46 | 2.10 | 4.52 | 2.20 | 4.34 | 1.90 | .890 | .911 | .651 | .613 |
| Trail making A, time in seconds | 36.79 | 14.30 | 37.30 | 14.26 | 36.90 | 11.99 | 38.04 | 17.76 | .738 | .947 | .584 | .624 |
| Digit symbol testb | 52.73 | 11.18 | 52.15 | 12.02 | 53.62 | 11.33 | 49.45 | 12.85 | .640 | .517 | .062 | .033 |
| NAB driving scenesc | 46.55 | 6.78 | 46.65 | 6.74 | 46.44 | 7.05 | 47.05 | 6.15 | .888 | .895 | .620 | .583 |
| Language domain | 0.00 | 0.85 | −0.07 | 0.82 | −0.06 | 0.76 | −0.09 | 0.91 | .410 | .531 | .469 | .813 |
| Boston naming testd | 26.60 | 3.30 | 26.38 | 3.00 | 26.39 | 2.76 | 26.38 | 3.43 | .528 | .577 | .662 | .988 |
| Category fluencye | 21.27 | 5.02 | 20.85 | 4.91 | 20.96 | 4.88 | 20.66 | 4.99 | .438 | .614 | .418 | .703 |
| Executive functioning domain | 0.00 | 0.67 | −0.12 | 0.64 | −0.05 | 0.56 | −0.25 | 0.77 | .091 | .507 | .020 | .065 |
| Trail making B, time in seconds | 85.77 | 50.62 | 92.51 | 53.39 | 86.83 | 47.21 | 102.90 | 62.27 | .229 | .861 | .035 | .065 |
| Controlled oral word association testf | 43.83 | 13.87 | 40.28 | 12.46 | 40.97 | 12.40 | 39.02 | 12.58 | .013 | .081 | .019 | .338 |
| NAB figure drawing organizationg | 7.96 | 1.82 | 8.01 | 1.81 | 8.09 | 1.79 | 7.86 | 1.84 | .796 | .549 | .719 | .433 |
| Learning and memory domain | 0.00 | 0.78 | −0.04 | 0.79 | −0.06 | 0.79 | −0.00 | 0.78 | .620 | .502 | .996 | .620 |
| Logical memory Ih | 13.33 | 3.74 | 13.20 | 3.89 | 13.18 | 3.97 | 13.22 | 3.77 | .743 | .748 | .852 | .944 |
| Logical memory IIi | 11.92 | 4.04 | 12.10 | 4.23 | 11.86 | 4.24 | 12.55 | 4.23 | .686 | .895 | .309 | .317 |
| NAB list A immediate recallj | 23.43 | 4.91 | 22.88 | 5.16 | 22.57 | 4.99 | 23.45 | 5.45 | .305 | .151 | .985 | .296 |
| NAB list B immediate recallk | 4.58 | 1.96 | 4.55 | 1.74 | 4.57 | 1.60 | 4.53 | 1.98 | .912 | .961 | .886 | .912 |
| NAB list A short delay recalll | 7.58 | 2.52 | 7.46 | 2.59 | 7.44 | 2.69 | 7.48 | 2.42 | .650 | .660 | .792 | .926 |
| NAB long delaym | 7.86 | 2.54 | 7.64 | 2.50 | 7.62 | 2.54 | 7.67 | 2.45 | .425 | .451 | .627 | .903 |
| Visual-spatial domain | 0.00 | 1.00 | 0.09 | 0.97 | 0.04 | 1.04 | 0.17 | 0.84 | .419 | .754 | .237 | .403 |
| NAB figure drawing copyn | 25.95 | 4.19 | 26.31 | 4.08 | 26.11 | 4.35 | 26.79 | 3.51 | .419 | .754 | .237 | .403 |
NOTE. Patient case domain scores standardized using control mean and SD as referent standard. With the exception of Trail making A and B, higher raw scores reflect better performance.
Abbreviations: NAB, Neuropychological Assessment Battery; SD, standard deviation.
No. of sequences correctly recalled.
No. of symbols produced in allowed time.
No. of new or missing items correctly identified.
No. of objects correctly named.
No. of animals named in allowed time.
No. of words given in allowed time.
Sum of fragmentation and planning scores.
No. of pieces of story recalled immediately.
No. of pieces of story recalled after delay.
Sum of three trials of word recall.
No. of words recalled.
No. of words recalled after short delay.
No. of words recalled after long delay.
Accuracy of figure copy.
Table A2.
Standardized Baseline Pretreatment Neuropsychological Test Results for Older Patients With Breast Cancer
| Test | Unadjusted Standardized z Score* |
Adjusted Standardized z Score† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stages 0 and I |
Stages II and III |
P | Stages 0 and I |
Stages II and III |
P | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Attention, working memory, and processing speed domain | 0.02 | 0.06 | −0.11 | 0.09 | .23 | 0.14 | 0.11 | 0.03 | 0.11 | .25 |
| NAB digits forward | 0.03 | 0.10 | −0.18 | 0.13 | .20 | 0.30 | 0.19 | 0.10 | 0.20 | .23 |
| NAB digits backward | 0.01 | 0.09 | −0.07 | 0.13 | .61 | 0.01 | 0.19 | −0.02 | 0.20 | .85 |
| Trail making A | −0.01 | 0.10 | −0.09 | 0.13 | .62 | 0.14 | 0.18 | 0.05 | 0.19 | .56 |
| Digit symbol test | 0.08 | 0.10 | −0.29 | 0.14 | .03 | 0.14 | 0.19 | −0.14 | 0.19 | .10 |
| NAB driving scenes | −0.02 | 0.10 | 0.07 | 0.13 | .58 | 0.11 | 0.18 | 0.16 | 0.19 | .74 |
| Language domain | −0.06 | 0.08 | −0.09 | 0.11 | .81 | 0.13 | 0.14 | 0.14 | 0.14 | .80 |
| Boston naming test | −0.06 | 0.09 | −0.07 | 0.12 | .99 | 0.16 | 0.15 | 0.20 | 0.16 | .74 |
| Category fluency | −0.06 | 0.10 | −0.12 | 0.13 | .70 | 0.09 | 0.18 | 0.09 | 0.18 | .96 |
| Executive function domain | −0.05 | 0.06 | −0.25 | 0.08 | .06 | 0.05 | 0.12 | −0.16 | 0.12 | .05 |
| Trail making B | −0.02 | 0.10 | −0.34 | 0.14 | .07 | 0.14 | 0.19 | −0.18 | 0.19 | .054 |
| Controlled oral word association test | −0.21 | 0.09 | −0.35 | 0.12 | .34 | −0.12 | 0.17 | −0.26 | 0.18 | .34 |
| NAB figure drawing | 0.07 | 0.10 | −0.05 | 0.13 | .43 | 0.11 | 0.19 | −0.04 | 0.20 | .38 |
| Learning and memory domain | −0.06 | 0.08 | −0.00 | 0.10 | .62 | −0.08 | 0.14 | 0.06 | 0.14 | .26 |
| Logical memory I | −0.04 | 0.10 | −0.03 | 0.14 | .94 | −0.12 | 0.20 | −0.00 | 0.21 | .50 |
| Logical memory II | −0.02 | 0.10 | 0.16 | 0.14 | .32 | −0.17 | 0.20 | 0.11 | 0.20 | .12 |
| NAB list A immediate recall | −0.18 | 0.10 | 0.00 | 0.14 | .30 | −0.12 | 0.18 | 0.16 | 0.18 | .07 |
| NAB list B immediate recall | −0.01 | 0.09 | −0.02 | 0.12 | .91 | 0.17 | 0.16 | 0.20 | 0.16 | .84 |
| NAB list A short delay recall | −0.06 | 0.10 | −0.04 | 0.14 | .93 | −0.12 | 0.18 | −0.06 | 0.19 | .71 |
| NAB long delay | −0.09 | 0.10 | −0.07 | 0.13 | .90 | −0.09 | 0.18 | −0.04 | 0.18 | .72 |
| Visual-spatial domain | 0.04 | 0.09 | 0.17 | 0.13 | .40 | 0.05 | 0.19 | 0.24 | 0.20 | .24 |
| NAB figure drawing copy | 0.04 | 0.09 | 0.17 | 0.13 | .40 | 0.05 | 0.19 | 0.24 | 0.20 | .24 |
Abbreviation: NAB, Neuropychological Assessment Battery.
Patient case scores standardized using control mean and standard deviation as referent standard.
Adjusted for age, race (white v nonwhite), education (in years), recruitment site, and surgery (mastectomy and lumpectomy).
Footnotes
Supported by National Institutes of Health (NIH) National Cancer Institute (NCI) Grant No. R01CA129769 and in part by NIH NCI Grants No. U10 CA 84131, R01CA 127617, and K05CA096940 (J.S.M.); NIH NCI Grant No. P30CA51008 to Lombardi Comprehensive Cancer Center (J.S.M., J.V.M.); Clinical and Molecular Epidemiology (now Non Therapeutic Subject Registry) and Biostatistics and Bioinformatics Shared Resources at Lombardi Comprehensive Cancer Center, funded by NIH NCI Grant No. P30CA51008; NIH Clinical and Translational Science Award No. UL1 TR000101 to Georgetown and Howard Universities; NIH Grant No. P30-AG13846 to Boston University Alzheimer's Disease Center (R.A.S.); and NIH NCI Grants No. P30AG10133, R01 AG19771, and R01 LM01136 (A.S.).
Presented in part at the Annual Meeting of the International Society of Geriatric Oncology, Copenhagen, Denmark, October 24-26, 2013.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Robert A. Stern, Athena Diagnostics (C); Arti Hurria, Seattle Genetics (C), Genentech (C); Tiffany A. Traina, Genentech (C), Celgene (C), Merck (C), Eisai (C), ProStrakan (C) Stock Ownership: None Honoraria: Claudine Isaacs, Genentech Research Funding: Arti Hurria, GlaxoSmithKline, Abraxis BioScience, Celgene; Tiffany A. Traina, Medivation, AstraZeneca, Eisai, ZIOPHARM Oncology, Janssen Pharmaceuticals, Genentech, Novartis Expert Testimony: None Patents, Royalties, and Licenses: Robert A. Stern, Neuropsychological Assessment Battery Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jeanne S. Mandelblatt, Robert A. Stern, Tim Ahles
Collection and assembly of data: Jeanne S. Mandelblatt, Meghan McGuckin, Jonathan D. Clapp, Claudine Isaacs, Neelima Denduluri, Brandon Gavett, Patricia Johnson, John W. Van Meter, Tim Ahles
Data analysis and interpretation: Jeanne S. Mandelblatt, Robert A. Stern, Gheorghe Luta, Jonathan D. Clapp, Arti Hurria, Paul B. Jacobsen, Leigh Anne Faul, Claudine Isaacs, Tiffany A. Traina, Rebecca A. Silliman, R. Scott Turner, Darlene Howard, John W. Van Meter, Andrew Saykin, Tim Ahles
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Breast cancer treatment guidelines. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast.
- 3.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J Clin Oncol. 28:3784–3796. doi: 10.1200/JCO.2009.26.3756. 2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender CM. Chemotherapy may have small to moderate negative effects on cognitive functioning. Cancer Treat Rev. 2006;32:316–319. doi: 10.1016/j.ctrv.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(suppl):S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins B, MacKenzie J, Tasca GA, et al. Cognitive effects of chemotherapy in breast cancer patients: A dose-response study. Psychooncology. 2013;227:1517–1527. doi: 10.1002/pon.3163. [DOI] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 9.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 10.Koppelmans V, Breteler MM, Boogerd W, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 11.Eberhardt B, Dilger S, Musial F, et al. Short-term monitoring of cognitive functions before and during the first course of treatment. J Cancer Res Clin Oncol. 2006;132:234–240. doi: 10.1007/s00432-005-0070-8. [DOI] [PubMed] [Google Scholar]
- 12.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment–associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen SJ, Otten W, Baas-Thijssen MC, et al. Explaining differences in attitude toward adjuvant chemotherapy between experienced and inexperienced breast cancer patients. J Clin Oncol. 2005;23:6623–6630. doi: 10.1200/JCO.2005.07.171. [DOI] [PubMed] [Google Scholar]
- 15.Anderson-Hanley C, Sherman ML, Riggs R, et al. Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 16.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 17.Stewart A, Bielajew C, Collins B, et al. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20:76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 18.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognititve deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 19.Phillips KA, Aldridge J, Ribi K, et al. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1-98 trial. Breast Cancer Res Treat. 2011;126:221–226. doi: 10.1007/s10549-010-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermelink K, Henschel V, Untch M, et al. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: Results of a multicenter, prospective, longitudinal study. Cancer. 2008;113:2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer VA, Merkle EC, Fagerlin A, et al. Chemotherapy was not associated with cognitive decline in older adults with breast and colorectal cancer: Findings from a prospective cohort study. Med Care. 2012;50:849–855. doi: 10.1097/MLR.0b013e31825a8bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ording AG, Jensen AB, Cronin-Fenton D, et al. Null association between tamoxifen use and dementia in Danish breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2013;22:993–996. doi: 10.1158/1055-9965.EPI-13-0139. [DOI] [PubMed] [Google Scholar]
- 23.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wefel JS, Lenzi R, Theriault R, et al. “Chemobrain” in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 25.Scherling C, Collins B, Mackenzie J, et al. Prechemotherapy differences in response inhibition in breast cancer patients compared to controls: A functional magnetic resonance imaging study. J Clin Exp Neuropsychol. 2012;34:543–560. doi: 10.1080/13803395.2012.666227. [DOI] [PubMed] [Google Scholar]
- 26.Cimprich B, Reuter-Lorenz P, Nelson J, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 27.Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: Promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- 28.Cimprich B, So H, Ronis DL, et al. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen LS, Johnson T, Kuipers HM, et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson T, Monk T, Rasmussen LS, et al. Posteroperative cognitive dysfunction in middle aged patients. Anesthesiology. 2002;96:1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Moretti R, Torre P, Antonello RM, et al. Vitamin B12 and folate depletion: Clinical evidence in a neurological population. Neurologist. 2004;10:338–343. doi: 10.1097/01.nrl.0000145597.28008.2e. [DOI] [PubMed] [Google Scholar]
- 32.Wefel JS, Meyers CA. Cancer as a risk factor for dementia: A house built on shifting sand. J Natl Cancer Inst. 2005;97:788–789. doi: 10.1093/jnci/dji167. [DOI] [PubMed] [Google Scholar]
- 33.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition: The case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 34.Joshi G, Aluise CD, Cole MP, et al. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: Implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blasiak J, Arabski M, Krupa R, et al. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutat Res. 2004;554:139–148. doi: 10.1016/j.mrfmmm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Laws SM, Clarnette RM, Taddei K, et al. APOE-epsilon4 and APOE -491A polymorphisms in individuals with subjective memory loss. Mol Psychiatry. 2002;7:768–775. doi: 10.1038/sj.mp.4001083. [DOI] [PubMed] [Google Scholar]
- 37.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis—APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 38.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 39.Schröder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84:1348–1353. doi: 10.1054/bjoc.2001.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velarde MC, Demaria M, Campisi J. Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol. 2013;38:17–27. doi: 10.1159/000343572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 44.Hurria A, Goldfarb S, Rosen C, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res Treat. 2006;98:343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- 45.Freilich RJ, Delattre JY, Monjour A, et al. Chemotherapy without radiation therapy as initial treatment for primary CNS lymphoma in older patients. Neurology. 1996;46:435–439. doi: 10.1212/wnl.46.2.435. [DOI] [PubMed] [Google Scholar]
- 46.Yamada TH, Denburg NL, Beglinger LJ, et al. Neuropsychological outcomes of older breast cancer survivors: Cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boston University School of Medicine. TLC study. http://www.bumc.bu.edu/tlcstudy/
- 48.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: Neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- 49.Head D, Snyder AZ, Girton LE, et al. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- 50.Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: New evidence from neuroimaging of the aging brain. Curr Opin Neurol. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- 51.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald BC, Conroy SK, Smith DJ, et al. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav Immun. 2013;30(suppl):S117–S125. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wefel JS, Vardy J, Ahles T, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 54.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern RA, White T. Neuropsychological Assessment Battery (NAB) Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 56.Hurria A, Lachs M. Is cognitive dysfunction a complication of adjuvant chemotherapy in the older patient with breast cancer? Breast Cancer Res Treat. 2007;103:259–268. doi: 10.1007/s10549-006-9383-9. [DOI] [PubMed] [Google Scholar]
- 57.Reitan R, Wolfson D. Tucson, AZ: Neuropsychology Press; 1985. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. [Google Scholar]
- 58.Wechsler D. Wechsler Adult Intelligence Scale (ed 3) San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- 59.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test: Experimental Edition (ed 2) Boston, MA: Kaplan and Goodglass; 1983. [Google Scholar]
- 60.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): Part I—Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 61.Benton AL. Neuropsychological assessment. Annu Rev Psychol. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs SR, Jacobsen PB, Booth-Jones M, et al. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33:13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Mandelblatt JS, Hurria A, McDonald BC, et al. Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Semin Oncol. 2013;40:709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern RA, Silva SG, Chaisson N, et al. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol. 1996;53:148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- 65.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 66.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Pscyhol Meas. 1977;1:385–401. [Google Scholar]
- 67.Dozeman E, van Schaik DJF, van Marwijk HWJ, et al. The Center for Epidemiological Studies Depression Scale (CES-D) is an adequate screening instrument for depressive and anxiety disorders in a very old population living in residential homes. Int J Geriatr Psychiatry. 2011;26:239–246. doi: 10.1002/gps.2519. [DOI] [PubMed] [Google Scholar]
- 68.Spielberger CD. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory (STAI: Form Y) [Google Scholar]
- 69.Cella D. Factors influencing quality of life in cancer patients: Anemia and fatigue. Semin Oncol. 1998;25(suppl 7):43–46. [PubMed] [Google Scholar]
- 70.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy–Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 72.Schilling V, Jenkins V, Morris R. The effects of adjuvant chemotherapy on cognition in women with breast cancer: Preliminary results of an observational study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Schilder CM, Seynaeve C, Linn SC, et al. The impact of different definitions and reference groups on the prevalence of cognitive impairment: A study in postmenopausal breast cancer patients before the start of adjuvant systemic therapy. Psychooncology. 2010;19:415–422. doi: 10.1002/pon.1595. [DOI] [PubMed] [Google Scholar]
- 74.Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog–methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Res Treat. 2006;95:125–129. doi: 10.1007/s10549-005-9055-1. [DOI] [PubMed] [Google Scholar]
- 75.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 76.Jansen CE, Cooper BA, Dodd MJ, et al. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 77.Ottati A, Feuerstein M. Brief self-report measure of work-related cognitive limitations in breast cancer survivors. J Cancer Surviv. 2013;7:262–273. doi: 10.1007/s11764-013-0275-9. [DOI] [PubMed] [Google Scholar]
- 78.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index: A self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 79.Mehlsen M, Pedersen AD, Jensen AB, et al. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psychooncology. 2009;18:248–257. doi: 10.1002/pon.1398. [DOI] [PubMed] [Google Scholar]
- 80.Schilder CM, Seynaeve C, Linn SC, et al. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: Findings from the neuropsychological TEAM side-study. Psychooncology. 2012;21:479–487. doi: 10.1002/pon.1928. [DOI] [PubMed] [Google Scholar]
- 81.Schilder CM, Seynaeve C, Linn SC, et al. Cognitive functioning of postmenopausal breast cancer patients before adjuvant systemic therapy, and its association with medical and psychological factors. Crit Rev Oncol Hematol. 2010;76:133–141. doi: 10.1016/j.critrevonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7:511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 85.van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer's disease: Results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arcidiacono B, Iiritano S, Nocera A, et al. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp Diabetes Res. doi: 10.1155/2012/789174. [epub ahead of print on June 4, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12. doi: 10.1186/1758-5996-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parekh AK, Barton MB. The challenge of multiple comorbidity for the US health care system. JAMA. 2010;303:1303–1304. doi: 10.1001/jama.2010.381. [DOI] [PubMed] [Google Scholar]
- 89.Chang SS, Weiss CO, Xue QL, et al. Patterns of comorbid inflammatory diseases in frail older women: The Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65:407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salive ME. Multimorbidity in older adults. Epidemiol Rev. doi: 10.1093/epirev/mxs009. [epub ahead of print on January 31, 2013] [DOI] [PubMed] [Google Scholar]
- 91.Yancik R, Ries LA. Cancer in older persons: An international issue in an aging world. Semin Oncol. 2004;31:128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 92.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 93.Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer's disease: Results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saadat M. Apolipoprotein E (APOE) polymorphisms and susceptibility to breast cancer: A meta-analysis. Cancer Res Treat. 2012;44:121–126. doi: 10.4143/crt.2012.44.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bishop NA, Lu T, Yankner BA. Neural mechanisms of aging and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brainerd CJ, Reyna VF, Petersen RC, et al. Is the apolipoprotein e genotype a biomarker for mild cognitive impairment? Findings from a nationally representative study. Neuropsychology. 2011;25:679–689. doi: 10.1037/a0024483. [DOI] [PubMed] [Google Scholar]