Abstract
The cerebral vasculature ensures proper cerebral function by transporting oxygen, nutrients, and other substances to the brain. Distribution of oxygenated blood throughout the neuroaxis takes place at the level of the circle of Willis (CW). While morphologic and functional alterations in CW arteries and its main branches have been reported in cerebrovascular and neurodegenerative diseases, accompanying changes in protein expression profiles remain largely uncharacterized. In this study, we performed proteomics to compile a novel list of proteins present in mouse CW arteries and its ramifications. Circle of Willis arteries were surgically removed from 6-month-old wild-type mice, proteins extracted and analyzed by two proteomics approaches, gel-free nanoLC-mass spectrometry (MS)/MS and gel-based GelLC-MS/MS, using nanoAcquity UPLC coupled with ESI-LTQ Orbitrap XL. The two approaches helped maximize arterial proteome coverage. Six biologic and two technical replicates were performed. In all, 2,188 proteins with at least 2 unique high-scoring peptides were identified (6,630 proteins total). Proteins were classified according to vasoactivity, blood–brain barrier specificity, tight junction and adhesion molecules, membrane transporters/channels, and extracellular matrix/basal lamina proteins. Furthermore, we compared the identified CW arterial proteome with the published brain microvascular proteome. Our database provides a vital resource for the study of CW cerebral arterial protein expression profiles in health and disease.
Keywords: circle of Willis, cerebral artery, cerebral microvessels, proteomics, vascular reactivity
Introduction
A healthy cerebral circulation is essential for maintaining brain perfusion and function.1 In human, oxygenated blood to the brain is delivered by the internal carotid and vertebrobasilar arteries, and is distributed throughout the neuroaxis at the level of the circle of Willis (CW), a ringlike arterial structure located in the subarachnoid space at the base of the brain. The CW is formed by the confluence of the anastomotic branches of the two internal carotid arteries, rostral portion of the vertebrobasilar artery, and the anterior and posterior communicating arteries.2 The arterial wreath allows for communications between the anterior and posterior circulations providing blood to the forebrain and the hindbrain, respectively, and insures redundancies in the cerebral circulation.3 The three principal brain arteries arising from the CW are the left and right anterior, middle, and posterior cerebral arteries, cortical branches of which penetrate the brain parenchyma to irrigate the cerebral cortex and deep structures of the brain.2 In mice, the CW is similarly located along the ventral aspect of the brain extending from the pons–midbrain junction to the anterior cerebrum and involves the same major arteries.4
Several studies have reported morphologic and protein expression changes in the CW and its surface branches in healthy aging5 as well as pathologic conditions, such as cerebrovascular and neurodegenerative diseases (e.g., Alzheimer's disease).5, 6 Arterial wall thickening, loss of elasticity, and alterations in perivascular innervation are common changes observed in aged cerebral arteries.5 Pathology-associated alterations observed in brain arteries/arterioles resemble an admix of accelerated vascular aging and disease-specific hallmarks, such as amyloid deposits in Alzheimer's disease. To date, in comparison with the plethora of structural and functional changes documented in CW arteries of human and animal models, only a limited number of differentially expressed proteins have been identified. The main aim of our study was to generate, and compile a database of the mouse CW arterial proteome that will provide a valuable resource to study differential protein expression patterns in healthy and diseased brain arteries. In this paper, we have developed methodologies for efficient, mass spectrometry (MS)-compatible arterial protein extraction and peptide detection, and compared the CW arterial proteome with a previously published7 brain microvascular proteome.
Materials and methods
Mice
Eighteen 6-month-old C57BL/6J wild-type (WT) mice from two different breedings (B1 and B2) of nine mice each were used in this study. Furthermore, two 6-month-old WT mice from a third breeding were used for protein validation. To eliminate potential gender-related differences in brain vasculature only males were used. Mice were housed under a 12-hour light-dark cycle, in a temperature (23°C) and humidity (50%) controlled room, with food and tap water available ad libitum. All experiments were performed in compliance with the Animal Ethics Committee of the Montreal Neurological Institute and the Canadian Council on Animal Care guidelines.
Surgical Extraction of Circle of Willis Cerebral Arteries
Mice were anesthetized (65 mg/kg sodium pentobarbital, intraperitoneal) and transcardially perfused (0.9% saline, 4°C) for 5 minutes to clear the brain vasculature of blood contaminants. After each perfusion, brain was extracted, immersed in a precooled (4°C) plexiglass-dissection bath containing 0.9% saline, and secured (ventral side up first) with two pins to the transparent silicone cushion at the bottom of the bath. The CW, cerebral arteries (anterior, middle, and posterior), and their main ramifications were surgically removed from the pia mater under a dissecting microscope. Thereafter, isolated arteries underwent a second round of attached pia mater removal. Similarly prepared ‘pure' cerebral arteries from three mice were combined (constituted one biologic replicate) and stored at −80°C. A total of six (B1a, B1b, B1c, B2a, B2b, and B2c) biologic replicates were prepared for this study.
Protein Extraction from Circle of Willis Arteries
Proteins were extracted from surgically isolated arteries using Rapigest SF (Waters, Milford, MA, USA), a MS-compatible acid-labile surfactant that enhances solubilization and tryptic digestion efficiencies of proteins in complex biologic mixtures.8 To each surgically isolated sample, we added 100 μL of 0.2% (w/v) Rapigest SF in 50 mmol/L ammonium bicarbonate. The mixture was then exposed to three 20-second pulses of sonication (550 Sonic Dismembrator; Fisher Scientific, Ottawa, ON, Canada) to further facilitate protein extraction. The above steps were all performed on ice. After sonication, each preparation was incubated at 95°C for 10 minutes, followed by a 20-minute centrifugation (Eppendorf Centrifuge 5415D; Brinkmann Instruments, Westbury, NY, USA) at 10,000 g. The supernatant (S0), containing soluble proteins, was transferred to a fresh tube and used as follows: 5 μL for protein concentration measurement (using Bradford assay; Bio-Rad Corporation, Philadelphia, PA, USA), 80 μL for gel-free proteomics (strong cation exchange (SCX)-LC-MS/MS), and the remaining for gel-based (GelLC-MS/MS) proteomics (see below). The pellet, however, was collagenase treated to recover unextracted/insoluble proteins. Collagenase treatment alters the structure of the collagen fiber network within the arterial wall,9 loosening the extracellular matrix (ECM) and promoting protein solubility. Each pellet was resuspended in 50 μL of buffered collagenase (0.1 mg/mL crude collagenase, 50 mmol/L Tris, pH 7.5, 2 mmol/L CaCl2) and incubated for 60 minutes at 37°C. As crude collagenase also contains active proteases, these samples could only be used for gel-free analysis and not gel-based methods (as the latter requires proteins to be intact). Thus, the collagenase-treated sample was mixed back with 80 μL of its saved S0 fraction, and the entire mixture sonicated three times (30 seconds each), followed by a 10-minute 95°C incubation in a dry bath. Thereafter, the mixture was centrifuged at 10,000 g for 20 minutes to remove debris and the supernatant (S1) collected for gel-free proteomics using ion exchange separation (see below). A total of six biologic replicates were collected for gel-free analysis.
Proteomics Methods for Analyses of Cerebrovascular Proteins
We used two different approaches, gel-based and gel-free, to maximize CW arterial proteome coverage. Both approaches reduced sample complexity by fractionating either (1) at the level of the protein or (2) at the peptide level, respectively. It should be noted that while all six biologic replicates underwent gel-free proteomics, only three samples (B1a, B1b, and B1c) were additionally subjected to the gel-based method.
Gel-based approach (GelLC-MS/MS)
Each soluble protein extract S0 was separated on a 12% one-dimensional SDS-PAGE. A gel cutter was used to consistently cut each sample lane into 29 contiguous pieces and allocated to 10 fractions. Each fraction was allotted 3 contiguous gel pieces, except for fractions 1 and 10. Fraction 1 containing the highest molecular weight proteins was assigned the top two contiguous gel pieces, while fraction 10 containing the lowest molecular weight proteins was apportioned the four contiguous pieces at the bottom of the gel. Each fraction was subjected to in-gel tryptic digestion, and the tryptic peptides were analyzed by nanoLC-MS/MS.
Gel-free approach (strong cation exchange-LC-MS/MS)
This approach involved protein deglycosylation and digestion followed by SCX-based separation of peptides. The proteins from soluble and collagenase-digested extracts (i.e., S1) were first denatured (10 minutes at 95°C in 100 mmol/L 2-mercaptoethanol) and then deglycosylated using N-Glycosidase F (Roche; Cat. # 11365185001, Laval, QC, Canada) at 37°C overnight to increase accessibility to trypsin in later step. Each sample was then reduced in 4 mmol/L dithiothreitol (10 minutes at 95°C) followed by alkylation in the dark in 10 mmol/L iodoacetic acid (30 minutes, at room temperature (RT)). Subsequently, each sample was trypsin digested (Trypsin Gold, MS grade, Promega, Madison, WI, USA; Cat. # V5280) by overnight incubation at 37°C. The resulting peptides were fractionated by SCX using a protocol described previously.10 In brief, an ICAT Cation Exchange Cartridge and Buffer Pack (Applied Biosystems, Carlsbad, CA, USA) were used to elute and collect peptide fractions in a stepwise manner, from low to high salt-containing SCX elution buffer concentration, by slowly injecting (∼1 drop/s) 0.5 mL of each elute buffer. Peptide fractions were collected at 20%, 40%, and 100% elution buffer concentrations.10 The approach is similar to the MUDPIT methodology used for microvessels,7 both being gel-free and label-free methods involving strong SCX separation followed by reverse phase nanoLC-MS/MS. However, unlike MUDPIT, our approach involved off-line SCX separation.
NanoLC-MS/MS Analysis
For each of the six biologic replicates, each SCX and/or in-gel-digest fraction was analyzed twice (technical replicates) by nanoLC-MS/MS. This culminated to a total of 96 nanoLC-MS/MS runs. In detail, each fraction was analyzed using ESI-LTQ-Orbitrap-XL mass spectrometer (Thermo) coupled to a NanoAcquity UPLC system (Waters). Samples were loaded on a NanoAcquity symmetry C18 trap (Waters) and separated on a 10 cm × 100 μm I.D. C18 column (Waters, 1.7 μm BEH130C18) at 250 nL/min using a 68-minutes gradient: 0% to 45% solution B (100% ACN/0.1% formic acid) over 66 minutes and 45% to 95% solution B over 2 minutes. Ten-minute washes in 40% solution B were followed by 30 minutes blank gradients between each sample to minimize carryover effects. The mass spectrometer was set to automated MS/MS analysis with an MS scan at 60 k resolution in the Orbitrap analyzer and data-dependent turbo MS/MS scans on the top three ions in the trap with dynamic exclusion (180 seconds).
Protein Identification and Bioinformatics
The raw data generated from LTQ-Orbitrap were converted to mzXML format using ReAdW program (http://tools.proteomecenter.org). MGF files were then generated from the mzXML file using MzXML2Search from the Trans Proteomics Pipeline project (http://tools.proteomecenter.org) and the resulting MS/MS spectra were searched against Mus musculus Swiss-Prot database using Mascot v2.2.0 (Matrix Science, Boston, MA, USA) search engine for protein identification with the following parameters: enzyme=trypsin; modifications=carbamidomethyl (C, fixed), oxidation (M, variable); peptide tolerance=1.5 Da; fragment tolerance=1.5 Da; 1 missed cleavage allowed). Decoy database searches were used to estimate the false positive identification rate. Search results were subsequently filtered to remove peptides with a delta mass of >15 p.p.m. or a score of <35. Under these conditions, the false positive identification rate based on decoy database searches was 1%. Peptides with a score of >50 had a false positive rate of 0.2%. As an independent statistical measure of peptide identification, Peptide Prophet probabilities (P scores) were also measured. All identified peptides had P scores ⩾0.95. For proteins identified based on only one peptide, additional peptides corresponding to the same proteins within the same gel band with P scores >0.9 were also examined and reported where indicated. To extract quantitative MS data, align all runs, and integrate protein search results, in-house software MatchRx10 version QnD-2.0 was used. MatchRx defines peptide peaks, extracts peptide abundance values from LC-MS data, allows quantitative comparison of peptide levels in two or more samples, and overlays the quantitative differences of peptides and LC-MS/MS identification results (from Mascot) into MSight images.10 Peptide intensity validation was performed using MSight software version 1.0 (http://www.expasy.ch/MSight), a visualization tool that allows graphical representation of the LC-MS and LC-MS/MS data.
Characterization of Arterial Protein List
Two protein lists (List 1 and List 2) of varying stringency were generated. All proteins with P scores ⩾0.95 were included in List 1. The second, more stringent List 2 only contained proteins identified with at least two peptide hits and P scores ⩾0.95. Subcellular localizations and function of proteins were determined using a combination of Gene Ontology (www.geneontology.org) and the Universal Protein Resource (UniProt, www.uniprot.org) databases. The PANTHER Classification System (www.pantherdb.org) was used to classify proteins into pathways. Only pathways with >2 proteins were accounted. Proteins were classified as endothelial cell (EC) proteins if present in an in-house EC database compiled through proteomics/literature mining, and as vascular smooth muscle cell (SMC) proteins if present in an in-house vascular SMC database compiled via literature mining of cultured arterial SMC proteomics data sets.
Western Blotting
Western blots were performed to validate List 1 proteins discoidin domain receptor tyrosine kinase 2 (DDR2) and hyperpolarization-activated cyclic nucleotide-gated cation channel 2 (HCN2), both known to be present in peripheral but not in cerebral arteries. DDR2 is a collagen receptor implicated in the regulation of SMC-mediated collagen turnover in atherosclerosis.11 The ion channel HCN2 is suggested to have a role in the control of vascular tone and dysregulation of its expression may contribute to vascular pathology.12
Frozen CW arteries from each mouse (n=2) were digested in 35 μL Laemmli buffer.13 In brief, each preparation was repeatedly exposed to a pulse of vortexing (20 seconds, RT), followed by incubation (10 minutes, RT) for 2 hours. Thereafter, each preparation was incubated (10 minutes, 100°C), centrifuged (2 minutes, RT), and the supernatant retained. In all, 9 μL of the supernatant was loaded in 5% to 10% Tris/tricine SDS-PAGE gradient gels, separated by electrophoresis, and transferred onto nitrocellulose membranes. Membranes were incubated (1.5 hours, RT) in a blocking buffer containing 5% skim milk and then (overnight, 4°C) with rabbit anti-HCN2 (1:100; Alomone Labs, Jerusalem, Israel) or -DDR2 (1:150; Abcam, Cambridge, MA, USA). Actin was used as the loading control. Membranes were further incubated (1 hour, RT) with horseradish peroxidase-conjugated secondary antibodies (1:1,500 and 1:1,000, respectively) and proteins visualized by chemiluminescence (ECL plus kit; GE Healthcare) using a phosphorImager (Scanner STORM 860; GE Healthcare, Mississauga, ON, Canada).
Comparison with Microvascular Proteome
List 2 arterial proteins were compared with the previously released cerebral microvessel protein data set.7 Inclusion criteria used for microvascular proteins were the following: (1) the presence of ⩾2 unique tryptic peptides, (2) the presence of protein in all technical replicates, and in at least one biologic replicate, and (3) an average spectral count of ⩾5. Common and unique proteins to each data set were identified. The PANTHER Classification System was used to classify proteins by pathways. Proteins were listed as (1) blood–brain barrier (BBB)-specific cell type proteins, (2) tight junction (TJ) and adhesion proteins, (3) membrane transporter and channel proteins, and (d) ECM and basal lamina proteins, if they were (1) identified as such in the microvascular proteomics data set,7 (2) classified accordingly by Gene Ontology or UniProt databases, and were (3) present in either the mouse14 or rat15 BBB transcriptome repositories.
Results
Extraction and Solubilization of Cerebral Arteries
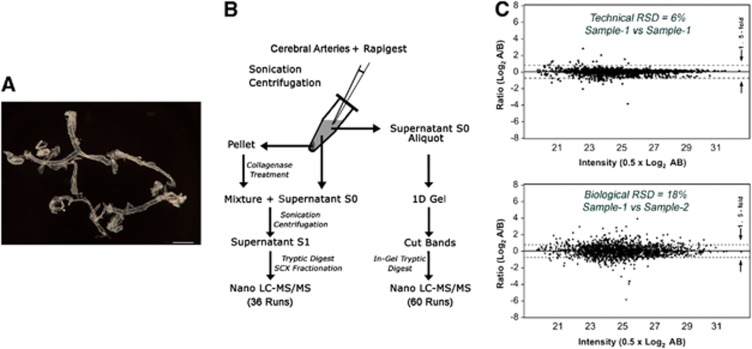
The CW and its ramifications (Figure 1A) were extracted in a reproducible manner using the surgical procedure described (see Materials and methods). Figure 1B outlines the protein solubilization and digest workflow developed in-house for efficient protein extraction from CW cerebral arteries. On average, 87 μg (ranged from 70 to 110 μg) proteins were extracted per biologic replicate. Figure 1C shows the reproducibility of two technical and biologic replicates, where the median relative standard deviation between technical replicates is 6% and between biologic replicates is 18%, values similar to that published for gel-free analysis.16
Figure 1.
Generation of the circle of Willis (CW) cerebral arterial database. (A) Surgically extracted fresh unfixed CW and its major ramifications from one mouse. Scale bar: 1 mm. (B) Simplified outline of the proteomics workflow used for the study. (C) Reproducibility of two technical (sample 1 versus sample 1) and two biologic (sample 1 versus sample 2) replicates. RSD, relative standard deviation; vs, versus; SCX, strong cation exchange.
Proteomic Profile
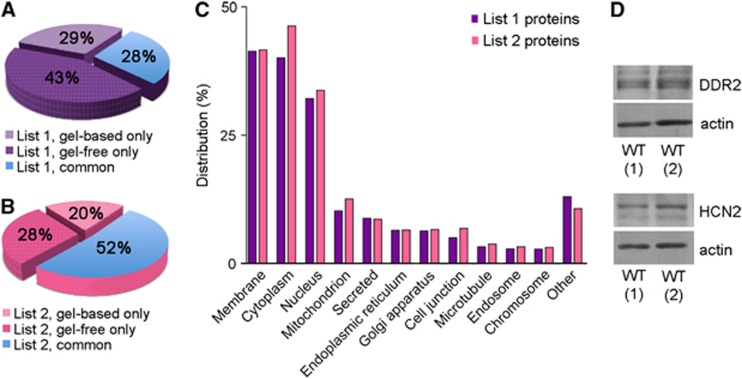
A total of 6,630 CW cerebral arterial proteins (List 1 proteins, Supplementary Table 1) were identified at P scores ⩾0.95. Of these proteins, 1,911 (28.8%) were detected using gel-based approach only, 2,875 (43.4%) using gel-free approach only, while 1,844 proteins (27.8%) were detected using both methods (Figure 2A). The latter was considered as ‘common CW cerebral arterial proteins'. Subcellularly, the top five locations List 1 proteins corresponded to membrane (41.3%), cytoplasm (40.1%), nucleus (32.1%), mitochondrion (10.2%), and secreted (8.8%) (Figure 2C). In addition, List 1 proteins DDR2 and HCN2 were validated using western blots (Figure 2D).
Figure 2.
Circle of Willis (CW) cerebral arterial protein database. (A) Percentage of List 1 proteins uniquely and commonly detected using gel-based and gel-free proteomics approaches. (B) Percentage distribution of the stringent List 2 proteins uniquely and commonly detected using gel-based and gel-free proteomics approaches. Approximately, half of the proteins present in List 2 were detected using both methods. (C) Distribution (in percentage) of subcellular localizations of proteins in List 1 and 2 proteins. (D) Detection of List 1 proteins, discoidin domain receptor tyrosine kinase 2 (DDR2) and hyperpolarization-activated cyclic nucleotide-gated cation channel 2 (HCN2), in CW arteries showed using western blots. Actin was used as the loading control. WT, wild type.
A total of 2,188 List 1 proteins (33%) were identified with ⩾2 peptides each and were cataloged as List 2 proteins (Supplementary Table 1). Approximately half (52%) of these were detected using both gel-based and gel-free approaches (Figure 2B). The top five subcellular locations of List 2 proteins were cytoplasm (46.3%), membrane (41.7%), nucleus (33.7%), mitochondrion (12.6%), and secreted (8.6%) (Figure 2C). A total of 466 proteins (21% of List 2 proteins) belonged to curated pathways in the PANTHER database. Ten pathways with >20 proteins were observed. In descending number of proteins they were as follows: integrin signaling (89 proteins), inflammation mediated by chemokine and cytokine signaling (53 proteins), heterotrimeric G-protein signaling (49 proteins), Wnt signaling (41 proteins), angiogenesis (36 proteins), platelet-derived growth factor signaling (36 proteins), cytoskeletal regulation by Rho GTPase (34 proteins), epidermal growth factor receptor signaling (27 proteins), cadherin signaling (23 proteins), and phosphatidylinositol 3-kinase pathway (23 proteins).
Vasoactivity
We detected 78 proteins shown to regulate the cerebral circulation or exert vasomotor effects on cerebral arteries (Table 1; Supplementary Table 2 for references and additional information). A few of these, specifically, neuronal mediators of vasoactivity and their vascular receptors detected in our arterial data set have been highlighted in the subsections below, and are illustrated in Figure 3.
Table 1. Proteins involved in vasomotricity.
| Protein ID | Symbol | Protein name |
|---|---|---|
| P97718*** | ADRA1A | Alpha-1A adrenergic receptor (ADRA1C) |
| Q8R429*** | ATP2A1 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 (SERCA1) |
| O55143*** | ATP2A2 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) |
| Q64518*** | ATP2A3 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 (SERCA3) |
| Q9ERZ4*** | CHRM2 | Muscarinic acetylcholine receptor M2 |
| P97445*** | CACNA1A | Voltage-dependent P/Q-type calcium channel subunit alpha-1A |
| P62204*** | CALM1 | Calmodulin |
| P35363*** | HTR2A | 5-Hydroxytryptamine receptor 2A |
| P34968*** | HTR2C | 5-Hydroxytryptamine receptor 1 or 2C |
| O55222*** | ILK | Integrin-linked protein kinase |
| Q9Z329*** | ITPR2 | Inositol 1,4,5-trisphosphate receptor type 2 (IP3R2) |
| P70227*** | ITPR3 | Inositol 1,4,5-trisphosphate receptor type 3 (IP3R3) |
| P63143*** | KCNAB1 | Voltage-gated potassium channel subunit beta-1 ( Kvb1) |
| P63143*** | KCNC2 | Potassium voltage-gated channel subfamily C member 2 (Kv3.2) |
| Q14B80*** | KCNH5 | Potassium voltage-gated channel subfamily H member 5 (Kv10.2) |
| P59111*** | KCNH8 | Potassium voltage-gated channel subfamily H member 8 (Kv12.1) |
| P97794*** | KCNJ8 | ATP-sensitive inward rectifier potassium channel 8 (Inward rectifier K(+) channel Kir6.1) |
| Q08460*** | KCNMA1 | Calcium-activated potassium channel subunit alpha-1 (BK channel) |
| O89109*** | KCNN4 | Intermediate conductance calcium-activated potassium channel protein 4 |
| Q8K3F6*** | KCNQ3 | Potassium voltage-gated channel subfamily KQT member 3 (Kv7.3) |
| Q8CFS6*** | KCNV2 | Potassium voltage-gated channel subfamily V member 2 (Kv8.2) |
| Q64133*** | MAOA | Amine oxidase (flavin-containing) A |
| Q8BW75*** | MAOB | Amine oxidase (flavin-containing) B |
| Q3THE2*** | MYL12B | Myosin regulatory light chain 12B (myosin regulatory light chain 20 kDa) |
| Q6PDN3*** | MYLK | Myosin light-chain kinase, smooth muscle (MLCK) |
| Q9Z0J4*** | NOS1 | Neuronal nitric oxide synthase |
| P29477*** | NOS2 | Inducible nitric oxide synthase |
| Q8K4S1*** | PLCE1 | 1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase epsilon-1 (phospholipase C-epsilon-1) |
| Q4KWH5*** | PLCH1 | 1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase eta-1 (phospholipase C-eta-1) |
| A2AP18*** | PLCH2 | 1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase eta-2 (phospholipase C-eta-2) |
| P62137*** | PPP1CA | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit |
| P62141*** | PPP1CB | Serine/threonine-protein phosphatase PP1-beta catalytic subunit |
| Q9DBR7*** | PPP1R12A | Protein phosphatase 1 regulatory subunit 12A (myosin phosphatase-targeting subunit 1 or MYPT1) |
| Q8BG95*** | PPP1R12B | Protein phosphatase 1 regulatory subunit 12B (myosin phosphatase-targeting subunit 2 or MYPT2) |
| Q3UMT1*** | PPP1R12C | Protein phosphatase 1 regulatory subunit 12C (protein phosphatase 1 myosin-binding subunit of 85 kDa or MBS85) |
| Q61056*** | TRPC1 | Short transient receptor potential channel 1 |
| Q9WVC5*** | TRPC7 | Short transient receptor potential channel 7 |
| Q7TN37*** | TRPM4 | Transient receptor potential cation channel subfamily M member 4 |
| O70176** | ADCYAP1 | Pituitary adenylate cyclase-activating polypeptide |
| Q01815** | CACNA1C | Voltage-dependent L-type calcium channel subunit alpha-1C (Cav1.2) |
| Q8R3Z5** | CACNB1 | Voltage-dependent L-type calcium channel subunit beta-1 |
| Q8BIG7** | COMTD1 | Catechol O-methyltransferase domain-containing protein 1 |
| P11881** | ITPR1 | Inositol 1,4,5-trisphosphate receptor type 1 (IP3R1) |
| P97382** | KCNAB3 | Voltage-gated potassium channel subunit beta-3 ( Kvb3) |
| Q17ST2** | KCNA7 | Potassium voltage-gated channel subfamily A member 7 (Kv1.7) |
| O88932** | KCNJ15 | Inward rectifying potassium channel Kir4.2 |
| Q9Z351** | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 (Kv7.2) |
| Q9JK97** | KCNQ4 | Potassium voltage-gated channel subfamily KQT member 4 (Kv7.4) |
| Q9JK45** | KCNQ5 | Potassium voltage-gated channel subfamily KQT member 5 (Kv7.5) |
| P70313** | NOS3 | Endothelial nitric oxide synthase |
| P30549** | TACR2 | Neurokinin A receptor |
| P24529** | TH | Tyrosine 3-monooxygenase |
| Q9R244** | TRPC2 | Short transient receptor potential channel 2 |
| Q9QX29** | TRPC5 | Short transient receptor potential channel 5 |
| P97751** | VIPR1 | Vasoactive intestinal polypeptide receptor1 |
| Q01337* | ADRA2C | Alpha-2C adrenergic receptor |
| Q9R0K7* | ATP2B2 | Plasma membrane calcium-transporting ATPase 2 (PMCA2) |
| Q99246* | CACNA1D | Voltage-dependent L-type calcium channel subunit alpha-1D (Cav1.3) |
| Q9JIS7* | CACNA1F | Voltage-dependent L-type calcium channel subunit alpha-1F (Cav1.4) |
| O88427* | CACNA1H | Voltage-dependent T-type calcium channel subunit alpha-1H (Cav3.2) |
| Q03059* | CHAT | Choline O-acetyltransferase (ChAT) |
| P16390* | KCNA3 | Potassium voltage-gated channel subfamily A member 3 (Kv1.3) |
| Q61762* | KCNA5 | Potassium voltage-gated channel subfamily A member 5 (Kv1.5) |
| Q61923* | KCNA6 | Potassium voltage-gated channel subfamily A member 6 (Kv1.6) |
| P58390* | KCNN2 | Small conductance calcium-activated potassium channel protein 2 |
| P97414* | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 (Kv7.1) |
| P70180* | NPR3 | Atrial natriuretic peptide receptor 3 (NPRC) |
| Q61212* | NPY6R | Neuropeptide Y receptor type 6 (NPY5R) |
| Q9Z1M0* | P2RX7 | P2X purinoceptor 7 |
| Q9Z1B3* | PLCB1 | 1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-1 (phospholipase C-beta-1) |
| P51432* | PLCB3 | 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-3 (phospholipase C-beta-3) |
| P63087* | PPP1CC | Serine/threonine-protein phosphatase PP1-gamma catalytic subunit |
| Q91VC7* | PPP1R14A | Protein phosphatase 1 regulatory subunit 14A or 17 kDa PKC-potentiated inhibitory protein of PP1 (CPI17) |
| P20444* | PRKCA | Protein kinase C alpha type |
| P63318* | PRKCG | Protein kinase C gamma type |
| P70414* | SLC8A1 | Sodium/calcium exchanger 1 (NCX) |
| Q9QUQ5* | TRPC4 | Short transient receptor potential channel 4 |
| Q61143* | TRPC6 | Short transient receptor potential channel 6 |
Protein ID refers to those assigned by UniProt (www.uniprot.org). Cerebral arterial proteins identified with (1) *** peptides ⩾2, with at least two peptides exhibiting Peptide Prophet scores ⩾0.95, (2) ** peptides ⩾2, with one peptide exhibiting Peptide Prophet scores ⩾0.95, and (3) * 1 peptide with Peptide Prophet scores ⩾0.95.
Figure 3.
Neuronal mediators of vasoactivity. Perivascular nerve fibers originating from various peripheral ganglia release vasoactive mediators that act on specific receptors in the vascular wall, many of which were detected in our arterial data set and have been highlighted with colored circles. Protein IDs in figure refer to those assigned by UniProt (www.uniprot.org). In alphabetical order, detected proteins were as follows: ADCYAP1 or PACAP, pituitary adenylate cyclase-activating polypeptide; ADRA1A and 2C, the α-1A and -2C adrenergic receptors; CACNA1A, alpha1A subunit of the voltage-dependent P/Q-type calcium channel; CHAT (or ChAT), choline o-acetyltransferase; CHRM2 (or mAChR2), muscarinic acetylcholine receptor M2; HTR2A and 2C, serotonin receptors 2A and 2C; NOS1, neuronal nitric oxide synthase; NPR3, natriuretic peptide receptor C (also known to bind VIP); NPY6R, neuropeptide Y receptor 6; VIPR1, vasoactive intestinal polypeptide receptor 1; TACR2, neurokinin A receptor. 5-HT, 5-hydroxytryptamine; ACh, acetylcholine; CGRP, calcitonin gene-related peptide; CNS, central nervous system; NA, noradrenaline; NKA, neurokinin A; NO, nitric oxide; NPY, neuropeptide Y; PNS, peripheral nervous system; SCG, superior cervical ganglion; SP, substance P; SPG/OG, sphenopalatine and otic ganglia; TG, trigeminal ganglion; and VIP, vasoactive intestinal polypeptide. Adapted from Hamel1 with permission from author (EH).
Neuronal proteins
Ultrastructural investigations of cerebral arteries show a rich neuronal network at the adventitia–media junction.17 Originating from the peripheral nervous system, these ‘extrinsic' nerve fibers are well-established modulators of vasoactivity.1 Of the classic vasoactive substances released by these nerves, we detected the pituitary adenylate cyclase-activating polypeptide (ADCYAP1 or PACAP) (Figure 3). We also detected eight other neuronal facilitators of vasomotricity, in particular, neuronal nitric oxide (NO) synthase (NOS1), muscarinic acetylcholine (ACh) receptor M2 (CHRM2), alpha1A subunit of the voltage-dependent P/Q-type calcium channel (CACNA1A), and choline O-acetyltransferase (ChAT) represented in Figure 3, as well as, tyrosine 3-monooxygenase (or tyrosine hydroxylase, TH), monoamine oxidases type A and B (MAOA, B), and catechol O-methyltransferase domain-containing protein 1 (COMTD1).
Arterial proteins
We also detected receptors, known to be present on the vessel wall, that bind vasoactive molecules released by the extrinsic nerve fibers. These included the α-1A and α-2C adrenergic receptors (ADRA1A and ADRA2C), vasoactive intestinal polypeptide receptor 1 (VIPR1 or VPAC1), neurokinin A receptor (TACR2 or NK2R), and the 5-hydroxytryptamine receptors 2A and 2C (HTR2A and HTR2C) (Table 1; Figure 3).
In addition, we detected various smooth muscle and endothelial mediators of vasoactivity (Table 1; Supplementary Table 2 for references and additional information), including endothelial NO synthase (NOS3 or eNOS) that generates NO, a potent vasodilator. A cartoon diagram summarizing how some of Table 1 proteins mediate arterial smooth muscle motricity is presented in Figure 4.
Figure 4.
Protein effectors of arterial smooth muscle contraction and dilation. Simplified representation of proteins and protein pathways involved in vascular smooth muscle contraction. Protein effectors present in our arterial database are indicated in italics and are listed in Table 1. It should be noted that while protein effectors of vascular smooth muscle relaxation (in particular, K+ channels, PMCAs, Na+/Ca2+ exchanger, and SERCAs) are present in this cartoon diagram, they are not in their activated states. ADP, adenosine diphosphate; ATP, adenosine triphosphate; Ca2+, calcium; CaM, calmodulin; G, G protein; LC20 (or MYL12B), myosin 20 kDa light chain; LC20.P (or MYL12B.P), phosphorylated myosin 20 kDa light chain; MLCK (or MYLK), myosin light-chain kinase; MLCP, myosin light-chain phosphatase; NO, nitric oxide; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PMCAs, plasma membrane calcium ATPases; ROCCs, receptor-operated calcium channels; SERCAs, smooth endoplasmic reticulum calcium ATPases; SOCCs, store-operated calcium channels; VGCCs, voltage-gated calcium channels. Inspired from Horowitz et al19 with permission from last author (KGM).
Cerebral Arterial Versus Microvascular Proteomes
A total of 3,411 unique proteins were present in the arterial (List 2, 2,188 proteins total) and microvascular (1,824 proteins total) data sets combined (Figure 5A). Of these, 1,587 (47%) and 1,223 (36%) proteins were unique to the arterial and microvascular data sets, respectively, whereas 601 (18%) were common to both (Figure 5A). These common proteins comprised 28% of the List 2 CW cerebral arterial data set and 33% of the microvascular data set. Analysis of common proteins yielded a total of 62 PANTHER pathways, the top 5 being integrin signaling (35 proteins), heterotrimeric G-protein signaling (26 proteins), inflammation mediated by chemokine and cytokine signaling (23 proteins), cytoskeletal regulation by Rho GTPase (17 proteins), and Wnt signaling (13 proteins). A total of 58 pathways were identified for proteins solely present in either arteries or microvessels. Of these, 2 and 7 pathways were unique to arterial and microvascular proteomes, respectively, whereas 49 were common. Listed are data set-specific pathways with ⩾4 proteins: (1) hypoxia response via hypoxia-inducible factor activation pathway (unique to arterial data set) and (2) 5HT4 type receptor mediated signaling pathway, corticotropin releasing factor receptor signaling pathway, opioid (prodynorphin, proenkephalin, and proopiomelanocortin) pathways, β3 adrenergic receptor signaling pathway, and androgen/estrogen/progesterone biosynthesis pathway (unique to microvascular data set). In the following subsections, we compare protein distributions of (1) smooth muscle and endothelial components and (2) BBB and associated categories in the arterial and microvascular data sets.
Figure 5.
Cerebral arterial versus microvascular proteomes. (A) Distribution of unique and common proteins present in the arterial and microvascular databases. (B) Percent distribution of unique vascular smooth muscle and endothelial proteins present in the two-abovementioned data sets. Proteins were classified as belonging to one of the two categories based on their presence in the in-house compiled endothelial cell (EC) and SM cell (SMC) databases. Distribution of arterial and microvascular proteins present in four different categories, namely, (C) BBB-specific cell types, (D) tight junction and adhesion proteins, (E) membrane transporter and channel proteins, and (F) ECM and basal lamina proteins. BBB, blood–brain barrier; ECM, extracellular matrix; SM, smooth muscle.
Smooth muscle cell and endothelial cell protein distribution
We identified 230 (11%) SMC- and 1,035 (47%) EC-classified proteins uniquely present in the arterial data set containing a total of 2,188 proteins (Figure 5B). In all, 204 (9%) of these proteins were common to both cell types. In comparison, we established that 122 (7%) SMC- and 623 (34%) EC-classified proteins were unique to the microvascular data set containing a total of 1,824 proteins (Figure 5B). In all, 107 (6%) of these proteins were common to both cell types. In addition, we identified 218 SMC- and 515 EC-classified proteins common to both arterial and microvascular data sets (containing a total of 3,411 proteins), with 206 (6%) belonging to both cell types.
Blood–brain barrier-specific proteins
In the arterial and microvascular data sets combined, we detected 86 BBB-specific proteins, 28 (33%) of which were detected in both sample types (Table 2; Figure 5C). While 52 BBB-enriched proteins were microvascular data set specific, we detected only 6 such proteins exclusive to our arterial data set. These included two transporters (ABCB1A and SLC2A1), three transcriptional regulators (EBF1, FOXQ1, and LEF1), and the DNA repair protein RAD54B.
Table 2. Cerebral artery proteome classified into BBB-specific proteins, tight junction and adhesion molecules, membrane transporters and channels, and ECM and basal lamina proteins.
| UniProt ID | Symbol | Protein name | M |
|---|---|---|---|
| BBB-specific proteins | |||
| P20152*** | VIM | Vimentin | y |
| P04370*** | MBP | Myelin basic protein | y |
| Q91ZX7*** | LRP1 | Prolow-density lipoprotein receptor-related protein 1 | y |
| P21279*** | GNAQ | Guanine nucleotide-binding protein G(q) subunit alpha | y |
| Q61490*** | ALCAM | CD166 antigen | y |
| Q9CZJ2*** | HSPA12B | Heat shock 70 kDa protein 12B | y |
| P48678*** | LMNA | Lamin-A/C | y |
| Q8BTM8*** | FLNA | Filamin-A | y |
| Q60634*** | FLOT2 | Flotillin-2 | y |
| P08228*** | SOD1 | Superoxide dismutase [Cu-Zn] | y |
| P10833*** | RRAS | Ras-related protein R-Ras | y |
| Q9R0P5*** | DSTN | Destrin | y |
| Q8R2Z5*** | VWA1 | von Willebrand factor A domain-containing protein 1 | y |
| P49817*** | CAV1 | Caveolin-1 | y |
| Q3UMF0*** | COBLL1 | Cordon-bleu protein-like 1 | y |
| P15208*** | INSR | Insulin receptor | y |
| Q8CIZ8*** | VWF | von Willebrand factor | y |
| P27600*** | GNA12 | Guanine nucleotide-binding protein subunit alpha-12 | y |
| P03995*** | GFAP | Glial fibrillary acidic protein | y |
| Q8R0Y6*** | ALDH1L1 | 10-formyltetrahydrofolate dehydrogenase | y |
| Q9QUP5*** | HAPLN1 | Hyaluronan and proteoglycan link protein 1 | y |
| P62737*** | ACTA2 | Actin, aortic smooth muscle | y |
| P63094*** | GNAS | Guanine nucleotide-binding protein G(s) subunit alpha isoform short | y |
| Q60675*** | LAMA2 | Laminin subunit alpha-2 | y |
| Q61830*** | MRC1 | Macrophage mannose receptor 1 | y |
| Q61696*** | HSPA1A | Heat-shock 70-kDa protein 1A | y |
| Q8VHY0*** | CSPG4 | Chondroitin sulfate proteoglycan 4 | y |
| P05622*** | PDGFRB | Beta-type platelet-derived growth factor receptor | y |
| P21447*** | ABCB1A | Multidrug resistance protein 3 | — |
| Q07802*** | EBF1 | Transcription factor COE1 | — |
| O70220*** | FOXQ1 | Forkhead box protein Q1 | — |
| P27782*** | LEF1 | Lymphoid enhancer-binding factor 1 | — |
| Q6PFE3*** | RAD54B | DNA repair and recombination protein RAD54B | — |
| P17809*** | SLC2A1 | Solute carrier family 2, facilitated glucose transporter member 1 | — |
| Tight junction and/or adhesion proteins | |||
| Q61490*** | ALCAM | CD166 antigen | y |
| P15379*** | CD44 | CD44 antigen | y |
| P26231*** | CTNNA1 | Catenin alpha-1 | y |
| Q61301*** | CTNNA2 | Catenin alpha-2 | y |
| Q02248*** | CTNNB1 | Catenin beta-1 | y |
| P30999*** | CTNND1 | Catenin delta-1 | y |
| O35927*** | CTNND2 | Catenin delta-2 | y |
| Q62108*** | DLG4 | Disks large homolog 4 | y |
| Q3V3R4*** | ITGA1 | Integrin alpha-1 | y |
| Q62470*** | ITGA3 | Integrin alpha-3 | y |
| Q61738*** | ITGA7 | Integrin alpha-7 | y |
| P43406*** | ITGAV | Integrin alpha-V | y |
| P09055*** | ITGB1 | Integrin beta-1 | y |
| O54890*** | ITGB3 | Integrin beta-3 | y |
| Q02257*** | JUP | Junction plakoglobin | y |
| Q6RHR9*** | MAGI1 | Membrane-associated guanylate kinase, WW & PDZ domain-containing protein 1 | y |
| Q9EQJ9*** | MAGI3 | Membrane-associated guanylate kinase, WW & PDZ domain-containing protein 3 | y |
| Q8R2Y2*** | MCAM | Cell surface glycoprotein MUC18 | y |
| Q8VBX6*** | MPDZ | Multiple PDZ domain protein | y |
| P70290*** | MPP1 | 55 kDa erythrocyte membrane protein | y |
| Q810U4*** | NRCAM | Neuronal cell adhesion molecule | y |
| Q08481*** | PECAM1 | Platelet endothelial cell adhesion molecule | y |
| Q80 × 82*** | SYMPK | Symplekin | y |
| P39447*** | TJP1 | Tight junction protein ZO-1 | y |
| Q9Z0U1*** | TJP2 | Tight junction protein ZO-2 | y |
| P29533*** | VCAM1 | Vascular cell adhesion protein 1 | y |
| P83741*** | WNK1 | Serine/threonine-protein kinase WNK1 | y |
| Q8K371*** | AMOTL2 | Angiomotin-like protein 2 | — |
| Q9WTR5*** | CDH13 | Cadherin-13 | — |
| Q99PF4*** | CDH23 | Cadherin-23 | — |
| Q9Z0S3*** | CLDN14 | Claudin-14 | — |
| Q65CL1*** | CTNNA3 | Catenin alpha-3 | — |
| Q8BJ42*** | DLGAP2 | Disks large-associated protein 2 | — |
| Q63ZW7*** | INADL | InaD-like protein | — |
| Q00651*** | ITGA4 | Integrin alpha-4 | — |
| P11688*** | ITGA5 | Integrin alpha-5 | — |
| A2ARA8*** | ITGA8 | Integrin alpha-8 | — |
| P05555*** | ITGAM | Integrin alpha-M | — |
| A2A863*** | ITGB4 | Integrin beta-4 | — |
| Q9Z0T9*** | ITGB6 | Integrin beta-6 | — |
| P97350*** | PKP1 | Plakophilin-1 | — |
| Q9QY23*** | PKP3 | Plakophilin-3 | — |
| P32507*** | PVRL2 | Poliovirus receptor-related protein 2 | — |
| A2ALU4*** | SHROOM2 | Protein Shroom2 | — |
| Q4G0F8*** | UBN1 | Ubinuclein-1 | — |
| Q3UH66*** | WNK2 | Serine/threonine-protein kinase WNK2 | — |
| Q80XP9*** | WNK3 | Serine/threonine-protein kinase WNK3 | — |
| Membrane transporter and channel proteins | |||
| P41233*** | ABCA1 | ATP-binding cassette sub-family A member 1 | y |
| P21447*** | ABCB1A | Multidrug resistance protein 3 | y |
| Q61102*** | ABCB7 | ATP-binding cassette sub-family B member 7 | y |
| P41234*** | ABCA2 | ATP-binding cassette sub-family A member 2 | — |
| Q8R420*** | ABCA3 | ATP-binding cassette sub-family A member 3 | — |
| Q8K441*** | ABCA6 | ATP-binding cassette sub-family A member 6 | — |
| Q91V24*** | ABCA7 | ATP-binding cassette sub-family A member 7 | — |
| Q5SSE9*** | ABCA13 | ATP-binding cassette sub-family A member 13 | — |
| P21440*** | ABCB4 | Multidrug resistance protein 2 | — |
| Q9QY30*** | ABCB11 | Bile salt export pump | — |
| Q8VI47*** | ABCC2 | Canalicular multispecific organic anion transporter 1 | — |
| Q9R1 × 5*** | ABCC5 | Multidrug resistance-associated protein 5 | — |
| P61222*** | ABCE1 | ATP-binding cassette subfamily E member 1 | — |
| Q64343*** | ABCG1 | ATP-binding cassette subfamily G member 1 | — |
| Q8VDN2*** | ATP1A1 | Sodium/potassium-transporting ATPase subunit alpha-1 | y |
| Q6PIE5*** | ATP1A2 | Sodium/potassium-transporting ATPase subunit alpha-2 | y |
| Q6PIC6*** | ATP1A3 | Sodium/potassium-transporting ATPase subunit alpha-3 | y |
| P14094*** | ATP1B1 | Sodium/potassium-transporting ATPase subunit beta-1 | y |
| P14231*** | ATP1B2 | Sodium/potassium-transporting ATPase subunit beta-2 | y |
| P97370*** | ATP1B3 | Sodium/potassium-transporting ATPase subunit beta-3 | y |
| Q8R429*** | ATP2A1 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 | y |
| O55143*** | ATP2A2 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 | y |
| Q64518*** | ATP2A3 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 | y |
| Q64436*** | ATP4A | Potassium-transporting ATPase alpha chain 1 | y |
| Q03265*** | ATP5A1 | ATP synthase subunit alpha, mitochondrial | y |
| P56480*** | ATP5B | ATP synthase subunit beta, mitochondrial | y |
| Q91VR2*** | ATP5C1 | ATP synthase subunit gamma, mitochondrial | y |
| Q9CQQ7*** | ATP5F1 | ATP synthase subunit b, mitochondrial | y |
| Q9DCX2*** | ATP5H | ATP synthase subunit d, mitochondrial | y |
| P97450*** | ATP5J | ATP synthase-coupling factor 6, mitochondrial | y |
| P56135*** | ATP5J2 | ATP synthase subunit f, mitochondrial | y |
| Q9CPQ8*** | ATP5L | ATP synthase subunit g, mitochondrial | y |
| Q9DB20*** | ATP5O | ATP synthase subunit O, mitochondrial | y |
| P50516*** | ATP6V1A | V-type proton ATPase catalytic subunit A | y |
| P62814*** | ATP6V1B2 | V-type proton ATPase subunit B, brain isoform | y |
| Q8BVE3*** | ATP6V1H | V-type proton ATPase subunit H | y |
| P70704*** | ATP8A1 | Probable phospholipid-transporting ATPase IA | y |
| Q9Z1W8*** | ATP12A | Potassium-transporting ATPase alpha chain 2 | y |
| A7L9Z8*** | ATP2C2 | Calcium-transporting ATPase type 2C member 2 | — |
| Q06185*** | ATP5I | ATP synthase subunit e, mitochondrial | — |
| Q3UHK1*** | SLC2A13 | Proton myo-inositol cotransporter | y |
| Q761V0*** | SLC6A5 | Sodium- and chloride-dependent glycine transporter 2 | y |
| Q8BLV3*** | SLC9A7 | Sodium/hydrogen exchanger 7 | y |
| P57787*** | SLC16A3 | Monocarboxylate transporter 4 | y |
| Q8VEM8*** | SLC25A3 | Phosphate carrier protein, mitochondrial | y |
| P48962*** | SLC25A4 | ADP/ATP translocase 1 | y |
| P51881*** | SLC25A5 | ADP/ATP translocase 2 | y |
| Q9CR62*** | SLC25A11 | Mitochondrial 2-oxoglutarate/malate carrier protein | y |
| Q8BH59*** | SLC25A12 | Calcium-binding mitochondrial carrier protein Aralar1 | y |
| Q5U680*** | SLC25A26 | S-adenosylmethionine mitochondrial carrier protein | y |
| Q8R0Y8*** | SLC25A42 | Solute carrier family 25 member 42 | y |
| Q80SU6*** | SLC34A3 | Sodium-dependent phosphate transport protein 2C | y |
| P43006*** | SLC1A2 | Excitatory amino-acid transporter 2 | — |
| O35544*** | SLC1A6 | Excitatory amino-acid transporter 4 | — |
| P17809*** | SLC2A1 | Solute carrier family 2, facilitated glucose transporter member1 | — |
| P10852*** | SLC3A2 | 4F2 cell-surface antigen heavy chain | — |
| Q8K0E3*** | SLC5A11 | Sodium/myo-inositol cotransporter 2 | — |
| O88576*** | SLC6A18 | Sodium- and chloride-dependent transporter XTRP2 | — |
| Q8BUE1*** | SLC9A4 | Sodium/hydrogen exchanger 4 | — |
| Q6UJY2*** | SLC9A10 | Sodium/hydrogen exchanger 10 | — |
| Q91Y63*** | SLC13A3 | Solute carrier family 13 member 3 | — |
| Q8VC69*** | SLC22A6 | Solute carrier family 22 member 6 | — |
| Q8BMD8*** | SLC25A24 | Calcium-binding mitochondrial carrier protein SCaMC-1 | — |
| Q8VC69*** | SLC26A1 | Solute carrier family 22 member 6 | — |
| P97441*** | SLC30A3 | Zinc transporter 3 | — |
| Q60825*** | SLC34A1 | Sodium-dependent phosphate transport protein 2A | — |
| Q9DBP0*** | SLC34A2 | Sodium-dependent phosphate transport protein 2B | — |
| Q8CD26*** | SLC35E1 | Solute carrier family 35 member E1 | — |
| ECM and basal lamina proteins | |||
| A2ASQ1*** | AGRN | Agrin | y |
| P07356*** | ANXA2 | Annexin A2 | y |
| P02463*** | COL4A1 | Collagen alpha-1(IV) chain | y |
| P08122*** | COL4A2 | Collagen alpha-2(IV) chain | y |
| P11276*** | FN1 | Fibronectin | y |
| Q9QUP5*** | HAPLN1 | Hyaluronan and proteoglycan link protein 1 | y |
| Q05793*** | HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein | y |
| P97927*** | LAMA4 | Laminin subunit alpha-4 | y |
| Q61001*** | LAMA5 | Laminin subunit alpha-5 | y |
| Q61292*** | LAMB2 | Laminin subunit beta-2 | y |
| P02468*** | LAMC1 | Laminin subunit gamma-1 | y |
| Q9R0B6*** | LAMC3 | Laminin subunit gamma-3 | y |
| P10493*** | NID1 | Nidogen-1 | y |
| O88322*** | NID2 | Nidogen-2 | y |
| Q60675*** | LAMA2 | Laminin subunit alpha-2 | y |
| Q9R001*** | ADAMTS5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 | — |
| Q811B3*** | ADAMTS12 | A disintegrin and metalloproteinase with thrombospondin motifs 12 | — |
| P59384*** | ADAMTS15 | A disintegrin and metalloproteinase with thrombospondin motifs 15 | — |
| Q69Z28*** | ADAMTS16 | A disintegrin and metalloproteinase with thrombospondin motifs 16 | — |
| P59509*** | ADAMTS19 | A disintegrin and metalloproteinase with thrombospondin motifs 19 | — |
| P59511*** | ADAMTS20 | A disintegrin and metalloproteinase with thrombospondin motifs 20 | — |
| Q80T21*** | ADAMTSL4 | ADAMTS-like protein 4 | — |
| Q99MQ4*** | ASPN | Asporin | — |
| P28653*** | BGN | Biglycan | — |
| Q8R2G6*** | CCDC80 | Coiled-coil domain-containing protein 80 | — |
| Q61245*** | COL11A1 | Collagen alpha-1(XI) chain | — |
| Q64739*** | COL11A2 | Collagen alpha-2(XI) chain | — |
| Q60847*** | COL12A1 | Collagen alpha-1(XII) chain | — |
| Q80 × 19*** | COL14A1 | Collagen alpha-1(XIV) chain | — |
| O35206*** | COL15A1 | Collagen alpha-1(XV) chain | — |
| Q8BLX7*** | COL16A1 | Collagen alpha-1(XVI) chain | — |
| Q07563*** | COL17A1 | Collagen alpha-1(XVII) chain | — |
| P39061*** | COL18A1 | Collagen alpha-1(XVIII) chain | — |
| Q0VF58*** | COL19A1 | Collagen alpha-1(XIX) chain | — |
| P11087*** | COL1A1 | Collagen alpha-1(I) chain | — |
| Q01149*** | COL1A2 | Collagen alpha-2(I) chain | — |
| Q30D77*** | COL24A1 | Collagen alpha-1(XXIV) chain | — |
| Q5QNQ9*** | COL27A1 | Collagen alpha-1(XXVII) chain | — |
| Q2UY11*** | COL28A1 | Collagen alpha-1(XXVIII) chain | — |
| P28481*** | COL2A1 | Collagen alpha-1(II) chain | — |
| P08121*** | COL3A1 | Collagen alpha-1(III) chain | — |
| Q9QZS0*** | COL4A3 | Collagen alpha-3(IV) chain | — |
| O88207*** | COL5A1 | Collagen alpha-1(V) chain | — |
| Q3U962*** | COL5A2 | Collagen alpha-2(V) chain | — |
| Q04857*** | COL6A1 | Collagen alpha-1(VI) chain | — |
| Q02788*** | COL6A2 | Collagen alpha-2(VI) chain | — |
| A2AX52*** | COL6A4 | Collagen alpha-4(VI) chain | — |
| A6H584*** | COL6A5 | Collagen alpha-5(VI) chain | — |
| Q8C6K9*** | COL6A6 | Collagen alpha-6(VI) chain | — |
| Q63870*** | COL7A1 | Collagen alpha-1(VII) chain | — |
| Q00780*** | COL8A1 | Collagen alpha-1(VIII) chain | — |
| P25318*** | COL8A2 | Collagen alpha-2(VIII) chain | — |
| Q05722*** | COL9A1 | Collagen alpha-1(IX) chain | — |
| Q07643*** | COL9A2 | Collagen alpha-2(IX) chain | — |
| Q8R555*** | CRTAC1 | Cartilage acidic protein 1 | — |
| P28654*** | DCN | Decorin | — |
| P54320*** | ELN | Elastin | — |
| Q91VF5*** | EMID1 | EMI domain-containing protein 1 | — |
| Q99K41*** | EMILIN1 | EMILIN-1 | — |
| Q08879*** | FBLN1 | Fibulin-1 | — |
| P37889*** | FBLN2 | Fibulin-2 | — |
| Q61554*** | FBN1 | Fibrillin-1 | — |
| Q61555*** | FBN2 | Fibrillin-2 | — |
| P50608*** | FMOD | Fibromodulin | — |
| O35367*** | KERA | Keratocan | — |
| Q61789*** | LAMA3 | Laminin subunit alpha-3 | — |
| Q61087*** | LAMB3 | Laminin subunit beta-3 | — |
| Q61092*** | LAMC2 | Laminin subunit gamma-2 | — |
| P16045*** | LGALS1 | Galectin-1 | — |
| Q9D1H9*** | MFAP4 | Microfibril-associated glycoprotein 4 | — |
| Q9R1A3*** | NTN3 | Netrin-3 | — |
| Q9JI33*** | NTN4 | Netrin-4 | — |
| Q62009*** | POSTN | Periostin | — |
| Q9JK53*** | PRELP | Prolargin | — |
| P04202*** | TGFBI | Transforming growth factor beta-1 | — |
| Q8R2Z5*** | VWA1 | von Willebrand factor A domain-containing protein | — |
| Q8CIZ8*** | VWF | von Willebrand factor | — |
| P70701*** | WNT10A | Protein Wnt-10a | — |
| P22725*** | WNT5A | Protein Wnt-5a | — |
Cerebral arterial proteins were identified with ⩾2 peptides each and with at least two peptides exhibiting Peptide Prophet scores ⩾0.95 (indicated with ***). Note that proteins present only in the microvascular data set are not listed. In column M (or microvascular), proteins detected in the study by Chun et al7 and present in our arterial data set are indicated as ‘y'. y, yes.
Tight junction and adhesion proteins
We identified 83 TJ and/or adhesion proteins. Of these proteins, 19 and 37 were specific to arteries and microvessels, respectively, while 27 proteins were common to both data sets (Figure 5D; Table 2). In descending order, common proteins belonged to the following groups: nine TJ plaque proteins: DLG4, MAGI1, MAGI3, MPDZ, MPP1, SYMPK, TJP1, TJP2, and WNK1; six immunoglobulin superfamily cell adhesion molecules (ALCAM, CD44, MCAM, NRCAM, PECAM1, and VCAM1), six integrins (α1, 3, 7, V and β1, 3), and five catenins (α1 and 2, β1, and δ1, 2) (Supplementary Table 3—for protein function and cellular component details).
Membrane transporter and channel proteins
We detected 138 proteins in the arterial and microvascular data sets involved in transport across the plasma membrane. Sixty-eight of these proteins were present in our arterial data set and include 28 solute carrier proteins (SLCs), 14 adenosine 5′-triphosphate (ATP)-binding cassette proteins (ABCs), and 26 ATPases (Na+/K+, H+/K+, and Ca2+) (Table 2). Twenty-nine of the above-mentioned proteins were unique to our data set, while thirty-nine were also present in the microvascular data set (Table 2; Figure 5E). In comparison, a larger number (70) of transporters were found exclusively in microvascular data set (Figure 5E).
Extracellular matrix and basal lamina proteins
A total of 94 ECM and/or basal lamina proteins were identified in the two data sets combined (Figure 5F). Of these 64 were present in our arterial data set only (Table 2), while a smaller number (15) were microvascular specific. Fifteen proteins were common to both data sets (Table 2). These included six laminins (subunits α2, α4, α5, β2, γ1, and γ3), two collagens (IVα1 and IVα2), two nidogens (1 and 2), agrin, annexin A2, fibronectin, hyaluronan/proteoglycan link protein 1, and basement membrane-specific heparan sulfate proteoglycan core protein.
Discussion
We have generated the first extensive database of 6,630 proteins expressed in the wall of fresh cerebral arteries. An advantage of using freshly isolated over cultured tissue is that it allows for maximal preservation of the tissue microenvironment experience in vivo in the absence of potential tissue culture-induced artifacts, such as phenotypic drift, that can result in altered protein expression.18 Of the 6,630 proteins in our database, 33% were detected with ⩾2 peptides at P scores ⩾0.95, and the remaining 67% with at least 1 peptide and the same stringent P scores cutoff. We established that approximately half (56%) of the proteins in our arterial databank are of known vascular origin, being copresent in the EC and/or the vascular SMC protein databases. We further established that almost a third of these proteins are robustly expressed in cerebral arteries and can be detected by both gel-based and gel-free approaches.
Arterial Proteome Coverage
To generate a comprehensive arterial protein database, we developed highly reproducible methodologies that allowed for (1) rapid surgical isolation of fresh cerebral arteries, (2) efficient protein extraction using the MS-compatible surfactant Rapigest, with yields ∼3 × higher compared with SDS-based extraction (unpublished data), and (3) optimal detection and quantification of low-abundant peptides. Concomitant use of gel-based and gel-free approaches contributed to increased detection of peptides (and proteins) as showcased by (1) the presence of EC layer proteins, which are harder to detect since unlike the SMC and adventitia layers, the EC layer is a single layer of cells and (2) good agreement between the breakdown of detected arterial proteins by subcellular localization and the relative sizes of cellular compartments (e.g., membrane>mitochondria).
Cerebral Arteries and Vasoactivity
Large arteries as well as smaller arterioles at the brain surface constitute resistance vessels that control blood flow and influence global perfusion.5 Vascular resistance is essential for pushing blood through the cerebral circulation, and is increased through contraction of the vessel muscular wall, and decreased via vasodilation stemming from the relaxation of SMCs.5 Interestingly, 3 of the top 10 PANTHER pathways detected in our arterial data set, namely heterotrimeric G-proteins (e.g., Figure 4), integrin signaling (e.g., Figure 4), and cytoskeletal regulation by Rho GTPases, are established modulators of vasomotricity.19, 20, 21 In addition to signaling cascade proteins, we also identified several perivascular and vascular mediators of vasoactivity that are discussed in the following two sections.
Neuronally released vasoactive substances and their receptors
Nerve fibers originating in the peripheral nervous system, specifically, the superior cervical ganglion (sympathetic innervation), the sphenopalatine and otic ganglia (parasympathetic innervation), and the trigeminal ganglion exert vasomotor control on cerebral arteries via release of neurotransmitters and other vasoactive substances.1 We detected vascular receptors for five of these substances in our arterial data set, in particular, the α-1A and α-2C adrenergic receptors (ADRA1A and ADRA2C); the neuropeptide Y receptor, NPY6R; serotonin receptors, HTR2A (or 5-HT2A) and HTR2C (or 5-HT2C); the established vasoactive intestinal polypeptide receptor, VIPR1 (or VPAC1); the lesser known intestinal polypeptide receptor, NPR3 (or NPRC (natriuretic peptide receptor C)); and finally, the neurokinin A receptor TACR2 (or NKR2) (Figure 3). The presence of all above-mentioned receptors, with the exception of NPY6R and HTR2C, has been previously shown in cerebral arteries (for references, see Supplementary Table 2). Moreover, the detected receptors are G protein-coupled and modulate vasoactivity via heterotrimeric G-protein signaling, interestingly, a top 10 PANTHER pathway detected in our study.
We also detected nine neuronal mediators of vasoactivity (Table 1; Supplementary Table 2 for references; and Figure 3). Of these, the pituitary adenylate cyclase activating peptide (ADCYAP1 or PACAP), released by perivascular nerve fibers originating in the trigeminal ganglion, is an established vasodilator.22 The calcium channel CACNA1A facilitates release of the calcitonin gene-related protein,23 another vasodilator compound released by trigeminal ganglion originating nerves.1 The presynaptic muscarinic ACh receptor CHRM2 (or mAChR2), located on ACh releasing nitric oxidergic nerves originating in sphenopalatine and otic ganglia, is known to mediate inhibition of neurogenic vasodilation in cerebral arteries via reduction in NOS activity and the production and release of the vasodilator NO.24 Both ChAT and NOS1 (or nNOS) enzymes involved in the synthesis of ACh and NO, respectively, were present in our arterial data set. In addition, we detected four enzymes (TH, MAOA, MAOB, and COMTD1) involved in the formation and degradation of superior cervical ganglion released vasoactive substance, norepinephrine (or noradrenaline).25
Proteins involved in vascular smooth muscle contractility and relaxation
Among the key effectors of smooth muscle contraction we detected calmodulin (CALM1), the 20-kDa myosin light chain (MYL12B or LC20), as well as the myosin light chain-specific kinase (MYLK or MLCK) and phosphatase (PPP1 or MLCP). Both catalytic (PPP1CA, B, and C) and myosin-targeting (PPP1R12A, B, and C) subunits of the phosphatase were detected, with only PPP1R12A previously reported in cerebral arteries (Supplementary Table 2 for references). It is well established that intracellular-free calcium (iCa2+) binds with calmodulin, which then binds and activates the myosin light-chain kinase.19 The activated kinase complex then phosphorylates myosin on the 20-kDa light chains, resulting in smooth muscle contraction.19 Conversely, dephosphorylation of myosin light chains induces relaxation (Figure 4). Activated protein kinase C (PKC) and integrins are known to inhibit dephosphorylation under physiologic conditions via the PKC-potentiated inhibitory protein of MLCP (PPP1R14A) and the integrin linked kinase, respectively20 (Figure 4). We detected two classic PKCs (PRKCA and PRKCG), PPP1R14A and integrin linked kinase in our arterial data set, with all except for PRKCG previously reported in cerebral arteries (Supplementary Table 2 for references), as well as several integrins (see Table 2).
Increases in iCa2+ are a major determinant of arterial smooth muscle contractility.19 In SMCs, rise in iCa2+ is facilitated by (1) Ca2+ release from intracellular stores in the smooth sarcoplasmic reticulum and (2) Ca2+ entry from the extracellular space via plasma membrane Ca2+ channels (Figure 4). Ca2+ release from sarcoplasmic reticulum requires hydrolysis of plasma membrane phosphatidylinositol 4,5-bisphosphate by phospholipase C (PLC).21 We detected five PLC isozymes belonging to β (B1, 3), ɛ (E1), and η (H1, 2) subtypes, of which, PLC-β, and -ɛ are activated by agonist (including vasoactive substances) binding to plasma membrane G protein-coupled receptors.21 All PLC isozymes detected are known generators of second messengers, inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol.21 Binding of IP3 to sarcoplasmic reticulum surface receptors (IP3Rs or ITPRs) activates Ca2+ release.26 We detected three ITPRs, namely ITPR1, 2, and 3, all previously showed to be present in rat cerebral artery SMCs with ITPR1 being the most abundant.27 In addition, the second messenger 1,2-diacylglycerol has been shown to activate PKC, which in turn increases smooth muscle contractility via several different mechanisms such as MLCP inhibition and increased actin availability.20
Several pathways of Ca2+ entry from the extracellular space have been suggested, and include passive leak Ca2+ channels, as well as channels activated by membrane depolarization (voltage gated), agonist binding (receptor operated), depletion of Ca2+ from intracellular stores (store operated), or stretch.28 Voltage-gated Ca2+ channels enriched in SMCs of major cerebral arteries and arterioles are of L, and to a lesser extent, T type.29 We detected four α-1 subunits belonging either to the high-voltage-activated L-type (CACNA1C, D, and F) or to the low-voltage-activated T-type (CACNA1H) channels. Of these, mRNAs of CACNA1C (or Cav1.2) and CACNA1H (or Cav3.2) have been previously detected in rat cerebral artery SMC and mouse coronary artery, respectively (Supplementary Table 2 for references). We also detected a known β subunit isoform (CACNB1) of the L-type channel. Interestingly, the α1-subunit forms the ion-conducting pore of voltage-gated Ca2+ channels, while the β subunit is involved in the modulation of gating.30 Receptor-operated Ca2+ channels frequently detected in SMC plasmalemma belong to the P2X purinoreceptor family and activate upon binding of extracellular ATP.28, 31 Of these, we detected the P2RX7 receptor, expression of which has been previously shown in cerebral arteries (Supplementary Table 2 for references). In addition, emerging evidence suggests relatedness between receptor- and store-operated Ca2+ channel types, both being formed by proteins of the transient receptor potential channel (TRPC) family.32 We identified six proteins (TRPC1, 2, 4, 5, 6, and 7) belonging to this family, all of which can form Ca2+ permeable cation channels, and except for TRPC2 have been previously shown to be present in cerebral arteries (Supplementary Table 2 for references). Mechanical stretch-induced Ca2+ influx is known to occur via opening of nonspecific cation channels in SMC membranes. We detected one such channel, specifically, the transient receptor potential cation channel TRPM4, an inherently mechanosensitive channel33 shown to be present in cerebral arterial myocytes.
In contrast to smooth muscle contraction, its relaxation is accompanied by a decrease in iCa2+ levels to resting values. Decrease in iCa2+ is facilitated by (1) reuptake into intracellular stores by Ca2+ ATPase pumps (smooth endoplasmic reticulum calcium ATPases, SERCAs) on the sarcoplasmic reticulum membrane and (2) extrusion from the cell by the plasma membrane Ca2+ ATPases (PMCAs) and the Na+/Ca2+ exchanger (Figure 4). We detected three SERCAs (ATP2A1, A2, and A3), one PMCA (ATP2B2), and one Na+/Ca2+ exchanger (SLC8A1 or NCX) in our arterial data set. To date, only mRNAs of ATP2B2 and SLC8A1 have been reported in cerebral arteries (Supplementary Table 2 for references). The SMC relaxation is further promoted by the activation of multiple arterial smooth muscle potassium (K+) channels. These channels provide an opposing hyperpolarizing influence reducing Ca2+ channel activity. We detected multiple K+ channels in our arterial data set, in particular, large (KCNMA1 or BKCa), intermediate (KCNN4), and small (KCNN2 or SKCa) conductance Ca2+-activated K+ channels, as well as ATP-sensitive (KATP channel subunits KCNJ8 and 15), and voltage-activated K+ channels (KCNA3, A5-7, C2, H5 and H8, Q1-5, V2, AB1 and AB3). The majority of these K+ channels have been previously observed in cerebral arteries (Supplementary Table 2 for references). Particularly, the peptide calcitonin gene-related peptide potently dilates brain cerebral arteries through activation of SMC K+ channels.34 Each of the endothelium-derived relaxing factors, namely NO, prostacyclin, and endothelium-derived hyperpolarizing factor, can activate one or several types of SMC K+ channels.35 We detected three NOSs in our arterial data set, specifically the previously mentioned NOS1 (or nNOS), as well as NOS2 (or iNOS) and NOS3 (or eNOS).
Insights into the Proteomes of Arteries and Microvessels
The recent release of the cerebral microvessel proteomics study7 provided an opportunity to compare and contrast protein-based findings in pial/extracerebral CW arteries (this study) and intraparenchymal brain vessels. Vessel diameters in our sampling of mouse cerebral arteries were ∼100 μm and lower, with the majority being large diameter vessels. The wide range of arterial diameters in our sampling is representative of the varied vessel sizes present at the brain surface, starting with the CW and its major arteries ranging from 75 to 100 μm in diameter.36 Furthermore, it has been shown that peripheral branches of the major arteries range between 50 and 70 μm in diameter, while their ramifications coursing over the brain surface, reach 35 μm or lower in diameter, just before perforating as penetrating intracerebral arterioles.36, 37 In comparison with our arterial sample, vessel diameters in the microvascular sampling7 ranged between >20 μm and <100 μm. Thus, it can be concluded that while the majority of vessels in the microvascular sample tended toward small diameters, some overlap in vessel diameter size exists between the two studies.
Comparatively, the most striking difference between the arterial and microvascular data sets was the ∼9 × increase in the number of unique BBB-specific cell types in the latter. Disparities in unique protein numbers in ‘TJ and adhesion proteins' and ‘membrane transporter and channel proteins' categories, while lesser (∼2x more in the microvascular data set), were still present. Overall, our findings of protein number inequalities in the above-mentioned categories are in line with the knowledge that at the level of cerebral arteries, the BBB is not as developed, nor specialized, as in intracortical microvessels. Incomplete barrier characteristic of cerebral arteries has been shown in smaller diameter surface vessels using endothelial barrier antigen staining.38 Compared with the uniform staining present in intracortical microvessels, those on the cerebral surface showed heterogeneity in endothelial barrier antigen expression.38 Moreover, two populations of interendothelial TJs have been shown in these surface microvessels, (1) those with the BBB-type fusion of adjacent membranes and (2) those with discernible gap between them.39 Unlike the three above-mentioned protein categories, the arterial data set housed higher numbers (∼5 × ) of ‘ECM and basal lamina proteins' compared with intracortical microvessels, probably as a consequence of an adventitia layer present in the former. This layer, largely composed of ECM proteins, is absent in intracortical microvessels.40
In summary, we have compiled a novel resource database for proteins present in the arterial wall of mouse CW and its surface ramifications. To ensure detection and identification of an extensive and comprehensive list of CW cerebral arterial proteins, we used fresh unfixed tissue and employed two parallel, custom proteomic approaches. Our database provides an excellent resource for the study of protein expression profile in healthy cerebral arteries and its perturbations in cerebrovascular diseases. Furthermore, we shed light on the difference and the similarities between brain arteries and microvessels at the level of the proteome.
Acknowledgments
The authors thank Drs A Pshezhetsky and E Kanshin (CHU Sainte-Justine, Research Center, Montréal, QC, Canada) for initial protein extraction and detection trials, Ms CE Delaney and Mr L Tessier (IBS-National Research Council of Canada, Proteomics and Mass Spectrometry group, Ottawa, ON, Canada) for their technical assistance with protein isolation and mass spectrometry, Dr Xinkang Tong (Montreal Neurological Institute, Montréal, QC, Canada) for actin western blots, and Mr I Markovic for help with graphic illustrations.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by grants (EH) from the Canadian Institutes of Health Research (CIHR, MOP-84275 and MOP-126001) and Takeda Pharmaceuticals USA, Inc., and a CIHR Banting and Best Canada Graduate Scholarship (AB).
Supplementary Material
References
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Carpenter MB. Core Text of Neuroanatomy. Williams & Wilkins: Baltimore, MD; 1985. [Google Scholar]
- Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999;30:2671–2678. doi: 10.1161/01.str.30.12.2671. [DOI] [PubMed] [Google Scholar]
- Dorr A, Sled JG, Kabani N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage. 2007;35:1409–1423. doi: 10.1016/j.neuroimage.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Nixon AM, Gunel M, Sumpio BE. The critical role of hemodynamics in the development of cerebral vascular disease. J Neurosurg. 2010;112:1240–1253. doi: 10.3171/2009.10.JNS09759. [DOI] [PubMed] [Google Scholar]
- Chun HB, Scott M, Niessen S, Hoover H, Baird A, Yates J, 3rd, et al. The proteome of mouse brain microvessel membranes and basal lamina. J Cereb Blood Flow Metab. 2011;31:2267–2281. doi: 10.1038/jcbfm.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klammer AA, MacCoss MJ. Effects of modified digestion schemes on the identification of proteins from complex mixtures. J Proteome Res. 2006;5:695–700. doi: 10.1021/pr050315j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadgar L, Marois Y, Deng X, Guidoin R. Arterial wall mechanical characteristics after treatment in collagenase: an in vitro aneurysm model. Clin Invest Med. 1997;20:25–34. [PubMed] [Google Scholar]
- Haqqani AS, Kelly JF, Stanimirovic DB. Quantitative protein profiling by mass spectrometry using label-free proteomics. Methods Mol Biol. 2008;439:241–256. doi: 10.1007/978-1-59745-188-8_17. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang BW, Kuan P, Chang H. RNA interference for discoidin domain receptor 2 attenuates neointimal formation in balloon injured rat carotid artery. Arterioscler Thromb Vasc Biol. 2008;28:1447–1453. doi: 10.1161/ATVBAHA.108.165993. [DOI] [PubMed] [Google Scholar]
- Kefaloyianni E, Coetzee WA. Transcriptional remodeling of ion channel subunits by flow adaptation in human coronary artery endothelial cells. J Vasc Res. 2011;48:357–367. doi: 10.1159/000323475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer's disease. J Neurosci. 2005;25:11165–11174. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerson BE, Drewes LR. The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab. 2006;26:959–973. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- Haqqani AS, Kelly J, Baumann E, Haseloff RF, Blasig IE, Stanimirovic DB. Protein markers of ischemic insult in brain endothelial cells identified using 2D gel electrophoresis and ICAT-based quantitative proteomics. J Proteome Res. 2007;6:226–239. doi: 10.1021/pr0603811. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, MacKenzie ET, McCulloch J. Cerebral blood flow and metabolism. Raven Press: New York, NY; 1993. [Google Scholar]
- Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol. 2009;297:H1–H7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, et al. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Baeres FM, Moller M. Origin of PACAP-immunoreactive nerve fibers innervating the subarachnoidal blood vessels of the rat brain. J Cereb Blood Flow Metab. 2004;24:628–635. doi: 10.1097/01.WCB.0000121234.42748.F6. [DOI] [PubMed] [Google Scholar]
- Akerman S, Williamson DJ, Goadsby PJ. Voltage-dependent calcium channels are involved in neurogenic dural vasodilatation via a presynaptic transmitter release mechanism. Br J Pharmacol. 2003;140:558–566. doi: 10.1038/sj.bjp.0705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Evans MS, Lee TJ. Presynaptic muscarinic M(2)-receptor-mediated inhibition of N-type Ca(2+) channels in cultured sphenopalatine ganglion: direct evidence for acetylcholine inhibition of cerebral nitrergic neurogenic vasodilation. J Pharmacol Exp Ther. 2002;302:397–405. doi: 10.1124/jpet.302.1.397. [DOI] [PubMed] [Google Scholar]
- Nielsen KC, Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res. 1967;6:773–776. doi: 10.1016/0006-8993(67)90134-5. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil RA. Regulation of vascular smooth muscle function. Morgan & Claypool Life Sciences: San Rafael, CA; 2010. [PubMed] [Google Scholar]
- Cribbs LL. T-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium. 2006;40:221–230. doi: 10.1016/j.ceca.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. A short history of voltage-gated calcium channels. Br J Pharmacol. 2006;147 (Suppl 1:S56–S62. doi: 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima B, Weir BK, Macdonald RL, Zhang H. Extracellular nucleotide-induced [Ca2+]i elevation in rat basilar smooth muscle cells. Stroke. 1997;28:2053–2058. doi: 10.1161/01.str.28.10.2053. [DOI] [PubMed] [Google Scholar]
- McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, et al. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci. 2007;103:417–426. doi: 10.1254/jphs.fp0061332. [DOI] [PubMed] [Google Scholar]
- Kitazono T, Heistad DD, Faraci FM. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am J Physiol. 1993;265:H581–H585. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- Waldron GJ, Cole WC. Activation of vascular smooth muscle K+ channels by endothelium-derived relaxing factors. Clin Exp Pharmacol Physiol. 1999;26:180–184. doi: 10.1046/j.1440-1681.1999.03006.x. [DOI] [PubMed] [Google Scholar]
- Myojin K, Taguchi A, Umetani K, Fukushima K, Nishiura N, Matsuyama T, et al. Visualization of intracerebral arteries by synchrotron radiation microangiography. AJNR Am J Neuroradiol. 2007;28:953–957. [PMC free article] [PubMed] [Google Scholar]
- Coyne EF, Ngai AC, Meno JR, Winn HR. Methods for isolation and characterization of intracerebral arterioles in the C57/BL6 wild-type mouse. J Neurosci Methods. 2002;120:145–153. doi: 10.1016/s0165-0270(02)00197-8. [DOI] [PubMed] [Google Scholar]
- Cassella JP, Lawrenson JG, Lawrence L, Firth JA. Differential distribution of an endothelial barrier antigen between the pial and cortical microvessels of the rat. Brain Res. 1997;744:335–338. doi: 10.1016/S0006-8993(96)00974-2. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Is the pial microvessel a good model for blood-brain barrier studies. Brain Res Brain Res Rev. 1997;24:67–76. doi: 10.1016/s0165-0173(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Dahl E.Microscopic observations on cerebral arteriesIn: Cervos-Navarro J, Betz E, Matakas F, Wullenweber R, eds. The Cerebral Vessel Wall Raven Press: New York, NY; 1976 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.