Abstract
Ischemic preconditioning is emerging as an innovative and novel cytoprotective strategy to counter ischemic vascular disease. At the root of the preconditioning response is the upregulation of endogenous defense systems to achieve ischemic tolerance. Identifying suitable biomarkers to show that a preconditioning response has been induced remains a translational research priority. Preconditioning leads to a widespread genomic and proteonomic response with important effects on hemostatic, endothelial, and inflammatory systems. The present article summarizes the relevant preclinical studies defining the mechanisms of preconditioning, reviews how the human preconditioning response has been investigated, and which of these bioresponses could serve as a suitable biomarker. Human preconditioning studies have investigated the effects of preconditioning on coagulation, endothelial factors, and inflammatory mediators as well as on genetic expression and tissue blood flow imaging. A biomarker for preconditioning would significantly contribute to define the optimal preconditioning stimulus and the extent to which such a response can be elicited in humans and greatly aid in dose selection in the design of phase II trials. Given the manifold biologic effects of preconditioning a panel of multiple serum biomarkers or genomic assessments of upstream regulators may most accurately reflect the full spectrum of a preconditioning response.
Keywords: biomarkers, ischemia, preconditioning
In the practice of medicine it is inevitable that some patients respond to prescribed treatments while others do not. Differentiating responders from nonresponders remains one of the challenges not only of clinical medicine but also of drug discovery and development, that is, how can we be certain that a specific treatment induces the targeted biologic activity and subsequently the desired clinical effect? The former may not necessarily imply the latter. Take for instance the widespread administration of aspirin for the prevention of vascular disease. The effect of aspirin on platelet function can readily be measured (desired biologic activity), as can the clinical effect (prevention of vascular events). However, we now know that not everybody who takes aspirin responds biologically, that is, platelet function is not inhibited (aspirin resistance), which in turn may identify a subset of patients at higher risk for vascular outcomes.1 Conversely, achieving the targeted biologic response, in this case platelet inhibition, does not guarantee prevention of vascular events, in the setting of poorly controlled vascular risk factors such as hypertension, diabetes, or dyslipidemia. Many factors determine the individual response to treatment with genetic factors accounting for much of this variability.2
Developing easily measurable end points of biologic activity to facilitate the translational success of novel cardioprotective and neuroprotective therapies has been a recommendation of the 2010 NHBL (National Heart Blood and Lung Institute) Workshop on cardioprotection and the STAIR (Stroke Therapy Academic Industry Roundtable) on neuroprotection.3, 4 A novel treatment strategy to counter ischemic cardiac disease and stroke is ischemic preconditioning. Ischemic preconditioning rests on the basic premise that brief durations of ischemia induce innate defense systems, and lead to tolerance of subsequent, more severe ischemia. In direct preconditioning, for example, brief occlusions of a coronary or carotid artery protect from longer durations of ischemia and reduce infarct volumes. In remote ischemic preconditioning inducing brief ischemia in one organ, subsequently protects a distant organ from more severe ischemia, that is, typically an easily accessible tissue such as a limb is transiently made ischemic to protect the brain or the heart from subsequent more noxious ischemia.
There is extensive laboratory evidence of the protective effects of ischemic preconditioning, which spans across many different species and organs, including heart, lung, brain, intestines, and kidneys.5, 6, 7, 8, 9, 10 This attests to the generalizability of the preconditioning response. At the root of this phenomenon is the stimulation of the body's own anti-ischemic defense systems and the induction of a state of ischemic tolerance. Ischemic preconditioning, particularly remote ischemic preconditioning, through transient cycles of limb ischemia, has been clinically investigated in myocardial and to a lesser extent in cerebral ischemia.11, 12, 13 In cardiac medicine preliminary clinical trials have not consistently shown efficacy of ischemic preconditioning, which raises the possibility that some subjects respond to the preconditioning stimulus, whereas others may not.14
Finding the responders and the factors that may influence this biologic response remains a highly desirable objective as ischemic preconditioning moves forward into clinical testing. In a recent workshop on preconditioning, such biomarker development was thought to be a priority in translating the successful protection achieved by preconditioning in laboratory models of ischemia to clinical medicine.15, 16 Similarly, the 2010 NHBL Workshop recommended the development of biomarkers, showing the existence of a cardioprotective state, as a basic science research priority for the development of new cytoprotective therapies.3
The characterization of such a systemic state of ischemic tolerance is the objective of biomarker development for preconditioning, particularly remote preconditioning. The ideal biomarker should be easily obtained, with satisfactory sensitivity and specificity, allowing repeated testing over time. It should show an early change in response to the stimulus, show a dose–response relationship, and be linked to the mechanism of protection. A biomarker would facilitate individual dose titration and clinical trial design, but ultimately needs validation of clinical efficacy. Several clinical studies have examined the biologic response to preconditioning. In the present article, we will review the current data on how the human preconditioning response has been investigated and whether these assessments allow for suitable biomarker development.
Preconditioning: mechanism and mediators of protection
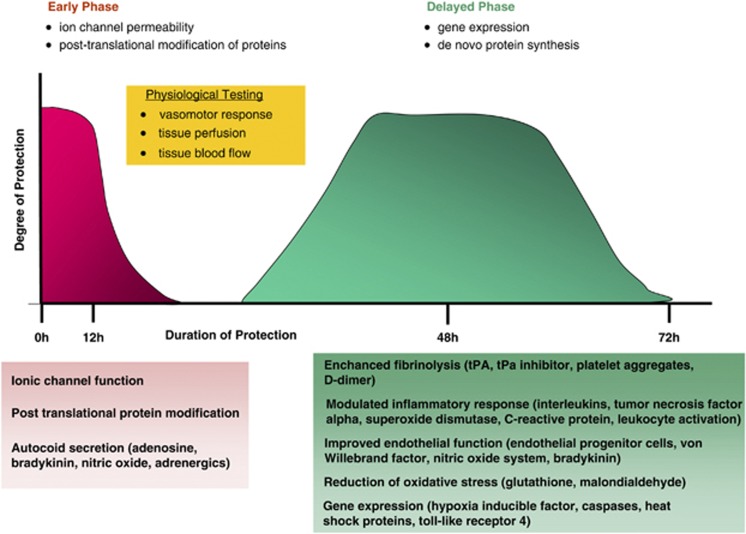
The preconditioning response is divided into an early immediate phase that persists for several hours and a delayed phase, with a later onset but longer duration, lasting 2 to 3 days. The two phases confer protection through different mechanisms. The early phase affects ion channel permeability and the posttranslational modification of proteins, with release of autocoids such as adenosine, nitric oxide (NO), and bradykinin. The later phase depends on gene expression and de novo synthesis of proteins involved in endothelial health, hemostasis, immune response, and cellular energy metabolism (Figure 1).17 While it is generally held that the delayed response is more robust, there has been very little direct comparison of the two preconditioning phases.18
Figure 1.
Overview of the preconditioning response that comprises an early and delayed phase. Effector pathways mediating the preconditioning response and potential targets of biomarker development are depicted in the boxes. tPA, tissue plasminogen activator.
The synthesis of new proteins during the late-response phase may make biomarker discovery more feasible in this setting. Most clinical studies of ischemic preconditioning have rendered a limb, typically the arm, transiently ischemic to protect the heart or the brain. The exact mechanisms of protection in remote ischemic preconditioning remain uncertain. The fact that this protection can be transferred from a conditioned animal to a naïve animal by whole blood transfusion suggests the involvement of humeral factors.16, 19 This strongly supports development of a serum-based biomarker as a suitable candidate to measure whether a preconditioning response has occurred. The exact nature of this serum transferable factor is unknown. Remote ischemic precondition activates multiple bioactive substances such as adenosine, prostaglandin, opioids, erythropoietin, and their respective signaling pathways.20 While clearly important in mediating the protective response these substances may not necessarily represent the transferable factors that confer actual protection.20
Laboratory preconditioning response
Many preclinical studies have shown an effect of preconditioning by directly examining the targeted tissue. In a rat model of focal cerebral ischemia, preconditioning with 10 minutes of middle cerebral artery occlusion significantly reduced the postischemic expression in the preconditioned hemisphere of many inflammatory genes including cytokines, chemokines, adhesion molecules, and proinflammatory transcription factors, and prevented the infiltration of neutrophils and macrophages into the ipsilateral cortex.21 Others have shown a multitude of responses such as genetic expression of heat-shock protein (HSP), upregulation of adenosine receptors, glutamate transporters and NO metabolism in the targeted tissue such as the rat brain and heart, and correlated this with tissue protection.22, 23 A large part of the genetic response to preconditioning involves mitochondrial proteins, leading to more efficient energy homeostasis.17 Measuring the preconditioning response in this manner is of little clinical value for biomarker development as this would require direct tissue examination, which is invasive and not feasible in human organs such as the brain. These preclinical studies have however defined important signaling pathways of the preconditioning response and in this manner serve as a basis and rationale for further clinical biomarker development and testing. Laboratory investigations into the effect of preconditioning on hemostatic, endothelial, and inflammatory mediators have been the most investigated targets in clinical trials of human preconditioning.
Hemostasis
In animal studies ischemic preconditioning, by transient middle cerebral artery occlusion, affected the expression of many genes involved in blood coagulation and lead to prolonged bleeding times in mice.24 It has been suggested that the effects of preconditioning include activation of the endogenous fibrinolytic system. In a rodent model of caerulein pancreatitis, direct ischemic conditioning, by transient clamping of the celiac artery, accelerated fibrinolysis and increased D-dimer levels.25 D-dimer is generated by plasmin from fibrin and elevated levels are a marker of fibrinolysis and the formation of fibrin products.25 Interestingly, fibrin-derived peptides are cardioprotective in animal models of myocardial ischemia.26 The major activator of the fibrinolytic system is tissue plasminogen activator (tPA) that generates plasmin from plasminogen. This reaction is in turn inhibited by tissue plasminogen inhibitor. Of note is that tPA has been shown to induce ischemic tolerance and was found to be an endogenous neuroprotectant in a mouse model of focal cerebral ischemia.27
Endothelial and Vasomotor Function
Preconditioning's preservation of endothelial function and vasomotor health are important biologic effects. In large part these are mediated through the NO system, vascular endothelial growth factor (VEGF), and the release of vasoactive substances, including adenosine and bradykinin.28 Increased immunoreactivity of NO synthase (NOS) has been shown in the cerebral vasculature 24 hours after ischemic preconditioning.29 Blockade of NOS or knock-out mice deficient in endothelial or inducible NOS abolishes the protective effect of preconditioning.30 Increased NO and NOS activity was showed in the rat CA1 hippocampal region after temporary clamping of the femoral artery.31 This was accompanied by a rise in serum NO and NOS. Nitric oxide mediates the effects of preconditioning in multiple organ systems including myocardium, brain, retina, and liver supporting it's role in the preconditioning response.9, 32 In mice, endothelial progenitor cells have been proposed to mediate preconditioning effects by serving as reservoirs for cytokine, NOS, and vasoactive substances and are recruited to tissues under ischemic stress.33 Ischemic preconditioning enhances VEGF mRNA expression in a rat model of myocardial ischemia and serum levels increased after 1 and 3 hours after transient abdominal aortic occlusion in mice and were associated with protection from myocardial ischemia.34 Particularly in remote ischemic preconditioning the release of vasoactive substances such as adenosine and bradykinin is thought to mediate the protective effects.35 The net physiologic effects on vasomotor function include improved cerebral perfusion and penumbral blood flow, with enhanced recovery of cerebral blood flow in the postischemic period.36, 37, 38
Inflammatory Response
Preconditioning has multiple effects on the inflammatory system including cytokine and chemokine expression. Inflammation in acute ischemia leads to secondary damage and further tissue injury. The protective effects of ischemic preconditioning include a downregulation of inflammation and reduction in the activation of neutrophils and leukocyte–endothelial interactions.39, 40 The modulation of immune response includes a decrease in proinflammatory cytokines interleukin-1 (IL-1) and IL-6 expression and upregulation of antiinflammatory cytokines such as IL-10.25, 41 In a rat model of myocardial ischemia, C-reactive protein levels were decreased particularly when assessed during the late phase of preconditioning.42 Important signaling pathways may occur through tumor necrosis factor alpha (TNF-α), which has been implicated in both conferring ischemic tolerance and neurotoxic effects.40 Tumor necrosis factor alpha activation of NF-kB has numerous proinflammatory and cytotoxic actions as well as anti-inflammatory and cytoprotective effects.43
Genetic Expression
Preconditioning reprograms the genetic response to ischemia and leads to widespread molecular and cellular changes.44 Microarray analysis has shown that in preconditioned animals over 60% of genes expressed in response to stroke are not present in animals only exposed to stroke.44 An important transcriptional response to hypoxic stress is conferred by hypoxia-inducible factor-1 alpha. Under hypoxic conditions, hypoxia-inducible factor-1 alpha activates genes involved in oxygen metabolism (erythropoietin), vasomotor health (VEGF), cell survival, and metabolic protection (glucose transporters and glycolytic enzymes).18
Technological advances have now made it possible to monitor alterations in the expression of thousands of genes in response to a particular stimulus, through high-density gene microarrays.23 The induction of genes currently believed to be at the root of the preconditioning response such as hypoxia-inducible factor, toll-like receptor 4, or HSP may be of particular interest. An example is the activation of sirtuins through hypoxia-inducible factor.45 Sirtuins are a family of stress response proteins that increase cellular longevity across several species and increase lifespan and protect against cardiovascular disease.46
Biomarker assessment in clinical preconditioning
Clinical studies have begun to assess a human biologic response to preconditioning based on the above reviewed signaling pathways identified in preclinical studies. These studies have been primarily conducted in healthy volunteers and in patients presenting for cardiac surgery or percutaneous coronary intervention.11, 47 In cardiac surgery troponin has frequently been used as a biomarker for a surrogate outcome of efficacy, rather than showing the induction of a preconditioning response. While beneficial effect on troponin—that is, diminished postsurgical troponin increase—implies myocardial protection and a state of ischemic tolerance, the converse is not necessarily the case. Some patients may respond to the preconditioning stimulus, yet may not achieve sufficient protection in the setting of other adversarial factors such as peri-operative complications, for example, blood loss and infection. Most clinical studies to date have assessed a human preconditioning response through investigations of serum markers of hemostasis, endothelial function, and inflammation and have analyzed the direct physiologic effects of these systems by assessing vasomotor function and tissue perfusion.13, 33, 48, 49
Serum Biomarkers
Serum biomarkers are of particular interest given their historical use in clinical medicine. With the multitude of biologic effects of preconditioning, the identification of a serum protein, characterizing the presence of a state of ischemic tolerance, seems likely. The fact that ischemic tolerance can be transferred with whole blood transfusion between animals strongly supports the existence of such a humeral marker. In a recent study of remote ischemic preconditioning a global proteonomic response was stimulated in the serum of healthy volunteers undergoing repetitive cycles of arm preconditioning, providing further impetus to the study of serum biomarkers.50 Serum biomarkers are easily obtained, can be serially repeated, and may already be commercially available. Clinical studies to date have predominately analyzed the effects of preconditioning on the hemostatic, endothelial, and inflammatory systems. These studies are outlined in Table 1.
Table 1. Clinical studies assessing serum biomarker response to preconditioning.
| Serum biomarker | Population | Preconditioning stimulus | Early phase Assessment<24 hours | Delayed phasea Assessment⩾24 hours |
|---|---|---|---|---|
| Hemostatic | ||||
| tPA tPA inhibitor antigen and activity | Healthy volunteers52 | 3 × 5 min arm ischemia | No effect | |
| Platelet aggregates | Healthy volunteers53 | 3 × 5 min arm ischemia | Decreased | |
| Endothelial | ||||
| VEGF | Healthy Volunteers49 Healthy volunteers55 | 6 cycles arm ischemia daily for one month 3 × 5 min bilateral leg ischemia | No effect | Increase No effect |
| Endothelial progenitor cells | Healthy volunteers55 | 3 × 5 min leg ischemia | Increase | Increase |
| von Willebrand | Healthy volunteers56 | Bradykinin 5 mcg/mL | No effect | |
| NO2, NO3 markers of NO metabolism | Healthy volunteers56 Healthy volunteers55 | Bradykinin 5 mcg/mL 3 × 5 min bilateral leg ischemia | Preserved NO availability No effect on NO | No effect on NO |
| Inflammatory | ||||
| CRP | Prior to PCI54 | 3 × 3 min arm ischemia | No effect | |
| IL-6, IL-8, IL-10, TNF | Healthy volunteers55 | 3 × 5 min leg ischemia | Increased IL-8 | Increased IL-8 |
| Infants with congenital heart disease57 | 3 × 5 min arm ischemia twice over 24 hours | Attenuated | ||
| Infants with congenital heart58 | 3 × 5 min leg ischemia | Modulated | ||
| Orthopedic surgery59 | 3 × 5 min leg ischemia | Decreased IL-6 and IL-8 No effect IL-10 | ||
| Stroke population40 | Preceding TIA | Increased TNF/IL-6 index | ||
| Neutrophil activation | Healthy volunteers68 | No effect | ||
| Superoxide dismutase malondialdehyde | Infants with congenital heart disease57 | 3 × 5 min arm ischemia twice over 24 hours | Attenuated coronary sinus levels | |
| Genomic Response | ||||
| Heat shock protein (HSP) | Infants with congential heart disease57 | 3 × 5 min arm ischemia | Increased expression of HSP-70 in myocytes obtained during surgery | |
| Inflammatory gene expression (caspase, HSP-70, TLR 4, TNF) | Healthy volunteers68 | 3 × 5 min arm ischemia | Modified inflammatory gene expression | Modified inflammatory gene expression |
| Proteonomics | Healthy volunteers50 | 4 × 5 min arm ischemia | Expression of multiple proteins modulated | Expression of multiple proteins modulated |
CRP, C-reactive protein; HSP, heat shock protein; IL, interleukin; NO, nitric oxide; NOS, NO synthase; PCI, percutaneous coronary interventions; TIA, transient ischemic attack; TLR, toll like receptor; TNF, tumor necrosis factor; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor.
24 h was used as an arbitrary cutoff for identifying the immediate and delayed phases of preconditioning.
Hemostatic Markers
Proteonomic studies in healthy volunteers, preconditioned with 4 × 5 minutes cycles of arm ischemia, showed that the expression of many proteins involved in hemostasis is altered.50 Animal studies have shown widespread effects of preconditioning on the expression of genes involved in hemostasis and fibrinolysis supporting proteins involved in the coagulation cascade as potential biomarkers of the preconditioning response. tPA levels and tPA inhibitor are commercially available and D-dimer levels are already in widespread clinical use. Only a few clinical studies have assessed the effects of preconditioning on these proteins. In subjects with subarachnoid hemorrhage four limb conditioning sessions, each consisting of 3 to 4 cycles of 5 to 10 minutes leg ischemia cycles, were correlated with a small, but statistically significant, prolongation of prothrombin time and international normalized ratio.51 In a study of healthy volunteers 3 × 5 minutes cycles of arm ischemia did not affect serum tPA and tPA inhibitor antigen and activity assessed within ∼30 minutes of the preconditioning stimulus.52 However, the time interval between the preconditioning stimulus and the serum analysis was relatively short, and a delayed effect of preconditioning on these proteins remains a possibility. In a similar study, the same preconditioning protocol reduced platelet activation.53 Whether markers of platelet activation reliably measure a preconditioning response requires further validation from additional studies.
Endothelial and Vasoactive Factors
Several clinical studies have evaluated the impact of preconditioning on vasoactive and endothelial factors. In healthy volunteers, daily repetitive cycles of arm preconditioning for 1 month increased VEGF levels and endothelial progenitor cells, which are markers of endothelial health and function. Only the latter was correlated with improved endothelial brachial vasomotor function to acetylcholine.49 This was not replicated in a study of patients presenting for percutaneous intervention, in whom 3 × 3 minutes cycles of upper arm ischemia immediately before the procedure did not change endothelial progenitor cell counts within the first 24 hours.54 Incidentally, no effect was noted on C-reactive protein in this study either. However in healthy volunteers progenitor cell counts increased during the first 24 hours after 3 × 5 minutes cycles of leg ischemia, which correlated with IL-8 levels but not with VEGF and serum nitrate (NO3), and nitrite (NO2) concentrations.55
Such differences are difficult to explain, for example, why does limb preconditioning, in subjects presenting for elective percutaneous coronary interventions, not lead to circulating progenitor cell increase, whereas in healthy volunteers it does? Differences in the type of conditioning stimulus applied and time of biomarker assessment may contribute to such discrepancies. Is preconditioning with 3 × 3 minutes cycles of arm ischemia (as done in patients presenting for coronary interventions) likely to result in the same biologic response as 3 × 5 minutes cycles of leg ischemia (healthy volunteers)? Does preconditioning the larger volume of tissue in a leg lead to a stronger biologic response? Would progenitor cell recruitment be expected to occur within hours or likely require more time? The assessment in patients presenting for percutaneous coronary intervention occurred within hours of the conditioning stimulus while in healthy volunteers progenitor cell analysis occurred much later. Such differences are likely to be factors as biomarker development proceeds.
Several vasoactive substances have been proposed to mediate the preconditioning response. These include bradykinin and adenosine and activation of NO. Little clinical data are available in regards to how preconditioning affects these levels, in part due to difficulties measuring some of these substances. Bradykinin preconditioning in healthy volunteers preserved brachial vasomotor function and plasma nitrate levels, while no effect on von Willebrand factor, a structural marker of endothelial integrity, was found, when assessed shortly after the conditioning stimulus was administered.56
Inflammatory Markers
Most of the clinical work in assessing a human preconditioning response has involved the immune and inflammatory systems. In a clinical study of infants undergoing cardiac surgery delayed arm preconditioning affected postoperative levels of IL-6, IL-8, IL-10, and TNF-α.57 Myocardial tissue obtained intraoperatively showed increased expression of HSP-70 in preconditioned infants; however, such an invasive investigation into the biologic response is not practical in most instances. In a similar study in children undergoing congenital heart defect surgery, leg preconditioning just before surgery did not affect IL-6, IL-8, IL-10, or TNF-α levels, but did increase the variance of IL-10 and TNF-α, suggestive of an acute modulation of inflammatory pathways.58 Of interest is that a positive TNF-α/IL-6 ratio has been proposed as a marker for ischemic tolerance in humans. In a study of 283 patients with stroke and assessing the protective effects of transient ischemic attack (TIA) before stroke, a positive TNF-α/IL-6 index was present in 92% of patients with prior TIA compared with 1% of patients without prior TIA. An increased TNF-α/IL-6 index was associated with improved stroke outcome.40 In a similar manner leg preconditioning reduced postoperative rise in malondialdehyde, IL-6, and IL-8 within 24 hours after orthopedic surgery.59 No effect on C-reactive protein was seen in a clinical study of patients undergoing arm conditioning right before coronary interventions.54
Imaging biomarkers
Aside from serum analysis, the preconditioning response has also been evaluated through blood flow and perfusion imaging, as well as vasomotor testing (Table 2).
Table 2. Clinical studies assessing physiologic responses to preconditioning.
| Physiologic Testing | Population | Preconditioning Stimulus | Early Phase Assessment<24 h | Delayed Phase Assessment⩾24 ha |
|---|---|---|---|---|
| Endothelial function | ||||
| Brachial artery vasomotor function | Healthy volunteers48 | 3 × 5 min arm ischemia | Improved flow after ischemic reperfusion injury | Improved flow after ischemia reperfusion injury |
| Healthy volunteers and patients with atherosclerosis66 | 2 × 3 × 5 min arm or leg ischemia | Improved flow after ischemic reperfusion injury (not all groups) | ||
| Healthy volunteers49 | 5 min arm ischemia 6 × daily | Improved vasomotor response to acetylcholine | ||
| Healthy volunteers67 | 3 × 5 min arm ischemia | Improved vasomotor response to acetylcholine | ||
| Imaging Blood flow or perfusion imaging | ||||
| SPECT | Symptomatic intracranial stenosis13 | 5 × 5 min arm ischemia twice daily for 300 days | Improved SPECT perfusion | |
| TCD | Symptomatic intracranial stenosis13 Subarachnoid hemorrhage65 | 5 × 5 min arm ischemia twice daily for 300 days 4 × 5 min leg ischemia repeated 3–4 times over 10 days | Vasodilation | Improved TCD velocities No effect |
| Coronary angiography | Prior to elective percutaneous intervention64 | Direct coronary preconditioning 3 × 5 min arm ischemia | No effect on coronary flow or resistance | |
| Transthoracic Doppler echocardiography | Healthy volunteers63 | Remote preconditioning (stimulus unclear) | Improved coronary blood flow | |
| Metabolic Assessment | ||||
| Cerebral microdialysis | Subarachnoid hemorrhage65 | 4 × 5 min leg ischemia repeated 3–4 times over 10 days | No effect on lactate: pyruvate ratio Reduction in glycerol | Reduced lactate: pyruvate ratio Reduction in glycerol |
SPECT, single photon emission computed tomography; TCD, transcranial Doppler.
24 h was used as an arbitrary cut off for identifying the immediate and delayed phases of preconditioning.
Blood Flow
Important physiologic effects of ischemic preconditioning include improved blood flow in the target organ during and after ischemia. In animal models of focal cerebral ischemia, preconditioning improves blood flow in the ischemic penumbrae, in comparison with animals not preconditioned.60 In a porcine model, remote preconditioning reduced coronary resistance and improved coronary blood flow.61 Similarly, coronary postischemic blood flow is preserved after preconditioning in dogs.62 Several clinical studies have assessed blood flow changes in response to preconditioning. Improvements in cerebral perfusion and blood flow by single photon emission computed tomography and transcranial Doppler have been shown with repetitive bilateral arm preconditioning for secondary prevention of stroke in patients with intracranial stenosis.13 After 300 days of daily arm preconditioning, 76% of subjects (versus 53% controls) showed improved cerebral perfusion by single photon emission computed tomography. While preconditioning reduced the incidence of recurrent stroke the authors did not report any possible correlation between stroke prevention and improved cerebral hemodynamics. Similarly, left anterior descending coronary artery flow measured by transthoracic Doppler echocardiography improved immediately after remote ischemic preconditioning in healthy volunteers.63 However, no effect on coronary resistance or flow was found by either direct coronary artery preconditioning or remote arm preconditioning in patients presenting for elective coronary percutaneous intervention.64
In a small study of subjects with subarachnoid hemorrhage, remote ischemic limb preconditioning, improved transcranial Doppler measured cerebral hemodynamics and cerebral microdialysis showed a reduction in the lactate/pyruvate ratio and glycerol—indicating cell membrane preservation.65 This opens the possibility of carefully monitoring metabolic responses to preconditioning in a subset of critically ill patients requiring invasive monitoring for clinical purposes.
Physiologic testing
Several clinical studies have assessed a preconditioning response on endothelial function in a model of brachial ischemic reperfusion injury. In this model, flow-mediated vasodilation was preserved, after 20 minutes of brachial ischemia, when healthy subjects were preconditioned with cycles of arm or leg ischemia.48, 66, 67 There was no difference in the degree of protection on brachial vasomotor function when both the early and late phases of preconditioning were examined.48 In a similar manner, six cycles of daily arm preconditioning for a month improved ipsilateral forearm blood flow responses to acetylcholine.49 These physiologic studies have largely been completed in healthy subjects and it is unknown whether a similar response can be elicited in patients with vascular disease.
Proteonomics and genomics
Very few studies have been done investigating the human genetic and proteonomic response to preconditioning. In a small study of healthy volunteers, preconditioned with 3 × 5 minutes cycles of repetitive arm ischemia, over 150 genes were differentially expressed.68 This included inflammatory genes, genes involved in apoptosis [caspase], cell survival [HSP-70], innate immunity [TLR4] among many others, when measured at 15 minutes and at 24 hours after the preconditioning stimulus.68 At 24 hours this activation of genes involved in the immune response, correlated with decreased serum neutrophil activation. Of particular interest was the varied genetic expression between individuals, when measured at 15 minutes, while a more consistent response was found between subjects when measured at 24 hours. This confirms the existence of interindividual differences in the response to the conditioning stimulus, that is, possibly identify responders from nonresponders. In a very similar study of proteonomics, preconditioning upregulated multiple proteins involved in acute phase response signaling, and physiologic molecular and cellular functions.50
It is easy to envision that a particular genetic or proteonomic expression profile, obtained in serum, in response to the preconditioning stimulus, could identify individuals who have achieved a state of ischemic tolerance. The challenge now lies in identifying the genomic or proteonomic profile associated with ischemic tolerance. New techniques such as serial analysis of gene expression, Gene Chips and genome wide association studies may greatly facilitate study of the preconditioning response.39 Similar exploratory approaches are now being used to assess the response to treatment in subjects undergoing chemotherapy to determine the likelihood to respond to the prescribed therapy.69 However, the complexities and vast number of genes expressed in response to preconditioning may prove to be overwhelming and an initial focused approach, assessing just a few early stress response and cell survival genes may prove to be more practical.
Biomarkers for preconditioning: special considerations
Biomarker development for preconditioning poses unique challenges. Preconditioning does not target one specific mechanism of action, as is often the case with other therapeutics and many of the cellular effects of preconditioning, for example, on mitochondrial function, are not amenable to conventional biomarker assessment. With preconditioning's effect including many different response systems a single serum biomarker may not capture the full extent of the preconditioning response. A panel of biomarkers, including representatives from each of the major systems affected by preconditioning, that is, hemostatic, endothelial, and inflammatory, may be more suitable to reflect the full spectrum of the preconditioning response. Alternatively assessing the effects of preconditioning further upstream, at the level of genomic expression, may lead to discovery of an expression profile indicative of ischemic tolerance and allow differentiating responders from nonresponders. Ideal biomarkers should be easily obtained and capable of being repeated, thus allowing an examination of both the early and delayed preconditioning response. This supports serum biomarkers, rather than an imaging marker, which tend to be more labor intensive and have additional expense in terms of equipment and technical assistance. The ideal marker should change immediately or early after preconditioning. Gene expression or de novo synthesized proteins may take some time to change and if a marker is altered early after preconditioning an opportunity exists to modify the precondition stimulus to each patient as needed. This would allow individual dose titration and may also identify a threshold or plateau effect, beyond which no further protective response can be achieved.
The need for developing a biomarker for preconditioning is particularly relevant as many questions remain surrounding the optimal conditioning stimulus. The conventional stimulus in clinical studies has been three cycles of 5 minutes arm preconditioning. A recent study, analogous to a drug dose finding study, has assessed the safety of increasing durations of leg ischemia times as a preconditioning stimulus, with conditioning cycles varying from 5 to 10 minutes.70 In laboratory preconditioning studies, particularly those showing cerebral protection, preconditioning cycles have been typically longer than those of clinical studies. In addition, preclinical studies induce hindlimb ischemia, whereas in clinical studies the arm is usually conditioned. Whether longer conditioning times and conditioning a larger volume of tissue such as the leg is more effective in inducing a preconditioning response is uncertain. Examining such a dose response effect through the use of a favorable biomarker profile would allow defining the optimal conditioning stimulus and allow a comparison of the effectiveness of a variety of different types of stimuli, that is, ischemic versus pharmacological.
In clinical practice many other factors not typically addressed in laboratory studies of preconditioning modulate the preconditioning response. These include the effects of age, comorbidities such as diabetes or infections, and medications. Some evidence suggests that ischemic tolerance is less likely achieved in the aged heart and brain. In animal studies transient ischemia leads to a different gene expression in older when compared with younger animals.39 Diabetes, hypertension, and intercurrent infections also lead to systemic stress. The effects of inducing additional stress, as done in preconditioning, remain poorly understood. It is conceivable that in the presence of such conditions, a lower intensity preconditioning stimulus is required to induce protection. Similarly, the preconditioning response is altered by medications commonly used in the prevention of cardiovascular disease, such as statins.71 Development of a biomarker would greatly facilitate our understanding of how these factors influence the preconditioning response and help titrate stimulus intensity needed to induce a protective response for each individual. In this manner, phase II clinical trial design would be optimized particularly in regards to dose finding studies.
Strategies for biomarker development
The development of biologic markers, characterizing a state of ischemic tolerance, remains highly exploratory. Substantial preclinical and clinical effort has been invested in defining the triggering and signaling pathways of the preconditioning response. Some of these pathways have been selected for biomarker development in exploratory proof-of-principle clinical studies, for example, TNF-α/IL-6 and VEGF as molecular indicators that a state of ischemic cytoprotection has been achieved. More clinical studies of this nature are needed, investigating a wider panel and combination of such markers.
In this manner, two strategies may be utilized. One approach takes advantage of patient populations, who through the natural course of their disease have achieved a state of ischemic tolerance. Evidence supports that a TIA or angina shortly before stroke or myocardial infarction lessens the severity of either, as determined by both surrogate markers of clinical outcomes, for example final infarct size on imaging, creatine kinase elevations, development of malignant arrhythmia, as well as hard clinical outcomes such as mortality and neurologic disability.40, 72, 73, 74, 75 This approach was successful in a study of 283 subjects with first stroke, in whom a preceding TIA was found to reduce infarct size on computed tomography and functional outcome at 3 months. On the basis of existing experimental data showing the immunomodulatory effects of ischemic preconditioning, the authors hypothesized and showed that this state of neuroprotection highly correlated with an increased TNF-α/IL-6 ratio.40 Other populations that may have undergone preconditioning may include patients with sleep apnea, who have repetitive, and at times severe, nocturnal hypoxic events or patients with peripheral occlusive vascular disease.76, 77 In a recent observational study of ischemic stroke patients, preexisting peripheral vascular disease resulted in a better functional outcomes and small stroke volumes. The authors attributed this neuroprotection to a preconditioning effect induced by chronic limb ischemia.77 The preconditioning effects of physical exercise have also been described and may contribute to the beneficial effects of exercise in the prevention of vascular disease. Subjects who regularly exercise may therefore also constitute a group of individuals readily accessible for the study of preconditioning biomarkers.78, 79
Such preconditioned populations may provide a fruitful substrate for hypothesis driven exploratory genomic or proteonomic, highly exploratory studies in biomarker development and need to include validation of efficacy through either surrogate markers or clinical outcomes. This strategy would preliminary identify markers of interest that would require further prospective testing.
Alternative approaches, which are more labor intensive, include prospectively investigating the effects of the preconditioning intervention on a preselected set of biomarkers. This would require the inclusion of a control group undergoing a sham preconditioning intervention. This approach would also lend itself well to study dose effects of the preconditioning intervention, for example, the effect of varying durations of limb ischemia used for preconditioning on the selected candidate marker(s). Virtually all studies listed in Tables 1 and 2 have been conducted in this manner. Ideally, this strategy would include a marker of surrogate outcome that would allow preliminary validation of the biomarker. This may be more readily achieved in cardiac medicine where a correlation of the biomarker with a surrogate maker of clinical outcome, that is, troponin levels is relatively more straightforward. In cerebrovascular disease final infarct size or measures of cerebral perfusion or blood flow may be used as surrogate markers, but are less validated and harder to obtain.
The challenge with either strategy is to determine which marker, or set of markers, to investigate. In the absence of a clear candidate marker, characterizing a state of ischemic tolerance, this would require a highly exploratory approach, which in our opinion, should include the screening of a panel of markers, from the major signaling pathways (hemostatic, endothelial/vasoactive, and inflammatory) affected by preconditioning. We also advocate investigating the genomics of the preconditioning response, by assessing the early basic stress response and cellular survival genes to determine whether a particularly genetic expression profile to the preconditioning stimulus allows characterization of a state of cytoprotection.
Ultimately both the preconditioning intervention and a preconditioning biomarker will need to be tested in definitive phase III clinical trials showing clinical efficacy. Ideally, these trials should include a genetic and proteonomic biorepository, which would allow extensive biomarker testing, as well as retrospective investigations as additional candidate markers become available.
Conclusion
Many challenges remain in finding the appropriate biomarker to accurately measure the ischemic preconditioning response. The identification of such a state of endogenous ischemic tolerance would significantly advance the field of cytoprotection in vascular disease. A panel of biomarkers would greatly aid in the dose selection design of phase II clinical trials and proof of concept phase IIb trials. However given the complexities of this phenomenon much work lies ahead to determine the best set of biomarkers to help accurately measure responses to ischemic preconditioning and identify those factors that influence this response.
The authors declare no conflict of interest.
References
- Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch Intern Med. 2007;167:1593–1599. doi: 10.1001/archinte.167.15.1593. [DOI] [PubMed] [Google Scholar]
- Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–289. doi: 10.1097/00008571-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, et al. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, et al. 'Ischemic tolerance' phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Ates E, Genc E, Erkasap N, Erkasap S, Akman S, Firat P, et al. Renal protection by brief liver ischemia in rats. Transplantation. 2002;74:1247–1251. doi: 10.1097/00007890-200211150-00009. [DOI] [PubMed] [Google Scholar]
- Abu-Amara M, Yang SY, Seifalian AM, Fuller B, Davidson BR. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury. Ann Surg. 2011;254:178–180. doi: 10.1097/SLA.0b013e318221ff34. [DOI] [PubMed] [Google Scholar]
- Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, et al. Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transpl Int. 2008;21:364–371. doi: 10.1111/j.1432-2277.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- Waldow T, Alexiou K, Witt W, Albrecht S, Wagner F, Knaut M, et al. Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia. Transpl Int. 2005;18:198–205. doi: 10.1111/j.1432-2277.2004.00005.x. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- Koch S, Gonzalez N. Preconditioning the human brain: proving the principle in subarachnoid hemorrhage. Stroke. 2013;44:1748–1753. doi: 10.1161/STROKEAHA.111.000773. [DOI] [PubMed] [Google Scholar]
- Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- Brevoord D, Kranke P, Kuijpers M, Weber N, Hollmann M, Preckel B. Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. PLoS ONE. 2012;7:e42179. doi: 10.1371/journal.pone.0042179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Perez-Pinzon M. Proceedings of the 2nd Translational Preconditioning Meeting Miami. Transl Stroke Res. 2013;4:1–2. doi: 10.1007/s12975-012-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S. Preconditioning the human brain: practical considerations for proving cerebral protection. Transl Stroke Res. 2010;1:161–169. doi: 10.1007/s12975-010-0025-5. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–327. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis. 1999;8:123–129. doi: 10.1023/a:1008911101951. [DOI] [PubMed] [Google Scholar]
- Lim SY, Hausenloy DJ. Remote ischemic conditioning: from bench to bedside. Front Physiol. 2012;3:27. doi: 10.3389/fphys.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen KK, Naylor M, Vemuganti R. Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int. 2006;49:127–135. doi: 10.1016/j.neuint.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Dave KR, Lange-Asschenfeldt C, Raval AP, Prado R, Busto R, Saul I, et al. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005;82:665–673. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Marjoram P, Poizat C, Kedes L, Kloner RA. Brief episode of ischemia activates protective genetic program in rat heart: a gene chip study. Cardiovasc Res. 2003;59:450–459. doi: 10.1016/s0008-6363(03)00399-7. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Kusnierz-Cabala B, et al. Influence of ischemic preconditioning on blood coagulation, fibrinolytic activity and pancreatic repair in the course of caerulein-induced acute pancreatitis in rats. J Physiol Pharmacol. 2007;58:303–319. [PubMed] [Google Scholar]
- Roesner JP, Petzelbauer P, Koch A, Mersmann J, Zacharowski PA, Boehm O, et al. The fibrin-derived peptide Bbeta15-42 is cardioprotective in a pig model of myocardial ischemia-reperfusion injury. Crit Care Med. 2007;35:1730–1735. doi: 10.1097/01.CCM.0000269035.30231.76. [DOI] [PubMed] [Google Scholar]
- Haile WB, Wu J, Echeverry R, Wu F, An J, Yepes M. Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-alpha. J Cereb Blood Flow Metab. 2012;32:57–69. doi: 10.1038/jcbfm.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi A, Yano S, Morioka M, Hamada J, Ushio Y, Takeuchi Y, et al. Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2004;24:271–279. doi: 10.1097/01.WCB.0000110539.96047.FC. [DOI] [PubMed] [Google Scholar]
- Cho S, Park E-M, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- Tokuno S, Chen F, Pernow J, Jiang J, Valen G. Effects of spontaneous or induced brain ischemia on vessel reactivity: the role of inducible nitric oxide synthase. Life Sci. 2002;71:679–692. doi: 10.1016/s0024-3205(02)01711-3. [DOI] [PubMed] [Google Scholar]
- Zhao HG, Sun XC, Xian XH, Li WB, Zhang M, Li QJ. The role of nitric oxide in the neuroprotection of limb ischemic preconditioning in rats. Neurochem Res. 2007;32:1919–1926. doi: 10.1007/s11064-007-9381-2. [DOI] [PubMed] [Google Scholar]
- Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005;19:1155–1157. doi: 10.1096/fj.04-3220fje. [DOI] [PubMed] [Google Scholar]
- Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via "imported" nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- Kawata H, Yoshida K, Kawamoto A, Kurioka H, Takase E, Sasaki Y, et al. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ Res. 2001;88:696–704. doi: 10.1161/hh0701.088842. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- Vlasov TD, Korzhevskii DE, Polyakova EA. Ischemic preconditioning of the rat brain as a method of endothelial protection from ischemic/repercussion injury. Neurosci Behav Physiol. 2005;35:567–572. doi: 10.1007/s11055-005-0095-0. [DOI] [PubMed] [Google Scholar]
- Hoyte LC, Papadakis M, Barber PA, Buchan AM. Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning. Brain Res. 2006;1121:231–237. doi: 10.1016/j.brainres.2006.08.107. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Katsumata T, Nishiyama Y, Otori T, Katsura K, Katayama Y. Effect of ischemic preconditioning on cerebral blood flow after subsequent lethal ischemia in gerbils. Life Sci. 2006;78:1713–1719. doi: 10.1016/j.lfs.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Guadagni F, Palmirotta R, Ferroni P, Testa G, Cacciatore F, et al. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13:1741–1757. doi: 10.2217/pgs.12.157. [DOI] [PubMed] [Google Scholar]
- Castillo J, Moro MA, Blanco M, Leira R, Serena J, Lizasoain I, et al. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54:811–819. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- Petcu EB, Kocher T, Kuhr A, Buga AM, Kloting I, Herndon JG, et al. Mild systemic inflammation has a neuroprotective effect after stroke in rats. Curr Neurovasc Res. 2008;5:214–223. doi: 10.2174/156720208786413424. [DOI] [PubMed] [Google Scholar]
- Valtchanova-Matchouganska A, Gondwe M, Nadar A. C-reactive protein in acute and delayed preconditioning of the rat heart. Cardiovasc J S Afr. 2005;16:118–123. [PubMed] [Google Scholar]
- Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22:142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38 (2 Suppl:680–685. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- Chen R, Dioum EM, Hogg RT, Gerard RD, Garcia JA. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem. 2011;286:13869–13878. doi: 10.1074/jbc.M110.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Kimura M, Ueda K, Goto C, Jitsuiki D, Nishioka K, Umemura T, et al. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans: role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:1403–1410. doi: 10.1161/ATVBAHA.107.143578. [DOI] [PubMed] [Google Scholar]
- Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, et al. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS ONE. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor F, Bilgin-Freiert A, Connolly M, Katsnelson M, Dusick JR, Vespa P, et al. Effects of remote ischemic preconditioning on the coagulation profile of patients with aneurysmal subarachnoid hemorrhage: a case-control study. Neurosurgery. 2013;73:808–815. doi: 10.1227/NEU.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Pedersen CM, Barnes G, Schmidt MR, Botker HE, Kharbanda RK, Newby DE, et al. Ischaemia-reperfusion injury impairs tissue plasminogen activator release in man. Eur Heart J. 2012;33:1920–1927. doi: 10.1093/eurheartj/ehr380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CM, Cruden NL, Schmidt MR, Lau C, Botker HE, Kharbanda RK, et al. Remote ischemic preconditioning prevents systemic platelet activation associated with ischemia-reperfusion injury in humans. J Thromb Haemost. 2011;9:404–407. doi: 10.1111/j.1538-7836.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- Prasad A, Gossl M, Hoyt J, Lennon RJ, Polk L, Simari R, et al. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013;81:930–936. doi: 10.1002/ccd.24443. [DOI] [PubMed] [Google Scholar]
- Czeiger D, Dukhno O, Douvdevani A, Porat Y, Shimoni D, Fulga V, et al. Transient extremity ischemia augments CD34+ progenitor cell availability. Stem Cell Rev. 2011;7:639–645. doi: 10.1007/s12015-011-9234-x. [DOI] [PubMed] [Google Scholar]
- Liuba P, Batra S, Pesonen E, Werner O. Bradykinin preconditions postischemic arterial endothelial function in humans. J Card Surg. 2005;20:420–424. doi: 10.1111/j.1540-8191.2005.2004120.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu P, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31:22–29. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Lin LN, Wang LR, Wang WT, Jin LL, Zhao XY, Zheng LP, et al. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia-reperfusion. Anesth Analg. 2010;111:539–543. doi: 10.1213/ANE.0b013e3181e368d2. [DOI] [PubMed] [Google Scholar]
- Zhao L, Nowak TS., Jr. CBF changes associated with focal ischemic preconditioning in the spontaneously hypertensive rat. J Cereb Blood Flow Metab. 2006;26:1128–1140. doi: 10.1038/sj.jcbfm.9600269. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Konstantinov IE, Kharbanda RK, Cheung MH, Redington AN. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol (Oxf) 2007;190:103–109. doi: 10.1111/j.1748-1716.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- Loke KE, Woodman OL. Preconditioning improves myocardial function and reflow, but not vasodilator reactivity, after ischaemia and reperfusion in anaesthetized dogs. Clin Exp Pharmacol Physiol. 1998;25:552–558. doi: 10.1111/j.1440-1681.1998.tb02250.x. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yang B, Zhou XM, Tan CM, Zhao Y, Huang C, et al. Effects of remote ischemic preconditioning on the flow pattern of the left anterior descending coronary artery in normal subjects. Int J Cardiol. 2007;122:250–251. doi: 10.1016/j.ijcard.2006.11.079. [DOI] [PubMed] [Google Scholar]
- Hoole SP, Heck PM, White PA, Khan SN, O'Sullivan M, Clarke SC, et al. Remote ischemic preconditioning stimulus does not reduce microvascular resistance or improve myocardial blood flow in patients undergoing elective percutaneous coronary intervention. Angiology. 2009;60:403–411. doi: 10.1177/0003319708328921. [DOI] [PubMed] [Google Scholar]
- Gonzalez NR, Hamilton R, Bilgin-Freiert A, Dusick J, Vespa P, Hu X, et al. Cerebral hemodynamic and metabolic effects of remote ischemic preconditioning in patients with subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:193–198. doi: 10.1007/978-3-7091-1192-5_36. [DOI] [PubMed] [Google Scholar]
- Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation. 2007;116:1386–1395. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MMH, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- Wolf B, Schwarzer A, Cote AL, Hampton TH, Schwaab T, Huarte E, et al. Gene expression profile of peripheral blood lymphocytes from renal cell carcinoma patients treated with IL-2, interferon-alpha and dendritic cell vaccine. PLoS ONE. 2012;7:e50221. doi: 10.1371/journal.pone.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis GF, Pipis J, Fekete V, Kovacs-Simon A, Odendaal L, Molnar E, et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol. 2008;294:H2406–H2409. doi: 10.1152/ajpheart.00862.2007. [DOI] [PubMed] [Google Scholar]
- Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect. Neurology. 2000;54:2089–2094. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart. J Am Coll Cardiol. 1997;30:947–954. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- Lorgis L, Gudjoncik A, Richard C, Mock L, Buffet P, Brunel P, et al. Pre-infarction angina and outcomes in non-ST-segment elevation myocardial infarction: data from the RICO survey. PLoS ONE. 2012;7:e48513. doi: 10.1371/journal.pone.0048513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Zuniga S, Rabinstein AA, Romano JG, Nolan B, Chirinos J, et al. Signs and symptoms of sleep apnea and acute stroke severity: is sleep apnea neuroprotective. J Stroke Cerebrovasc Dis. 2007;16:114–118. doi: 10.1016/j.jstrokecerebrovasdis.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Connolly M, Bilgin-Freiert A, Ellingson B, Dusick JR, Liebeskind D, Saver J, et al. Peripheral vascular disease as remote ischemic preconditioning, for acute stroke. Clin Neurol Neurosurg. 2013;115:2124–2129. doi: 10.1016/j.clineuro.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevaidis IA, Iliodromitis EK, Mavrogeni S, Karavolias GK, Theodorakis GN, Georgiadis M, et al. Repeated exercise stress testing identifies early and late preconditioning. Int J Cardiol. 2005;98:221–226. doi: 10.1016/j.ijcard.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Zdrenghea D, Bodizs G, Ober MC, Ilea M. Ischemic preconditioning by repeated exercise tests involves nitric oxide up-regulation. Rom J Intern Med. 2003;41:137–144. [PubMed] [Google Scholar]