Abstract
Objectives
Nicotine is a natural alkaloid and insecticide in tobacco leaves. Green tobacco sickness (GTS) is known as a disease of acute nicotine intoxication among tobacco farmers. Until now, GTS has been recognized globally as a disease that results from nicotine absorption through the skin. However, we assumed that GTS might also result from nicotine inhalation as well as absorption. We aimed to measure the airborne nicotine concentrations in various work environments of Korean tobacco farmers.
Methods
We measured the nicotine concentrations in the tobacco fields, private curing barns, and joint curing barns of farmers from July to October 2010. All sampling and analyses of airborne nicotine were conducted according to the National Institute for Occupational Safety and Health manual of analytic methods.
Results
The airborne nicotine concentrations (geometric mean [geometric standard deviation]) in the tobacco field were 83.4 mg/m3 (1.2) in the upper region and 93.3 mg/m3 (1.2) in the lower region. In addition, the nicotine concentration by personal sampling was 150.1 mg/m3. Similarly, the nicotine concentrations in the private curing barn, workers in curing barns, the front yard of the curing barn, and in the joint curing barn were 323.7 mg/m3 (2.0), 121.0 mg/m3 (1.5), 73.7 mg/m3 (1.7), and 610.3 mg/m3 (1.0), respectively.
Conclusions
The nicotine concentration in the workplaces of tobacco farmers was very high. Future studies should measure the environmental concentration of nicotine that is inhaled by tobacco farmers.
Keywords: Nicotine, Inhalation, Poisoning, Green tobacco sickness
INTRODUCTION
Nicotine is well known for its relationship with smoking, secondhand smoke, and environmental tobacco smoke; however, the fact that nicotine is a naturally alkaloid and insecticide in tobacco leaves is less widely discussed [1,2]. Green tobacco is cultivated globally in more than 100 countries. The worldwide production of green tobacco was estimated at about 5.93 million tons annually. Green tobacco is grown in China (39.5%), Brazil (7.2%), India (8.7%), the US (9.0%) as well as other countries, but these four countries occupy 65% or more of total production [3]. In Korea, an estimated 10 000 tobacco farmers actively grow tobacco, and approximately 7.0 million kg of tobacco leaves were produced in 2013 [4].
However, there is health risks associated with tobacco farming. Tobacco farmers can be exposed to nicotine through skin absorption or inhalation of airborne nicotine. Nicotine is a major causal factor of headache, dizziness, nausea, and vomiting as well as a high respiratory rate, heart rate, and blood pressure because the liquid alkaloid concentration in the tobacco leaves is typically 1% to 6%. Absorbed nicotine directly affects the central nervous system and raises the gag reflex [2]. The lethal dose was estimated as 40 to 60 mg (0.6 to 0.9 mg/kg), and the workplace threshold was 0.5 mg/m3 using the threshold limit value time weighted average (TLV-TWA) [5,6]. This disease is known as green tobacco sickness (GTS). Weizenecker and Deal [7] first reported this disease among tobacco farmers in 1970.
Until recently, GTS has been known globally as a disease that occurs due to the absorption of nicotine through the skin [8,9,10]. However, recent studies conducted by us [11] and others [12] have suggested that absorption may also occur through the respiratory system. Airborne nicotine concentrations in a private curing barn as well as in the tobacco field were very high; therefore, these tobacco farmers may have been exposed to nicotine during harvesting and while curing the tobacco leaves [11]. To supplement our previous study, this study was performed to measure the airborne nicotine concentrations of all processes related to tobacco farming including harvesting and curing tobacco.
METHODS
Subjects
Tobacco farmers living in Cheongsong-gun, a rural city located in Gyeongsangbuk-do, Korea were recruited for this study. We measured the nicotine concentrations in the tobacco field, private curing barn, and joint curing barn from July to October 2010. All sampling and analyses of airborne nicotine were conducted according to the National Institute for Occupational Safety and Health manual of analytic methods (method number 2551) [13]. Sampling areas were designated at 23 points in the tobacco field, 30 points in the private curing barn, 3 points in the joint curing barn, and 20 points in the local schoolyard, which served as the control. The sampling in the tobacco field was conducted simultaneously with personal sampling in one farmer. The sampling in the curing barn of tobacco leaves was conducted simultaneously with personal sampling in 10 farmers, and area sampling at 10 front yards in private curing barn. The institutional review board approved this study.
Sampling and Analysis
The authors sampled each area using XAD-4 sorbent tubes (80/40 mg; SKC, Eighty Four, PA, USA) and personal air samplers (GilAir-3; Sensidyne, St. Petersburg, FL, USA), and all samples were collected at 0.5 L/min flow rate. In addition, a correction for personal air samplers was conducted before and after sampling using a flow calibration system (Gilibrator-2; Sensidyne). Sampling in the tobacco field was conducted in the upper and lower regions of the tobacco tree separately; the upper region was 15 to 20 cm from the upper leaves, and the lower region was 30 cm from the ground. Samples in the tobacco field were collected within 180 to 280 minutes, except air sampling in one tobacco farm lasted only 120 minutes (Figure 1). Sampling in the joint curing barn was conducted at 1.5 m from the ground for 150 minutes (Figure 2), whereas sampling in the private curing barn was conducted in the barn and in the front yard outside the curing rooms for 120 minutes, respectively. The collected samples were kept away from sunlight, wrapped in foil, and then refrigerated. Sampling temperature and humidity, and were measured using appropriate equipment, and wind speed was measured with the VelociCALC Plus (TSI, Shoreview, MN, USA) hot wire anemometer.
Figure 1.
Samplers used to measure airborne nicotine concentrations in the tobacco field (A) and on the farmer (B). (a) Upper region, (b) lower region, (c) air sampler.
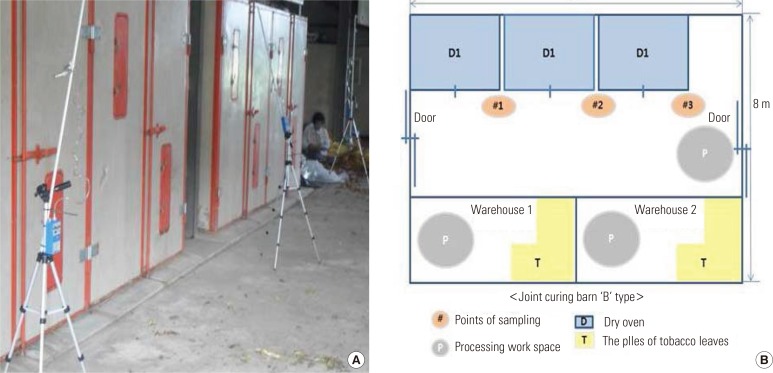
Figure 2.
Measurements of airborne nicotine concentrations in the joint curing barn. (A) Joint curing barn, (B) joint curing barn layout
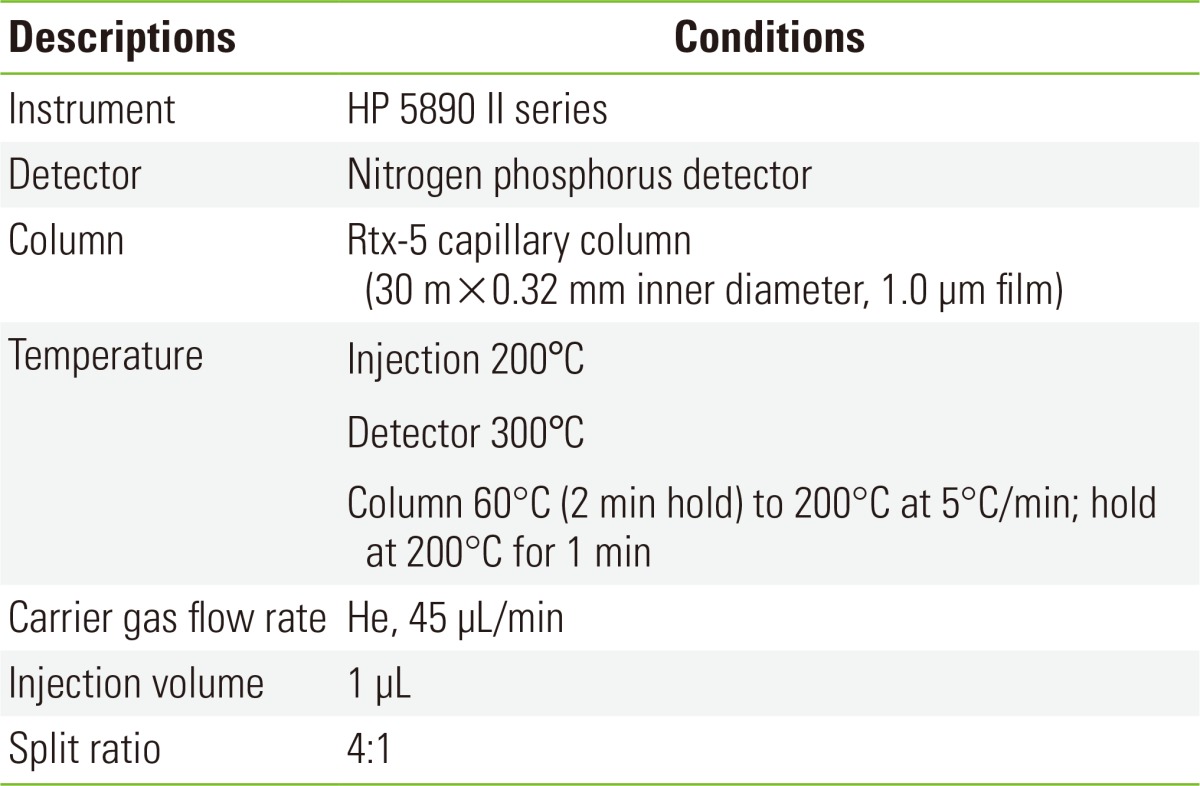
The front and back sections of each XAD-4 tube were desorbed separately in 1 mL of modified ethyl acetate. After the desorption solvent and quinoline internal standard were added, all samples were allowed to desorb for at least 30 minutes before analysis. To improve the ratio of nicotine to quinoline during quantitation, 10 µL of the quinoline secondary standard was used as the internal standard. The samples were then analyzed by a gas chromatography-nitrogen phosphorous detector (HP5890 II; Hewlett Packard, Wilmington, DE, USA) equipped with a 30-M RTX-5 (0.32 mm inner diameter, 1.0 µm film) fused silica capillary column. The separation of the analyte was achieved using the temperature program reported in Table 1. Nicotine analysis was performed using in the regression equation Y=10.67502X-12.0759, and the correlation coefficient (R) was 0.9998. Nicotine desorption efficiency was verified at the concentrations 5, 10, 20, 50, and 100 µg/mL by adding nicotine, and the average desorption efficiency was 89.8%. The detection limit was 3.3×standard deviation=0.1061 µg/mL.
Table 1.
The operating conditions of gas chromatography
RESULTS
Nicotine Concentrations in the Tobacco Fields
The average concentration of nicotine in the lower region of the field (93.3 mg/m3 [1.2]) was higher than that in the upper region (83.4 mg/m3 [1.3]), but the difference was not significant. The nicotine concentration of one farmer by personal sampling was found to be 150.1 mg/m3. The range of nicotine concentrations in the upper and lower regions of the sampled fields were 56.6 to 125.7 mg/m3 and 65.6 to 166.2 mg/m3, respectively (Table 2).
Table 2.
Nicotine concentrations in the tobacco fields
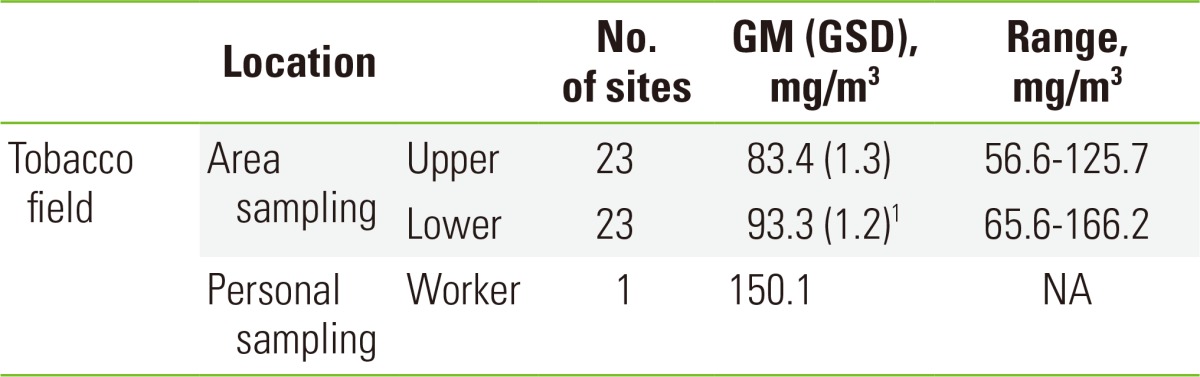
GM, geometric mean; GSD, geometric standard deviation; NA, not applicable. 1p=0.087 by Mann-Whitney U-test between the upper and lower regions.
Nicotine Concentrations in the Private Curing Barns and Joint Curing Barns
The average nicotine concentrations at the 30 points in the private curing barns and the 10 points in the yards of the private curing barns were 323.7 mg/m3 (2.0), 73.7 mg/m3 (1.7) respectively. Moreover, the average nicotine concentrations of the 10 farmers during working was 121.0 mg/m3 (1.5). At the 3 points in the joint curing barn, the average concentration was 610.3 mg/m3 (1.0) (Table 3).
Table 3.
The nicotine concentrations in the curing barns and the schoolyard (control)
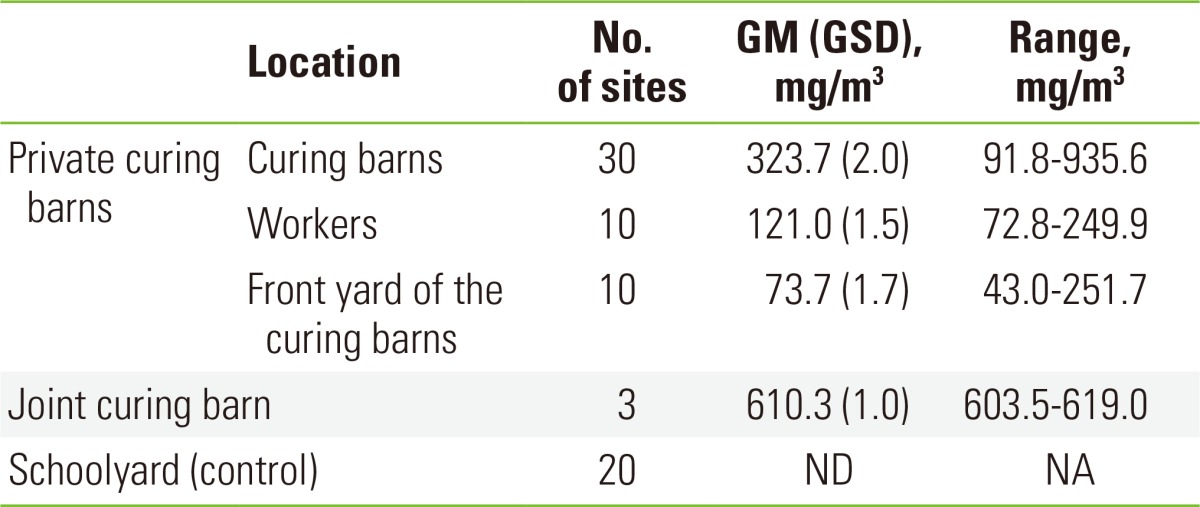
GM, geometric mean; GSD, geometric standard deviation; ND, not detectable; NA, not applicable.
DISCUSSION
This study expands the findings of our previous study [11]. In our previous study, we measured only the nicotine concentration in tobacco field and one private curing barn. The number of samples was insufficient and the lack of a control group was a major limitation. Therefore, in this study, we measured the nicotine concentration at each step in the tobacco farming process including harvesting and curing tobacco leaves. In addition, we increased the number of samples, and compared our findings with a control group by collecting samples in the tobacco fields (lower and upper regions), private curing barns, joint curing barns, the front yard of the private curing barns, and in the schoolyard. By measuring the nicotine concentration in various points, we were able to understand when tobacco farmers are exposed to nicotine throughout all of the process required in tobacco farming. This information also provides insight as to whether preventive measures or strategies should be performed differently or more specifically in each area according to the concentration of nicotine.
Generally, nicotine exposure has been measured according to the level of environmental tobacco smoke, and measurements of nicotine exposure during tobacco farming are not well known. The nicotine concentrations in our previous study were 43.4 mg/m3 (1.4) in tobacco field and 224.4 mg/m3 (1.2) in curing barn and were similar to the concentrations in this study. In another recent study of Korean tobacco farmers, the nicotine concentrations by area sampling were 0.8 µg/m3 (2.0) during harvesting and 246.7 µg/m3 (1.4) during weaving [12]. In addition, in a study performed outside of Korea, the nicotine concentrations were from 50 to 150 µg/m3 in the post tobacco curing process [14], which are very low level compared to our results. It is possible that airborne nicotine concentration is affected by various conditions including wind or a ventilation system as well as the methods and periods of measurement, which could explain these discrepancies. The measurement in previous studies might have been conducted when few tobacco leaves were present in the field, and the curing barn may have had several windows. In our previous study, we found the nicotine concentrations in the sampled tobacco fields to vary over different periods [11]. We suppose these differences may because the nicotine concentration in the leaves may change as they mature [15]. In addition, almost all farmers from previous studies worked outside of their barns such as in the road or in their yards. In the latter case, airborne dust present in the yard was collected, and then the nicotine concentration within the dust was measured. Therefore, it may be difficult to compare our findings directly with those of previous studies. Another study measured the nicotine concentration of a bus experimentally exposed to 78 cigarettes over a 2 hour period was 110 mg/m3 [16]. However, almost all nicotine concentrations of environmental tobacco smoke have been measured under 0.5 mg/m3 [17,18]. Moreover, the nicotine concentrations of these studies vary substantially according to the country, place of measurement, and smoking-related policies in the public facilities.
In the present study, the nicotine concentrations in the tobacco fields were a thousand fold compared with other studies and a hundred fold of the TLV-TWA (0.5 mg/m3). Interestingly, the nicotine concentration of one farmer's field while harvesting tobacco leaves was 150.1 mg/m3; this concentration is similar to the average concentration (120.1 mg/m3) of nicotine in the private curing barns. In addition, the nicotine concentrations of the private curing barns and joint curing barns were 600 to 1000 folds higher than that of the exposure threshold measured in the workplace. Therefore, farmers working in their private curing barn or joint curing barn might be exposed to a level of nicotine that considerably exceeds the fatal concentration. According to the American Conference of Industrial Hygienists recommendation, a considerably fatal exposure should be anticipated in these environments [6]. However, despite the high concentration of nicotine exposure, few fatalities have been reported [19]. One reason for this could be that all of the measured nicotine in the environment is not absorbed by the farmers. Even if a high amount of nicotine is absorbed, of the farmers may be resist to nicotine, have different levels of susceptibility, and different smoking habits, which may influence the occurrence of symptoms. In addition, GTS symptoms including nausea, vomiting, headaches, and dizziness typically occur before a fatal condition; therefore, tobacco farmers tend to stop working and subsequently decrease their exposure to nicotine.
In this study, no significant different was found between the nicotine concentration of the upper and lower regions of the tobacco fields; however, we believe that the nicotine concentration was higher in lower region than in the upper region. The upper region is more open and may be exposed to more wind than the lower region is. In addition, the high nicotine concentration found in the tobacco fields may have been due to high levels of heat during measurement, which would have increased the amount of vaporization. For example, moisture present in the morning may evaporate alongside any present nicotine [20]. Therefore, when we measured the nicotine concentration in the tobacco fields, we considered the effects of weather such as moisture and wind velocities. Nevertheless, detailed analysis of each weather condition was not possible because of the small sample size.
In most private curing barns, only one gate door and one ventilation window are available to circulate the air. We believe that this ventilation system is insufficient and more should be added to the ventilation systems. However, during curing, tobacco leaves must be stored at a proper humidity; therefore, a ventilation system that does not decrease the humidity in the curing barn is needed. These facts should be considered when installing ventilation systems in barns used for curing tobacco.
GTS in South Korea is common during the harvesting season (from June to August). However, according to our results that indicate the nicotine concentration in the yard during tobacco curing was 73.7 mg/m3, we think that GTS may occur until the end of October. Thus, tobacco farmers may be continuously exposed to high concentrations of nicotine for 5 to 6 months a year.
We were not able to measure the concentration of metabolites to estimate actual nicotine exposure among the tobacco farmers because nicotine disappears rapidly from the blood, with a half-life of 2 to 3 hours in humans; however, cotinine is a well-known biomarker of nicotine absorption [21]. Generally, tobacco farmers perform many tasks related to tobacco farming; therefore, it is difficult to measure nicotine exposure throughout the day, even if cotinine is measured. Moreover, because tobacco farming is strenuous labor, it is difficult to measure nicotine concentration accurately throughout the day. Furthermore, because nicotine concentration in the environment may differ from the concentration absorbed in humans, cotinine should be measured in future studies to understand the relationship between one's work environment and health status [14].
In our previous study, we were the first to measure nicotine concentration in the tobacco field [11]. Measurements of nicotine concentrations in curing barns as well as tobacco fields are still not common in other countries. Understanding the history and scope of harvesting tobacco, we suspect that many GTS cases happen every year not only in Korea but also in China and India. In Korea, Lim and Lee reported the first case of GTS [22]. Since, several studies have been conducted [23,24,25,26], but more are needed worldwide [27].
Our research suggests that GTS may result from exposure to the respiratory system and skin; therefore, o nicotine concentration should be measured in the workplaces of tobacco farmers for the prevention of GTS. Furthermore, future studies are needed to investigate the main route of nicotine absorption, and personal protective equipment that protect the skin and respiratory system are needed.
Footnotes
The authors have no conflicts of interest with the material presented in this paper.
References
- 1.Soloway SB. Naturally occurring insecticides. Environ Health Perspect. 1976;14:109–117. doi: 10.1289/ehp.7614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karaconji IB. Facts about nicotine toxicity. Arh Hig Rada Toksikol. 2005;56(4):363–371. [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations. Food and agricultural commodities production-tobacco, unmanufactured. [cited 2014 Mar 13]. Available from: http://faostat.fao.org/site/339/default.aspx.
- 4.Korea Tobacco Growers Organization. In 2013, the status of tobacco leaf contract in Korea. [cited 2013 Nov 25]. Available from: http://ktgo.or.kr/04/07.asp (Korean)
- 5.Ellenhorn MJ, Schonwald S, Ordog G, Wasserberger J. Natural toxins. In: Ellenhorn MJ, editor. Ellenhorn's medical toxicology: diagnosis and treatment of human poisoning. 2nd ed. Baltimore: Williams and Wilkins; 1997. pp. 912–917. [Google Scholar]
- 6.American Conference of Governmental Industrial Hygienists. Documentation of the threshold limit values and biological exposure indices. 7th ed. Cincinnati: American Conference of Governmental Industrial Hygienists; 2007. p. 43. [Google Scholar]
- 7.Weizenecker R, Deal WB. Tobacco cropper's sickness. J Fla Med Assoc. 1970;57(12):13–14. [PubMed] [Google Scholar]
- 8.Hipke ME. Green tobacco sickness. South Med J. 1993;86(9):989–992. doi: 10.1097/00007611-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Arcury TA, Quandt SA, Preisser JS, Bernert JT, Norton D, Wang J. High levels of transdermal nicotine exposure produce green tobacco sickness in Latino farmworkers. Nicotine Tob Res. 2003;5(3):315–321. doi: 10.1080/1462220031000094132. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh SK, Gokani VN, Doctor PB, Parikh JR. Intervention studies against "green symptoms" among Indian tobacco harvesters. Arch Environ Health. 1991;46(5):316–317. doi: 10.1080/00039896.1991.9934396. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Kim JS, Kim JS, Lee K, Lim HS. Airborne nicotine concentrations in harvesting and the processing of tobacco leaves. J Korean Soc Occup Environ Hyg. 2010;20(1):47–52. (Korean) [Google Scholar]
- 12.Kim HC, Lee KS, Chae HS, Park YS, Min KD. A research on airborne nicotine exposure during harvest and weaving tasks in tobacco farms. J Korean Soc Occup Environ Hyg. 2012;22(3):217–223. (Korean) [Google Scholar]
- 13.National Institute for Occupational Safety and Health (NIOSH) NIOSH Manual of Analytical Methods (NMAM), 4th edition. Nicotine: methods 2551, issue 1. [cited 2014 Mar 13]. Available from: http://www.cdc.gov/niosh/nmam/pdfs/2551.pdf.
- 14.Trikunakornwongs A, Kongtip P, Chantanakul S, Yoosook W, Loosereewanich P, Rojanavipart P. Assessment of nicotine inhalation exposure and urinary cotinine of tobacco processing workers. J Med Assoc Thai. 2009;92(Suppl 7):S121–S127. [PubMed] [Google Scholar]
- 15.Jeong KT, Kim SB, Cho SH, Bock JY, Lee JR. Effect of soil factors, cultural practices and climatic conditions on some chemical components of flue-cured tobacco. J Korean Soc Tobacco Sci. 2006;28(1):17–22. (Korean) [Google Scholar]
- 16.Willers S, Skarping G, Dalene M, Skerfving S. Urinary cotinine in children and adults during and after semiexperimental exposure to environmental tobacco smoke. Arch Environ Health. 1995;50(2):130–138. doi: 10.1080/00039896.1995.9940890. [DOI] [PubMed] [Google Scholar]
- 17.Park YS, Roh Y, Kim CN. Urinary cotinine concentration by passive smoking in the PC game room. Korean J Environ Health Soc. 2002;28(1):11–20. (Korean) [Google Scholar]
- 18.Ellingsen DG, Fladseth G, Daae HL, Gjolstad M, Kjaerheim K, Skogstad M, et al. Airborne exposure and biological monitoring of bar and restaurant workers before and after the introduction of a smoking ban. J Environ Monit. 2006;8(3):362–368. doi: 10.1039/b600050a. [DOI] [PubMed] [Google Scholar]
- 19.Swinker M, Meredith JT. A seizure in the tobacco field. Green tobacco sickness. N C Med J. 2000;61(1):390–392. [PubMed] [Google Scholar]
- 20.Higgins J, Ewing PL, McGuigan HA. The effect of evaporation and radiation on nicotine solutions. J Pharmacol Exp Ther. 1931;42(2):213–216. [Google Scholar]
- 21.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 22.Lim HS, Lee K. Cases of green tobacco sickness: occupational nicotine poisoning in tobacco harvesters in Korea. Korean J Rural Med. 2001;26(2):7–14. (Korean) [Google Scholar]
- 23.Lim HS, Lee K. Green tobacco sickness on tobacco harvesters in a Korea village. Korean J Epidemiol. 2002;24(1):29–36. (Korean) [Google Scholar]
- 24.Lee JS, Bae SH, Lim HS, Lee K. Epidemiological characteristics and changes of prevalence for green tobacco sickness among Korea tobacco harvesters. Korean J Epidemiol. 2004;26(1):39–49. (Korean) [Google Scholar]
- 25.Lim HS, Lee K, Nam SH. Prevalence and risk factors of green tobacco sickness among Korean tobacco harvesters. Korean J Prev Med. 2004;37(1):37–43. (Korean) [PubMed] [Google Scholar]
- 26.Lee K, Lim HS. Prevalence and risk factors of green tobacco sickness among Korean tobacco harvesters, 2003. Korean J Epidemiol. 2005;27(1):129–139. (Korean) [PubMed] [Google Scholar]
- 27.Lee K, Lim HS. Proposal of the global network for the study of green tobacco sickness. Cien Saude Colet. 2013;18(6):1859–1860. [PubMed] [Google Scholar]