Abstract
Blood ejected from the left ventricle perfuses the brain via central elastic arteries, which stiffen with advancing age and may elevate the risk of end-organ damage. The purpose of this study was to determine the impact of central arterial aging on cerebral hemodynamics. Eighty-three healthy participants aged 22 to 80 years underwent the measurements of cerebral blood flow (CBF) and CBF velocity (CBFV) using magnetic resonance imaging (MRI) and transcranial Doppler, respectively. The CBF pulsatility was determined by the relative amplitude of CBFV to the mean value (CBFV%). Central arterial stiffness (carotid-femoral pulse wave velocity), wave reflection (carotid augmentation index), and pressure were measured using applanation tonometry. Total volume of white-matter hyperintensity (WMH) was quantified from MR images. Total CBF decreased with age while systolic and pulsatile CBFV% increased and diastolic CBFV% decreased. Women showed greater total CBF and lower cerebrovascular resistance than men. Diastolic CBFV% was lower in women than in men. Age- and sex-related differences in CBF pulsatility were independently associated with carotid pulse pressure and arterial wave reflection. In older participants, higher pulsatility of CBF was associated with the greater total volume of WMH. These findings indicate that central arterial aging has an important role in age-related differences in cerebral hemodynamics.
Keywords: age, arterial wave reflection, central arterial stiffness, cerebral hemodynamics, sex difference
Introduction
Cerebrovascular disease (CVD) is a leading cause of death and the major determinant of disability and cognitive impairment.1 With rapid aging of the population, the prevalence of CVD is growing.2 However, the pathophysiologic mechanism(s), in particular the relation between the risk of CVD and age-related changes in cerebral hemodynamics, remains poorly understood.3 Age is the most important risk factor for CVD.4 It directly contributes to the disease pathogenesis but also the manifestation of its risk factors.4
Blood ejected from the left ventricle perfuses the brain via central elastic arteries, which stiffen with advancing age and may increase the risk of end-organ damage.5 The brain is a high flow, low resistance organ that is continuously exposed to the mechanical forces of cardiac pulsations.3 In healthy young individuals, central elastic arteries (e.g., aorta and carotid artery) expand and recoil effectively within each cardiac cycle, providing a Windkessel effect to dampen hemodynamic pulsatility and facilitate a continuous blood flow in the capillaries.6 In contrast, age-related increases in central arterial stiffness may lead to a less effective Windkessel function, enhance cerebral hemodynamic pulsatility, and thus elevate the risk of CVD.6, 7 Indeed, population-based epidemiologic studies have shown that an age-related increase in central arterial stiffness is an important risk factor for white-matter damage and cognitive decline in older adults.8
Previous studies of cerebral hemodynamics with age were limited by a lack of understanding of the association with central hemodynamics, particularly the dynamic relation between pulsatile changes in arterial pressure and cerebral blood flow (CBF). Accordingly, the primary aims of the present study were twofold: (1) to characterize age-related differences in pulsatile cerebral hemodynamics in healthy human subjects and (2) to determine the impact of central arterial aging on cerebral hemodynamics. We hypothesized that while total CBF decreases with age, CBF pulsatility increases due to the elevations in central arterial stiffness, wave reflection, and pressure pulsatility. In addition, given the reported differences in the risk of CVD between men and women,9, 10 we explored sex-related differences in cerebral hemodynamics and its relation to central hemodynamics.
Materials and methods
Study Participants
Eighty-three participants aged 22 to 80 years old (37 men and 46 women) were recruited through flyers and newspaper advertisements from the Dallas/Fort Worth metropolitan area. Subjects were rigorously screened using a 12-lead electrocardiogram, 24-hour ambulatory blood pressure, and echocardiogram to exclude cardiovascular disease. Carotid ultrasonography was performed to exclude individuals with atherosclerotic plaque or stenosis that occluded the common and/or internal carotid artery by >50%.11 Participants were excluded with the following criteria: blood pressure (⩾140/90 mm Hg), fasting blood glucose (>126 mg/dL), body mass index (>35 kg/m2), smoking, pregnancy, and the presence or history of cerebrovascular (e.g., stroke), metabolic (e.g., diabetes), neurologic, psychiatric, or inflammatory diseases, brain damage or trauma, hypothyroidism, active alcoholism, or drug abuse. Individuals who are taking antihypertensive medications or participating in regular exercise were excluded for their potential impact on cerebral and central hemodynamics.12, 13, 14 This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas, and was performed in accordance with the guidelines of the Declaration of Helsinki and Belmont Report. All subjects gave informed written consent before participation.
Procedures
Study visit 1
All data were collected in an environmentally controlled laboratory with an ambient temperature of 22°C. Subjects abstained from caffeinated beverages, alcohol, and vigorous exercise for ⩾24 hours before the study. After subjects rested in the supine position for ⩾10 minutes, intermittent brachial cuff blood pressure was measured at least three times using an ECG-gated electrosphygmomanometer (Suntech, Morrisville, NC, USA) and averaged to obtain systolic and diastolic blood pressures. Heart rate was monitored using a 3-lead ECG (Hewlett-Packard, Palo Alto, CA, USA). Middle cerebral artery blood flow velocity was obtained using a transcranial Doppler (TCD) (Multi-Dop X2; Compumedics/DWL, Singen, Germany) and end-tidal CO2 was recorded via a capnograph (Capnogard; Novamatrix, Wallingford, CT, USA). All of these variables were continuously recorded throughout data collection. Beat-by-beat blood pressure waveforms from the common carotid, brachial, femoral, and radial arteries were recorded continuously for >10 seconds using an applanation tonometry (SphygmoCor 8.0; AtCor Medical, West Ryde, NSW, Australia). During the tonometric measurements, a pressure sensor was directly placed on skin and pressed on the arteries at a location where the strongest pulse was felt and the vessel was well supported by an underlying bone structure.15 Cerebral blood flow velocity (CBFV) was measured over the temporal window ipsilateral to the carotid pressure measurement using a 2-MHz TCD probe, which was fixated at a constant angle by a probe holder (Spencer Technologies, Seattle, WA, USA). The insonation depth and angle were adjusted to optimize the signal quality and strength according to a standard procedure.16 End-tidal CO2 and breathing frequency were monitored by a nasal cannula. During data collection, subjects were instructed to breathe normally and avoid body movement and swallow maneuvers. To ensure stable hemodynamics, intermittent brachial cuff and continuous finger blood pressures (Finapres; Ohmeda, Boulder, CO, USA) were recorded throughout data collection. Arterial blood pressure and CBFV waveforms were inspected visually to obtain high quality signals and to exclude artifacts. Arterial blood pressure and CBFV waveforms were collected with a sampling frequency of 1,000 Hz and analyzed offline using the data analysis software (Acknowledge, BIOPAC Systems, Goleta, CA, USA & DADiSP, Newton, MA, USA). Carotid-femoral (cfPWV) and carotid-radial pulse wave velocities (i.e., measures of central and peripheral arterial stiffness, respectively) and the tonometric indices of carotid pulse wave were recorded using a standard procedure described in detail elsewhere (SphygmoCor 8.0, AtCor Medical).17, 18
Study visit 2
On a separate day, participants underwent a magnetic resonance (MR) imaging (MRI) after abstaining from caffeinated beverage, alcohol, and vigorous exercise for ⩾24 hours. There were 29±34 days between study visits 1 and 2. The MRI data were collected by a 3T MRI system (Philips Medical Systems, Best, The Netherlands) using an 8-channel transmit/receive head coil. Four imaging protocols were performed. First, a 3D time-of-flight MR angiographic image of neck vessels was collected with the following parameters: repetition time=23 ms, echo time=3.5 ms, flip angle=18°, field of view=160 × 160 mm, number of slices=47, resolution=0.3 × 0.3 × 1.5 mm3, a venous saturation slab placed above the imaging slab, and scan duration=1 minute 15 seconds. An imaging plane for 2D phase-contrast MRI (PC-MRI) was determined using the coronal and sagittal maximum-intensity projections of the time-of-flight MR angiographic image. Care was taken to select the imaging plane at the straight and perpendicular portion of vessels above the bifurcations of internal and external carotid arteries and below the bends of vertebral arteries (Supplementary Figure S1a). Second, nongated PC-MRI was collected four times at different stages of the cardiac cycle (time interval=6.5 seconds) and averaged to generate a single image. The acquisition parameters of PC-MRI were as follows: repetition time=20 ms, echo time=6.9 ms, flip angle=15°, field of view=230 × 230 mm, resolution=0.45 × 0.45 mm2, slice thickness=5 mm, maximum velocity encoding=80 cm/s, and scan duration=30 seconds. Third, magnetization-prepared rapid acquisition gradient echo images were acquired with the following parameters: repetition time=8.1 ms, echo time=3.7 ms, flip angle=12°, field of view=256 × 256 mm, number of slices=160 (no gap), resolution=1 × 1 × 1 mm3, SENSE factor=2, and scan duration=4 minutes. Fourth, fluid-attenuated inversion recovery images were collected using the following parameters: repetition time=11,000 ms, echo time=125 ms, inversion time=2,800 ms, field of view=230 × 230 mm, number of slices=64 (no gap), slice thickness=2.5 mm, resolution=0.45 × 0.45 mm2, SENSE factor=2, and scan duration=3 minutes 40 seconds.
Data Analysis
Carotid pressure waveform obtained by applanation tonometry was calibrated to brachial blood pressure using a standard procedure described in detail elsewhere.19, 20 Briefly, beat-by-beat recording of brachial arterial pressure waveform was first calibrated to systolic and diastolic brachial blood pressures measured by electrosphygmomanometer. Brachial mean arterial pressure was calculated from area under curve of the brachial arterial pressure waveform. Carotid pressure waveform was then calibrated to brachial mean and diastolic blood pressures.
Carotid pulse wave analysis was performed to measure the timing and magnitude indices of the reflected pressure wave (Supplementary Figure S2). Specifically, left ventricular ejection time (LVET), time to inflection point (TR), augmented pressure, and augmentation index (AIx) were obtained. Because heart rate influences the relative timing of reflected pressure wave that collides with an incident pressure wave during systole18 and advancing age prolongs LVET,19 relative inflection time was calculated by dividing TR by LVET times 100.21 Carotid-femoral and carotid-radial pulse wave velocities were calculated using a standard procedure described in detail elsewhere.17, 18
Cerebral hemodynamic data were analyzed in both the mean and pulsatile components. The former was obtained from total CBF and time-averaged CBFV measured by PC-MRI and TCD, respectively. The pulsatile component of CBF was estimated from the waveform of CBFV recorded by TCD. To minimize the baseline effect of mean CBFV on the pulsatile indices of CBF, the following procedure was performed. First, continuous CBFV waveforms, consisting of ∼10 cardiac cycles without artifact, were extracted with the simultaneous recordings of carotid arterial pressure. Second, time-averaged CBFV was used to rescale the waveform in percentage (CBFV%). The main rationales to normalize CBFV were twofold: (1) age-related reduction in the mean CBF or CBFV may confound the interpretation of the concurrent alterations in CBF pulsatility22, 23 and (2) the waveform contour of CBFV resembles that of volumetric flow.6 The pulsatile indices of CBF were calculated from the following equations:
Systolic CBFV%=
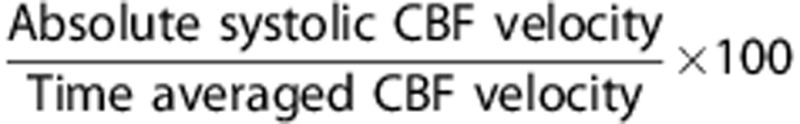
Diastolic CBFV%=
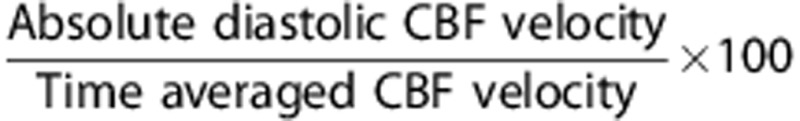
Pulsatile CBFV%=
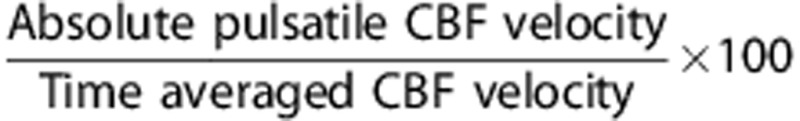
Phase-contrast MRI data were analyzed using a region of interest (ROI) method described in detail elsewhere.24 Briefly, ROIs were manually drawn on each of the bilateral internal carotid and vertebral arteries using the magnitude images. Vessel masks generated from the magnitude image (Supplementary Figure S1b) were then applied to the phase image (Supplementary Figure S1c) to obtain mean blood flow velocity in each of the arteries. Cerebral blood flow was calculated by multiplying mean blood flow velocity by area of the corresponding vessels and added together to obtain total CBF. Total CBF was corrected for individual differences in the brain size and expressed as blood flow (mL/min) per 100 gram of brain tissue. Brain volume was converted into brain mass by assuming a density of 1.06 g/mL.25 Total brain volume was calculated by adding the volumes of cerebrum, cerebellum, and brain stem (FreeSurfer, https://surfer.nmr.mgh.harvard.edu/fswiki). In addition, normalized total brain volume was calculated relative to the intracranial volume.
Total volume of white-matter hyperintensity (WMH) in the supratentorial region was quantified on fluid-attenuated inversion recovery images using a procedure described in detail elsewhere (MRIcro, www.mricro.com).26 First, ROI corresponding to WMH was created using a semiautomated technique that applies individually determined intensity thresholding. Second, gross manual outlining of WMH was performed to create ROI map. Third, the intersection of ROIs created from the first and second steps was identified, visually inspected, and produced the final WMH volume. To account for individual differences in head size, total volume of WMH was normalized to the intracranial volume.
All data analyses were performed blind to clinical and personal information of participants.
Statistical Analyses
Subject characteristics were presented separately for men and women. Sex-related differences in demographic and physiologic variables were examined using independent t-test. Analysis of covariance was performed to control the effect of potential covariate(s). Simple correlation between hemodynamic variables was assessed by Pearson's product moment correlation analysis. Multiple linear regression analysis was used to determine the association of cerebral hemodynamic variables with age, sex, and central hemodynamic measures. In the model, age, age squared, and sex were first entered sequentially. The curvilinear effect of age was examined due to the reported nonlinear relationships between age and central and peripheral hemodynamics,27 and confirmed if age squared made a significant improvement in R2. Sex was dummy-coded as ‘0' for men and ‘1' for women. Central hemodynamic variable(s) that significantly correlated with a dependent, cerebral hemodynamic variable was subsequently entered using the stepwise method. All data are reported as mean±s.d. unless otherwise stated. All statistical analyses were performed using SPSS 17.0 (Chicago, IL, USA). Statistical significance was set a priori at P<0.05 for all tests.
Results
Subject Characteristics
Table 1 presents subject characteristics in men and women. Age, heart rate, cfPWV, and carotid systolic blood pressure were not different between the groups. Height, body mass, and body mass index were smaller in women than in men. Compared with men, women showed lower diastolic and mean arterial pressure and higher carotid pulse pressure. Carotid AIx and AP were greater and relative inflection time was shorter in women than in men. After covarying the effect of carotid AIx, AP, or relative inflection time using analysis of covariance, sex-related difference in carotid pulse pressure became nonsignificant.
Table 1. Subject characteristics and hemodynamics.
| Men | Women | t-test P values | |
|---|---|---|---|
| n | 37 | 46 | |
| Age (years) | 49±13 | 49±16 | 0.99 |
| Height (cm) | 177±7 | 163±6 | <0.001 |
| Body mass (kg) | 85±11 | 66±10 | <0.001 |
| Body mass index (kg/m2) | 27±3 | 25±4 | <0.01 |
| Heart rate (b.p.m.) | 60±9 | 63±9 | 0.13 |
| Brachial blood pressures (mm Hg) | |||
| Systolic pressure | 114±9 | 111±10 | 0.20 |
| Mean pressure | 89±8 | 85±8 | 0.04 |
| Diastolic pressure | 73±8 | 68±6 | 0.001 |
| Pulse pressure | 40±7 | 43±8 | 0.13 |
| Daytime systolic pressurea | 132±8 | 124±10 | <0.001 |
| Daytime diastolic pressurea | 78±6 | 73±7 | 0.001 |
| Night time systolic pressurea | 114±9 | 112±9 | 0.28 |
| Night time diastolic pressurea | 65±6 | 63±7 | 0.16 |
| 24-hour systolic pressurea | 128±8 | 121±9 | <0.001 |
| 24-hour diastolic pressurea | 76±5 | 71±7 | 0.001 |
| Carotid pulse wave measures | |||
| Systolic pressure (mm Hg) | 108±10 | 107±12 | 0.52 |
| Pulse pressure (mm Hg) | 35±5 | 38±9 | 0.03 |
| LVET (ms) | 323±22 | 329±23 | 0.19 |
| Time to inflection point (ms) | 155±17 | 140±22 | 0.001 |
| Relative inflection time (%) | 48±6 | 43±8 | 0.001 |
| Augmented pressure (mm Hg) | 3±8 | 7±8 | <0.01 |
| Augmentation index (%) | 8±18 | 18±18 | 0.01 |
| Augmentation index at 75 b.p.m. (%) | 0±17 | 12±17 | <0.01 |
| Pulse wave velocities (m/s) | |||
| Carotid-femoral | 8.9±1.8 | 8.7±2.4 | 0.68 |
| Carotid-radial | 9.6±1.5 | 9.2±1.3 | 0.20 |
| Cerebrovascular parameters | |||
| End-tidal CO2 (mm Hg) | 39±3 | 38±3 | 0.39 |
| Total CBF (mL/min) | 505±130 | 658±131 | <0.001 |
| Cerebrovascular resistance (mm Hg/mL per minute) | 0.19±0.06 | 0.14±0.04 | <0.001 |
| Total brain volume (mL) | 1,304±107 | 1,158±104 | <0.001 |
| Normalized total brain volume (%ICV) | 79±3 | 81±3 | 0.049 |
| Intracranial volume (mL) | 1,648±131 | 1,437±109 | <0.001 |
| Total WMH (%ICV) | 0.035±0.033 | 0.079±0.101 | 0.02 |
| Absolute cerebral blood flow velocities (cm/s) | |||
| Systolic velocity | 74±15 | 93±18 | <0.001 |
| Mean velocity | 49±11 | 61±13 | <0.001 |
| Diastolic velocity | 32±9 | 39±11 | <0.01 |
| Pulsatile velocity | 42±8 | 54±10 | <0.001 |
| Cerebrovascular resistance index (mm Hg/cm per second) | 1.89±0.44 | 1.48±0.41 | <0.001 |
CBF, cerebral blood flow; CO2, carbon dioxide; ICV, intracranial volume; LVET, left ventricular ejection time; WMH, white-matter hyperintensity.
Normalized total brain volume was calculated by dividing the absolute total brain volume by ICV times 100. Total WMH was normalized to the ICV.
Values are mean±s.d.
Ambulatory blood pressure. Bold values represent P<0.05.
Age- and Sex-Related Differences in Cerebral Hemodynamics
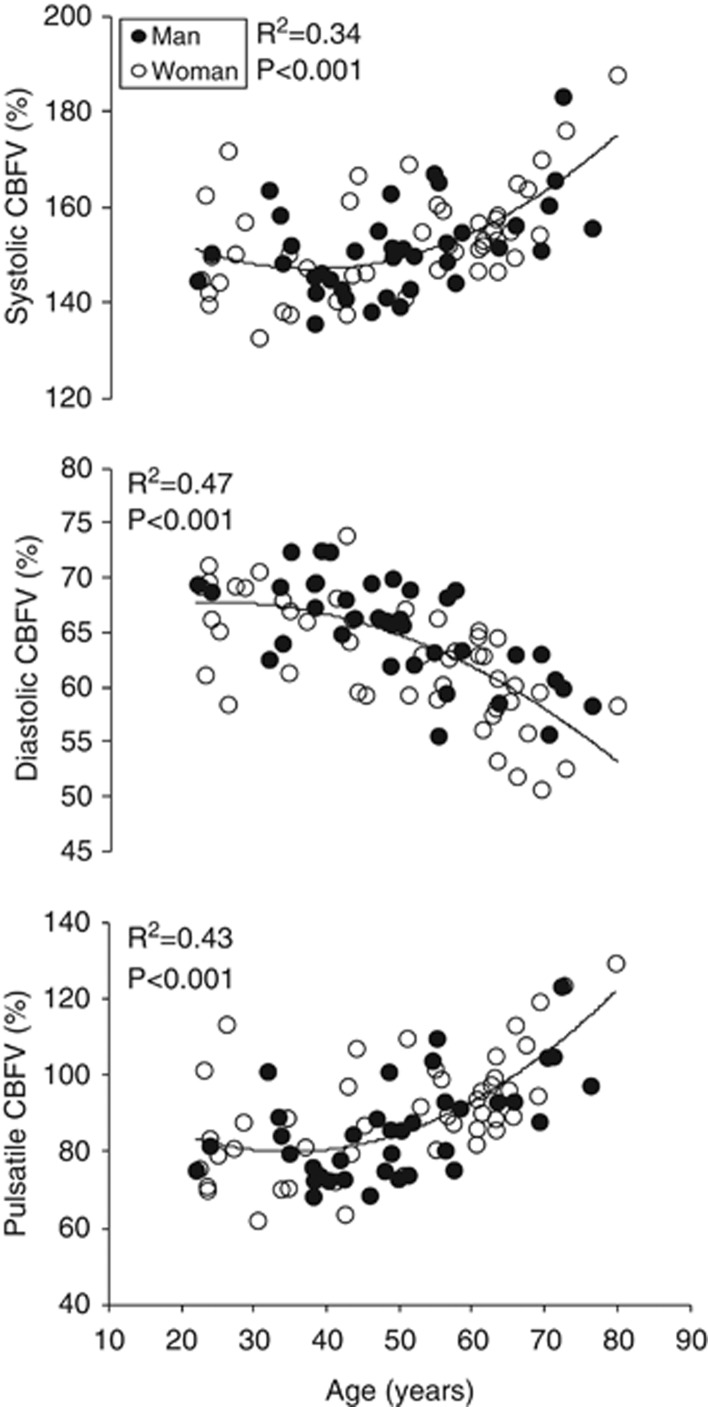
Associations between age and cerebral hemodynamic variables are presented in Table 2. Age was linearly related to the lower total CBF and mean CBFV (Figure 1a). In addition, the lower total CBF and mean CBFV were positively correlated with each other (Figure 1b). There were nonlinear relationships between age and the pulsatile indices of CBF, as indicated by the significant age and age squared terms. Specifically, systolic and pulsatile CBFV% showed a curvilinear elevation while diastolic CBFV% exhibited a curvilinear reduction with advancing age (Figure 2).
Table 2. Multiple linear regression analysis of cerebral hemodynamics.
| Model | Variable | Overall model | Model improvement | B |
95% CI |
|
|---|---|---|---|---|---|---|
| R2 (P value) | ΔR2 (P value) | Lower | Upper | |||
| Dependent variable: Total cerebral blood flow (mL/100 g per minute) | ||||||
| 1 | Age | 0.10 (<0.01) | 0.10 (<0.01) | −0.26 | −0.44 | −0.09 |
| 2 | Age2 | 0.11 (0.01) | 0.01 (0.49) | −0.004 | −0.016 | 0.008 |
| 3 | Sex (female) | 0.62 (<0.001) | 0.51 (<0.001) | 17.8 | 14.3 | 21.4 |
| 4 | Diastolic blood pressure | 0.64 (<0.001) | 0.02 (0.03) | −0.29 | −0.56 | −0.03 |
| Dependent variable: Systolic cerebral blood flow velocity (%) | ||||||
| 1 | Age | 0.22 (<0.001) | 0.22 (<0.001) | 0.32 | 0.19 | 0.46 |
| 2 | Age2 | 0.34 (<0.001) | 0.12 (<0.001) | 0.016 | 0.008 | 0.024 |
| 3 | Sex (female) | 0.34 (<0.001) | 0.001 (0.81) | 0.47 | −3.41 | 4.34 |
| 4 | Carotid pulse pressure | 0.39 (<0.001) | 0.05 (0.02) | 0.34 | 0.06 | 0.61 |
| Dependent variable: Diastolic cerebral blood flow velocity (%) | ||||||
| 1 | Age | 0.42 (<0.001) | 0.42 (<0.001) | −0.22 | −0.28 | −0.16 |
| 2 | Age2 | 0.47 (<0.001) | 0.05 (0.01) | −0.005 | −0.009 | −0.001 |
| 3 | Sex (female) | 0.52 (<0.001) | 0.05 (<0.01) | −2.41 | −4.06 | −0.76 |
| 4 | Carotid pulse pressure | 0.59 (<0.001) | 0.07 (0.001) | −0.20 | −0.32 | −0.09 |
| 5 | Carotid-femoral PWV | 0.61 (<0.001) | 0.02 (0.03) | 0.64 | 0.06 | 1.22 |
| Dependent variable: Pulsatile cerebral blood flow velocity (%) | ||||||
| 1 | Age | 0.32 (<0.001) | 0.32 (<0.001) | 0.54 | 0.37 | 0.72 |
| 2 | Age2 | 0.43 (<0.001) | 0.11 (<0.001) | 0.021 | 0.010 | 0.031 |
| 3 | Sex (female) | 0.44 (<0.001) | 0.01 (0.25) | 2.88 | −2.09 | 7.84 |
| 4 | Carotid pulse pressure | 0.50 (<0.001) | 0.06 (<0.01) | 0.54 | 0.20 | 0.89 |
| 5 | Carotid AIx | 0.55 (<0.001) | 0.05 (<0.01) | −0.26 | −0.45 | −0.08 |
AIx, augmentation index; B, unstandardized beta coefficient; CI, confidence interval; PWV, pulse wave velocity; R2, coefficient of determination.
Age, age2, and sex were forced to enter sequentially. Stepwise method was used to enter central hemodynamic variables.
Figure 1.
(A) Association between age and total cerebral blood flow (CBF) (top) and mean CBF velocity (CBFV) (bottom), and (B) the relation between total CBF and mean CBFV. Total CBF was normalized to the tissue mass. Mean CBFV was measured from the middle cerebral artery. Linear trend was observed from the relations among age, total CBF, and mean CBFV.
Figure 2.
Association between age and systolic (top), diastolic (middle), and pulsatile (bottom) cerebral blood flow velocities (CBFVs). Quadratic trend was observed from the relations between age and the pulsatile indices of cerebral blood flow.
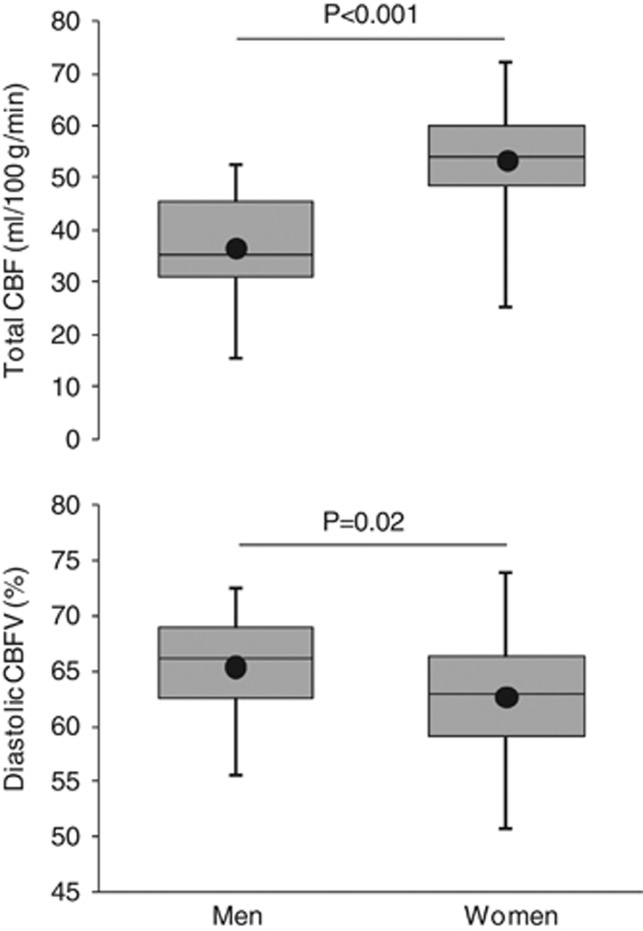
Figure 3 shows the key sex-related differences in cerebral hemodynamics. Total CBF was higher (53±9 versus 37±9 mL/100 g per minute, P<0.001) while cerebrovascular resistance was lower in women than in men. Compared with men, diastolic CBFV% was lower in women (63±5% versus 65±5%, P=0.02) while systolic (154±11% versus 152±10%, P=0.42) and pulsatile (91±16% versus 86±16%, P=0.15) CBFV% were not different. These sex-related differences in total CBF and diastolic CBFV% persisted after adjusting for the effects of age and age squared (Table 2).
Figure 3.
Whisker box plots exhibiting sex-related differences in total cerebral blood flow (CBF) (top) and diastolic CBF velocity (CBFV) (bottom). A solid line inside each box represents a median value, whereas a dot represents the mean value.
Association between Central and Cerebral Hemodynamics
With increasing age, cfPWV (R2=0.60, P<0.001) and carotid pulse pressure (R2=0.20, P<0.001) exhibited a curvilinear elevation while carotid AIx (R2=0.41, P<0.001) showed a linear increase (Supplementary Figure S3). Table 3 presents a correlation matrix of central and cerebral hemodynamic variables. Carotid pulse pressure was correlated positively with systolic and pulsatile CBFV% and negatively with diastolic CBFV%. After controlling for the effects of age, age squared, and sex, carotid pulse pressure remained as an independent predictor of systolic, diastolic, and pulsatile CBFV% (Table 2).
Table 3. Pearson's product-moment correlation coefficients and (P values) illustrating the association between central and cerebral hemodynamic variables.
|
Central hemodynamics |
Cerebral hemodynamics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cSBP | MAP | DBP | cPP | cAIx | cfPWV | sCBFV% | dCBFV% | pCBFV% | Total CBF | |
| cSBP | 0.91 | 0.74 | 0.75 | 0.54 | 0.64 | 0.22 | −0.35 | 0.28 | −0.32 | |
| MAP | (<0.001) | 0.94 | 0.43 | 0.44 | 0.58 | −0.02 | −0.12 | 0.03 | −0.42 | |
| DBP | (<0.001) | (<0.001) | 0.11 | 0.35 | 0.46 | −0.14 | 0.04 | −0.12 | −0.46 | |
| cPP | (<0.001) | (<0.001) | (0.33) | 0.44 | 0.49 | 0.46 | −0.56 | 0.53 | −0.02 | |
| cAIx | (<0.001) | (<0.001) | (0.001) | (<0.001) | 0.45 | 0.12 | −0.49 | 0.26 | 0.07 | |
| cfPWV | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | 0.41 | −0.47 | 0.46 | −0.43 | |
| sCBFV% | (0.048) | (0.87) | (0.21) | (<0.001) | (0.30) | (<0.001) | −0.69 | 0.97 | −0.27 | |
| dCBFV% | (0.001) | (0.26) | (0.69) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | −0.85 | 0.08 | |
| pCBFV% | (<0.01) | (0.78) | (0.30) | (<0.001) | (0.02) | (<0.001) | (<0.001) | (<0.001) | −0.22 | |
| Total CBF | (<0.01) | (<0.001) | (<0.001) | (0.83) | (0.54) | (<0.001) | (0.02) | (0.48) | (0.054) | |
cAIx, carotid augmentation index; CBF, cerebral blood flow; cfPWV, carotid-femoral pulse wave velocity; cPP, carotid pulse pressure; cSBP, carotid systolic blood pressure; DBP, diastolic blood pressure; dCBFV%, diastolic cerebral blood flow velocity (%); MAP, mean arterial pressure; pCBFV%, pulsatile cerebral blood flow velocity (%); sCBFV%, systolic cerebral blood flow velocity (%).
Total CBF was normalized to the brain tissue mass.
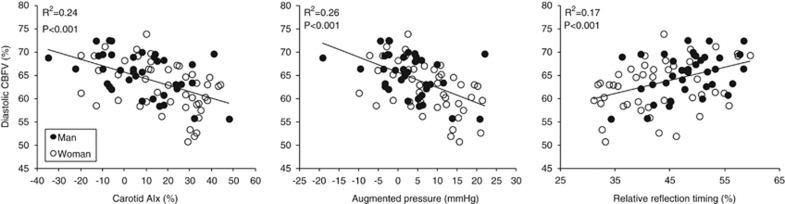
To explore whether sex-related difference in diastolic CBFV% is explained by central hemodynamics, analysis of covariance was performed. Adjusting for the effect of carotid pulse pressure, AIx, AP, or relative inflection time, the difference in diastolic CBFV% became nonsignificant. Furthermore, lower diastolic CBFV% was correlated with higher carotid AIx and AP and shorter relative inflection time (Figure 4).
Figure 4.
Association between diastolic cerebral blood flow velocity (CBFV) and augmentation index (AIx) (left), augmented pressure (middle), and relative inflection time (right). Linear trend was observed from the relations between diastolic CBFV and the indices of carotid arterial wave reflection.
Association between White-Matter Hyperintensity and Cerebral Hemodynamics
As presented in Supplementary Figure S4, there was a trend of increasing total volume of WMH after the age of 60 years. Thus, we performed a preliminary analysis to explore the association between WMH volume and cerebral hemodynamics in participants older than 60 years. Supplementary Table S1 presents characteristics of the selected subjects (n=24). As shown in Supplementary Figure S5, the greater total volume of WMH was correlated with higher systolic (R2=0.25, P=0.01) and pulsatile (R2=0.38, P=0.001) CBFV% and lower diastolic CBFV% (R2=0.44, P<0.001). Total CBF was not correlated with the WMH volume (R2=0.03, P=0.42).
Discussion
The major findings from the present study are as follows. First, advancing age is associated with higher pulsatility of CBF, which was accompanied by the concurrent reductions in total CBF. Second, diastolic CBF was lower while total CBF was higher in women compared with men. Third, age- and sex-related differences in CBF pulsatility were independently associated with carotid pulse pressure. Fourth, higher pulsatility of CBF was correlated with the greater total volume of WMH in older subjects. Below we discuss the potential underlying mechanism(s) as well as clinical implications of these findings.
Association between Cerebral and Central Hemodynamics
To our knowledge, this is the first study to show curvilinear elevations in the pulsatile indices of CBF with advancing age, which was further explained by the concurrent alterations in central hemodynamics. Notably, steeper changes in CBF pulsatility occurred after middle age, which also correspond accelerated changes in central hemodynamics during the similar age (Supplementary Figure S3).27 In addition to the greater total volume of CBF in women which has been reported in the literature,22, 28 women showed lower diastolic CBF compared with men. Sex-related difference in diastolic CBF was further explained by the difference in central hemodynamics (i.e., carotid pulse pressure and arterial wave reflection). Therefore, these findings collectively suggest that CBF pulsatility is, at least in part, determined by the central–cerebral hemodynamic interactions.
With advancing age, left ventricular afterload increases as a result of elevated total peripheral resistance and stiffer central elastic arteries.6 Consequently, the amplitude of left ventricular ejection pressure increases during early systole which would not be dampened effectively by Windkessel function.6 Such increase in central pulse pressure would be transmitted into the high flow, low resistance organs such as the brain and the kidney.28, 29, 30 In support of this, our data showed that age-related increase in CBF pulsatility was accompanied by the concurrent elevations in carotid pulse pressure. Moreover, carotid pulse pressure was an independent predictor of CBF pulsatility.
Arterial wave reflections returning from the peripheral resistance vessels may augment the pulsatility of CBF yet attenuate diastolic CBF.31 It is likely that arterial wave reflections from low resistance vascular beds, such as brain, may be small, thus CBF pulsatility would be determined mainly by wave reflections returning from other vascular beds with high resistance.3 With increasing age, faster cfPWV may cause premature wave reflections. Also, arterial wave reflection is greater in women as observed in the present and previous studies due, in part, to shorter body height.32 Therefore, advancing age and female sex may augment central pulse pressure, which in turn may attenuate diastolic yet elevate pulsatile CBF. Several observations from the present study support this hypothesis. First, age-related reductions in diastolic CBF and elevations in pulsatile CBF were independently associated with the concurrent increases in carotid pulse pressure (Table 2). Second, sex-related difference in diastolic CBF (Figure 3) disappeared after adjusting for the effect of carotid pulse pressure or arterial wave reflection. Third, lower diastolic CBF was correlated with higher carotid AIx and AP and shorter relative inflection time (Figure 4). Taken together, these data suggest that carotid pulse pressure, as well as its components including the timing and magnitude indices of arterial wave reflection, influences the pulsatility of CBF.
Clinical Implications
Advancing age is the strongest risk factor for CVD.4 Furthermore, cerebral small vessel disease has long been hypothesized to result from mechanical insult to the microcirculation due to hemodynamic pulsatility.33 In the present study, we conducted a preliminary analysis to explore the association between WMH and cerebral hemodynamics. White-matter hyperintensity, which represents brain injury related to cerebral small vessel disease, is an established risk factor for CVD and dementia.34, 35 In a subset of participants older than 60 years, the greater total volume of WMH was associated with higher systolic and pulsatile CBFV% and lower diastolic CBFV% (Supplementary Figure S5). While limited by a small sample, our observations are, at least in part, consistent with the findings by Webb et al who reported the associations of WMH with higher CBF pulsatility and lower diastolic blood pressure in patients within 6 weeks of a transient ischemic attack or minor stroke.28 The present study further extends clinical implications of this study by showing that falling diastolic CBF and elevations in CBF pulsatility are related to the severity of cerebral small vessel disease in normal subjects without a history of transient ischemic attack and stroke. Therefore, these findings suggest that prevention and treatment of central arterial aging may attenuate the detrimental hemodynamic impact of increases in pressure and CBF pulsatility on cerebral circulation and lower the risks of CVD and cognitive impairment.
Study Strengths and Limitations
Our study is strengthened by using the noninvasive measurements of MRI and TCD to characterize cerebral hemodynamics and applanation tonometry to measure central hemodynamics in healthy human subjects. It also documented for the first time a close link between the age-related differences in the central and cerebral hemodynamics. Furthermore, our subject population was rigorously screened to exclude cerebral and cardiovascular diseases, and thus is a representation of normal aging.
There are several study limitations that need to be discussed. First, CBF pulsatility was estimated from the amplitude of CBFV waveform relative to the mean value. While this method allowed a direct comparison of CBF pulsatility among individuals with different levels of CBF or CBFV, whether the relative or absolute amplitude of CBF pulsatility is more clinically relevant to brain structure and function remains to be determined. Second, arterial pressure was not measured during MR imaging. Hence, cerebrovascular resistance was calculated from arterial pressure measured under laboratory conditions. Nonetheless, PC-MRI was conducted under similar supine resting conditions as those during pressure measurements in the laboratory. In addition, a 24-hour ambulatory blood pressure further confirmed age-related differences in the laboratory blood pressure measurements. Third, sample size of the present study was small relative to the age range of the participants. Thus, small differences in the upper extreme of age may have influenced the sex-related differences in hemodynamics although these differences are still present after making statistical adjustments for age and age squared (Table 2). In addition, the association between cerebral hemodynamics and WMH volume was limited by a small sample of older subjects. Thus, the results are preliminary and need to be tested using a larger sample and a more sensitive measure of white-matter damage (e.g., diffusion tensor MR imaging).36 Finally, a crosssectional nature of the present study precludes our understanding of causal relationships among age and hemodynamic variables. Longitudinal study that assesses central and cerebral hemodynamics and brain structures in the same individuals after middle age may provide insights into the causal role of central arterial stiffness in the development of CVD.
Conclusions
To the best of our knowledge, this is the first study to show in humans that age-related increase in CBF pulsatility is independently associated with central hemodynamics. Specifically, advancing age is associated with higher systolic and pulsatile CBF and lower diastolic CBF. In addition, women showed greater total CBF but lower diastolic CBF than men. Both age- and sex-related differences in CBF pulsatility are independently associated with carotid pulse pressure and arterial wave reflection. In a subset of older participants, higher pulsatility of CBF was positively correlated with the greater total volume of WMH. These findings collectively highlight the importance of central arterial aging in age-related differences in cerebral hemodynamics and provide new insights for the understanding of physiologic mechanisms by which central arterial stiffness increases the risk of CVD.
Acknowledgments
The authors thank all our study participants for their willingness, time, and effort devoted to this study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH R01HL102457-01 and NIH R01AG033106-01.
Supplementary Material
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: a report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke ME. Mcdonald's blood flow in arteries. New York: Oxford University Press; 2005. [Google Scholar]
- Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:1–8. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol. 2012;11:82–91. doi: 10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991;22:1485–1490. doi: 10.1161/01.str.22.12.1485. [DOI] [PubMed] [Google Scholar]
- Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, et al. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke. 2013;44:3235–3238. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension. 2005;46:244–248. doi: 10.1161/01.HYP.0000166723.07809.7e. [DOI] [PubMed] [Google Scholar]
- Protogerou AD, Blacher J, Aslangul E, Le Jeunne C, Lekakis J, Mavrikakis M, et al. Gender influence on metabolic syndrome's effects on arterial stiffness and pressure wave reflections in treated hypertensive subjects. Atherosclerosis. 2007;193:151–158. doi: 10.1016/j.atherosclerosis.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113 (Pt 1:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (cmro2) by MRI. Magn Reson Med. 2009;62:141–148. doi: 10.1002/mrm.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water. J Cereb Blood Flow Metab. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham heart study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–846. doi: 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62:542–549. doi: 10.1161/HYPERTENSIONAHA.113.01318. [DOI] [PubMed] [Google Scholar]
- London GM, Guerin AP, Pannier B, Marchais SJ, Stimpel M. Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension. 1995;26:514–519. doi: 10.1161/01.hyp.26.3.514. [DOI] [PubMed] [Google Scholar]
- Soros P, Whitehead S, Spence JD, Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol. 2013;9:174–178. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.