Abstract
Preserving mitochondrial pools of nicotinamide adenine dinucleotide (NAD) or nicotinamide phosphoribosyltransferase (Nampt), an enzyme involved in NAD production, maintains mitochondrial function and confers neuroprotection after ischemic stress. However, the mechanisms involved in regulating mitochondrial-localized Nampt or NAD have not been defined. In this study, we investigated the roles of protein kinase C epsilon (PKCɛ) and AMP-activated protein kinase (AMPK) in regulating mitochondrial pools of Nampt and NAD after resveratrol or ischemic preconditioning (IPC) in the cortex and in primary neuronal-glial cortical cultures. Using the specific PKCɛ agonist ψɛRACK, we found that PKCɛ induced robust activation of AMPK in vitro and in vivo and that AMPK was required for PKCɛ-mediated ischemic neuroprotection. In purified mitochondrial fractions, PKCɛ enhanced Nampt levels in an AMPK-dependent manner and was required for increased mitochondrial Nampt after IPC or resveratrol treatment. Analysis of intrinsic NAD autofluorescence using two-photon microscopy revealed that PKCɛ modulated NAD in the mitochondrial fraction. Further assessments of mitochondrial NAD concentrations showed that PKCɛ has a key role in regulating the mitochondrial NAD+/nicotinamide adenine dinucleotide reduced (NADH) ratio after IPC and resveratrol treatment in an AMPK- and Nampt-dependent manner. These findings indicate that PKCɛ is critical to increase or maintain mitochondrial Nampt and NAD after pathways of ischemic neuroprotection in the brain.
Keywords: antioxidants, brain ischemia, energy metabolism, ischemic preconditioning and induced tolerance, neuroprotection
Introduction
Nicotinamide adenine dinucleotide (NAD) is an essential coenzyme used by the tricarboxylic acid cycle and electron transport chain in the maintenance of mitochondrial membrane potential and production of ATP.1 In the brain, reductions in NAD after oxidative stress or ischemic events cause mitochondrial depolarization, decreased respiration, apoptotic signaling, and neuronal death.2, 3, 4 Conversely, increases in cellular NAD have been shown to prevent mitochondrial dysfunction and neurodegeneration after cerebral ischemia.5, 6 Ischemic preconditioning (IPC), a paradigm where a brief ischemic insult protects the brain against a subsequent lethal ischemic injury, or treatment with the polyphenol resveratrol, has been shown to protect mitochondria and enhance levels of NAD in the brain.7, 8, 9, 10
The mechanism by which resveratrol and IPC regulate NAD levels has not been clearly defined. However, two key signaling pathways that may be involved are protein kinase C epsilon (PKCɛ) and AMP-activated protein kinase (AMPK), enzymes that regulate mitochondrial ischemic neuroprotection after IPC and resveratrol treatment.9, 11 Protein kinase C epsilon activity has been associated with increased expression of aspartate amino transferase and malate dehydrogenase, two major components of the malate-aspartate shuttle.12 This redox shuttle is responsible for maintaining electron transfer between cytoplasmic and mitochondrial pools of NAD.13 AMP-activated protein kinase enhances whole-cell levels of NAD by increasing the expression of nicotinamide phosphoribosyltransferase (Nampt), the rate-limiting enzyme in the major biosynthetic pathway for NAD production.14 Nicotinamide phosphoribosyltransferase overexpression has been shown to maintain mitochondrial function and protect the brain against ischemic injury through its ability to produce NAD.6, 15
The mitochondrial NAD pool is distinct and is regulated separately from the rest of the cell.16, 17, 18, 19 In the brain, the mitochondria contain a large portion of the total cellular NAD,16 indicating the importance of mitochondrial pools of NAD to neuronal and astrocyte function. In neurons, enhancements in mitochondrial NAD after oxidative stress preserved mitochondrial membrane potential, enhanced respiration, and prevented the release of apoptosis-inducing factor.18 Similarly, increased mitochondrial NAD content protected the heart from damage after postischemic reperfusion.17 Maintenance of mitochondrial-localized Nampt was shown to enhance mitochondrial NAD and preserve liver cell viability after genotoxic-induced exhaustion of cytoplasmic NAD.19 Collectively, this evidence indicates an important role for mitochondrial-specific Nampt and NAD in promoting mitochondrial function and cytoprotection against lethal stress. However, the mechanisms regulating mitochondrial pools of NAD are not well defined.
The goal of the present study was to determine whether PKCɛ and AMPK work together to regulate mitochondrial pools of Nampt and NAD after preconditioning paradigms in the cortex. AMP-activated protein kinase has previously been linked to the PKC family in IPC-mediated cardioprotection in the heart,20 but no specific PKC isoform has been identified. Therefore, we first hypothesized that PKCɛ regulates AMPK activity in the brain. We next investigated whether PKCɛ and AMPK are involved in modulating mitochondrial-localized Nampt. Finally, since previous studies showed that IPC and resveratrol regulate mitochondrial NAD7, 8 and that PKCɛ is involved in improving mitochondrial function,9 we tested the hypothesis that PKCɛ enhances mitochondrial pools of Nampt and NAD after IPC or treatment with resveratrol.
Materials and methods
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by the Animal Care and Use Committee of the University of Miami.
Mixed Cortical Neuron/Astrocyte Cell Cultures
Neuronal-glial cocultures were prepared from Sprague-Dawley rats as previously described.21 For glial astrocytes, 1- to 2-day-old pups were anesthetized by intraperitoneal injection of ketamine (1.0 mg/pup), killed, and the brains were quickly removed. The cerebral cortices of the pups were isolated and the dissociated astrocytes were plated at 1.5 cortical hemispheres/24-well plate with MEM (minimum essential medium) (Life Technologies, Grand Island, NY, USA) containing 10% fetal bovine serum, 10% equine serum, 2 mmol/L glutamine, and 1% penicillin-streptomycin. After 2 weeks, 18- to 19-day pregnant Sprague-Dawley rats were anesthetized by isoflurane and embryos were quickly removed and decapitated. The cerebral cortices of the embryos were isolated and the dissociated cortical neurons were plated in MEM containing 5% fetal bovine serum and 2 mmol/L glutamine on the confluent monolayer of astrocytes prepared 2 weeks previously. Every 3 to 4 days, half of the media was changed with normal maintenance media consisting of MEM containing 2 mmol/L glutamine. The mixed cortical neuron/astrocyte cultures were kept in an incubator containing 5% CO2 at 37°C and used after 2 weeks in vitro.
Induction of Oxygen-Glucose Deprivation
To mimic ischemia, we subjected cultures to oxygen-glucose deprivation (OGD). Cultures were washed twice with glucose-free Hank's balanced salt solution (pH 7.4) of the following constitution (in mmol/L): 1.26 CaCl2·2H2O, 5.37 KCl, 0.44 KH2PO4, 0.49 MgCl2, 0.41 MgSO4·7H2O, 136.9 NaCl, 4.17 NaH-CO3, 0.34 Na2HPO4·7H2O, and 10 HEPES (Sigma, St Louis, MO, USA). The cell cultures were then transferred to an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI, USA) gassed with 90% N2/5% CO2/5% H2 at 37°C. Ischemic preconditioning was induced by exposing cultures to 1 hour of OGD, after which the cultures were removed from anoxic conditions and media was replaced with normal maintenance media with glucose. Cultures were used for experiments 30 minutes or 48 hours after IPC. For lethal OGD, cultures were subjected to 4 hours of OGD, after which the media was replaced with normal maintenance media and placed back into the incubator.
Pharmacological Treatments
For in vivo experiments, 3-month-old male Sprague-Dawley rats underwent intraperitoneal injection with 0.5 mg/kg of Tat-conjugated ψɛRACK (PKCɛ agonist) or the Tat peptide (KAI Pharmaceuticals, San Francisco, CA, USA). Cortices were collected for analysis 1 hour or 48 hours after injection. For in vitro experiments, cultures were exposed for 1 hour to 25 μmol/L resveratrol (Sigma), 100 nmol/L of Tat-conjugated ψɛRACK, or 0.5 mmol/L AICAR (AMPK activator) (Sigma) with or without 15 μmol/L Compound C (CC) (AMPK inhibitor) (EMD Millipore, Billerica, MA, USA) or 100 or 250 nmol/L of Tat-conjugated ɛV1-2 (PKCɛ antagonist) (KAI Pharmaceuticals). Cultures were used for experimental analyses 30 minutes or 48 hours after pharmacological preconditioning treatment.
Assessment of Neuronal Death
Forty-eight hours after pharmacological treatment, cultures were subjected to 4 hours of OGD (lethal OGD), after which the media was replaced with normal maintenance media and placed back into the incubator. To determine neuronal death, cytotoxicity was measured by lactate dehydrogenase (LDH) released for 48 hours into culture medium after lethal OGD using a Cytotoxicity Detection Kit (Roche Diagnostics Corporation, Indianapolis, IN, USA). Maximal neuronal LDH release was measured in the neuron-glial cocultures exposed to N-methyl-D-aspartate (500 μmol/L), an excitotoxin that preferentially kills neurons, for 48 hours. Lactate dehydrogenase release was measured at an absorbance of 340 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Values were expressed relative to LDH measurement from maximal neuronal LDH.
Isolation of Mitochondrial Fractions
Cortices or cultures were washed twice in cold (4°C) isolation medium consisting of (in mmol/L) 225 mannitol, 75 sucrose, 5 HEPES, and 1 EGTA, pH 7.4 and then homogenized in a hand-operated glass Teflon homogenizer in isolation medium. The homogenates were centrifuged at 1,300 × g for 5 minutes. The resulting supernatant was centrifuged at 17,000 × g for 10 minutes and the supernatant pellet was used as the source of the crude mitochondrial fraction. This pellet was resuspended in 15% Percoll and layered over a preformed gradient of 22% Percoll which was layered over 50% Percoll. The Percoll density gradient was centrifuged at 17,000 × g for 10 minutes and the purified mitochondria were collected at the interface between 50% and 22% gradients.22 The purified mitochondrial sample was centrifuged at 7,000 × g for 10 minutes and the final pellet resuspended in isolation medium without EGTA.
Western Blot Analysis
Cells or mitochondria were lysed in RIPA buffer pH 8.0 containing 150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris, supplemented with 1% protease and 1% phosphatase inhibitor cocktails (Sigma) and then centrifuged at 15,900 g for 15 minutes. Equal amounts of proteins were subjected to 8%, 10%, or 12% SDS–polyacrylamide gel electrophoresis and the separated proteins were electrophoretically transferred onto PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). The blot was blocked with 5% nonfat dried milk, incubated overnight at 4°C with phospho-AMPKα (Thr172) (1:250), AMPKα (1:1,000), phospho-acetyl-CoA carboxylase (Ser79) (1:500), acetyl-CoA carboxylase (1:1,000), CoxIV (1:1,000), β-actin (1:2,000) (Cell Signaling Technology, Danvers, MA, USA), or Nampt (1:125) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. Then, incubation was followed by horseradish peroxidase-conjugated specific secondary antibody for 1 hour at room temperature. The immunoreactive bands were revealed by ECL western blotting detection reagents (Pierce Thermo Scientific, Rockford, IL, USA). Western blot images were digitized by means of a CCD camera equipped with 50 mm NIKKOR lens (Nikon, Tokyo, Japan). The camera was interfaced to the Versadoc Imaging System (Bio-Rad). The digitized immunoblots were subjected to densitometric analysis using the Quantity One 1-D Analysis software (Bio-Rad).
Immunohistochemistry
Rats were perfused under anesthesia with fixative comprised of 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. Brains were removed and incubated in fixative overnight before being suspended in sucrose for 24 hours. Brains were quickly frozen on dry ice and 30-μm-thick sections were cut using a cryostat. The cortical sections were washed in PBS and then blocked in 5% goat serum albumin in PBS-T (0.8% Triton X-100 in PBS) overnight at 4°C. The sections were then incubated with CoxIV mouse antibody (1:50; Cell Signaling Technology) and Nampt rabbit antibody (1:150; Bethyl Laboratories, Montgomery, TX, USA) in 1% BSA in PBS-T at 4°C for 72 hours. After washing with PBS, cortical sections were incubated with anti-mouse Alexa Fluor 568 (1:500; Abcam, Cambridge, MA, USA) and anti-rabbit Hilytefluor 488 (1:500; AnaSpec, Fremont, CA, USA) for 2 hours in the dark at room temperature. The nuclei were stained with Hoechst 33342 (Life Technologies) for 10 minutes and then the sections were washed several times with PBS. The cortical sections were mounted on slides using the Prolong Antifade reagent (Molecular Probes, Carlsbad, CA, USA) and were visualized using confocal microscopy at × 150 magnification. Images were acquired with the FLUOVIEW FV1000 imaging system (Olympus, Center Valley, PA, USA) and then the Image J software (National Institute of Health, Bethesda, MD, USA) was used to quantify fluorescence. Colocalization of fluorescence was assessed using the colocalization._class plugin for Image J which defined areas of colocalization with white pixilation.
Two-Photon Imaging of Intrinsic Mitochondrial Nicotinamide Adenine Dinucleotide Reduced Fluorescence
Neuron-glial cocultures were grown onto glass coverslips in 24-well plates and cultured in vitro for 2 weeks as described above. Forty-eight hours after preconditioning, cultures were exposed to 250 nmol/L Mitotracker Red CMX Ros (Life Technologies) to label mitochondria. Coverslips were then placed into a petri dish containing normal maintenance media. Nicotinamide adenine dinucleotide exists in both an oxidized (NAD+) and reduced (NADH) form. Two-photon microscopy controlled by the Lasersharp 2000 software (Bio-Rad) was used to excite intrinsic mitochondrial NADH fluorescence at 740 nm.23, 24 Emission spectra of the two-photon excited intrinsic tissue fluorescence were obtained by scanning a region using a × 10 objective (Olympus NA 0.40 UPLASAPO) at a resolution of 1,280 × 1,024. Maximal intrinsic mitochondrial NADH fluorescence was measured 2 minutes after exposure to 1 mmol/L potassium cyanide (KCN), a potent reducing agent.
Measurement of Mitochondrial Nicotinamide Adenine Dinucleotide
Total NAD+ and NADH levels in purified mitochondrial samples were quantified using a NAD+/NADH quantification kit according to the manufacturer's instructions (Biovision, Milpitas, CA, USA). Total NAD+/NADH levels were assessed by converting all NAD+ in the samples to NADH using the NAD Cycling Enzyme Mix and NADH Developer included in the kit. Nicotinamide adenine dinucleotide reduced-only concentrations were achieved by heating the mitochondrial samples to 60°C for 30 minutes to decompose all NAD+. Nicotinamide adenine dinucleotide reduced was measured at OD450 nm using a spectrophotometer microplate reader (BioTek Instruments, Winooski, VT, USA). Known concentrations of purified NADH were used to generate standard curves and to calculate mitochondrial concentration. Values of NAD+ were generated by subtracting the NADH-only value from the total NAD+/NADH value.
Statistical Analysis
All data were expressed as the mean±s.e.m. Statistical significance was determined with Student's t-test for comparison between two groups or one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test for comparison between more than two groups. In all cases, a P value of <0.05 was considered as statistically significant.
Results
Protein Kinase C epsilon Is a Major Regulator of AMP-Activated Protein Kinase Activity
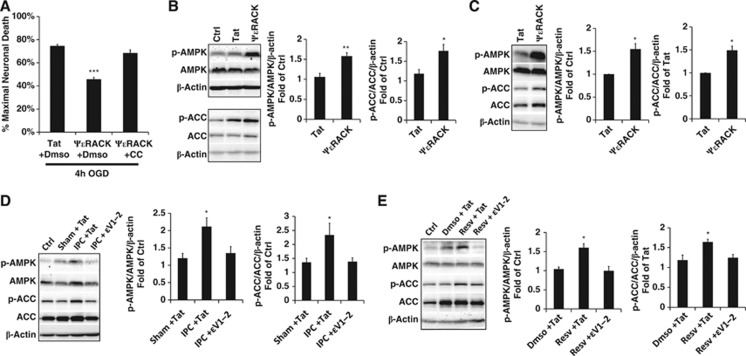
Previous studies in the heart have linked an AMPK-PKC pathway to ischemic protection,20 but the involvement of a specific PKC isoform has not been determined. Since both PKCɛ and AMPK protect mitochondria, we hypothesized that PKCɛ and AMPK functionally cooperate to provide ischemic protection in the brain. To test this, we first assessed neuronal death induced by 4 hours of OGD in neuronal-glial cortical cultures. One-hour pretreatment with the Tat-conjugated PKCɛ activator (ψɛRACK; 100 nmol/L) resulted in 45.8±1.5% neuronal death, which was significantly lower than the Tat-only treatment (74.6±1.2% P<0.001, n=6) (Figure 1A). However, this neuroprotection was reversed with the addition of the AMPK inhibitor CC (15 μmol/L), indicating that AMPK functions as a downstream target of the PKCɛ pathway.
Figure 1.
Protein kinase C epsilon (PKCɛ) is necessary and sufficient for AMP-activated protein kinase (AMPK) activation. (A) Bar graph representing neuronal death measured by lactate dehydrogenase (LDH) release at 48 hours of reperfusion after lethal oxygen/glucose deprivation (OGD). Neuronal-glial cortical cultures were exposed to ΨɛRACK (100 nmol/L), a PKCɛ agonist, with or without compound C (CC) (15 μmol/L), an AMPK inhibitor. (B–D) Western blot analysis of protein levels of phospho-AMPKThr172 and phospho-ACCSer79 in whole-cell lysates in neuronal-glial cortical cultures or rat cortex. Acetyl-CoA carboxylase (ACC) phosphorylation represented in the bar graphs was determined as phospho-ACCSer79 normalized by ACC/β-actin ratio. (B) Cortical lysates 30 minutes after 1 hour treatment with ΨɛRACK in cultures (C) or lysates from the cortex 1 hour after intraperitoneal injection of ΨɛRACK (0.5 mg/kg). (D, E) Cortical lysates from cultures 30 minutes after 1 hour exposure to ischemic preconditioning (IPC) or resveratrol (Resv) (25 μmol/L), with or without ɛV1-2 (100 or 250 nmol/L, respectively), a PKCɛ antagonist. *P<0.05, **P<0.01, ***P<0.001.
To determine whether PKCɛ regulates AMPK activity, we analyzed whole-cell lysates in neuronal-glial cortical cultures 30 minutes after 1 hour of ψɛRACK (100 nmol/L) exposure, which revealed significant increases in levels of phospho-AMPKThr172 (by 1.6±0.1-fold; P<0.01, n=5) (Figure 1B), the activated form of AMPK. To further confirm AMPK activity, we also assessed the phosphorylation of acetyl-CoA carboxylase (ACC), a direct downstream target of AMPK. ACCSer79 phosphorylation was also significantly increased after ψɛRACK treatment (by 1.8±0.2-fold; P<0.05, n=5) (Figure 1B). To determine whether PKCɛ activates AMPK in vivo, whole-cell lysates were collected from the cortex 1 hour after ψɛRACK intraperitoneal injection (0.5 mg/kg) which also resulted in significant increases in phospho-AMPKThr172 (by 1.6±0.1-fold; P<0.05, n=3), and phospho-ACCSer79 (by 1.5±0.1-fold; P<0.05, n=3) indicating that PKCɛ is sufficient to activate AMPK (Figure 1C).
Both IPC and resveratrol have been previously shown to enhance AMPK activity.8, 20 To investigate whether PKCɛ is the key signaling pathway required for AMPK activation during ischemic or resveratrol preconditioning, we exposed glial-neuronal cortical cultures to the Tat-conjugated PKCɛ inhibitor (ɛV1-2) at 250 nmol/L during IPC, or 100 nmol/L during resveratrol preconditioning. In previous studies, we have used the PKCɛ antagonist ɛV1-2 quite extensively9, 25, 26, 27 and treatment with this inhibitor alone showed no effect on neuroprotection or mitochondrial functioning. However, inhibition of PKCɛ activity during preconditioning treatment blocked increases in AMPKThr172 and ACCSer79 phosphorylation 30 minutes after IPC (P<0.05, n=5) and resveratrol preconditioning (P<0.05, n=5) (Figures 1D and 1E). Collectively, these results showed that PKCɛ is both sufficient and necessary for the induction of AMPK activity after ischemic and resveratrol preconditioning.
Protein Kinase C epsilon Regulates Mitochondrial Pools of Nampt
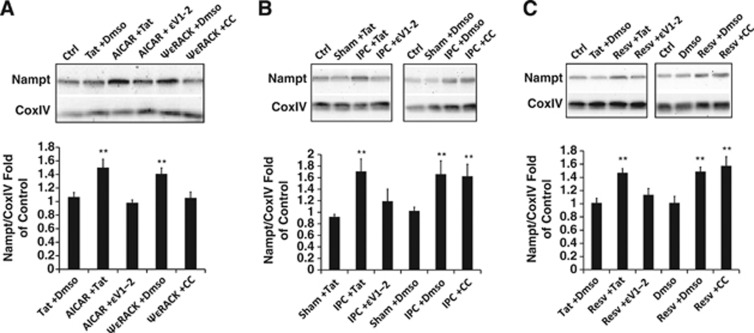
AMP-activated protein kinase has been previously shown to increase whole-cell levels of Nampt by enhancing Nampt mRNA expression.14 At this time it is unknown whether AMPK also regulates mitochondrial pools of Nampt, which are regulated separately from the cytoplasmic pools.19 Since our previous experiments indicated that PKCɛ and AMPK activity is linked, we next wanted to determine whether the PKCɛ-AMPK pathway is involved in regulating Nampt levels in mitochondria from neuronal-glial cortical cultures. We first assessed whether PKCɛ and AMPK require each other for the modulation of Nampt in isolated mitochondria from glial-neuronal cortical cultures. Protein levels of mitochondrial-localized Nampt were increased by 1.4±0.09-fold 48 hours after 100 nmol/L treatment with ψɛRACK (P<0.01, n=6). This increase was blocked with addition of CC (15 μmol/L), an AMPK inhibitor. Treatment with AICAR (AMPK activator) (0.5 mmol/L) also enhanced mitochondrial Nampt by 1.5±0.1-fold (P<0.01, n=6), but these increases were blocked with the addition of the PKCɛ inhibitor ɛV1-2 (100 nmol/L) (Figure 2A). These results show that there is reciprocal regulation of mitochondrial Nampt between PKCɛ and AMPK.
Figure 2.
Protein kinase C epsilon (PKCɛ) regulates mitochondrial nicotinamide phosphoribosyltransferase (Nampt) in neuronal-glial cortical cultures. (A–C) Bar graphs depict densitometric analysis from western blots of Nampt normalized to CoxIV from mitochondria isolated in neuronal-glial cortical cultures 48 hours after 1 hour treatment with (A) AICAR (0.5 mmol/L), an AMP-activated protein kinase (AMPK) activator, or ΨɛRACK (100 nmol/L), with ɛV1-2 (100 nmol/L) or compound C (CC) (15 μmol/L), respectively. (B, C) Ischemic preconditioning (IPC), or resveratrol (Resv) (25 μmol/L), with ɛV1-2 or CC. **P<0.01.
We next examined the role of PKCɛ and AMPK in regulating mitochondrial pools of Nampt after ischemic or resveratrol preconditioning in neuronal-glial cortical cultures. Ischemic preconditioning induced a 1.7±0.2-fold increase in Nampt levels 48 hours later, which was blocked with the addition of the PKCɛ inhibitor ɛV1-2 (250 nmol/L) (P<0.01, n=6). Exposure to the AMPK inhibitor CC (15 μmol/L) during IPC had no effect on mitochondrial Nampt levels (Figure 2B). Similarly, addition of ɛV1-2 (100 nmol/L), but not CC (15 μmol/L), reduced the resveratrol-mediated 1.5±0.2-fold increases in mitochondrial Nampt 48 hours later (P<0.01, n=6) (Figure 2C). These results provide evidence that PKCɛ, but not AMPK, is essential for increased mitochondrial Nampt after ischemic or resveratrol preconditioning.
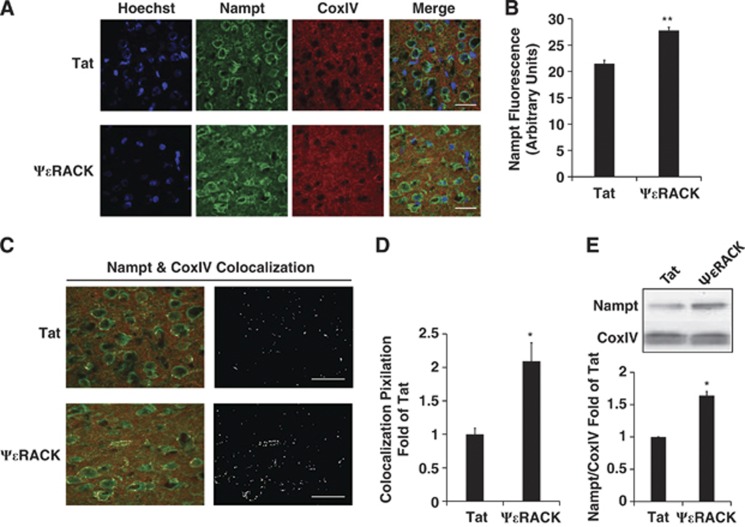
To determine whether the PKCɛ regulation of Nampt observed in vitro also occurs in adult cortex in vivo, we used immunohistochemical analysis to assess the nuclear marker Hoechst (blue), Nampt (green), and the mitochondrial marker CoxIV (red) in the cortex 48 hours after intraperitoneal injection of Tat or ψɛRACK (Figure 3A). Overall, Nampt fluorescence was increased by 30±0.1% (P<0.01, n=3) (Figure 3B). Further analysis of Nampt and CoxIV fluorescence revealed a 2.1±0.3-fold increase (P<0.05, n=3) in colocalization after ψɛRACK treatment, as indicated by the white areas of pixilation (Figures 3C and 3D). Western blotting of isolated mitochondria from the cortex 48 hours after intraperitoneal injection of Tat or ψɛRACK showed a 1.6±0.1-fold increase in Nampt levels (P<0.05, n=6) with PKCɛ activation (Figure 3E).
Figure 3.
Protein kinase C epsilon (PKCɛ) increases mitochondrial nicotinamide phosphoribosyltransferase (Nampt) in the cortex. (A) Immunohistochemical analysis of Hoechst (blue), Nampt (green), and CoxIV (red) fluorescence in the cortex 48 hours after intraperitoneal injection of Tat or ΨɛRACK (100 nmol/L). Scale bars 20 μm. (B) Quantification of Nampt fluorescence. (C) White pixilation denoting areas of colocalization between Nampt and CoxIV fluorescence. Scale bars, 20 μm. (D) Quantification of colocalization pixilation. (E) Western blot analysis of mitochondria isolated from cortex 48 hours after intraperitoneal injection of Tat or ΨɛRACK (0.5 mg/kg). Bar graphs depict densitometric analysis from western blots of Nampt normalized to CoxIV. *P<0.05, **P<0.01.
Protein Kinase C epsilon Modulates the Mitochondrial Nicotinamide Adenine Dinucleotide Oxidized to Reduced Ratio
Nicotinamide phosphoribosyltransferase is the rate-limiting enzyme in the major biosynthetic pathway for the production of NAD.13 Nicotinamide adenine dinucleotide exists in both an oxidized (NAD+) and a reduced (NADH) form. It has been reported that increases in Nampt expression increase NAD+ levels and the NAD+/NADH ratio.6, 14, 15 Since our previous experiments identified PKCɛ as a regulator of mitochondrial Nampt, we now wanted to determine whether PKCɛ has a role in regulating mitochondrial levels of NAD+, NADH, and the NAD+/NADH ratio in the cortex.
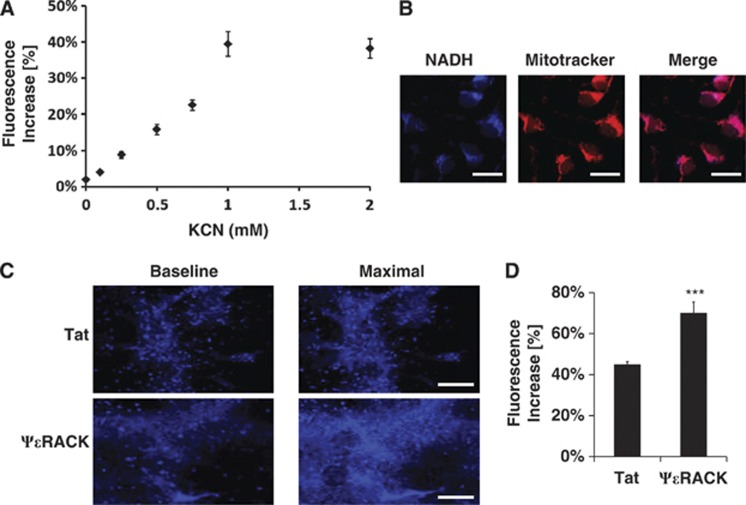
To assess mitochondrial NADH, we used two-photon microscopy to perform live-cell imaging of NADH autofluorescence in neuronal-glial cortical cultures. Two-minute exposures to KCN, a potent mitochondrial reduction agent, increased NADH fluorescence in a dose-dependent manner (Figure 4A). This verified that the observed fluorescence was derived from NADH. Since NADH fluorescence does not include its oxidized form NAD+, exposure to concentrations above 1 mmol/L KCN maximally reduced mitochondria to reveal total mitochondrial NAD (NAD+ and NADH) (Figure 4A). We further confirmed that the observed NADH autofluorescence is primarily of mitochondrial origin24 by showing colocalization of NADH fluorescence with mitotracker, a live-cell mitochondrial marker (Figure 4B). To determine whether PKCɛ enhances the NAD+/NADH ratio, we compared basal and maximal NADH fluorescence (induced with 1 mmol/L KCN) 48 hours after 1 hour treatment with Tat or ψɛRACK (100 nmol/L) (Figure 4C). Maximal NADH fluorescence increased by 45±1% in Tat-treated and 70±5% in ψɛRACK-treated cultures in comparison with baseline NADH fluorescence (P<0.001, n=6) (Figure 4D), indicating that PKCɛ activation increased the NAD+/NADH ratio.
Figure 4.
Protein kinase C epsilon (PKCɛ) enhances mitochondrial nicotinamide adenine dinucleotide reduced (NADH) fluorescence. (A–D) Live-cell imaging of NADH fluorescence using two-photon microscopy in neuronal-glial cortical cultures. (A) Nicotinamide adenine dinucleotide reduced fluorescence increases with exposure to potassium cyanide (KCN) in a dose-dependent manner. (B) Nicotinamide adenine dinucleotide reduced fluorescence colocalizes with Mitotracker Red. Scale bar 20 μm. (C) Comparison of basal versus KCN-induced maximal NADH fluorescence 48 hours after 1 hour treatment with Tat or ΨɛRACK (100 nmol/L). Scale bar 200 μm. (D) Quantification of the delta change in NADH fluorescence after KCN (1 mmol/L) exposure 48 hours after Tat or ΨɛRACK treatment. ***P<0.001.
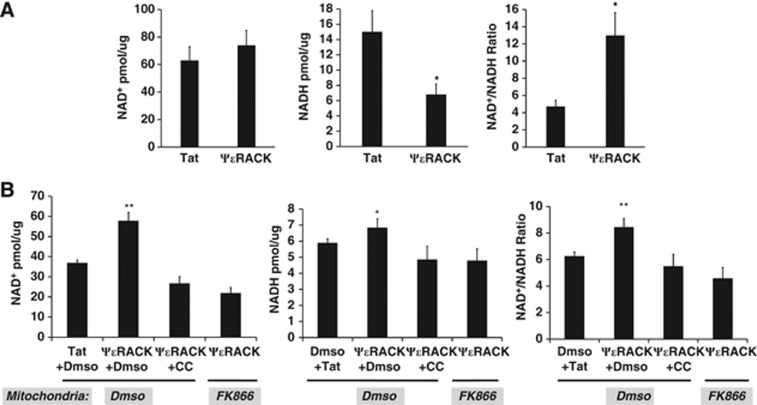
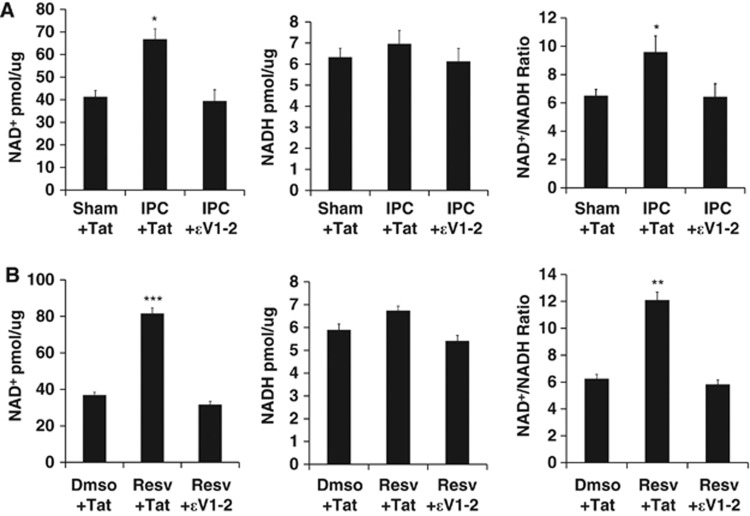
Since the previous experiment only allowed for the analysis of NADH fluorescence, we next wanted to directly assess how PKCɛ affects both NAD+ and NADH levels in mitochondria. In the next set of experiments, we measured the concentrations of NAD+ and NADH in mitochondria isolated from the cortex or neuronal-glial cortical cultures 48 hours after treatment. Analysis of intrinsic NADH in our previous experiment showed that PKCɛ regulates the NAD+/NADH ratio in neuronal-glial cortical cultures. We now wanted to determine whether PKCɛ regulates the NAD+/NADH ratio in cortical mitochondria in vivo. Mitochondria were isolated from the cortex 48 hours after intraperitoneal injection of ψɛRACK (0.5 mg/kg). There was no significant change in NAD+ levels. However, NADH concentrations were decreased by 65±2% (P<0.05, n=6) and the NAD+/NADH ratio was increased by 305±8% (P<0.05, n=6) (Figure 5A). To further delineate the pathways involved in PKCɛ regulation of mitochondrial NAD, we assessed NAD+, NADH, and NAD+/NADH ratio in neuronal-glial cortical cultures. One-hour ψɛRACK (100 nmol/L) exposure in cortical cultures increased mitochondrial concentrations of NAD+ by 58±4.2% (P<0.01, n=5), NADH by 16±0.5% (P<0.05, n=5), and the NAD+/NADH ratio by 35±0.7% (P<0.01, n=5) 48 hours later in comparison with control (Figure 5B). The significant ψɛRACK-mediated increases in NAD+ and NADH were blocked with the addition of the AMPK inhibitor CC (15 μmol/L). At this time it is unknown whether pathways other than Nampt may contribute to mitochondrial NAD levels. Therefore, to assess whether PKCɛ regulates mitochondrial NAD in a Nampt-dependent manner, isolated mitochondria from the ψɛRACK-treated glial-neuronal cortical cultures were also exposed to DMSO or the FK866 Nampt-specific inhibitor (25 nmol/L) for 30 minutes. Nicotinamide phosphoribosyltransferase inhibition significantly blocked PKCɛ-induced increases in mitochondrial concentrations of NAD+ and NADH (P<0.01, n=5) and the NAD+/NADH ratio (P<0.01, n=5) (Figure 5B). Since these experiments revealed PKCɛ activation was sufficient to regulate mitochondrial NAD+ and NADH, we next examined whether PKCɛ modulates mitochondrial NAD+/NADH, NADH, and the NAD+/NADH ratio 48 hours after ischemic and resveratrol preconditioning in glial-neuronal cortical cultures. Ischemic preconditioning increased the concentrations of NAD+ by 62±4.6% (P<0.05, n=5), NADH by 10±0.6% (P=0.4, n.s., n=5), and the NAD+/NADH ratio by 47±1.1% (P<0.05, n=5), which were blocked with the addition of the PKCɛ inhibitor, ɛV1-2 (250 nmol/L) (Figure 6A). Resveratrol increased the concentrations of NAD+ by 121±2.9% (P<0.001, n=5), NADH by 16%±0.2% (P=0.1, n.s., n=5), and the NAD+/NADH ratio by 93.5±0.6% (P<0.001, n=5), which was reversed with the addition of ɛV1-2 (100 nmol/L) (Figure 6B).
Figure 5.
Protein kinase C epsilon (PKCɛ) regulates the mitochondrial nicotinamide adenine dinucleotide oxidized (NAD+) to nicotinamide adenine dinucleotide reduced (NADH) ratio. (A, B) Concentrations of NAD+, NADH, and the NAD+/NADH ratio were assessed in cortical mitochondria isolated 48 hours after ΨɛRACK treatment. The NAD+/NADH ratio was calculated using these concentrations. (A) Mitochondria isolated from cortex after intraperitoneal injection ΨɛRACK (0.5 mg/kg). (B) Mitochondria isolated from neuronal-glial cortical cultures after ΨɛRACK (100 nmol/L) treatment with or without compound C (CC) (15 μM). Isolated mitochondria from cortical cultures were exposed to DMSO or FK866 (25 nmol/L), a specific inhibitor of nicotinamide phosphoribosyltransferase (Nampt). *P<0.05, **P<0.01.
Figure 6.
Ischemic preconditioning (IPC) and resveratrol (Resv) treatment increase the mitochondrial nicotinamide adenine dinucleotide oxidized (NAD+) to nicotinamide adenine dinucleotide reduced (NADH) ratio via protein kinase C epsilon (PKCɛ). (A, B) Concentrations of NAD+, NADH, and the NAD+/NADH ratio were assessed in mitochondria isolated from neuronal-glial cortical cultures 48 hours after IPC or Resv treatment. The NAD+/NADH ratio was calculated using these concentrations. (A) Cortical cultures exposed to IPC with or without ɛV1-2 (250 nmol/L). (B) Cortical cultures exposed to Resv (25 μM) with or without ɛV1-2 (100 nmol/L). *P<0.05, **P<0.01, ***P<0.001.
Discussion
Nampt and NAD have important roles in maintaining mitochondrial function against oxidative and ischemic stress.6, 15, 17 However, the pathways involved in regulating mitochondrial-localized Nampt and NAD have not been previously investigated. The purpose of this study was to determine whether PKCɛ and AMPK work together to enhance mitochondrial pools of Nampt, NAD, and the mitochondrial oxidized (NAD+) to reduced nicotinamide adenine dinucleotide reduced (NADH) ratio in the brain. We found that PKCɛ activation was both sufficient and necessary to induce AMPK activity after IPC and resveratrol treatment. AMP-activated protein kinase activity was required for PKCɛ-mediated ischemic neuroprotection, which suggested an important functional consequence of PKCɛ modulation of AMPK. Indeed, we showed that PKCɛ activation significantly enhanced mitochondrial pools of Nampt in an AMPK-dependent manner. Furthermore, PKCɛ had a critical role in increasing Nampt, NAD+, and the NAD+/NADH ratio in mitochondria after ischemic and resveratrol preconditioning. Collectively, these findings have identified PKCɛ as a major regulator of the mitochondrial localization of Nampt and NAD in the brain and after ischemic and resveratrol preconditioning.
Prevention of mitochondrial dysfunction after cerebral ischemia is a major target of neuroprotective pathways.28, 29, 30 Both PKCɛ and AMPK have established roles in ischemic neuroprotection and enhancing mitochondrial function. For example, our previous studies in the brain showed that PKCɛ mediates ischemic protection25 and translocates to mitochondria where it regulates the mitochondrial K+ATP channel,26 increases mitochondrial respiration, decreases reactive oxygen species production, and inhibits cytochrome c release.9 While PKCɛ has an established role in preventing brain ischemic injury, the role of AMPK in ischemic protection in the brain is less clear. For example, AMPK has been shown to promote apoptotic cell death through its interaction with p53.31 However, AMPK also provides protection against ischemic injury11, 15, 20 by regulating various transcription factors and coregulators (such as PGC1alpha, FOXO3a, and sirtuin 1 (SIRT1)) to induce mitochondrial biogenesis, enhance the expression of mitochondrial reactive oxygen species scavengers, and increase mitochondrial respiration.31 Previously, both PKC activity and an upstream activator of AMPK were shown to be important in preventing N-methyl-D-aspartate-induced neurodegeneration after cerebral ischemia.32 More recently, studies in the heart have linked AMPK to the PKC family in ischemic protection,20 but the involvement of a specific PKC isoform has not been determined. We showed here that PKCɛ activates AMPK in vitro and in vivo and that PKCɛ requires AMPK for neuroprotection against lethal OGD. Therefore, this study represents the first report linking AMPK to PKCɛ for activation and ischemic neuroprotection.
AMP-activated protein kinase has previously been shown to enhance mRNA expression of Nampt, the rate-limiting enzyme in the major biosynthetic pathway for NAD production.13, 14 Nicotinamide phosphoribosyltransferase converts nicotinamide to NMN (nicotinamide mononucleotide) which is then converted to NAD by Nmnat (NMN adenyl transferase).13 A major factor contributing to the requirement of AMPK to provide PKCɛ-mediated ischemic neuroprotection in our experiments may lie in the ability of AMPK to regulate Nampt and NAD levels. Ischemic preconditioning and resveratrol have been shown to enhance levels of Nampt,33, 34 which implicates Nampt as a major player in ischemic neuroprotection. Studies have shown that knockdown of Nampt increases susceptibility to cerebral ischemic infarct, while Nampt overexpression enhances neuronal survival after ischemic injury through AMPK activity.6, 15 Despite the involvement of AMPK in enhancing Nampt mRNA expression,14 no studies thus far have investigated the contribution of AMPK, or any other signaling pathway, in regulating mitochondrial-specific pools of Nampt.
Since we have previously shown that PKCɛ directly regulates mitochondrial function,9, 26, 27 and our current experiments linked PKCɛ to AMPK activity, we next determined whether PKCɛ and AMPK were involved in modulating mitochondrial Nampt. In purified mitochondrial fractions, we found that both AMPK and PKCɛ activation enhanced Nampt levels that were blocked with the addition of PKCɛ or AMPK inhibitors, respectively. These results were particularly interesting since AMPK has been previously shown to enhance whole-cell Nampt levels, but we now showed that AMPK enhances mitochondrial-localized Nampt in a PKCɛ-dependent manner (Figure 7). Similarly, both IPC and resveratrol required PKCɛ activity to increase mitochondrial-localized Nampt, indicating that PKCɛ has a crucial role in the maintenance or translocation of Nampt to the mitochondrial fraction. The fact that blockade of AMPK had no effect on the ability of IPC or resveratrol to enhance Nampt levels in the mitochondria further highlighted the importance of PKCɛ in regulation of mitochondrial levels. The dependence of PKCɛ on AMPK for increased mitochondrial Nampt may largely rest on the ability of AMPK to increase Nampt mRNA expression, whereas IPC and resveratrol may have AMPK-independent mechanisms for regulating hypoxia response elements in the Nampt promoter.35 Since there is a mitochondrial-specific Nmnat (Nmnat-3),36 there also remains the possibility that PKCɛ regulates a mitochondrial-specific isoform of Nampt. Nevertheless, PKCɛ regulation of mitochondrial Nampt levels provides a mechanism by which PKCɛ may mediate mitochondrial function and neuroprotection by enhancing mitochondrial NAD.
Figure 7.
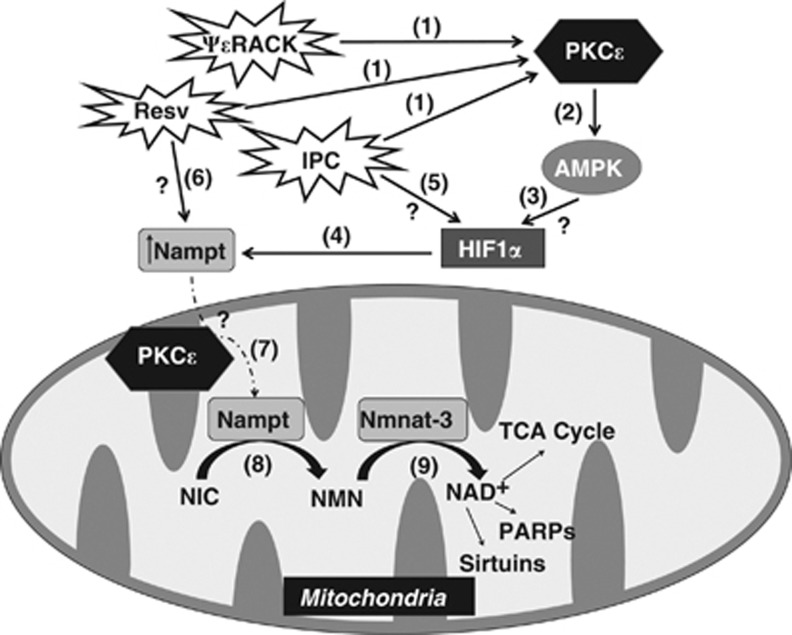
Protein kinase C epsilon (PKCɛ) is a major regulator of mitochondrial nicotinamide phosphoribosyltransferase (Nampt) and nicotinamide adenine dinucleotide (NAD). Schematic diagram of the proposed model. (1) PKCɛ is activated after treatment with ΨɛRACK, resveratrol (Resv), or ischemic preconditioning (IPC) (2) and enhances the activity of AMP-activated protein kinase (AMPK). (3) AMPK activation leads to the induction of hypoxia inducible factor 1α (HIF1α) (4) which upregulates the expression of Nampt. (5) The anoxic conditions during IPC allow AMPK-independent induction of HIF1α and Nampt expression. (6) Resv can also enhance Nampt levels independent of AMPK through an unknown pathway. (7) PKCɛ is required for the translocation or maintenance of Nampt in the mitochondria. (8) Nampt converts nicotinamide (NIC) to nicotinamide mononucleotide (NMN). (9) The mitochondrial-specific isoform Nmnat-3 converts NMN to NAD+, a critical cofactor for the tricarboxylic acid (TCA) cycle, sirtuin activity, and poly(ADP-ribose) polymerases (PARPs).
Nicotinamide adenine dinucleotide is an essential coenzyme used by the tricarboxylic acid cycle and electron transport chain in the maintenance of mitochondrial membrane potential and production of ATP.1 In the brain, the mitochondria contain a large portion of the cellular pool of NAD with up to one-third or half of NAD cellular content reported to reside in the mitochondria of astrocytes or neurons, respectively.16 Reductions in NAD during ischemic events cause DNA damage, energy depletion and lead to neurodegeneration.4, 5, 6, 37 Nicotinamide phosphoribosyltransferase overexpression has been shown to prevent mitochondrial dysfunction and neuronal death after ischemia through its ability to produce NAD.6, 15 Specifically enhancing mitochondrial-localized NAD after oxidative stress was shown to preserve mitochondrial membrane potential, enhanced respiration, and prevented the release of apoptosis-inducing factor in neurons.18 Several studies in the heart and the brain have indicated that mitochondrial NAD can be maintained despite substantial depletion of cytoplasmic NAD;16, 17, 18, 19 however, the mechanisms regulating mitochondrial NAD remain unclear. By blocking Nampt activity in isolated mitochondria, we have now revealed that PKCɛ regulates mitochondrial NAD in a Nampt-dependent manner. We also showed that PKCɛ activity after IPC and resveratrol treatment was responsible for enhanced mitochondrial NAD which further linked PKCɛ regulation of mitochondrial Nampt to modulation of mitochondrial NAD (Figure 7).
By assessing both the oxidized and reduced forms of NAD we found that the PKCɛ-regulated increases in mitochondrial Nampt correlated with increases in the NAD+/NADH ratio. These results mirrored previous findings where increased Nampt levels were associated with enhanced NAD+ levels.14, 15, 19, 33 Increases in the NAD+/NADH ratio have been linked to the activity of NAD+-dependent SIRTs,11, 14, 15, 33 which have been shown to provide protection to the mitochondria by regulating mitochondrial biogenesis and bioenergetics.38 The mitochondrial SIRTs, SIRT3 and SIRT4, have been shown to have integral roles in mitochondrial-Nampt-dependent protection against genotoxic stress.19 In a recent study, we showed that PKCɛ induces increases in SIRT1 mitochondrial levels.27 The results from our current study indicate that PKCɛ may also regulate mitochondrial SIRT activity by increasing the NAD+/NADH ratio in mitochondria (Figure 7). Another set of enzymes dependent on NAD+ are poly-ADP-ribose polymerases (PARPs), DNA-repair enzymes activated during cellular stress. During ischemia, overactivation of PARPs mediates NAD+ depletion and apoptotic signaling.3, 4 Protein kinase C epsilon-mediated increases in mitochondrial NAD+ availability may help to maintain pools of NAD+ during activity of mitochondrial-localized PARPs (Figure 7), thereby maintaining mitochondrial DNA integrity and preventing cell death.18, 39
The protective role of Nampt against neurodegenerative damage has recently become the focus of intense investigation.40 In this study, we have expanded the focus to understand how mitochondrial pools of Nampt, NAD, and the NAD+/NADH ratio are regulated by a PKCɛ-AMPK pathway during IPC and resveratrol treatment. The role of PKCɛ and AMPK crosstalk in mitochondrial protection should be further investigated since AMPK may also confer protection via pathways previously thought as PKCɛ-dependent-only and vice versa. Our findings also provide the initial steps in answering a previously unknown question of how mitochondria maintain NAD levels. Preservation of mitochondrial NAD during ischemic or oxidative stress may enhance neuronal viability by maintaining the activity of the electron transport chain, enhancing SIRT activity, and preventing PARP-mediated NAD+ depletion. Future research should focus on determining how Nampt is imported into the mitochondria and whether a mitochondrial Nampt isoform exists. Elucidating these gaps may provide enhanced mechanisms for protecting mitochondria against ischemic and oxidative injury.
The authors declare no conflict of interest.
Footnotes
This work was supported by AHA Grants 10PRE3050053, 13POST1672001, the Lois Pope Life Fellows Program, and NIH Grants NS45676, NS054147, and NS34773.
References
- Ying W. NAD+ and NADH in ischemic brain injury. Front Biosci. 2008;13:1141–1151. doi: 10.2741/2751. [DOI] [PubMed] [Google Scholar]
- Liu D, Pitta M, Mattson MP. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann NY Acad Sci. 2008;1147:275–282. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, Ishida J, et al. A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther. 2004;310:425–436. doi: 10.1124/jpet.104.066944. [DOI] [PubMed] [Google Scholar]
- Zheng C, Han J, Xia W, Shi S, Lui J, Ying W. NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett. 2012;512:67–71. doi: 10.1016/j.neulet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Bi J, Li H, Ye SQ, Ding S. Pre-B-cell colony-enhancing factor exerts a neuronal protection through its enzymatic activity and the reduction of mitochondrial dysfunction in in vitro ischemic models. J Neurochem. 2012;120:334–346. doi: 10.1111/j.1471-4159.2011.07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno JM, Orti M, Salom JB, Sick TJ, Perez-Pinzon MA. Nitric oxide is involved in anoxic preconditioning neuroprotection in rat hippocampal slices. Brain Res. 1999;836:62–69. doi: 10.1016/s0006-8993(99)01610-8. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Wang YJ, Cui M, Luo WJ, Wang XJ, Barber PA, et al. A dietary polyphenol resveratrol acts to provide neuroprotection in recurrent stroke models by regulating AMPK and SIRT1 signaling, thereby reducing energy requirements during ischemia. Eur J Neurosci. 2013;37:1669–1681. doi: 10.1111/ejn.12162. [DOI] [PubMed] [Google Scholar]
- Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI, et al. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J Mol Cell Cardiol. 2009;46:268–277. doi: 10.1016/j.yjmcc.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan TF, Tian WW, Viollet B, Rui YC, et al. Nicotinamide phosphoriboslytransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes and cardiac myocytes. J Neurosci Res. 2007;85:3378–3385. doi: 10.1002/jnr.21479. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Du L, Zhang X, Han YY, Burke NA, Kochanek PM, Watkins SC, et al. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 upregulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Raval AP, Perez-Pinzon MA. Preconditioning mediated by sublethal oxygen-glucose deprivation-induced cyclooxygenase-2 expression via the signal transducers and activators of transcription 3 phosphorylation. J Cereb Blood Flow Metab. 2008;28:1329–1340. doi: 10.1038/jcbfm.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian T, Hopkins IB, McKenna MC, Fiskum G. Isolation of mitochondria with high respiratory control from primary cultures of neurons and astrocytes using nitrogen cavitation. J Neurosci Methods. 2006;152:136–143. doi: 10.1016/j.jneumeth.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, et al. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook RW. Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles. Anal Biochem. 1962;4:231–245. doi: 10.1016/0003-2697(62)90006-4. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Raval AP, Dave KR, Lin HW, Perez-Pinzon MA. Post-ischemic activation of protein kinase C ɛ protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci Lett. 2011;487:158–162. doi: 10.1016/j.neulet.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, DeFazio RA, Perez-Pinzon MA. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–353. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Dave KR, Saul I, Narayanan SV, Perez-Pinzon MA. Epsilon PKC increases brain mitochondrial Sirt1 protein levels via heat shock protein 90 following ischemic preconditioning in rats. PLoS ONE. 2013;8:e75753. doi: 10.1371/journal.pone.0075753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning. Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Starkov A, Blass JP, Ratan RR, Beal MF. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Waxham MN, Grotta JC. Neuronal protection and preservation of calcium/calmodulin-dependent protein kinase II and protein kinase C activity by dextrorphan treatment in global ischemia. J Cereb Blood Flow Metab. 1993;13:550–557. doi: 10.1038/jcbfm.1993.72. [DOI] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HS, Sang WW, Wang YO, Liu W. Nicotinamide phosphoribosyltransferase/sirtuin 1 pathway is involved in human immunodeficiency virus type 1 Tat-mediated long terminal repeat transactivation. J Cell Biochem. 2010;110:1464–1470. doi: 10.1002/jcb.22704. [DOI] [PubMed] [Google Scholar]
- Segawa K, Fukuhara A, Hosogai N, Morita K, Okuno Y, Tanaka M, et al. Visfatin in adipocytes is upregulated by hypoxia through HIF1alpha-dependent mechanism. Biochem Biophys Res Commun. 2006;349:875–882. doi: 10.1016/j.bbrc.2006.07.083. [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39:2587–2595. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MN, Carbone M, Mostocotto C, Mancone C, Tripodi M, Maione R, et al. Mitochondrial localization of PARP-1 requires interaction with mitofilin and is involved in the maintenance of mitochondrial DNA integrity. J Biol Chem. 2009;284:31616–31624. doi: 10.1074/jbc.M109.025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]