Abstract
In vivo mapping of the full vasculature dynamics based on Ultrafast Doppler is showed noninvasively in the challenging case of the neonatal brain. Contrary to conventional pulsed-wave (PW) Doppler Ultrasound limited for >40 years to the estimation of vascular indices at a single location, the ultrafast frame rate (5,000 Hz) obtained using plane-wave transmissions leads to simultaneous estimation of full Doppler spectra in all pixels of wide field-of-view images within a single cardiac cycle and high sensitivity Doppler imaging. Consequently, 2D quantitative maps of the cerebro-vascular resistivity index (RI) are processed and found in agreement with local measurements obtained on large arteries of healthy neonates using conventional PW Doppler. Changes in 2D resistivity maps are monitored during recovery after therapeutic whole-body cooling of full-term neonates treated for hypoxic ischemic encephalopathy. Arterial and venous vessels are unambiguously differentiated on the basis of their distinct hemodynamics. The high spatial (250 × 250 μm2) and temporal resolution (<1 ms) of Ultrafast Doppler imaging combined with deep tissue penetration enable precise quantitative mapping of deep brain vascular dynamics and RI, which is far beyond the capabilities of any other imaging modality.
Keywords: angiography, brain imaging, cerebral hemodynamics, neurosonology, ultrasound
Introduction
An imaging modality that provides brain vascular anatomy and hemodynamic information mapping with high spatial and temporal resolution at bedside is today unavailable in clinics. Computed tomography and magnetic resonance angiography can rely on spatial resolutions typically down to 600 μm isotropic with deep penetration but are limited to morphologic analysis. Radiation exposure and the need of contrast agent injection are also serious limitations of computed tomography angiography for neonate imaging. Beyond vascular anatomy, the assessment of vascular hemodynamics with these approaches remains a bottleneck. Vascular hemodynamics is usually obtained by Doppler measurements in ultrasonography.1 Characterized by the time profile of the Doppler spectrum, this vascular hemodynamics assessment can only be performed at a single location (i.e., the focal region of the interrogating ultrasonic beam), as it requires a very high temporal resolution typically in the kHz range. This is the conventional pulse-wave (PW) Doppler mode. From the analysis of this time-dependent profile of the Doppler spectrum, several indices were introduced in an attempt to extract quantitative data from the Doppler waveform. Pourcelot et al2, 3 proposed to use the resistivity index (RI) that characterizes the blood vessel resistance, whereas Gosling and King4proposed a quite similar pulsatility index (PI). These indexes are defined by:
In these equations, the subscripts ‘syst' and ‘ed' indicate the peak systole and end diastole, respectively. They are widely used in clinics today as they reflect well the changes in the blood circulation. In the context of preterm cerebral vasculature, they are of particular interest as the immature cerebral vascular system has several vulnerabilities5 that contribute to the genesis of intraventricular hemorrhage and white-matter damage, the two main brain lesions observed in neonates leading to subsequent neurologic handicaps including cerebral palsy, neurocognitive impairment, and epilepsy. They also provide in general a good correlation with an increase in intracranial pressure,6, 7 as first described by Bada et al in 1982 in preterm infants with posthemorrhagic hydrocephalus. Indeed, the raised intracranial pressure leads to the compression of brain capillaries and increase in vascular resistance of cerebral arteries.8, 9, 10, 11 Gera et al12 proved the importance of the assessment of resistive index of anterior cerebral artery in newborns with hydrocephalus in relationship to the need of drainage procedure. As PI and RI are influenced by the heart rate, Hanlo et al introduced in 1995 a new Doppler index, the trans-systolic time, better correlated with intracranial pressure.13, 14 However, to date all these measurements remain limited to single locations in the main large arteries.
To build complete 2D maps of cerebral blood flow, conventional approaches based on line per line construction of a Doppler image have been developed known under the terminology Color Flow Imaging (CFI). Unfortunately, CFI does not allow the acquisition of enough successive time samples per line to estimate the full time profile of the Doppler spectrum required for vascular index assessment. This issue with the frame rate represents a major challenge for ultrasonography, as a compromise between spatial and temporal resolutions has always to be done in conventional approaches: ultrasonography can provide either full quantitative Doppler information on a limited sample volume (Spectral Doppler), or averaged Doppler velocity estimations on a large region of interest (ROI) (Doppler CFI). This limitation between imaging (CFI) and localized characterization (PW Doppler) remains today in clinical practice. For this reason, though Transcranial Doppler Ultrasound is widely used in research studies, its clinical use is today limited to single local measurements of velocities and resistive indices in large arteries such as of the anterior or middle cerebral arteries as an indirect measure of cerebral vascular resistance.
Recently, the concept of plane-wave transmissions rather than line-by-line scanning beams15, 16, 17 was developed to break the conventional limits of ultrasound imaging in the framework of multiwave imaging.18, 19, 20 By using such large field of view transmissions, the frame rate reaches the theoretical limit of physics dictated by the ultrasound speed and an ultrasonic map can be provided typically in tens of microseconds (giving several 1,000 frames per second). For blood flow measurements, ultrafast plane-wave Doppler imaging enables to break the limitation between imaging and quantification.21 The plane-wave transmission gives access to high-precision characterization of complex vascular and cardiac flows as all pixels of the 2D ultrasonic image rely simultaneously to a very high frame rate and a large number of temporal samples. In the brain, the highly improved sensitivity of Ultrafast Doppler paved the way for functional ultrasound imaging (fUltrasound) of brain activity with unprecedented spatial and temporal resolution compared with functional magnetic resonance imaging (fMRI).22
Such brain Ultrafast Doppler is here applied for the first time in clinics providing high sensitivity maps of neonate brain vasculature. Moreover, as the full Doppler spectrum is available simultaneously for each pixel during a plane-wave imaging sequence, it becomes possible to estimate the vascular indexes (PI, RI, and trans-systolic time) simultaneously everywhere in the imaged area. Here, we investigate the proof of concept of vascular resistivity imaging within a single cardiac cycle, called vascular index imaging. It provides clear discrimination between arterial and venous flow. Vascular resistivity imaging is investigated in vivo in the framework of transfontanellar imaging on neonates and provides maps of brain hemodynamics with key features such as high spatial resolution (∼250 μm), deep penetration (whole-brain imaging), and short acquisition time (<1 second).
Materials and methods
Clinical Protocol and Data Acquisition
A total of 14 neonates including 9 full-term and 5 preterm infants below 32 weeks' gestation were enrolled in a proof-of-concept prospective study at Robert Debré Children's tertiary perinatal center. This observational study was approved by the institutional review board (CCP: ‘Comité de Protection des Personnes', i.e., Committee for the Protection of Persons, CCP agreement N° 120601) and local ethical committee, and strictly complies with the ethical principles for medical research involving human subjects of the World Medical Association Declaration of Helsinki, and written consent was obtained from parents of participants. Transfontanellar ultrasound was performed at bedside on the awake infant using a programmable ultrasound scanner (Aixplorer; Supersonic Imagine, Aix-en-Provence, France) and a commercial linear array probe (SL10-2; Supersonic Imagine) (center frequency 6 MHz, pitch 0.2 mm, 192 elements, bandwidth 80%, elevation focus at 30 mm). The probe was applied against the anterior fontanel by an experienced neonatologist (OB) in both coronal and sagittal planes, and images were collected and processed in collaboration with physicists (CD, MP, and MT). Some acquisitions were performed transtemporally (sphenoidal fontanel) and through the mastoidal fontanel, to prove the feasibility of Ultrafast Doppler imaging through those windows, giving access respectively to the Willis polygon and posterior fossa. The entire procedure, including positioning of the probe with the help of standard B mode imaging and the UF Doppler acquisition itself (1 second) to obtain one Ultrafast Doppler image, lasted less than 2 minutes.
Dedicated Ultrafast ultrasound sequences relying on Compound Plane-Wave imaging23 were used to image the neonatal brain. Those sequences attempt to optimize the trade-off between frame rate and resolution. In the medial sagittal plane, large vessels such as the anterior cerebral artery, the callosomarginal artery, or the medial frontal arteries, with axial blood flow speeds reaching 40 cm/s, impose a Doppler frame rate of up to 3,500 Hz to avoid aliasing. In coronal planes and parasagittal planes, we image smaller vessels such as lenticulostriate arteries or cortical arteries where the blood flow is much slower (less than 10 cm/s) and a frame rate of 800 Hz is enough to correctly sample the data. As a consequence, with those two values of frame rate imposed by the blood flow itself, we choose a 3-angle (−3°, 0°, 3°) compound imaging for the big vessels in the median sagittal plane and a 6-angle (−5°, −3°, −1°, 1°, 3°, 5°) compound imaging for the slower blood flows. Those ultrasound sequences comply with the FDA (Food and Drug Administration) recommendations (Track 3).
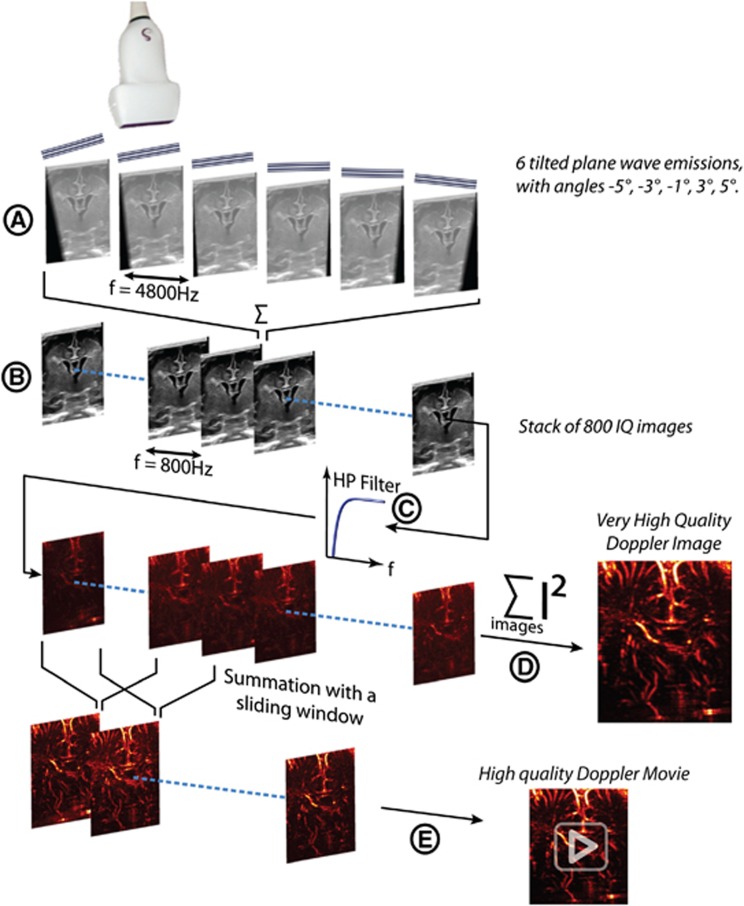
An Ultrafast Doppler sequence is made of the following steps (Figure 1): for each Compound frame, a set of tilted plane waves is emitted; for each tilted plane wave emitted, RF data are collected by the transducer and beam formed as an in-phase/quadrature (IQ) image. Those complex images are coherently summed together to generate a Compound frame. This operation is repeated at a frame rate of 3,500 Hz (high speeds) or 800 Hz (high resolution) during 1 second, to obtain a stack of Ultrafast Compound Frames (Figure 1) IQ(x,z,t) where x is the lateral dimension along the transducer array, z is the depth inside the medium, and t is the time. This stack of images is high pass filtered with a fourth-order Butterworth filter (cutoff frequency 60 Hz) along the temporal dimension (Equation 3), giving a high frequency in phase/quadrature frames stack 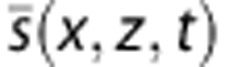 , which is the principal material for the hereinafter signal processing.
, which is the principal material for the hereinafter signal processing.
Figure 1.
Ultrafast Doppler acquisition pipeline: example of the high-resolution sequence. An SL 10-2 probe emits an ultrasound sequence composed of six tilted plane waves at the firing frequency of 4,800 Hz (A), the six complex images obtained being then coherently added to form one higher contrast B mode image. This sequence is repeated at 800 Hz (1/6 of 4,800 Hz) during 1 second (B), giving a stack of 800 compound B mode images. This stack is high pass filtered through the temporal dimension (C), with a fourth-order Butterworth high pass filter, giving a stack of high frequency images. These data can be processed in two ways described in the Power Doppler Processing part: either the summation of the intensity of all the Doppler frames (D) gives a final very high quality Ultrafast Doppler image, or the summation is done on a sliding window (typically 80 images) enabling the generation (E) of an Ultrafast Doppler movie describing the blood dynamics along the cardiac cycle.
Power Doppler Processing
To obtain a high quality Power Doppler Image PW (x,z) from the whole acquisition, the easiest way is to integrate the energy of the high frequency signal 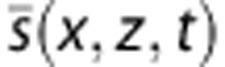 in each pixel (Equation 4).
in each pixel (Equation 4).
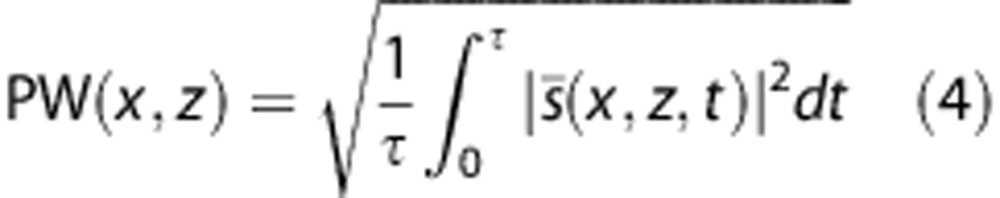 |
By doing so, a Power Doppler image is obtained, where the intensity in each pixel is proportional to the number of moving reflectors in this pixel, that is to say to the blood volume in this pixel. Depending on the duration of the acquisition, this gives an image of the blood volume averaged on 2 to 3 cardiac cycles (cardiac frequency is 140 to 180 b.p.m. for a preterm infant at rest and 100 to 140 b.p.m. for a full-term infant).
Moreover, the blood volume dynamics can be derived over one single cardiac cycle. If, instead of computing the mean energy in each pixel along the entire acquisition time, a sliding window is used to compute this integration, then it is possible to obtain a decent quality Power Doppler movie describing the evolution of blood volume in the imaging plane versus time. A typical sliding window is an 80-point (23 ms at a frame rate of 3,500 Hz) window with an overlap of 70 points, giving a good trade-off between the smoothing of the blood signal speckle pattern and the temporal resolution of the cardiac transient events during the cardiac cycle (see Supplementary Videos 1 to 3). It is important to underline that the Power Doppler signal, proportional to the blood volume in the pixel, does not increase in theory with the blood flow speed, but only with the number of red blood cells in the pixel. As a consequence, if a change in Power Doppler signal is visible during a cardiac cycle this is due to two main reasons. First, the diameter of the blood vessel imaged in this pixel can change, because of the changing blood pressure during the cardiac cycle. Second, the assumption that the Power Doppler does not change with a change in blood flow speed is not exactly satisfied. Indeed, it would only be exact if the energy of the blood signal was integrated over its entire bandwidth, but it is actually integrated over the high frequency band selected by the high pass filter. As a consequence, when the flow accelerates from a speed below the cutoff frequency to a speed above it, a change in Power Doppler is observed.
A higher step of processing can be easily reached with the energy of the signal: it is the directional Power Doppler. The intensity in each pixel is calculated the same way but a Fourier transform of the signal in each pixel is computed to determine in which half of the frequency domain is the energy mainly contained. This gives the main direction of the flow: up or down, which is coded in the image in red or blue (see Supplementary Video 4).
Spectral Processing
The huge advantage of plane-wave Ultrafast Doppler imaging lies in the instantaneous (single transmission) way of acquiring images, compared with a classic focused line per line approach where the Doppler image is acquired by blocks of lines. This enables to obtain a continuously sampled blood signal in each pixel through the entire acquisition and to do powerful filtering processing like the Butterworth filtering, which requires a relatively long signal to be efficient due to its impulse response.
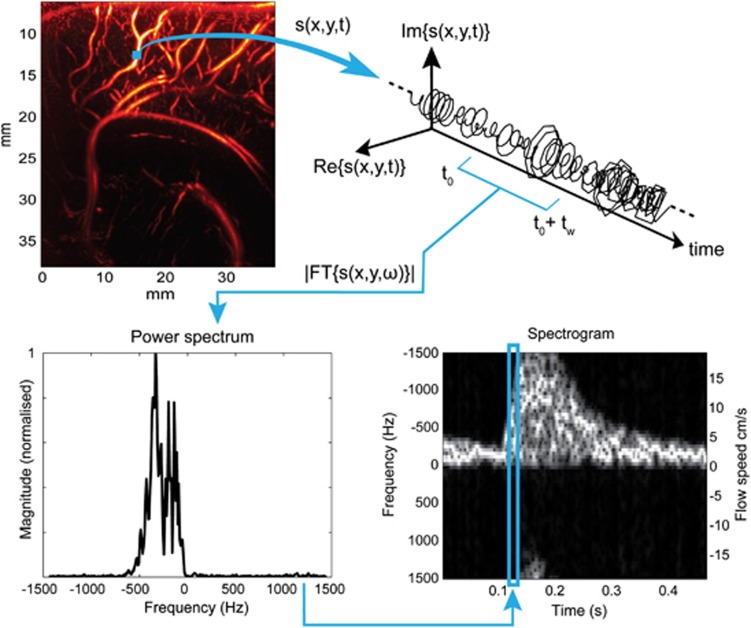
This also enables to do a complete spectral analysis of the signal in each pixel via a fast Fourier transform to calculate precisely blood flow speed (see Supplementary Video 5). In each pixel, the spectral content of the high frequency signal is linked to the Doppler frequency shift and the spectral broadening increasing with the flow speed (Figure 2). We can then compute the power spectral estimate of the signal in each pixel, calculate the mean frequency of the spectrum, that is, the Doppler frequency, and deduce the mean blood flow speed. Ultrafast Doppler imaging enables in fact to compute a retrospective ‘Pulse-Wave Doppler like' spectrogram in each pixel, giving a spectral and temporal description of the blood flow over the entire image (see Supplementary Video 6). The process to do such a calculation is illustrated in Figure 2. We can compute the power spectral density (PSD) estimation of this signal over a temporal window of length tw, short compared with the time scale of the cardiac dynamics but long enough to enable a correct spectral estimation.
Figure 2.
Extraction of the Doppler spectrum pixel per pixel from the Ultrafast Doppler acquisition. At each pixel (x,y) we have access to the high pass filtered in-phase/quadrature-phase (IQ) complex signal s(x,y,t). s(x,y,t) rotates in the complex plane with a speed related to the blood flow speed. Over a short temporal window (80 points, 27 ms), the power spectral estimation by Fourier Transform can be calculated and used as a gray-scale coded column in a spectrogram, completed by sliding the window over the entire temporal sample s(x,y,t).
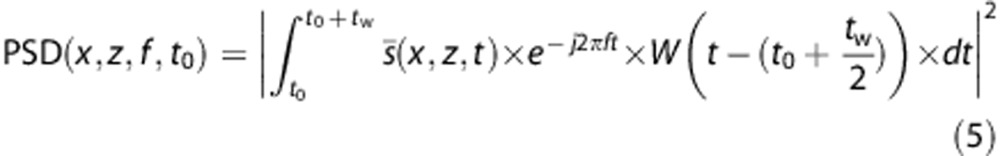 |
W(t) is a Hann Window centered on 0. This power spectral estimation is then used as a gray-scale row in the spectrogram, and the computation of all the rows is completed by increasing t0.
This ‘Pulse-Wave Doppler like' postprocessing on the Ultrafast Doppler data opens a totally new aspect of ultrasound Doppler acquisition, since from this spectrogram available in each pixel, blood flow parameters like the maximum speed, or the time to systolic peak, can be computed. The data can be further processed by calculating the mean frequency of the spectrum at each time step t0 using the first spectral moment.
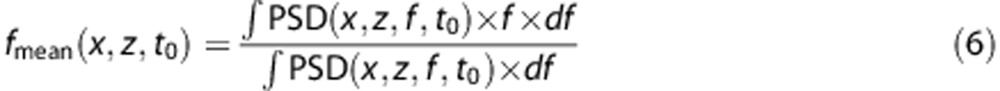 |
It is important to underline what really contribute to the spectral components of the signal inside a pixel. First, a moving target generates a broad spectrum centered on the Doppler frequency, whose shape and wideness are dependent on the acoustic field in this location and the speed vector (and not only its projection in the imaging plane) of the moving target.24 Then, a large number of red blood cells are imaged at the same time in one pixel, with different speeds, making that calculation an averaged spectrum over all the moving targets.
The computation of PSD (x,z,f,t) has been described in Equation 5 but has to be detailed here. As visible on the last spectrogram of Figure 6B, aliasing can be a problem with big vessels with high blood flow speeds. As a matter of fact as 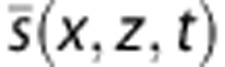 is a complex signal its Fourier Transform is not symmetric. Its energy lies mainly in one half of the Fourier domain depending on its direction of rotation in the IQ plane, that is to say the upward or downward blood flow direction. In the case of cerebral blood vessels, it is very unlikely to have both upward and downward blood flow in the same vessel, and this a priori on the uniqueness of the blood flow direction enables to have efficient bandwidth from −fs to fs with a frame rate of only fs. This is used in a step of dealiasing in the computation of the central frequency.
is a complex signal its Fourier Transform is not symmetric. Its energy lies mainly in one half of the Fourier domain depending on its direction of rotation in the IQ plane, that is to say the upward or downward blood flow direction. In the case of cerebral blood vessels, it is very unlikely to have both upward and downward blood flow in the same vessel, and this a priori on the uniqueness of the blood flow direction enables to have efficient bandwidth from −fs to fs with a frame rate of only fs. This is used in a step of dealiasing in the computation of the central frequency.
With the evaluation of the mean frequency over time in each pixel, it is possible to consider mapping blood flow parameters over the entire vascular network observed in the image. We present here examples of the mapping of the Pourcelot index (also called resistivity index), currently used by physicians on local Pulse-Wave Doppler data to characterize the blood flow dynamics in a particular vessel.
Obtaining a good quality resistivity map relies on the maximum improvement of the PW (x,z) Power Doppler Image, because it is the best way to discriminate between area with blood flow from area with only noise. Indeed, when PSD (x,z,f,t0) is calculated from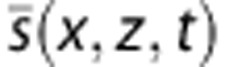 , there is no simpler way to determine whether its extent in the spectral domain is due to blood flow or just high frequency noise than computing the energy. The contrast of PW (x,z) is increased via histogram equalization. The difficulty then is to efficiently merge the Power Doppler information along with the resistivity information computed as:
, there is no simpler way to determine whether its extent in the spectral domain is due to blood flow or just high frequency noise than computing the energy. The contrast of PW (x,z) is increased via histogram equalization. The difficulty then is to efficiently merge the Power Doppler information along with the resistivity information computed as:
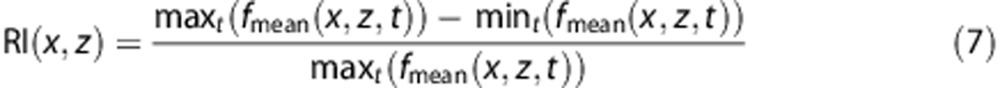 |
The color system hsv (hue saturation value) was used to achieve this merging: it enables to display an RI between 0 and 1 as a hue and to weight this RI by the value (that is to say the brightness). When a vessel is depicted the value will be high and the hue will be clearly visible, but when there is no vessel the value will darken the area and only black will be visible. The complete display pipeline is described in Supplementary Figure 1.
Results
Ultrasound Angiography in the Imaging Plane
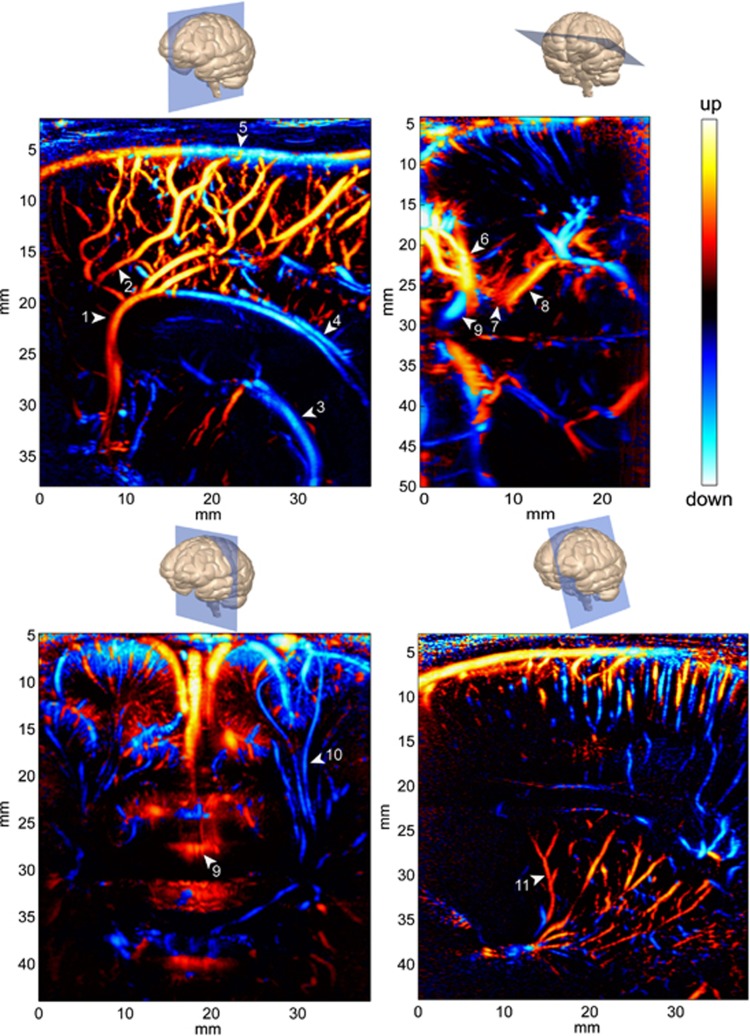
Increased sensitivity of Ultrafast Doppler revealed its ability to give high-resolution images of the vascular network in all the transfontanellar imaging planes (Figure 3). In the medial sagittal plane, large vessels like the pericallosal artery, the callosomarginal artery, and their ramifications are perfectly delineated. But smaller arterial ramifications, undetectable with conventional Doppler imaging are also visualized. Veins can also be observed such as the Galen vein, the straight sinus, the superior and inferior sagittal sinuses, and the smaller cortical veins. In parasagittal planes, small arteries irrigating the cortex, the white matter, and the basal ganglia are made visible. In the coronal plane, most branches from the anterior cerebral arteries are seen along their transverse section, between the two hemispheres and in the sulci. But cortical arteries and long medullar arteries penetrating inside the brain can be observed.
Figure 3.
Four typical directional Power Doppler images obtained in Ultrafast Doppler imaging in neonates. Top left: Medial Sagittal view, probe is longitudinally applied against the anterior fontanel; bottom left: coronal view, probe is iscoronally applied against the anterior fontanel; top right: transtemporal transverse view, probe is applied at the junction of the sphenoidal and mastoid fontanels; bottom right: tilted parasagittal view, probe is applied longitudinally against the anterior fontanel and slightly tilted. Vessel annotation: (1) Precallosal segment of the anterior cerebral artery, (2) Callosomarginal artery, (3) Internal cerebral vein, (4) Inferior sagittal sinus, (5) Superior sagittal sinus, (6) Middle cerebral artery, (7) Posterior communicating artery, (8) Posterior cerebral artery, (9) Anterior cerebral artery, (10) Medullar artery, and (11) Thalamostriate artery.
The smallest vessel size delineated by Ultrafast Doppler imaging is limited by the spatial resolution λ (wavelength) of 250 μm with our imaging system. That is to say the smallest vascular path will appear as a 250-μm wide vessel on the image. But this does not represent a threshold for detection, as the scatterers (red blood cells) are much smaller, and it may be possible to detect vessels smaller than 250 μm as long as the blood flow speed is high enough and the erythrocytes are numerous enough.
In some regions, the direction of the flow gives information on the nature of the vessel: artery or vein, given a particular anatomic location. For example on the bottom right image of Figure 3, in the cortical part we can assume that the small blue vessels are arteries (arterial flow penetrating the cortex) and the red one are veins, whereas in the basal ganglia red vessels likely depict arteries (thalamostriate arteries). However in some cases it is hard to determine whether a vessel is a vein or an artery using the sole information of the flow direction, and this is when the spectral processing of the Ultrafast Doppler data reveals its completeness.
Pulse-Wave Doppler-Like Profile Everywhere
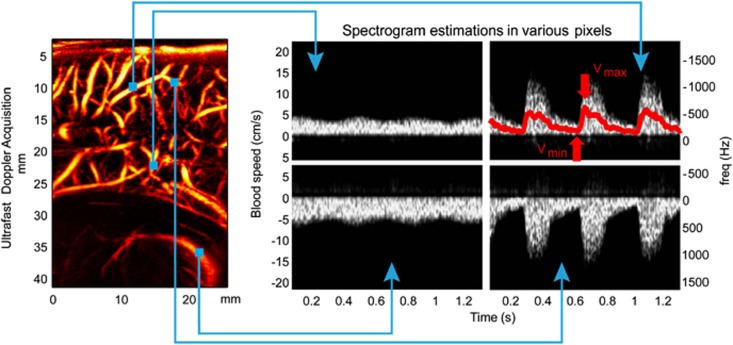
As shown in Figure 2, in each pixel of an Ultrafast Doppler acquisition a pulse-wave like spectrogram can be calculated. This is revolutionary for the radiologic practice that currently relies on the classic approach where the physician first uses CFI to get in position and then switch to Pulse-Wave Doppler to make a measurement by focusing the ultrasonic beam at a desired location in a blood vessel. In contrast, with Ultrafast Doppler imaging, every information in the imaging plane is recorded in one acquisition, and the physician can then a posteriori choose the location where to compute the blood speed profile (Figure 4).
Figure 4.
Once the location chosen in the image, and using a mean spectrogram over a small neighborhood (3 × 3 pixels) for better quality, a precise ‘Pulse Wave Doppler-like' blood flow profile is calculated. Top left: venous flow upward, bottom left: venous flow downward, top right: arterial flow upward, bottom right arterial flow downward. Top right: superimposed on the spectrogram is shown as a red line the central frequency determined from Equation (5).
Beyond the fact that a considerable amount of information is recorded in the same time, another aspect is that all these blood flow profiles are acquired synchronously, a thing that would not be possible with conventional focused ultrasound sequences. It is then possible to analyze the simultaneity of blood flow events.
As shown in the top right image of Figure 4, high end spectral calculation such as the determination of the Doppler central frequency along time can be done on every location of the image. From the central frequency profile, it is possible to extract the peak systole and end-diastole blood speed, and then to calculate the RI. The different steps to obtain a resistivity map from an Ultrafast Doppler acquisition are explained in online Supplementary Method. It is important to note that the high sensitivity Power Doppler image, after some image processing, is used as a weighting on the parametric data, because it is the best way to discriminate areas with blood flow from area without blood flow.
Quantitative Data Mapping with Ultrafast Doppler Imaging
On the 14 infants enrolled, Ultrafast Doppler acquisitions have been conducted in sagittal plane and parasagittal planes and resistivity maps have been obtained in several location, including basal ganglia, thalamus, and periventricular white matter (Figure 5).
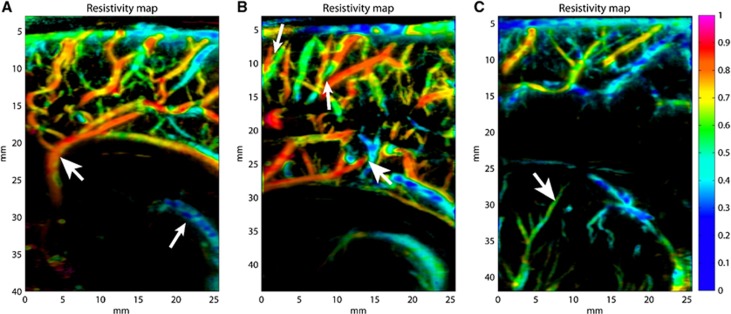
Figure 5.
Typical resistivity maps obtained in different imaging planes. In medial sagittal plane (A) (same acquisition than Figure 3, top left) resistivity values in big arteries are generally between 0.75 and 0.9 (large head arrow); in veins resistivity values are typically below 0.45 (small head arrow), with relatively strong fluctuations due to the slowness of blood flow. In this slightly parasagittal plane (B) (same acquisition than Figure 4), it can be observed that arteries and veins are clearly discriminated, even if they are really close (small head arrows) or intersecting (large head arrow); values of resistivity measured in the main afferent arteries are (0.84±0.03). In parasagittal plane (C), the lenticulostriate arteries show a lower resistivity (0.56±0.07) (arrow).
Typical resistivity values have been measured on those resistivity maps in six different locations and are gathered in Table 1. Measurements have been conducted by defining an ROI around the concerned arterial part (anterior cerebral artery (ACA) segments) or around an artery or a small group of arteries (medial frontal, lenticulostriate and cortical medullary arteries). On each ROI, the mean value of the resistivity and its standard deviation have been calculated. Those values are in good agreement with those given by Couture and Veyrac25 and Argollo et al,26 which however did neither describe the different segments of the ACA, nor the resistivity of the cortical medullary arteries.
Table 1. Typical resistivity values observed in various locations of the neonate brain.
| ROI location | ACA subcallosal segment | ACA pericallosal segment | ACA supracallosal segment | Medial frontal arteries | Lenticulostriate arteries | Cortical medullary arteries |
|---|---|---|---|---|---|---|
| Resistivity value | 0.83±0.06 | 0.81±0.06 | 0.72±0.07 | 0.78±0.05 | 0.63±0.08 | 0.45±0.08 |
| No of infants | 10 | 14 | 12 | 14 | 12 | 13 |
ACA, anterior cerebral artery; ROI, region of interest.
Effect of Therapeutic Hypothermia (for Hypoxo Ischemic Encephalopathy) and Rewarming on the Cerebral Resistivity
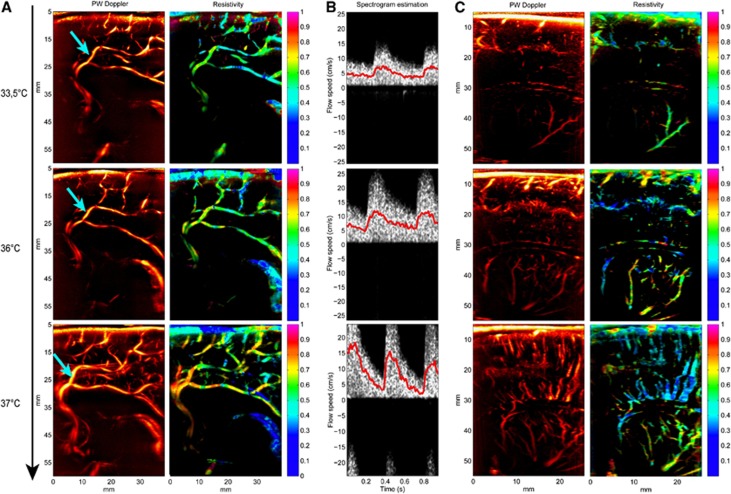
As a clinical illustration of the capability of resistivity maps to give easily readable clinical information on the resistivity of the vascular network, serial examinations have been performed on a full-term infant subjected to therapeutic hypothermia after neonatal hypoxo ischemic encephalopathy.27, 28 The patient was maintained under mild whole-body hypothermia (33.5°C) from 6 hours of life and for 72 hours. When the period of cooling concluded, rewarming was cautiously conducted and the servo-controlled rectal temperature was allowed to rise by no more than 0.5°C per hour, to a maximum of 36.5±0.2°C. We have monitored this infant during hypothermia (at the end of the hypothermia phase), after complete rewarming (24 hours after the end of the hypothermia phase) and 5 days later (after intensive care). Figure 6 shows increases in resistivity maps during this controlled rewarming.
Figure 6.
Monitoring of the cerebral vascular resistivity of a full-term neonate before the end of controlled hypothermia (temperature 33.5°C, day −1), just after the completion of the rewarming process (24 hours after the first acquisition, body temperature 36°C, day 0) and 5 days after (6 days after the first acquisition, body temperature 37°C, day +5). (A) In a medial sagittal plane, the first thing to observe is that the Power Doppler image itself already gives an information: the Doppler signal is really weak under hypothermia (day −1) making only the main big arteries visible, and gets really stronger on day +5 when the baby has recovered a normal temperature. Then, the resistivity map also reveals a really small resistivity index at 33.5°C (precallosal segment of the anterior cerebral artery 0.63±0.05, first segment of the callosomarginal artery 0.66±0.05, posterior pericallosal artery 0.45±0.06), which increases a bit at 36°C (0.68±0.06, 0.69±0.03, 0.57±0.04) and goes back to normal values at 37°C (0.80±0.04, 0.79±0.03, 0.69±0.04). (B) The spectrogram computed in the first segment of the callosomarginal artery (cyan arrow in the Power Doppler image), with speeds corrected with angles 29°, 33°, and 22°, respectively for images of days −1, 0, and +5. During hypothermia (day −1), blood flow is weakly pulsatile and speeds are very low for this kind of vessel. After a partial rewarming, a global increase in the blood flow speeds can be observed, during both systole and diastole. At day +5, the blood flow speed is back to a typical, very pulsatile, high systolic speed profile. (C) Power Doppler image and the corresponding resistivity maps in a parasagittal plane. During hypothermia, small vessels are hardly depicted. Resistivity changes are also observed in the thalamostriate arteries (day −1: 0.41±0.05, day 0: 0.63±0.11, day +5: 0.73±0.10).
Discussion
Cerebral vascular blood flow monitoring of preterm infants and neonates is currently limited in terms of prognostic and diagnostic value: X-ray angiography is an invasive procedure only performed for interventional purposes (vascular malformation). Computed tomography angiography also uses ionizing radiation and therefore is not the imaging modality of choice for neonates. Magnetic resonance imaging with time of flight sequences can depict vascular anatomy. Quantification of the cerebral blood flow relies on arterial spin labeling sequences or contrast of phase sequences, which are difficult to implement on small neonatal arteries, whereas perfusion MRI requires dynamic acquisition after bolus injection of a contrast agent. Neither of these techniques can provide valuable information for real-time assessment of the developing brain at the bedside. Finally, conventional Doppler ultrasonography, which is the most represented modality, presents a weak sensitivity limiting it to large vessels and has poor quantification ability (precise measure of a blood flow speed profile only at one selected point). Ultrafast Doppler imaging overcomes these limitations by giving access both to an ultrasensitive angiographic imaging modality, with precise delineation of even small arteriolar beds with blood flow speed as low as 1 cm/s, and to a quantitative vascular resistivity mapping.
Ultrafast Doppler imaging surpasses other angiographic modalities on several points. First of all, most of these modalities require a contrast agent, which qualifies it as invasive: gadolinium derivatives in the case of MRI, iodine or barium compounds in the case of computed tomography angiography. Some modalities such as Arterial Spin Labeling,27 Phase-contrast MR Angiography, or Cineangiography by MR global coherent-free precession29 avoid the use of contrast agent but their resolution is limited (800 μm for the last one) compared with our 250 μm Ultrafast Doppler resolution. The rapidity of the acquisition process must be underlined: a decent quality Power Doppler Image is obtained with 400 ms of acquisition (one preterm infant cardiac cycle), and 1 second of acquisition gives a very good quality image (the quality of the Doppler images generally increases with the duration of the acquisition, but after 1.5 seconds of acquisition movement artifact can occur and blur the vascular paths). Moreover, beyond ultrasound angiography without contrast agents, the time resolution of Ultrafast Doppler is high enough to give access to blood pulsatility within a single cardiac cycle and to the mapping of vascular resistivity.
Optical angiographic techniques have generally better spatial resolution: the NIR II vascular imaging method presented in Hong et al30 reports a 30 μm resolution, and the optical coherence tomography optical angiography technique described in Wang et al31 reports a 16-μm resolution. But both methods have a penetration depth limited to a few millimeters, whereas Ultrafast Doppler imaging can image easily the deep brain with a penetration depth of up to 80 mm with the 6-MHz Transducer used in this study, which makes it a whole-brain imaging modality.
Of particular interest, the anterior fontanel window is not exclusive and acquisitions have been done also through the sphenoid and mastoid fontanels, giving access to all the main brain vascular structures. This capability of Ultrafast Doppler has been illustrated by including an Ultrafast Doppler view of the Willis polygon (Figure 3). On the basis of these results, we envision that trans-temporal Ultrafast Doppler should be feasible and these preliminary results pave the way to fUltrasound as a future alternative to fMRI, at least for whole-brain functional imaging of children. Imaging through the occipital fontanel is technically difficult for two main reasons: the linear probe used in this study is slightly too large to fit longitudinally against the neck and hospitalized infants (especially intubated infants) must in most cases lie on their back. It has to be underlined that this study was performed with a linear probe, whereas Ultrafast Doppler imaging is also achievable with convex arrays that give a wider field of view. The development of an Ultrafast system compatible with 2D matrix of transducers is then the only technical challenge to be taken up to achieve 3D Ultrafast Doppler cerebral imaging.
Beyond these vascular imaging capabilities, Ultrafast Doppler offers what other angiographic modalities are unable to assess: the evaluation of blood flow speed at a subcardiac cycle time scale. This enables the unambiguous discrimination between venous and arterial flow based on their resistivity. It introduces the resistivity mapping illustrated here, but we can actually consider other parametric mapping: pulsatility mapping, angle-corrected maximum velocity mapping, trans-systolic time mapping, etc. Each new parametric imaging modality has brought its batch of clinical breakthrough: mapping the hemoglobin level of oxygenation with fMRI enables to study functional activity of the brain, mapping the elasticity of tissue with elastography enables for example to circumscribe tumor contour and improve the diagnostic specificity. There is no doubt that RI mapping will open new fields of application in the understanding of cerebral autoregulation and occurrence of pathologies in preterm and term infants. Finally, this cerebral vascular Ultrafast Doppler imaging represents the first step before 3D vascular Ultrafast Doppler imaging of the brain and ultrasound functional imaging of the human brain activity that could become an interesting alternative to neonate fMRI.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by LABEX WIFI (Laboratory of Excellence ANR-10-LABX-24) within the French Program ‘Investments for the Future' under reference ANR-10-IDEX-0001-02 PSL, and by the Assistance Publique-Hôpitaux de Paris.
Supplementary Material
References
- Aaslid R, Markwalder T-M, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Pourcelot L.Diagnostic ultrasound for cerebral vascular diseases Present and future in diagnostic ultrasound Kooyker: Amsterdam, Netherland; 1976. p141 [Google Scholar]
- Pourcelot L. Vélocimétrie Ultrasonore Doppler. INSERM: Paris, France; 1975. Applications cliniques de l'examen Doppler transcutané; pp. 213–240. [Google Scholar]
- Gosling R, King D.Continous wave ultrasound as an alternative and complement to X-rays in vascular examinations. In: (Reneman R, ed) Cardiovascular application of ultrasound. (Reneman R, ed.).North-Holland Publishing Company: Amsterdam, Netherland; 1974 [Google Scholar]
- Wigglesworth JS, Pape KE. An integrated model for haemorrhagic and ischaemic lesions in the newborn brain. Early Hum Dev. 1978;2:179–199. doi: 10.1016/0378-3782(78)90010-5. [DOI] [PubMed] [Google Scholar]
- Goh D, Minns RA, Hendry GMA, Thambyayah M, Steers AJW. Cerebrovascular resistive index assessed by Duplex Doppler sonography and its relationship to intracranial pressure in infantile hydrocephalus. Pediatr Radiol. 1992;22:246–250. doi: 10.1007/BF02019849. [DOI] [PubMed] [Google Scholar]
- Goh D, Minns RA. Intracranial pressure and cerebral arterial flow velocity indices in childhood hydrocephalus: current review. Childs Nerv Syst. 1995;11:392–396. doi: 10.1007/BF00717403. [DOI] [PubMed] [Google Scholar]
- Bada HS, Hajjar W, Chua C, Sumner DS. Noninvasive diagnosis of neonatal asphyxia and intraventricular hemorrhage by Doppler ultrasound. J Pediatr. 1979;95:775–779. doi: 10.1016/s0022-3476(79)80735-0. [DOI] [PubMed] [Google Scholar]
- Seibert JJ, McCowan TC, Chadduck WM, Adametz JR, Glasier CM, Williamson SL, et al. Duplex pulsed Doppler US versus intracranial pressure in the neonate: clinical and experimental studies. Radiology. 1989;171:155–159. doi: 10.1148/radiology.171.1.2648468. [DOI] [PubMed] [Google Scholar]
- Cosan TE, Gucuyener D, Dundar E, Arslantas A, Vural M, Uzuner K, et al. Cerebral blood flow alterations in progressive communicating hydrocephalus: transcranial Doppler ultrasonography assessment in an experimental model. J Neurosurg. 2001;94:265–269. doi: 10.3171/jns.2001.94.2.0265. [DOI] [PubMed] [Google Scholar]
- Hill A, Volpe JJ. Decrease in pulsatile flow in the anterior cerebral arteries in infantile hydrocephalus. Pediatrics. 1982;69:4–7. [PubMed] [Google Scholar]
- Gera P, Gupta R, Sailukar M, Pathak R, Agarwal P, Parelkar S, et al. Role of transcranial Doppler sonography and pressure provocation test to evaluate the need for cerebrospinal fluid drainage in hydrocephalic children. J Indian Assoc Pediatr Surg. 2002;7:174. [Google Scholar]
- Hanlo PW, Peters RJA, Gooskens RHJM, Heethaar RM, Keunen RWM, van Huffelen AC, et al. Monitoring intracranial dynamics by transcranial Doppler—a new Doppler index: Trans systolic time. Ultrasound Med Biol. 1995;21:613–621. doi: 10.1016/0301-5629(94)00147-6. [DOI] [PubMed] [Google Scholar]
- Leliefeld PH, Gooskens RHJM, Peters RJM, Tulleken CAF, Kappelle LJ, Han KS, et al. New transcranial Doppler index in infants with hydrocephalus: transsystolic time in clinical practice. Ultrasound Med Biol. 2009;35:1601–1606. doi: 10.1016/j.ultrasmedbio.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Tanter M, Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:102–119. doi: 10.1109/TUFFC.2014.6689779. [DOI] [PubMed] [Google Scholar]
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- Sandrin L, Tanter M, Catheline S, Fink M. Shear modulus imaging with 2-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:426–435. doi: 10.1109/58.996560. [DOI] [PubMed] [Google Scholar]
- Tanter M, Bercoff J, Athanasiou A, Deffieux T, Gennisson J-L, Montaldo G, et al. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol. 2008;34:1373–1386. doi: 10.1016/j.ultrasmedbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Fink M, Tanter M. Multiwave imaging and super resolution. Phys Today. 2010;63:28–33. [Google Scholar]
- Bavu É, Gennisson J-L, Couade M, Bercoff J, Mallet V, Fink M, et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 Hepatitis C Virus patients. Ultrasound Med Biol. 2011;37:1361–1373. doi: 10.1016/j.ultrasmedbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Bercoff J, Montaldo G, Loupas T, Savery D, Mézière F, Fink M, et al. Ultrafast compound Doppler imaging: providing full blood flow characterization. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:134–147. doi: 10.1109/TUFFC.2011.1780. [DOI] [PubMed] [Google Scholar]
- Macé E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M. Functional ultrasound imaging of the brain. Nat Methods. 2011;8:662–664. doi: 10.1038/nmeth.1641. [DOI] [PubMed] [Google Scholar]
- Montaldo G, Tanter M, Bercoff J, Benech N, Fink M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:489–506. doi: 10.1109/TUFFC.2009.1067. [DOI] [PubMed] [Google Scholar]
- Censor D, Newhouse VL, Vontz T, Ortega HV. Theory of ultrasound Doppler-spectra velocimetry for arbitrary beam and flow configurations. IEEE Trans Biomed Eng. 1988;35:740–751. doi: 10.1109/10.7275. [DOI] [PubMed] [Google Scholar]
- Couture AP, Veyrac C. Transfontanellar Doppler imaging in neonates. Springer; 2001. [DOI] [PubMed] [Google Scholar]
- Argollo N, Lessa I, Ribeiro S. Cranial Doppler resistance index measurement in preterm newborns with cerebral white matter lesion. J Pediatr (Rio J) 2006;82:221–226. doi: 10.2223/JPED.1488. [DOI] [PubMed] [Google Scholar]
- Wintermark P, Hansen A, Gregas MC, Soul J, Labrecque M, Robertson RL, et al. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. Am J Neuroradiol. 2011;32:2023–2029. doi: 10.3174/ajnr.A2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Rehwald WG, Chen E-L, Kim RJ, Judd RM. Noninvasive cineangiography by magnetic resonance global coherent free precession. Nat Med. 2004;10:545–549. doi: 10.1038/nm1027. [DOI] [PubMed] [Google Scholar]
- Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat Med. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express. 2007;15:4083–4097. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.