Abstract
Purpose
The aim of this study was to determine the effects of nutritional supplements on periodontal health and tooth mobility after surgery.
Methods
Patients were randomly assigned to an intervention group who consumed nutritional supplement drinks for 8 weeks, while the placebo group did not receive any such supplements. The gingival index (GI) and tooth mobility were measured at baseline and at 1, 4, and 8 weeks. In addition, the oral health impact profile-14 and anthropometric measurements along with loss of appetite and dietary intake were assessed at baseline and 8 weeks.
Results
At 1 week, GI values were reduced in the intervention group (P<0.05), and tooth mobility had increased, but to a lesser extent in the intervention group (P<0.05). At 8 weeks, the intakes of protein, vitamins A and B1, and niacin were increased in the intervention group.
Conclusions
These results demonstrate that nutritional supplementation improves early periodontal healing after surgery.
Graphical Abstract
Keywords: Nutrition therapy, Periodontal index, Periodontics, Wound healing
INTRODUCTION
Periodontitis is the most common chronic inflammatory disease, involving the inflammation and destruction of periodontal tissue, which occurs in a noticeable segment of the adult population [1,2]. Therapies for periodontal diseases routinely include cause-related therapies and nonsurgical or surgical interventions. A substantial number of patients usually suffer from discomfort following these treatments, particularly after periodontal surgery [3,4]. Among various types of discomfort, including bleeding, infection, pain, swelling, or decreased ability to masticate, the decreased masticatory function due to increased tooth mobility significantly deters the patients from continuing with adequate food consumption [5,6]. Alkan et al. [7] and Feller and Lemmer [8] reported that the mean tooth mobility peaked in the first week following periodontal surgery, and Kerry et al. [9] noted continuously increased tooth mobility at 1 month following periodontal surgery, which returned to the presurgical level only after 1 year. Therefore, the discomfort and impaired masticatory function due to hypermobile teeth can limit food choices and decrease the quality of life (QoL) over a significant period of time in patients subjected to periodontal treatment [10].
It is well established that adequate host nutrition is important to maintain resistance to periodontal disease [11,12], and has beneficial effects on the recovery from periodontal treatment [13,14]. In addition, specific nutrient deficiencies might lead to a disturbed wound-healing process, and prolonged nutrient deficiency could increase the risk of wound-related complications [15]. The provision of adequate nutrition following periodontal surgery may thus enhance the postsurgical healing process and minimize the associated discomfort by minimizing the tooth mobility.
Nutritional supplement drinks have been acknowledged as one of the greatest medical advances, providing essential nutrients and energy to patients who are unable to chew or digest regular food. Supplemental feeding serves to provide patients who have undergone surgery with sufficient calories, vitamins, and minerals, and allows time for healing [16]. Collins et al. [17] showed that improvements in some indicators of wound healing and cognition were observed in those who received energy-, protein-, and nutrient-dense supplements. However, there have been few or possibly no investigations of the effects of enhancing the nutritional intake of patients by administering nutritional supplement drinks on periodontal healing after periodontal surgery.
To the best of our knowledge, this is the first study to investigate whether the provision of nutritional supplement drinks helps the healing process for periodontitis patients after surgery. The aims of the study were thus to determine the effects of nutritional supplement drinks on nutritional status after periodontal surgery, and to elucidate any associated changes in periodontal health and tooth mobility over time.
MATERIALS AND METHODS
Study subjects
The subjects comprised 28 periodontitis patients (18 males and 10 females, aged 30-65 years) who visited the Department of Periodontology, Yonsei University College of Dentistry, between February and June 2012 for periodontal flap surgery. The criteria for inclusion in the study were (1) generalized moderate chronic periodontitis, (2) at least 18 remaining teeth, and (3) at least 2 sites with a probing depth of ≥6 mm. The exclusion criteria were the presence of pregnancy, kidney disease, diabetes mellitus, systemic disease, or disorders affecting wound healing, as well as participation in another clinical trial within the previous 3 months.
This study was approved by the Yonsei University Hospital Institutional Review Board (No. 2-2011-0062). All patients provided written informed consent to participate in the study. The study was an 8-week intervention trial in which 28 periodontitis patients were randomly assigned by using a random number table method with no restriction. The dentist allocated the patients right after the surgery either to an intervention group, each member of which was provided with nutritional supplement drinks to consume over an 8-week period after periodontal surgery (n=17), or to a control group, to which no such supplements were provided (n=11).
Intervention by nutritional supplement drinks
A commercially available nutritional supplement drink, Nucare High Protein, was provided by Daesang Co. (Seoul, Korea) in the form of a 200-mL drink containing 200 kcal of energy, 13 g of protein (24% of daily value), 24 g of carbohydrates, 6 g of fat, vitamins (A, D, E, K, B1, B2, B6, C, niacin, folate, pantothenic acid, and biotin), and minerals (sodium, potassium, calcium, phosphorous, iron, and zinc).
The subjects in the intervention group were asked to consume three 200-mL cans of this nutritional supplement drink per day for 8 weeks between their regular meals. Patients tapered their normal food intake according to the nutritional supplement drink provided. The intervention group received the nutritional supplement drink at home every 3 weeks. Compliance was verified on every visit to the hospital via an interview with the research dietitian. The compliance rate was calculated using the average number of cans consumed daily during the intervention period.
Study design
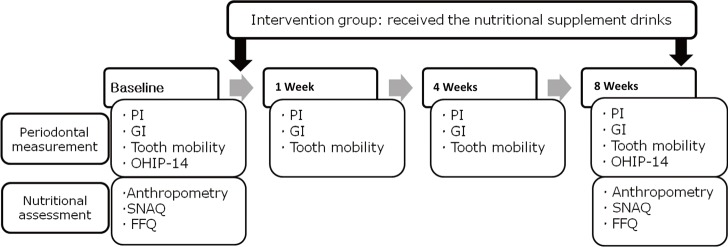
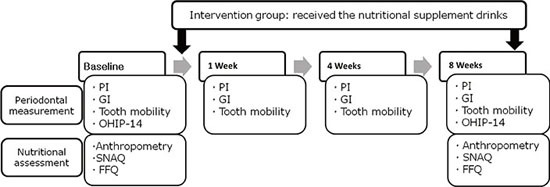
The intervention group consumed the nutritional supplement drink, as prescribed, for 8 weeks, while the control group did not receive any nutritional supplementation. At baseline (0 weeks), anthropometric measurements were made, and dietary intake and loss of appetite were assessed by the dietitian. Periodontal measurements were performed by the specialist. Conventional flap surgery was performed in both the intervention and the control group. Clinical periodontal measurements were made for all subjects at 1, 4, and 8 weeks after the surgery. At the final session (8 weeks), the health status of all subjects was checked by assessing the changes in their anthropometric data, nutritional status, and periodontal status (Fig. 1).
Figure 1.
Study design. PI: plaque index, GI: gingival index, OHIP-14: oral health impact profile-14, SNAQ: simplified nutritional appetite questionnaire, FFQ: food frequency questionnaire.
Clinical periodontal measurements
The Loe and Silness plaque index
The plaque index (PI) of Silness and Loe [18] was measured at baseline (point of surgery) and at 1, 4, and 8 weeks thereafter by assigning each of the four surfaces of the teeth (buccal, lingual, mesial, and distal) a score. The scores from these four areas of the tooth were then added and divided by 4 to obtain the PI for the tooth.
The Loe and Silness gingival index
The gingival index (GI) of Loe and Silness [19] was also evaluated at the same time points. The GI differentiates gingival inflammation into a score of 0 (normal), 1 (slight inflammation), 2 (moderate inflammation), and 3 (severe inflammation).
Tooth mobility
Measurements of tooth mobility were carried out using the Periotest M system (Medizintechnik Gulden, Modautal, Germany), which measures the damping characteristics of the periodontium. The duration of the contact of the tapping head on the tooth surface is measured by the instrument, which calculates the Periotest value to indicate tooth mobility [20].
Oral health impact profile-14
The oral-health-related QoL (OHRQoL) was evaluated using the oral health impact profile-14 questionnaire (OHIP-14; a validated short version of the original 49-item questionnaire) [21] at baseline (0 weeks) and at the final follow-up session (8 weeks). Responses to the OHIP-14 questions were scored on a 5-point Likert scale as 4, 3, 2, 1, or 0 if the problem had been experienced very often, fairly often, occasionally, hardly ever, or never, respectively, during the previous 2 months. The OHIP-14 scores were calculated by summing up the scores of the responses to 14 items. The total OHIP-14 score ranged from 0 to 56, with lower scores indicating a better OHRQoL.
Nutritional assessment
Anthropometric data
Anthropometric measurements were made at baseline (0 weeks) and the final session (8 weeks). A portable body composition analyzer (BIA-530, Jawon Medical, Gyeongsan, Korea) was used to measure body weight, body fat (%), and fat-free mass (FFM). Body mass index (BMI) was calculated using the standard formula (body weight (kg)/height (m2)). Waist circumference (WC) was measured between the top of the hip bone and the bottom of the rib cage with a flexible measuring tape. Blood pressure was measured with the patient in the sitting position after at least 5 minutes of rest (HEM-1000, OMRON, Kyoto, Japan).
Simplified nutritional appetite questionnaire
The simplified nutritional appetite questionnaire (SNAQ) was developed in 2005 from the Council on Nutrition Appetite Questionnaire using long-term care institutionalized and community-dwelling adults to assess loss of appetite [22]. It is a self-assessment nutritional screening tool that predicts weight loss and was used successfully to screen older people at risk of malnutrition or malnourishment. However, it was subsequently suggested that it would also be a useful assessment questionnaire for younger patients with a variety of chronic disorders associated with weight loss. The SNAQ includes four questions that were asked by the research dietitian during this study. The results were tallied on the basis of the following numerical scale: a=1, b=2, c=3, d=4, and e=5. The sum of the scores for the individual items constitutes the SNAQ score. A SNAQ score of ≤14 indicates a significant risk of at least 5% weight loss within 6 months. The research dietitian administered the SNAQ through interviews at baseline (0 weeks) and at the final session (8 weeks).
Dietary intake
Dietary intakes were assessed using the food frequency questionnaire (FFQ) that was developed for a community-based cohort of the Korean Genome and Health Study of the Korea National Genome Research Institute [23]. The FFQ includes 103 food items, and the frequency of servings was classified into 9 categories: never or seldom, once a month, 2-3 times a month, 1-2 times a week, 3-4 times a week, 5-6 times a week, once a day, twice a day, or 3 times or more daily. The portion size of each food item was classified as small, medium, or large. The subjects completed the FFQ at baseline (0 weeks) and at the final session (8 weeks).
Statistical analysis
Statistical analyses were performed using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). Patients who completed the entire clinical trial according to the protocol were counted towards the final results (Per Protocol Analysis). The means of the two groups were compared using an independent t-test, and the pre- and postintervention data were compared using a paired t-test. Periodontal data are presented in the main text as mean±standard error of mean values. The level of statistical significance was set at P<0.05.
RESULTS
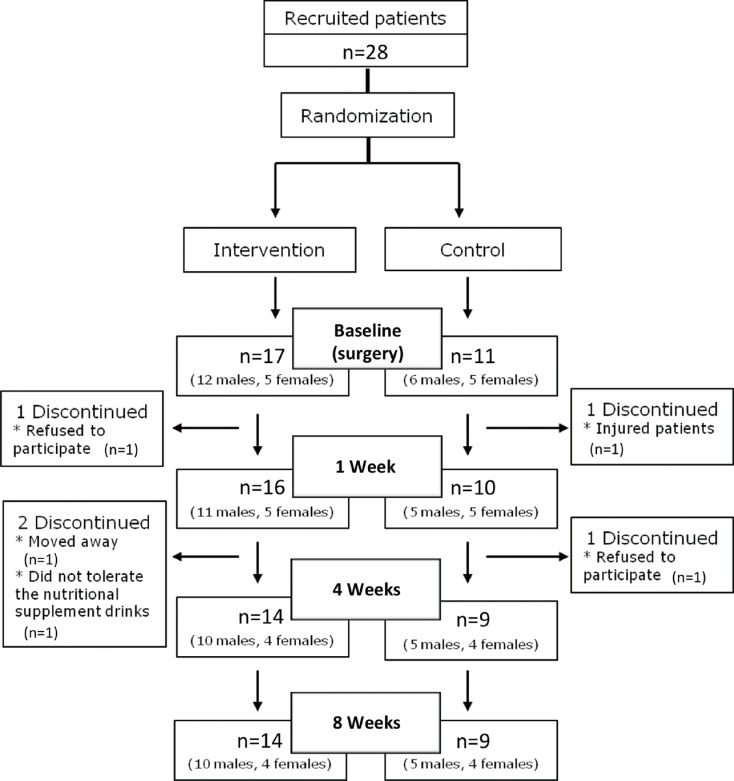
Among the 28 recruited patients, a total of 23 volunteers completed the study: 14 (10 males, 4 females) in the intervention group and 9 (5 males, 4 females) in the control group. Fig. 2 shows the participant flow chart. The mean ages in the intervention and control groups were 51.1 years (range, 42-59 years) and 46.9 years (range, 40-54 years), respectively, showing no significant differences at the start of study (P=0.068). The compliance rate was 73%, corresponding to an average daily consumption of 2.2 cans of nutritional supplement.
Figure 2.
Flow chart showing patient participation in the study.
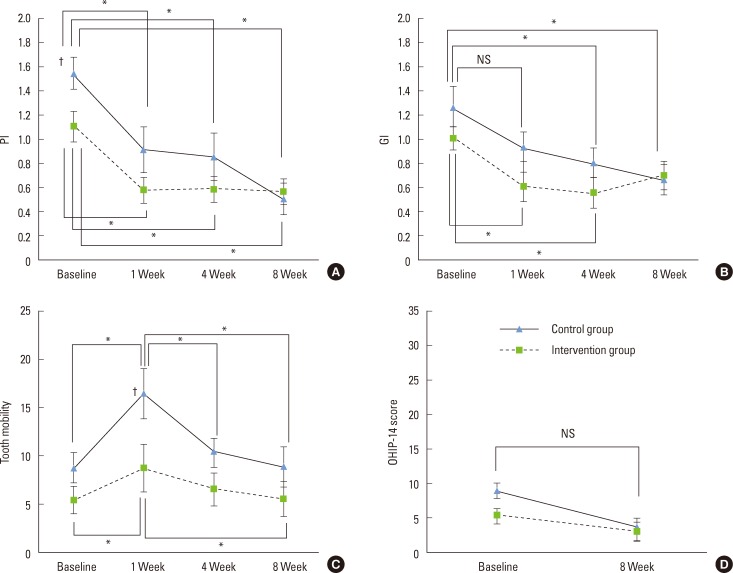
The PI values were significantly improved as compared to the baseline in both groups at 1, 4, and 8 weeks (P<0.05) (Fig. 3A). The inflammation marker (i.e., the GI value) did not differ between the groups at baseline but was reduced significantly after 1 week in the intervention group (0.61±0.12 vs. 1.01±0.09 at 0 weeks, P<0.05) but not in the control group. After 8 weeks, no significant differences were observed between the GI values of the intervention and control groups (P<0.05) (Fig. 3B).
Figure 3.
Changes in periodontal measurements for the intervention and control groups with respect to the intervention period. Data are mean and standard error of mean values. PI: plaque index, GI: gingival index, OHIP-14: oral health impact profile-14, NS: not statistically significant. *Statistically significant difference during the intervention period (P<0.05). †Statistically significant difference between the intervention and the control group (P<0.05).
The tooth mobility score did not differ significantly between the two groups at baseline (5.36±1.44 and 8.69±1.58 in the intervention and control groups, respectively) but, as expected, was significantly increased in both groups at 1 week after the surgery (P<0.05), although the extent of increase was less in the intervention group. Tooth mobility returned to baseline levels by 8 weeks after surgery in both groups (Fig. 3C).
There were no statistically significant differences in the evaluated OHIP-14 scores between the intervention and the control group at either baseline or 8 weeks postsurgery, and the OHIP-14 scores at 8 weeks did not differ significantly from the baseline values (Fig. 3D).
The anthropometric measurements during the intervention period are compared between the two groups in Table 1. In both groups, body weight, BMI, WC, body fat, FFM, systolic blood pressure, and diastolic blood pressure did not appear to change from the baseline measurements throughout the study period.
Table 1.
Anthropometric measurements of the subjects.
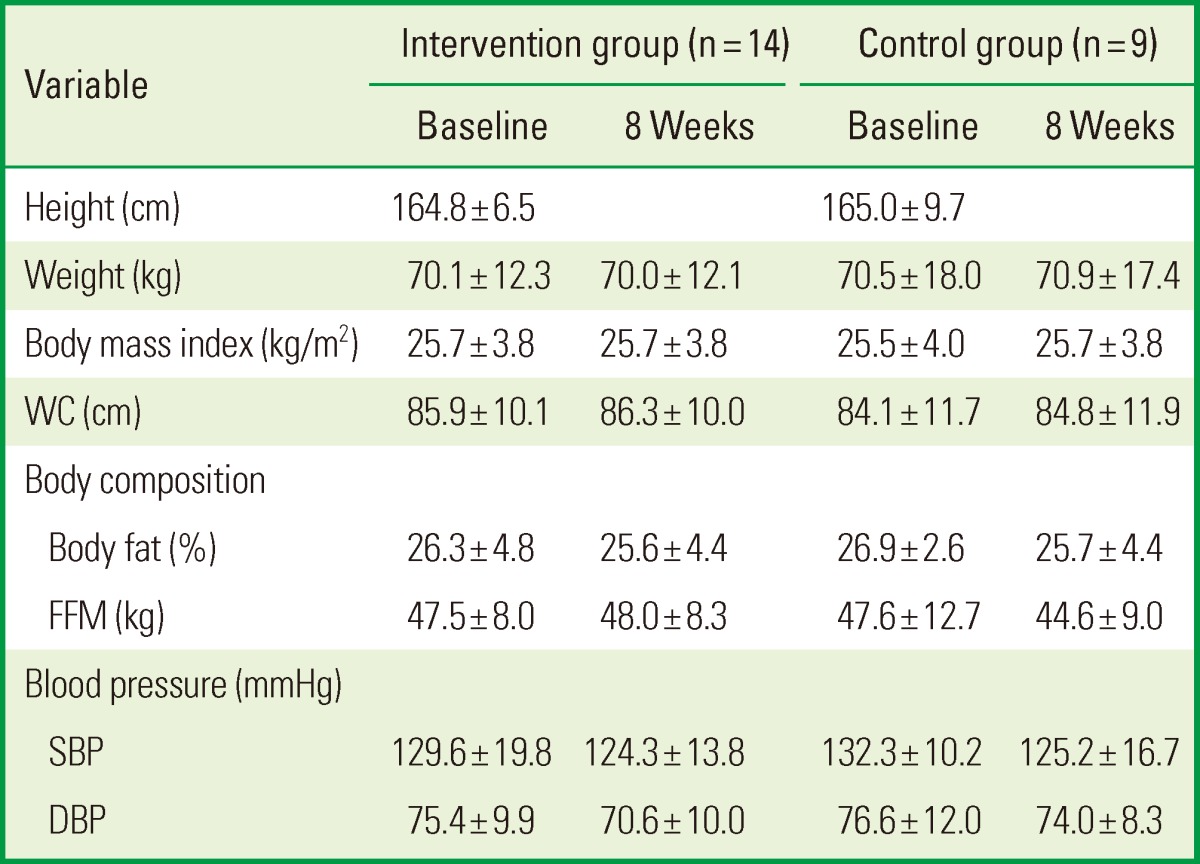
Values are presented as mean±standard deviation.
WC: waist circumference, FFM: fat-free mass, SBP: systolic blood pressure, DBP: diastolic blood pressure.
The mean SNAQ scores of the groups during the intervention period are given in Table 2. No differences were found between the intervention and the control group at baseline, and the mean SNAQ score at 8 weeks did not differ significantly from the baseline values. The nutrient intakes of the subjects during the intervention period are presented in Table 3. The intakes of energy; protein; fat; vitamins A, B1, B2, B6, and E; niacin; and calcium, phosphorous, iron, and zinc were significantly higher at 8 weeks than at baseline in the intervention group, while no significant changes in these parameters were observed in the control group between baseline and 8 weeks. At the 8-week time point, the intakes of vitamins A, B2, B6, C, and E, niacin, calcium, and iron were higher in the intervention group than in the control group.
Table 2.
Simplified nutritional appetite questionnaire (SNAQ) scores of the subjects.
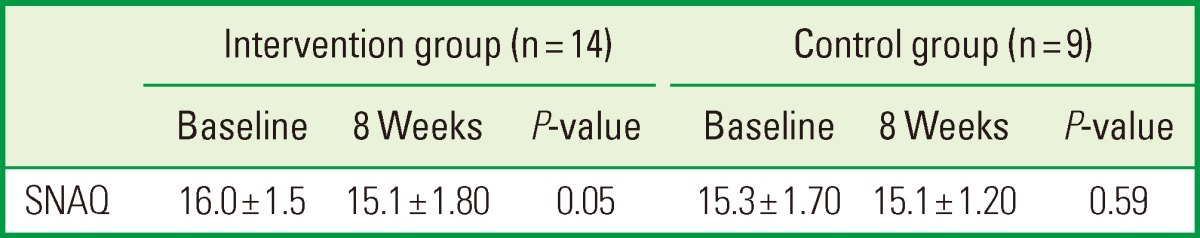
Values are presented as mean±standard deviation. Paired t-test.
Table 3.
Nutrient intakes (including the nutritional supplement) of the subjects.
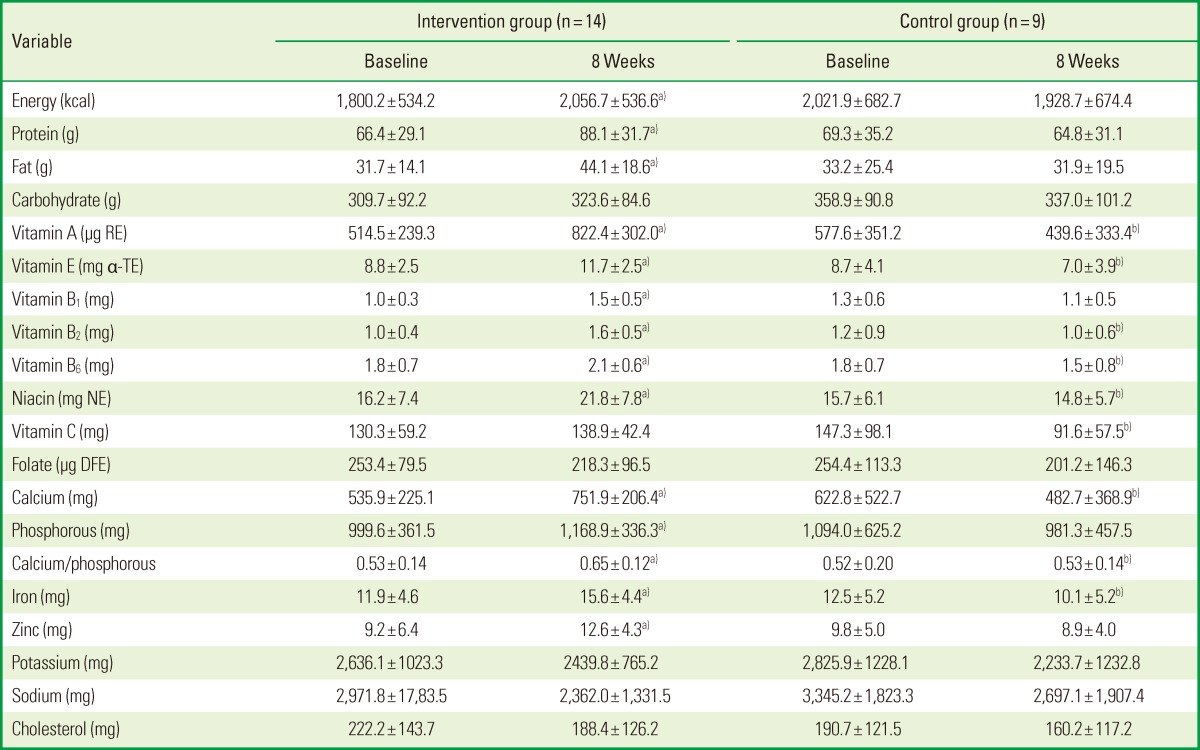
Values are presented as mean±standard deviation.
RE: retinol equivalents, α-TE: α-tocopherol equivalents, NE: niacin equivalents, DFE: dietary folate equivalents.
a)Statistically significant difference compared to the baseline (P<0.05). b)Statistically significant difference compared to the intervention group (P<0.05).
DISCUSSION
This study investigated the effects of postsurgical intake of nutritional supplement drinks on periodontal healing and tooth mobility for a period of 8 weeks after periodontal surgery. The results demonstrate that a regular intake of nutritional supplement drinks following periodontal surgery for 8 weeks significantly enhanced periodontal recovery and supported periodontal health in terms of the PI, GI, and tooth mobility. To the best of our knowledge, this is the first study to find the beneficial effects of nutritional supplement drinks on periodontal healing following periodontal surgery.
The role of nutrition in the field of periodontology has been discussed extensively, and recent studies on the interactions between nutrition, host defense, and infection have found a correlation between nutrition and the pathogenesis of periodontal disease [23]. In particular, a large body of literature has elucidated the mechanisms underlying the effects of each nutrient on the wound-healing process, and the role of nutrition on the outcome of periodontal treatment [24] has been extensively studied.
It is well established that the wound-healing process is highly dependent on nutrients associated with the synthesis of collagen and wound healing. The protein collagen is a major organic component of teeth, periodontal ligament, and muscle [12]. Since wound strength relies on the formation and deposition of collagen, an increased availability of amino acids may help healing [25,26]. Vitamin A, which is essential for the development of epithelial and bone tissue, as well as cellular differentiation, can control the rate of collagen cross-linkage [27]. There is considerable evidence for the benefits of vitamin A as a perioperative nutritional supplement. Likewise, vitamin C plays a role in collagen synthesis. This is relevant for the maintenance of oral tissue such as the periodontal ligament, the formation of bone matrix, and the integrity of blood vessel walls [28]. Leggott et al. [29] found that the GI values decreased after the supplementation period, suggesting that measurements of gingival inflammation are directly related to the ascorbic acid status.
Vitamin B-complex supplementation has also demonstrated positive effects on wound healing after periodontal surgery [30]. It has been reported that one of the B vitamins, thiamine (vitamin B1), may increase wound repair. Furthermore, niacin (vitamin B3) supplementation reportedly has a positive effect on the healing of repositioned flaps [31], suggesting that the vitamin creates a more balanced collagen structure during the wound-healing processes [32].
While periodontal disease is not directly caused by malnutrition, the provision of appropriate nutrition may decrease the susceptibility to periodontal disease, prevent the progression of pre-existing periodontal diseases, and improve the healing outcome after periodontal therapy. Among the previously mentioned benefits of appropriate nutrition, the enhanced healing process can greatly benefit those patients who receive periodontal treatment, since rapid wound healing will reduce the chance of infection, provide a more predictable outcome after periodontal treatment, and reduce the discomfort in these patients.
The authors of this study hypothesized that the nutritional supplementation of patients via a supplement drink enhanced the periodontal healing, as evaluated by measuring PI, GI, and tooth mobility. The findings regarding tooth mobility were the key outcome of this study and concurred with previous studies demonstrating the benefits of nutritional supplementation. Periodontal surgery removes or modifies etiologic agents, such as bacterial plaque and calculus [33], and consequently, a significant amount of periodontal tissue is removed during therapy. In addition, surgical trauma and the resolution of inflammation together increase the looseness of the periodontal tissue during the extended healing period, resulting in mobile teeth. In accordance with the previous results, the patients in our study also exhibited significantly increased tooth mobility after surgery [7,8,9]. This increased tooth mobility has repeatedly been shown to be associated with a decreased mastication ability [34], and difficulty in mastication after surgery can greatly limit the patient's food choice. Poor nutrition caused by limitations of food choice can delay recovery after periodontal surgery, and in the present study, the aim was to compensate for this insufficient nutritional intake by providing nutritional supplement drinks to support proper or enhanced periodontal wound healing, particularly in terms of tooth mobility.
The results of our study show that while tooth mobility increased significantly at 1 week postsurgery in both groups, the extent of this change was less in the intervention group than in the control group, suggesting that the consumption of nutritional supplement drinks contributed to the observed postsurgical reduction in tooth mobility in these patients. The enhanced formation and maturation of collagen fibers may have played a major role in wound stabilization, but the underlying mechanism remains to be elucidated. The increased tooth mobility of both groups significantly decreased after 1 month, plateauing thereafter to 2 months and finally returned to the presurgical levels. Thus, the effect of nutritional supplement drink intake appears to be limited to the early healing phase at 1 week postsurgery.
Both of the other clinical parameters (i.e., GI and PI) decreased significantly throughout the healing period in the two groups. This indicates that the oral hygiene status of all patients improved during the intervention period [35] and that the gingival inflammation subsided. However, it is not clear whether these results are attributable to the effect of periodontal surgery or the consumption of nutritional supplement drinks. Further clinical studies are required to address this issue.
OHIP-14 is a well validated measure of OHRQoL that detects dysfunction, discomfort, and disability attributable to oral conditions, based on the World Health Organization's "disease-impairment-disability-handicap" model [21]. The results show that there was no significant difference between the baseline and postsurgery OHIP-14 scores, although the scores of both groups concomitantly decreased from the baseline. However, these results should be interpreted with caution, and it should be noted that the initial values of both groups were lower than the average values measured in other studies [36]. The patients recruited in the present study appeared to have less discomfort and better functionality, and therefore, periodontal treatment with/without nutritional support did not seem to contribute much to the enhancement of OHIP-14 scores.
It has been reported that periodontitis is related to general malaise, weight loss, depression, and loss of appetite, and in response to metabolic cues such as pain signals, appetite can mediate the suppression of food intake [37]. The authors expected the patients' appetites and body weights to decrease because of the eating difficulties caused by mobile teeth after periodontal surgery. However, the SNAQ findings and anthropometric measurements did not differ significantly from the baseline values. This implies that the improved early healing shown in the supplementation group was achieved irrespective of the changes in appetite and weight. Recent studies have disclosed a strong association between obesity and periodontitis [38,39]. The findings of the present study corroborate those of previous studies; 60.9% of the patients studied were classified as obese (BMI of >25 kg/m2 for the Asian population).
This study was subject to some limitations. The effect of an intervention study is best evaluated when it has a blinded and placebo-controlled design. However, this study was conducted using an isolated soy protein-based drink for the intervention and an appropriate placebo was not established due to the difficulties of mimicking food materials. Second, the mean age of the subjects was around 50 years and there is a possibility that the baseline health and nutritional status of the subjects were optimal, which might have markedly diminished the effects of supplementation.
In conclusion, the consumption of nutritional supplement drinks may aid wound healing after periodontal surgery. In this study, while there were no changes in the OHRQoL or anthropometry, nutritional supplements ingested over an 8-week period improved early periodontal healing after surgery without changing any anthropometric parameters. Further studies are required to demonstrate the relationship between obesity and periodontitis and to expand this research to the older generation, whose nutritional status is most impacted by the integrity of oral health.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2011-0010739).
Footnotes
No potential conflict of interest relevant to this article was reported. The authors thank Daesang Co. (Seoul, Korea) for supplying the nutritional supplement drink.
References
- 1.Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H. Nutritional intervention in patients with periodontal disease: clinical, immunological and microbiological variables during 12 months. Br J Nutr. 2009;101:879–885. doi: 10.1017/S0007114508047776. [DOI] [PubMed] [Google Scholar]
- 2.Choi YK, Do SR, Park DY. Change in number of outpatients with periodontal diseases during recent 20 years based on patient survey. J Korean Acad Oral Health. 2011;35:331–339. [Google Scholar]
- 3.Canakci CF, Canakci V. Pain experienced by patients undergoing differentperiodontal therapies. J Am Dent Assoc. 2007;138:1563–1573. doi: 10.14219/jada.archive.2007.0105. [DOI] [PubMed] [Google Scholar]
- 4.Pereira LJ, Gazolla CM, Magalhaes IB, Dominguete MH, Vilela GR, Castelo PM, et al. Influence of periodontal treatment on objective measurement of masticatory performance. J Oral Sci. 2012;54:151–157. doi: 10.2334/josnusd.54.151. [DOI] [PubMed] [Google Scholar]
- 5.Fleszar TJ, Knowles JW, Morrison EC, Burgett FG, Nissle RR, Ramfjord SP. Tooth mobility and periodontal therapy. J Clin Periodontol. 1980;7:495–505. doi: 10.1111/j.1600-051x.1980.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang HL, Burgett FG, Shyr Y, Ramfjord S. The influence of molar furcation involvement and mobility on future clinical periodontal attachment loss. J Periodontol. 1994;65:25–29. doi: 10.1902/jop.1994.65.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Alkan A, Keskiner I, Arici S, Sato S. The effect of periodontal surgery on bite force, occlusal contact area and bite pressure. J Am Dent Assoc. 2006;137:978–983. doi: 10.14219/jada.archive.2006.0319. [DOI] [PubMed] [Google Scholar]
- 8.Feller L, Lemmer J. Tooth mobility after periodontal surgery. SADJ. 2004;59:407, 409–411. [PubMed] [Google Scholar]
- 9.Kerry GJ, Morrison EC, Ramfjord SP, Hill RW, Caffesse RG, Nissle RR, et al. Effect of periodontal treatment on tooth mobility. J Periodontol. 1982;53:635–638. doi: 10.1902/jop.1982.53.10.635. [DOI] [PubMed] [Google Scholar]
- 10.Budtz-Jorgensen E, Chung JP, Rapin CH. Nutrition and oral health. Best Pract Res Clin Gastroenterol. 2001;15:885–896. doi: 10.1053/bega.2001.0247. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka K, Miyake Y, Sasaki S, Ohya Y, Matsunaga I, Yoshida T, et al. Relationship between intake of vegetables, fruit, and grains and the prevalence of tooth loss in Japanese women. J Nutr Sci Vitaminol (Tokyo) 2007;53:522–528. doi: 10.3177/jnsv.53.522. [DOI] [PubMed] [Google Scholar]
- 12.Van der Velden U, Kuzmanova D, Chapple IL. Micronutritional approaches to periodontal therapy. J Clin Periodontol. 2011;38(Suppl 11):142–158. doi: 10.1111/j.1600-051X.2010.01663.x. [DOI] [PubMed] [Google Scholar]
- 13.Neiva RF, Steigenga J, Al-Shammari KF, Wang HL. Effects of specific nutrients on periodontal disease onset, progression and treatment. J Clin Periodontol. 2003;30:579–589. doi: 10.1034/j.1600-051x.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- 14.San Miguel SM, Opperman LA, Allen EP, Zielinski J, Svoboda KK. Bioactive antioxidant mixtures promote proliferation and migration on human oral fibroblasts. Arch Oral Biol. 2011;56:812–822. doi: 10.1016/j.archoralbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 15.De Marchi RJ, Hugo FN, Hilgert JB, Padilha DM. Association between oral health status and nutritional status in south Brazilian independent-living older people. Nutrition. 2008;24:546–553. doi: 10.1016/j.nut.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Wouters-Wesseling W, Van Hooijdonk C, Wagenaar L, Bindels J, de Groot L, Van Staveren W. The effect of a liquid nutrition supplement on body composition and physical functioning in elderly people. Clin Nutr. 2003;22:371–377. doi: 10.1016/s0261-5614(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 17.Collins CE, Kershaw J, Brockington S. Effect of nutritional supplements on wound healing in home-nursed elderly: a randomized trial. Nutrition. 2005;21:147–155. doi: 10.1016/j.nut.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 19.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka E, Ueki K, Kikuzaki M, Yamada E, Takeuchi M, Dalla-Bona D, et al. Longitudinal measurements of tooth mobility during orthodontic treatment using a periotest. Angle Orthod. 2005;75:101–105. doi: 10.1043/0003-3219(2005)075<0101:LMOTMD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Bae KH, Kim HD, Jung SH, Park DY, Kim JB, Paik DI, et al. Validation of the Korean version of the oral health impact profile among the Korean elderly. Community Dent Oral Epidemiol. 2007;35:73–79. doi: 10.1111/j.1600-0528.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82:1074–1081. doi: 10.1093/ajcn/82.5.1074. [DOI] [PubMed] [Google Scholar]
- 23.Ahn YJ, Lee JE, Paik HY, Lee HK, Jo IH, Kim KC. Development of a semi-quantitative food frequency questionnaire based on dietary data from the Korea National Health and Nutrition Examination Survey. Korean J Nutr. 2003;6:173–184. [Google Scholar]
- 24.Alfano MC. Controversies, perspectives, and clinical implications of nutrition in periodontal disease. Dent Clin North Am. 1976;20:519–548. [PubMed] [Google Scholar]
- 25.Woolfe SN, Kenney EB, Hume WR, Carranza FA., Jr Relationship of ascorbic acid levels of blood and gingival tissue with response to periodontal therapy. J Clin Periodontol. 1984;11:159–165. doi: 10.1111/j.1600-051x.1984.tb01319.x. [DOI] [PubMed] [Google Scholar]
- 26.Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11:278–296. doi: 10.1016/j.arr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Seifter E, Crowley LV, Rettura G, Nakao K, Gruber C, Kan D, et al. Influence of vitamin A on wound healing in rats with femoral fracture. Ann Surg. 1975;181:836–841. doi: 10.1097/00000658-197506000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staudte H, Sigusch BW, Glockmann E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br Dent J. 2005;199:213–217. doi: 10.1038/sj.bdj.4812613. [DOI] [PubMed] [Google Scholar]
- 29.Leggott PJ, Robertson PB, Rothman DL, Murray PA, Jacob RA. The effect of controlled ascorbic acid depletion and supplementation on periodontal health. J Periodontol. 1986;57:480–485. doi: 10.1902/jop.1986.57.8.480. [DOI] [PubMed] [Google Scholar]
- 30.Neiva RF, Al-Shammari K, Nociti FH, Jr, Soehren S, Wang HL. Effects of vitamin-B complex supplementation on periodontal wound healing. J Periodontol. 2005;76:1084–1091. doi: 10.1902/jop.2005.76.7.1084. [DOI] [PubMed] [Google Scholar]
- 31.Collins TM, Denish A, Sheffield J, Mitra A, Stueber K, Smith YR. Nicotinamide enhances skin flap survival. Scand J Plast Reconstr Surg Hand Surg. 1989;23:177–179. doi: 10.3109/02844318909075114. [DOI] [PubMed] [Google Scholar]
- 32.Vaxman F, Olender S, Lambert A, Nisand G, Aprahamian M, Bruch JF, et al. Effect of pantothenic acid and ascorbic acid supplementation on human skin wound healing process. A double-blind, prospective and randomized trial. Eur Surg Res. 1995;27:158–166. doi: 10.1159/000129395. [DOI] [PubMed] [Google Scholar]
- 33.Wikesjo UM, Nilveus RE, Selvig KA. Significance of early healing events on periodontal repair: a review. J Periodontol. 1992;63:158–165. doi: 10.1902/jop.1992.63.3.158. [DOI] [PubMed] [Google Scholar]
- 34.Alkan A, Keskiner I, Arici S, Sato S. The effect of periodontitis on biting abilities. J Periodontol. 2006;77:1442–1445. doi: 10.1902/jop.2006.060025. [DOI] [PubMed] [Google Scholar]
- 35.Lindhe J, Nyman S. Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol. 1984;11:504–514. doi: 10.1111/j.1600-051x.1984.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 36.Wong RM, Ng SK, Corbet EF, Keung Leung W. Non-surgical periodontal therapy improves oral health-related quality of life. J Clin Periodontol. 2012;39:53–61. doi: 10.1111/j.1600-051X.2011.01797.x. [DOI] [PubMed] [Google Scholar]
- 37.Bosley BN, Weiner DK, Rudy TE, Granieri E. Is chronic nonmalignant pain associated with decreased appetite in older adults? Preliminary evidence. J Am Geriatr Soc. 2004;52:247–251. doi: 10.1111/j.1532-5415.2004.52063.x. [DOI] [PubMed] [Google Scholar]
- 38.Wood N, Johnson RB, Streckfus CF. Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES III) J Clin Periodontol. 2003;30:321–327. doi: 10.1034/j.1600-051x.2003.00353.x. [DOI] [PubMed] [Google Scholar]
- 39.Kong YM, Han GS. Relationships between obesity types and periodontitis according to characteristics of subjects. J Dent Hyg Sci. 2012;12:279–286. [Google Scholar]