Abstract
Emerging evidence suggests that Alzheimer's disease (AD) and Parkinson's disease dementia (PDD) share neurodegenerative mechanisms. We sought to directly compare cerebral perfusion in these two conditions using arterial spin labeling magnetic resonance imaging (ASL-MRI). In total, 17 AD, 20 PDD, and 37 matched healthy controls completed ASL and structural MRI, and comprehensive neuropsychological testing. Alzheimer's disease and PDD perfusion was analyzed by whole-brain voxel-based analysis (to assess absolute blood flow), a priori specified region of interest analysis, and principal component analysis (to generate a network differentiating the two groups). Corrections were made for cerebral atrophy, age, sex, education, and MRI scanner software version. Analysis of absolute blood flow showed no significant differences between AD and PDD. Comparing each group with controls revealed an overlapping, posterior pattern of hypoperfusion, including posterior cingulate gyrus, precuneus, and occipital regions. The perfusion network that differentiated AD and PDD groups identified relative differences in medial temporal lobes (AD<PDD) and right frontal cortex (PDD<AD). In conclusion, the pattern of cerebral hypoperfusion is very similar in AD and PDD. This suggests closely linked mechanisms of neurodegeneration mediating the evolution of dementia in both conditions.
Keywords: Alzheimer's disease, arterial spin labeling, dementia, MRI, Parkinson's disease
Introduction
Alzheimer's disease (AD) and Parkinson's disease (PD) are the two most common neurodegenerative disorders. While AD is by definition a dementing process, it has also become increasingly apparent that cognitive deficits occur in PD, and longitudinal studies have shown that most PD patients will eventually develop dementia.1, 2 Formal criteria for the diagnosis of Parkinson's disease dementia (PDD) and PD-associated mild cognitive impairment now exist.3, 4 The fundamental AD pathologic changes include accumulation of beta-amyloid (plaques) and tau protein (neurofibrillary tangles),5 while alpha-synuclein containing Lewy bodies is the hallmark of PD.6 However, studies have shown a significant neuropathologic overlap between AD and PDD,7, 8 and current theories emphasize the role of the cholinergic rather than dopaminergic deficit in the development of PDD,9 a feature shared with AD. Indeed, it remains unclear whether the development of dementia in established PD is linked to the progressive spread of Lewy bodies, the development of Alzheimer's pathology, or a combination of the two.7 This has led some authors to suggest that the two diseases exist on a spectrum: while their initiating events may differ, the processes that drive ongoing neurodegeneration and the resultant clinical phenotype may be closely linked.10, 11
Arterial spin labeling magnetic resonance imaging (ASL-MRI) is a relatively new technique for measuring cerebral perfusion. Using magnetically labeled water as an endogenous tracer, ASL-MRI is an easily performed procedure that allows noninvasive voxel-by-voxel quantification of absolute brain perfusion (mL/100 g per minute),12 which can be combined with other MRI scan information (e.g., atrophy and white-matter lesions). It has been shown to correlate highly with fluoro-deoxy-glucose positron emission tomography measures of cerebral metabolism in AD.13 It is likely to have an important ongoing role as an assessment tool and biomarker in neurodegenerative diseases.14
Arterial spin labeling magnetic resonance imaging has revealed cerebral perfusion abnormalities in both AD and PDD,15, 16 but to our knowledge the two conditions have not been compared directly with this modality. Although a study using single-photon emission computed tomography with MRI (for atrophy analysis) found relative occipito-parietal hypoperfusion in PDD compared with AD,17 there is a significant overlap in each condition's perfusion abnormality, with precuneus, posterior cingulate, temporo-parietal, and occipito-parietal regions affected in both.15, 16 Given the possibility of shared neurodegenerative mechanisms between AD and PDD, and the emerging importance of ASL-MRI as a biomarker in each condition, we sought to directly compare these two groups' perfusion patterns. Specifically, we hypothesized that perfusion in the PDD group would be reduced compared with AD in parieto-occipital and frontal regions, and increased compared with AD in medial temporal lobe regions. We aimed to test this using two techniques—a voxel-based analysis (VBA) to compare absolute and regional blood flow in the two conditions, and a principal component analysis (PCA) to derive a perfusion network that discriminated AD and PDD subjects.
Materials and methods
Subjects
Twenty PDD subjects, seventeen AD subjects, and thirty-seven healthy controls (HCs) were included in the study. The PDD sample consisted of all subjects from within a larger study of PD undertaken at the New Zealand Brain Research Institute who met the Movement Disorders Society task force criteria for PDD.18 All subjects had a clinical diagnosis of PD confirmed by a neurologist specializing in movement disorders (TJA). The AD sample was recruited from the Princess Margaret Hospital Memory Clinic, Christchurch, New Zealand who met the NINCDS-ADRDA for probable AD of mild–moderate severity.19 The control sample consisted of healthy volunteers matched to the dementia groups for age and sex. Exclusion criteria included atypical parkinsonism; previous history of neurologic conditions including moderate or severe head injury, stroke, learning disability or other dementing process; major medical or psychiatric illness in the previous 6 months. A trained neuroradiologist (RJK) screened all images to exclude potentially confounding brain abnormalities (previous stroke, tumor, and hydrocephalus). All subjects gave written, informed consent to participate in the study, with additional consent obtained from a significant other when appropriate. The study was approved by the Upper South Regional Ethics Committee of the New Zealand Ministry of Health.
Diagnostic Criteria and Assessment
Neuropsychological testing assessed global cognitive status (Montreal Cognitive Assessment (MoCA), Mini Mental State Examination, and the Alzheimer's Disease Assessment Scale—Cognition), with additional measures for executive function, attention, working memory and processing speed, memory and visuospatial/visuoperceptual function. Motor impairment was measured in the PDD group using the Unified Parkinson's Disease Rating Scale (part III) with patients in the ‘on' state. Demographic details and results of neuropsychological testing are presented in Table 1. The only significant differences between the AD and PDD groups for these variables were impaired delayed recall in AD compared with PDD, and impaired PDD performance on the map search test of everyday attention compared with AD.
Table 1. Demographic and neuropsychological data.
| Controls | AD | PDD | P value (AD versus PDD) | |
|---|---|---|---|---|
| Number | 37 | 17 | 20 | — |
| Age (years) | 72.7 (5.5) | 72.4 (7.2) | 74.9 (6.0) | 0.54 |
| Male:Female | 23:14 | 8:9 | 15:5 | — |
| Education (years) | 13.5 (2.9) | 12.8 (3.1) | 12.3 (2.3) | 0.71 |
| UPDRS | — | — | 47.2 | — |
| Levodopa (mg/day) | — | — | 670 | — |
| Cholinesterase inhibitor global | — | 5/9a | 2/20 | — |
| MoCA | 27.0 (1.9) | 15.5 (5.2) | 17.3 (3.6) | 0.54 |
| MMSE (w) | 28.9 (1.0) | 19.7 (4.6) | 23.2 (3.1) | 0.29 |
| ADAS-cog | 5.6 (2.4) | 21.2 (8.5) | 19.4 (7.6) | 0.7 |
| CDR | — | 1 | 1.2 | 0.54 |
| Memory | ||||
| Word list recall (incorrect/10) | 3.2 (1.1) | 6.0 (1.6) | 5.9 (1.5) | 0.94 |
| Word recognition (incorrect/12) | 2.1 (1.7) | 6.2 (3.0) | 3.8 (2.9) | 0.14 |
| Delayed recall (correct/5) | 3.6 (1.3) | 0.3 (0.7) | 1.7 (1.5) | 0.02 |
| Visuospatial | 0.7 (0.5) | −0.2 (1.2) | −0.6 (1.1) | 0.54 |
| JLO | 0.6 (0.7) | −0.5 (1.3) | −0.5 (1.1) | 0.94 |
| VOSP | 0.9 (0.8) | 0.1 (1.5) | −0.5 (1.3) | 0.54 |
| Executive function | 0.7 (0.8) | −1.2 (1.3) | −1.5 (0.6) | 0.69 |
| Letter fluency | 0.8 (1.2) | −0.9 (1.4) | −0.8 (1.3) | 0.94 |
| Action fluency | 0.2 (1.0) | −0.9 (1.3) | −1.6 (0.9) | 0.29 |
| Category fluency | 1.2 (1.2) | −1.2 (1.7) | −1.1 (0.9) | 0.94 |
| Stroop inhibition | 0.5 (0.9) | −1.8 (1.8) | −2.2 (0.9) | 0.60 |
| Attention/working memory | 0.3 (0.6) | −1.3 (1.4) | −1.9 (0.8) | 0.39 |
| Map search (TEA) | 0.6 (1.0) | −1.4 (1.3) | −2.5 (0.6) | 0.03 |
| Stroop color | 0.2 (1.1) | −1.4 (1.8) | −1.6 (1.1) | 0.78 |
| Stroop word | 0.2 (0.9) | −0.9 (1.8) | −1.4 (1.3) | 0.54 |
AD, Alzheimer's disease; PDD, Parkinson's disease dementia; UPDRS, Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment; MMSE (w), Mini Mental State Examination (world); ADAS-cog, Alzheimer's Disease Assessment Scale—Cognition; CDR, clinical dementia rating; JLO, judgment of line orientation; VOSP, visual object and space perception battery; TEA, test of everyday attention; FDR, false discovery rate.
Standard deviations are indicated in parentheses. P values refer to Welch's t-test for unequal variances between the AD and PDD groups and were FDR corrected. Neuropsychological test values for specific domains are Z-scored and normalized for sex and age, and averaged by domain (shaded gray) except ‘Memory' domain tests, which show the raw number of items correct/incorrect. Immediate recall and word recognition were taken from the ADAS-cog, and delayed recall from MoCA.
Information on cholinesterase inhibitor use was available for nine AD subjects. Statistically significant comparisons are shown in bold.
Magnetic Resonance Imaging Acquisition
All subjects were imaged using a 3-tesla General Electric HDx scanner (Waukesha, WI, USA) with an eight channel head coil. The protocol included a T1-weighted, 3D spoiled gradient recalled echo acquisition (SPGR; echo time=2.8 ms, repetition time 6.6 ms, inversion time=400 ms, flip angle=15°, acquisition matrix=256 × 256 × 170, field of view=250 mm, slice thickness=1 mm, voxel size=0.98 × 0.98 × 1.0 mm3). A stack of spiral, fast spin echo acquired images were prepared with pseudo-continuous ASL and background suppression to measure whole-brain perfusion quantitatively (repetition time=6 seconds, echo spacing=9.2 ms, postlabeling delay=1.5 seconds, labeling duration=1.5 seconds, eight interleaved spiral arms with 512 samples at 62.5 kHz bandwidth and 30 phase encoded 5 mm thick slices, no slice gap, number of excitations=5, total scan time=6 minutes 46 seconds, units=mL/100 g per minute).20 Two MRI scanner software updates occurred during the course of the study. Subjects were instructed to close their eyes and remain as still as possible during scanning.
Magnetic Resonance Imaging Data Preprocessing
We used VBM8 (http://dbm.neuro.uni-jena.de/vbm/), a toolbox of SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm), and custom scripts in Matlab 7.6.0 (R2008a; Mathworks, Natick, MA, USA) to preprocess MRI data as follows: structural images were bias corrected, tissue classified, and normalized using linear and nonlinear transformations (diffeomorphic anatomical registration using exponentiated lie algebra), within a unified model.21 Gray-matter (GM) segments for each subject were modulated using nonlinear components of the normalization only, thereby preserving actual tissue values locally to account for individual brain size globally. Modulated, normalized GM segments were smoothed with a 10-mm FWHM Gaussian kernel to improve signal to noise and to minimize the effect of any residual misalignment. Perfusion images were quantified, coregistered to SPGR images, brain extracted, and normalized using the deformation fields generated during segmentation and normalization of the SPGR images. Normalized perfusion images were also smoothed with a 10-mm FWHM Gaussian kernel. All images were visually inspected. A study-specific GM mask, used to exclude nonGM contributions in subsequent analyses, was created by averaging the modulated, normalized, GM segment from all subjects. Values less than 0.15 were excluded from the mask, as were all slices inferior to the superior cerebellum as spiral artifacts were present in this area on perfusion images; periventricular white-matter regions misclassified as GM (partial voluming) were manually excluded from the GM mask.
Voxel-Based Analyses
We performed whole-brain VBA of the perfusion data using a permutation-based inference tool for nonparametric statistical thresholding (‘randomize' in FSL 5.0.2; www.fmrib.ox.ac.uk/fsl). Group differences were assessed (AD versus PDD; control versus AD; control versus PDD) with age, sex, years of education, MoCA, and scanner software version as covariates. Additionally, voxelwise GM concentration (the smoothed GM segment for each individual) was included in the model to covary for the effect of GM atrophy on perfusion. For each contrast, the null distribution was generated over 5,000 permutations and the α level set at P<0.05, corrected for multiple comparisons using threshold-free cluster enhancement.22 We also investigated GM differences across groups as above, with age, sex, years of education, MoCA, and scanner software version as covariates.
Region of Interest Analyses
We performed a region of interest (ROI) analysis comparing AD and PDD perfusion in a priori selected regions known to be relevant to each condition and/or previously shown to be affected in functional neuroimaging.15, 16 We defined these areas anatomically using the Harvard/Oxford cortical and subcortical GM atlases (available with FSL). Region of interest analyses were performed with R 3.0 using Ime4 package for models (http://cran.r-project.org/web/packages/lme4/). (See online Supplementary Material.) The primary predictors of interest included in the model were region and the region and group interaction. To control for other covariates, predictors of age, sex, GM concentration (for perfusion ROI analysis), and scanner software version were also included in the model. The variables age and GM concentration were centred on their mean values across the subjects, and ‘latest version' and ‘male' were used as the reference levels for scanner version and sex. P values were corrected to control for the false discovery rate.23
A PCA to assess the relative perfusion characteristics across the AD and PDD groups is described in the Supplementary Material.
Results
Global Cerebral Perfusion
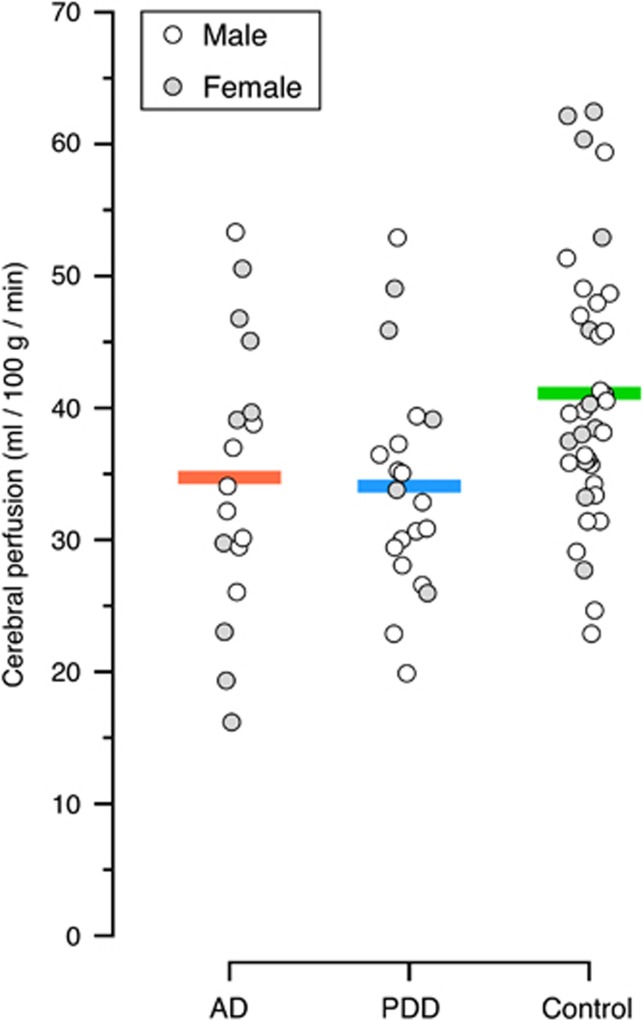
Global GM perfusion was similar in the AD and PDD groups (34.7 (s.d. 10.6) and 34.1 (s.d. 8.4) mL/100 g per minute, respectively), and both groups were significantly lower than HCs (41.1 (s.d. 10.0) mL/100 g per minute), P=0.03 (AD) and P=0.02 (PDD) (Figure 1).
Figure 1.
Global gray-matter perfusion (mL/100 g per minute) in each group, corrected for age, magnetic resonance imaging (MRI) scanner software version, and education. Males and females within each group are represented separately (although in subsequent analyses sex is accounted for).
Voxel-Based Analyses
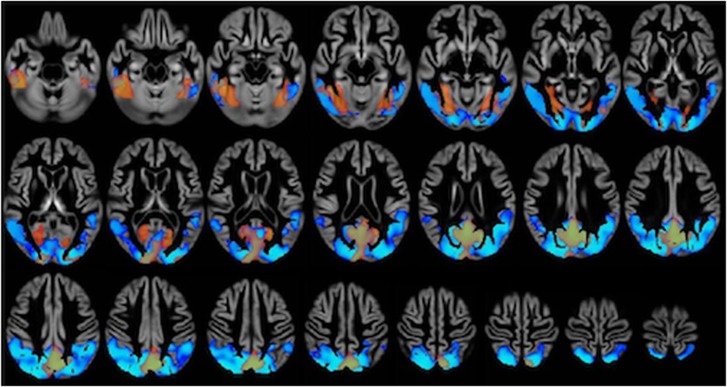
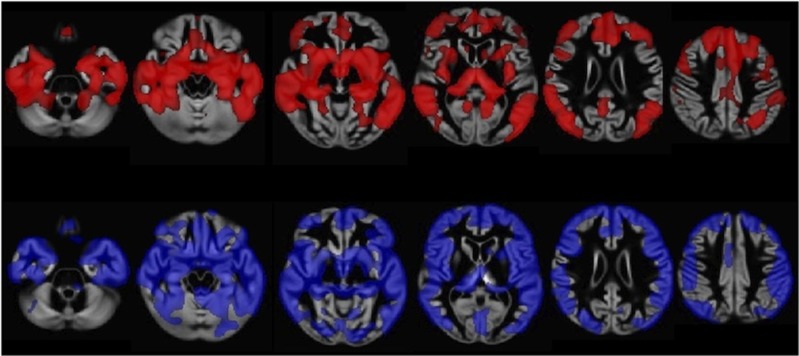
There were no significant differences in regional perfusion between AD and PDD groups on whole-brain VBA. The PDD and AD groups showed widespread areas of reduced perfusion compared with HCs (Figure 2). In the AD group, significantly reduced perfusion compared with HCs was evident in the medial temporal lobes, fusiform gyrus, intracalcarine cortex, cuneus, precuneus, posterior cingulate gyrus, and occipital pole. The PDD group had reduced perfusion compared with HCs in the inferior and middle temporal gyri, fusiform gyrus, inferior and superior lateral occipital cortex, supracalcarine and intracalcarine cortex, cuneus, precuneus, posterior cingulate gyrus, superior parietal lobule, and right middle frontal gyrus. There were no areas of increased perfusion in either group compared with controls. Direct comparison of degree of atrophy between AD and PDD revealed no differences between the groups, in contradistinction to the widespread cortical and subcortical atrophy in both groups compared with HCs (Figure 3).
Figure 2.
Whole-brain voxel-based analysis of absolute cerebral perfusion, comparing Alzheimer's disease (AD) with controls and Parkinson's disease dementia (PDD) with controls (results superimposed). Significantly (threshold-free cluster enhancement corrected P<0.05) reduced perfusion in AD (orange) and PDD (blue) is shown, covarying for gray-matter concentration, age, sex, education, and magnetic resonance imaging (MRI) scanner software version. Lighter areas represent areas with a greater statistical difference. Although the PDD perfusion deficit appears more widely distributed posteriorly than the AD one, this apparent difference is not significant on direct comparison between the two groups, as AD perfusion in these posterior areas is also reduced but did not reach a level of statistical significance on whole-brain voxel-based analysis (VBA) (this is better illustrated in the region of interest analysis).
Figure 3.
Whole-brain voxel-based analysis of gray-matter concentration, comparing Alzheimer's disease (AD) with controls and Parkinson's disease dementia (PDD) with controls. Significant (threshold-free cluster enhancement corrected P <0.05) atrophy in AD (red) and PDD (blue) is shown. Lighter areas represent regions with a greater statistical difference. Widespread atrophy is apparent in both groups.
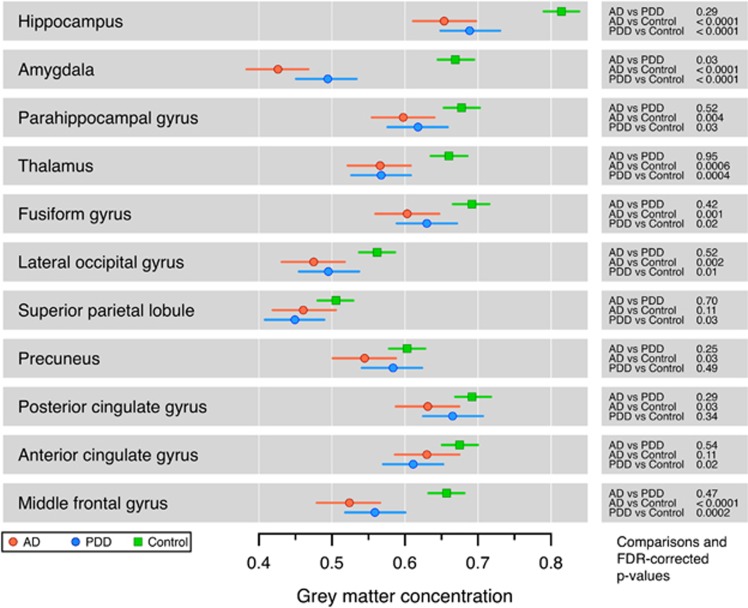
Region of Interest Analyses
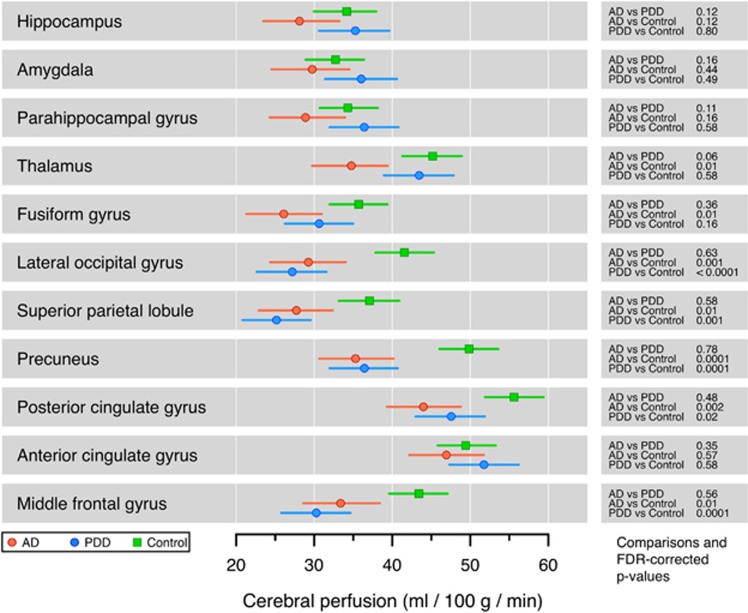
There were no statistically significant perfusion differences between AD and PDD groups in the ROI analysis (Figure 4). Alzheimer's disease trended toward reduced perfusion (compared with PDD) in the hippocampus, parahippocampal gyrus, amygdala, and thalamus. In both AD and PDD groups, perfusion was reduced compared with HCs in posterior cortical regions and in the middle frontal gyrus, but not in medial temporal lobes. The AD group, but not the PDD group, showed reduced perfusion in the thalamus compared with HCs.
Figure 4.
Region of interest analysis of cerebral perfusion, comparing controls, Alzheimer's disease (AD), and Parkinson's disease dementia (PDD). Bars indicate the 95% confidence interval (CI). There were no statistically significant differences between AD and PDD. In both AD and PDD groups, perfusion was reduced compared with controls in multiple regions. FDR, false discovery rate.
Region of interest analysis of GM atrophy revealed a significant reduction in GM concentration in medial temporal structures and the thalamus in both AD and PDD compared with HCs, but only the amygdala showed significantly more atrophy in AD compared with PDD (Figure 5).
Figure 5.
Region of interest analysis of gray-matter concentration, comparing controls, Alzheimer's disease (AD), and Parkinson's disease dementia (PDD). Bars indicate 95% confidence interval (CI). The amygdala showed a significant reduction in AD compared with PDD, but there were no other significant differences between the two groups. There were multiple reductions in each group compared with controls. FDR, false discovery rate.
Principal Component Analysis
A network differentiating AD and PDD was constructed from the (two) components that significantly differentiated the AD and PDD groups (together explaining 11.1% of the total variance in the data) (Supplementary Figure A, Supplementary Material). Broadly, the network was characterized by relatively reduced perfusion in AD subjects compared with PDD subjects in bilateral medial temporal lobes (parahippocampal gyrus, fusiform gyrus, amygdala, and hippocampus), and relatively reduced perfusion in PDD subjects compared with AD subjects in the right frontal pole and right inferior, middle and superior frontal gyri. Periventricular white-matter areas were manually excluded, but a remnant periventricular signal was evident.
Discussion
We identified widespread areas of hypoperfusion compared with controls in both AD and PDD, using both voxel-based and ROI-based analyses, but no statistically significant absolute differences in cerebral perfusion between AD and PDD. Importantly, perfusion data were corrected for atrophy, which was comparable between the AD and PDD groups. Using a PCA, we determined a perfusion network that maximally separated the two similar conditions. Taken together, these results show considerable overlap in the perfusion profile, and by inference, metabolism deficits in these two forms of dementia. Perhaps most striking is that this similarity in perfusion abnormality is apparent at a relatively early clinical dementia stage—the average clinical dementia rating in each group was around 1.0, corresponding to mild dementia.
Evidence is accumulating that the processes leading to dementia in PD follow a similar course to AD.10 While early executive and attention dysfunction in PD patients with otherwise normal cognition sometimes predicts eventual PDD, increasing evidence suggests that other cognitive changes may be more relevant.24 One longitudinal study found the presence of executive dysfunction in PD, while common, did not predict development of dementia. It was instead the presence of features suggesting more posterior cortical dysfunction (impaired semantic fluency and difficulty copying intersecting pentagons), increasing age and microtubule-associated protein tau gene H1/H1 genotype that predicted PDD development.25 This observation accords with previous functional imaging studies, which have shown that while frontal dysfunction is the prominent feature in PD and PD-associated mild cognitive impairment, a posterior pattern of abnormality affecting precuneus, posterior cingulate, and occipital regions is dominant in PDD.26 Similarly, while early medial temporal lobe changes (particularly in pathologic and atrophy studies) in AD are well described, the areas of marked hypoperfusion (as the disease progresses) exist more posteriorly.15 Our study is consistent with previous perfusion studies in AD and PDD, but extends prior observations to show the pattern of abnormality is essentially the same in both conditions.
This shared pattern raises the possibility of shared pathophysiologic mechanisms. There is good evidence that the synergistic combination of pathologic proteins may in itself be a powerful drive toward dementia in both PD and AD, and more broadly in other neurodegenerative conditions.11 Alpha-synuclein and tau interact to promote each other's aggregation and often colocalize within Lewy bodies.27, 28 Postmortem examination of PDD brains has shown a range of pathology, with a combination of Lewy body and Alzheimer's-type pathology more predictive of dementia than any single pathology.7 A recent study has suggested that amyloid deposition in Lewy body disease (PDD or Lewy body dementia) is associated with an ‘Alzheimer's disease pattern' of GM atrophy affecting in particular parahippocampal, lateral temporal, and parietal cortex.29 Additionally, accumulating white-matter lesions in many PD and AD subjects adds a further stressor that may mediate more intense neurodegeneration or overwhelm compensatory mechanisms.30 It is plausible that the differences between AD and PDD relate to earlier stages in their development, before a final shared pathway of neurodegenerative change has begun. This viewpoint is supported by our PCA—which identified relative perfusion differences in regions known to be affected early in each disease—and is concordant with recent work suggesting that neurodegenerative conditions begin within ‘functional networks' before becoming more widespread.31
We believe our study samples were representative of their respective populations. The observed reductions in perfusion were consistent with previous studies that compared HCs with AD and PDD groups.15, 17, 32 The similarity in neuropsychological deficits between the two groups, showing differences only in verbal memory and attention, was also in agreement with previous work.33 There were no significant group differences on global cognitive testing or clinical dementia rating. We did not assess Unified Parkinson's Disease Rating Scale in the AD group, although it should be noted that previous studies have commonly found extrapyramidal signs within AD patients.11 All PDD patients, but no AD patients, took levodopa therapy; however, we feel that this difference is unlikely to have changed our results. Although global cerebral blood flow does not change as a result of dopaminergic medication,34, 35 focal blood flow increases have been reported in basal ganglia and frontal regions.34 This may have reduced the significance of the differences identified on PCA, but should not have altered other factors. In particular, the greatest areas of hypoperfusion (and similarity) were more posterior, in regions where dopaminergic networks are felt to be less important. Other baseline demographic variables (including age and years of education) were very comparable between the two groups, and adjusted for in the analysis. Head motion is a potential confounder in ASL-MRI. As head tremor is not usually a feature of PD, it is unlikely that the two dementia groups exhibited significantly different amounts of motion; however, this remains a limitation of the study. Additional potential biases in the study are a change in MRI scanner software version during the course of the study and the different sex ratios in the groups, although it seems unlikely these issues had a strong influence on our results. The software change affected both groups to a similar extent, and we adjusted for software version and sex in VBA and ROI. We interpreted the absence of significant difference between AD and PDD groups as a general similarity between the two groups, but it is possible that we were unable to detect subtle differences because of the relatively small group sizes.
From a clinical perspective, this study provides important insights into the pathophysiology of two common dementias. It provides evidence for why both conditions respond to the same treatment (cholinesterase inhibitor therapy), despite (especially early) clinical differences. More broadly, it suggests that any therapy found to be effective in one condition should also be considered for trial in the other. A logical extension of this is that, for disease-specific therapies to be effective, treatment must begin early in the disease process, before widespread degeneration has occurred. One application of ASL imaging we did not utilize, ASL-fMRI, may be increasingly important in this respect. Although still a developing technique assessing change in regional cerebral perfusion in response to functional cognitive tasks is likely to be more sensitive at detecting subtle or early differences between individuals, potentially aiding both diagnosis and further research into the pathophysiology of neurodegenerative disorders.36
This study provides new evidence of strong similarities between two important dementia syndromes, while also showing some relative cerebral perfusion differences between them. The similarity in deficits, along with the novel AD/PDD network, requires confirmation in larger and separate cohorts, including mild cognitive impairment or at-risk populations. This study provides further insight into the evolution of these two neurodegenerative diseases, suggesting that while different inciting forces set up the neurodegenerative process in each condition, the mechanisms driving the eventual evolution to dementia may well be closely linked.
Acknowledgments
The authors would like to thank all subjects who gave their time to participate in this study. We would also like to thank Dr Matthew Croucher for assistance with AD data collection.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the New Zealand Brain Research Institute, the Neurological Foundation of New Zealand, The Neurology Trust, and the Canterbury Medical Research Foundation.
Supplementary Material
References
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important. Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Siebert GA, Kril JJ, Mellick GD. Knowing me, knowing you: can a knowledge of risk factors for Alzheimer's disease prove useful in understanding the pathogenesis of Parkinson's disease. J Alzheimers Dis. 2011;25:395–415. doi: 10.3233/JAD-2011-110026. [DOI] [PubMed] [Google Scholar]
- Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- Detre JA, Rao H, Wang DJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging. 2012;35:1026–1037. doi: 10.1002/jmri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wolk DA, Reddin JS, Korczykowski M, Martinez PM, Musiek ES, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–1985. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Detre JA. Arterial spin labeling MRI: an emerging biomarker for Alzheimer's disease and other neurodegenerative conditions. Curr Opin Neurol. 2012;25:421–428. doi: 10.1097/WCO.0b013e328354ff0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, Stern Y. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer's disease. J Cereb Blood Flow Metab. 2007;28:725–736. doi: 10.1038/sj.jcbfm.9600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pearson JF, Rueger S, Pitcher TL, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain. 2011;134 (Pt 3:845–855. doi: 10.1093/brain/awq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O'Brien JT. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage. 2003;20:1309–1319. doi: 10.1016/S1053-8119(03)00364-1. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psych. 2012;83:183–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia D, Clavero P, Gasca Salas C, Lamet I, Arbizu J, Gonzalez-Redondo R, et al. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson's disease. Eur J Nucl Med Mol Imaging. 2012;39:1767–1777. doi: 10.1007/s00259-012-2198-5. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Shimada H, Shinotoh H, Hirano S, Miyoshi M, Sato K, Tanaka N, et al. beta-Amyloid in Lewy body disease is related to Alzheimer's disease-like atrophy. Mov Disord. 2013;28:169–175. doi: 10.1002/mds.25286. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Seara MA, Mengual E, Vidorreta M, Aznárez-Sanado M, Loayza FR, Villagra F, et al. Cortical hypoperfusion in Parkinson's disease assessed using arterial spin labeled perfusion MRI. Neuroimage. 2012;59:2743–2750. doi: 10.1016/j.neuroimage.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Song IU, Kim JS, Yoo JY, Song HJ, Lee KS. Cognitive dysfunctions in mild Parkinson's disease dementia: comparison with patients having mild Alzheimer's disease and normal controls. Eur Neurol. 2008;59:49–54. doi: 10.1159/000109261. [DOI] [PubMed] [Google Scholar]
- Hirano S, Asanuma K, Ma Y, Tang C, Feigin A, Dhawan V, et al. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson's disease. J Neurosci. 2008;28:4201–4209. doi: 10.1523/JNEUROSCI.0582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, et al. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol. 1992;32:749–757. doi: 10.1002/ana.410320608. [DOI] [PubMed] [Google Scholar]
- Borogovac A, Asllani I. Arterial Spin Labeling (ASL) fMRI: advantages, theoretical constrains, and experimental challenges in neurosciences. Int J Biomed Imaging. 2012;2012:818456. doi: 10.1155/2012/818456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.