Abstract
Intraarterial therapy (IAT) in acute ischemic stroke is effective for opening occlusions of major extracranial or intracranial vessels. Clinical efficacy data are lacking pointing to a need for proper patient selection. We examined feasibility, clinical impact, and safety profile of magnetic resonance imaging (MRI) for patient selection before IAT. In this single-center study, we collected epidemiologic, imaging, and outcome data on all intraarterial-treated patients presenting with anterior circulation occlusions at our center from 2004 to 2011. Magnetic resonance imaging was the first imaging choice. Computer tomography (CT) was performed in the presence of a contraindication. We treated 138 patients. Mean age was 64 years and median National Institutes of Health Stroke Scale (NIHSS) was 17. Major reperfusion (thrombolysis in cerebral infarction (TICI) 2b+3) was achieved in 52% and good outcome defined as modified Rankin Scale (mRS) score 0 to 2 at 90 days was achieved in 41%. Mortality at 90 days was 10%. There was only one symptomatic hemorrhage. Recanalization, age, and stroke severity were associated with outcome. Preprocedure MRI was obtained in 83%. Good outcome was significantly associated with smaller diffusion-weighted imaging (DWI) lesion size at presentation and not with the size of the perfusion lesion. It is feasible to triage patients for IAT using MRI with acceptable rates of poor outcome and symptomatic hemorrhage.
Keywords: acute stroke, diffusion-weighted imaging, endovascular therapy, magnetic resonance imaging, outcome, selection
Introduction
Reperfusion therapy with intraveneous tissue plasminogen activator (tPA) is the only approved treatment for acute ischemic stroke.1 Intraarterial therapy (IAT) is increasingly used for major strokes secondary to proximal artery occlusions, given its higher rate of early recanalization compared with intraveneous tPA. Using intraarterial lytic or mechanical devices particularly the newest generation stent retrievers, revascularization rates can be doubled compared with intraveneous tPA.2, 3, 4, 5 However, after the failed results of recent randomized controlled trials,6, 7, 8 the clinical efficacy of IAT remains unproven compared with medical management alone including intraveneous tPA.
Although numerous factors likely account for the failed trials, patient selection appears to be critical.9 ‘Futile recanalization' occurs commonly10 and better imaging biomarkers may aid in identifying the best treatment candidates. In the major trials to date, noncontrast computer tomography (CT) scan has been employed largely to exclude intracranial hemorrhage (ICH). Given its high accuracy and reliability for characterizing ischemic tissue damage, magnetic resonance imaging (MRI) has been proposed as an important triage tool for IAT. Specifically, diffusion-weighted imaging (DWI) may be helpful to reliably exclude patients in whom the infarct is large or already completed and IAT would be futile and potentially dangerous.11 Furthermore, a recent study has suggested the value of perfusion imaging (PI) for selecting patients for IAT.12
Despite the promise of MRI, there are few data concerning IAT outcomes after MRI-based selection. In most centers, patients are selected for intraveneous tPA and IAT on the basis of a CT scan.13 At Aarhus University Hospital (Aarhus, Denmark) MRI scanning for patient evaluation for stroke reperfusion therapy was introduced in 2004 and from that time IAT was also offered in selected patients. We sought to review our single-center experience using MRI as the primary imaging selection tool for IAT, and examine our clinical outcomes and safety data in relation to imaging findings.
Materials and methods
At Aarhus University Hospital, data on acute ischemic stroke patients treated with IAT are collected prospectively. The present study is a retrospective analysis of clinical outcomes between 2004 and 2011, during which time 153 acute ischemic stroke patients (138 anterior circulation, 15 posterior circulation) were treated using a catheter-based approach.
In the early years treatment was limited, but from January 2010, IAT was offered in nearly all cases that satisfied our selection criteria. These were a patient deemed to be in reasonably good health (although comorbid conditions were not specified) with an occlusion of a large vessel that was possible to reach with a catheter: internal carotid artery (ICA), vertebral artery, basilar artery, or most often the middle cerebral artery (MCA) where M1 and M2 refer to the stem and the arteries after the first division, respectively. Furthermore, the patient had to have a substantial neurologic deficit—interpreted as a National Institutes of Health Stroke Scale (NIHSS) of ⩾10. A few patients with lower scores were also treated because it was judged that they were at risk of neurologic deterioration secondary to large vessel occlusion. We excluded patients with very large infarct sizes (e.g., >1/3 of the MCA territory estimated by visual inspection) from treatment. This assessment was made using MRI DWI whenever possible. Computer tomography was chosen if the patient had a contraindication to MRI (e.g., pacemaker), if the patient was too unstable (e.g., vomiting and respiratory concerns), or if the patient could not fit within the MRI scanner (e.g., obesity and severe cervical kyphosis). Information about, for example, a pacemaker was retrieved from the patient, or if the patient was aphasic, from relatives and from the Electronic Patient File that covers all Danish patients and where such a procedure would be logged. The patients were preferably treated with intraveneous tPA and taken directly for IAT without waiting for clinical response. Some (for the anterior circulation group 35/138=25.4%) had a contraindication for intraveneous tPA. In general, patients were treated within 6 hours for anterior circulation strokes and within 12 hours for posterior circulation strokes. However, 15 patients with anterior circulation stroke had groin puncture after the 6-hour time window from last seen well. They were treated based on a favorable imaging profile (a small DWI lesion) as judged by the treating clinician.
Because this analysis is focused on imaging selection, the study population was limited to anterior circulation occlusions, as criteria are inherently different for the posterior circulation. This study included all patients treated or in whom an attempt was made to treat with IAT at our hospital, that is, all cases where a groin puncture was performed with the intent of treating an acute stroke. Recanalization was assessed by the modified thrombolysis in cerebral infarction (mTICI) score where 0 denotes no reperfusion, 1 minimal flow past occlusion but no distal reperfusion, 2a reperfusion of <50% of the ischemic territory, 2b >50%, and 3 signifies full reperfusion of the ischemic territory.14 Clinical outcome was assessed at 90 days using the modified Rankin Scale (mRS); good outcome was defined as 90-day mRS 0 to 2 (i.e., functional independence). The study was conducted with the approval of the local Ethical Committee (Central Denmark Region).
Imaging Analysis
Of 138 study patients, 101 had an acute MRI scan with a DWI series and 80 had additional PI series, where mean transit time (MTT) maps were created. The MTT maps were calculated using the nordicICE software15 in the acute stroke setting. If MTT maps had not been saved, then they were calculated retrospectively using the in-house software. The arterial input function was selected semiautomatically around the M1/M2 segment on the unaffected side.16 It was deconvolved from the tissue curves using standard singular value decomposition. During the study period, 77 patients were scanned on 1 of 2 different 3-Tesla machines, and 24 on 1 of 3 different 1.5-Tesla magnets. Our scan protocol includes a scout, DWI, T2*, a T2-Fluid Attenuated Inversion Recovery, time-of-flight angiography, and PI giving a total scan time of 11 minutes and 51 seconds on the current 3-Tesla machine.
Statistical Analysis
Standard descriptive statistics were used to summarize the baseline clinical and imaging variables. For continuous variables, normality was tested using the Kolmogorov–Smirnov test. For univariate analysis of good outcome, the Chi-square test was used for categorical variables, the Mann–Whitney test for ordinal or nonnormal continuous data, and the Student's t-test was used for normal data. Significant univariate predictors were tested in multiple logistic regression. Statistical significance was taken at two-tailed P value <0.05. All analyses were performed using the MedCalc software (v.12.5.0.0, Ostend, Belgium).
Results
A total of 138 anterior circulation acute ischemic stroke patients were taken for IAT between 2004 and 2011. The baseline characteristics are similar to other reported cohorts (Table 1). The mean age was 64.0±13.9 years (range: 10 to 89 years). There were 90 (65.2%) men. The median NIHSS was 17 (interquartile range (IQR): 13 to 20) at presentation. The median DWI lesion volume at presentation was 14.4 mL (IQR: 6.4 to 45.0), and the median MTT lesion volume was 173.7 mL (IQR: 129.5 to 214.8). Intraveneous tPA was administered to 103 (74.6%) patients. The median time from stroke onset to groin puncture was ∼4 hours (IQR: 3.10 to 5.10).
Table 1. Baseline variables.
| Variable | Entire group (n=138) | Good outcome (n=57) | Poor outcome (n=81) | Univariate P | Multivariate P |
|---|---|---|---|---|---|
| Baseline variables | |||||
| Age | 64.0±13.9 | 60.7±14.8 | 66.3±12.8 | 0.02 | |
| Baseline NIHSS | 17 (13–20) | 14 (9–18) | 17 (15–21) | <0.0001 | 0.0003 |
| Men (%) | 90 (65.2%) | 34 (59.6%) | 56 (69.1%) | 0.33 | |
| Diabetes (%) | 20 (14.5%) | 4 (7.0%) | 16 (19.8%) | 0.06 | 0.02 |
| HTN (%) | 67 (48.6%) | 21 (36.8%) | 46 (56.8%) | 0.03 | <0.05 |
| AF (%) | 45 (32.6%) | 22 (38.6%) | 23 (28.4%) | 0.28 | |
| HL (%) | 74 (53.6%) | 30 (52.6%) | 44 (54.3%) | 0.98 | |
| CHF (%) | 14 (10.1%) | 7 (12.3%) | 7 (8.6%) | 0.68 | |
| Previous stroke (%) | 19 (13.8%) | 3 (5.3%) | 16 (19.8%) | 0.03 | |
| Current smoking (%) | 43 (31.2%) | 18 (31.6%) | 25 (30.9%) | 0.92 | |
| Smoking history (%) | 25/78 (32.1%) | 10/30 (33.3%) | 15/48 (31.3%) | 0.95 | |
| Imaging findings | |||||
| Intracranial occlusion (%) | 0.02 | ||||
| ICA | 16 (11.6%) | 1 (1.8%) | 15 (18.5%) | ||
| MCA M1+ACA | 1 (0.7%) | 0% | 1 (1.2%) | ||
| MCA M1 | 78 (56.5%) | 36 (63.2%) | 42 (51.9%) | ||
| MCA M2 | 21 (15.2%) | 11 (19.3%) | 10 (12.3%) | ||
| ACA | 2 (1.4%) | 2 (3.5%) | 0% | ||
| None (isolated cervical) | 20 (14.5%) | 7 (12.3%) | 13 (16.0%) | ||
| Cervical ICA occlusion (%) | 51 (37%) | 20 (35.1%) | 31 (38.3%) | 0.84 | |
| Baseline DWI lesion volume (mL) | 14.4 (6.4–45.0) (n=101) | 10.3 (4.4–19.3) (n=46) | 19.8 (8.7–52.2) (n=55) | 0.001 | |
| Baseline MTT lesion volume (mL) | 173.7 (129.5–214.8) (n=80) | 153.4 (113.2–214.8) (n=40) | 179.8 (155.1–219.0) (n=40) | 0.12 | |
| Treatment data | |||||
| IV tPA (%) | 103 (74.6%) | 47 (82.5%) | 56 (69.1%) | 0.12 | |
| LSW to groin (min) | 238.5 (190–310) | 240 (190–310) | 235 (194–310) | 0.73 | |
| Stent retriever (%) | 39/131 (29.8%) | 15/53 (28.3%) | 24/78 (30.8%) | 0.91 | |
| Cervical stent (%) | 32/137 (23.4%) | 16/56 (28.6%) | 16 (19.8%) | 0.32 | |
| Modified TICI | 2b (0-3) | 3 (2a-3) | 2a (0-2b) | <0.0001 | 0.0001 |
| mTICI 2b-3 | 72 (52.2%) | 42 (73.7%) | 30 (37.0%) | <0.0001 | |
| LSW to TICI 2a-3 (minutes) | 330 (276–370) (n=90) | 320 (271–360) (n=47) | 340 (288–389) (n=43) | 0.14 | |
| Admission to groin (minutes) | 149.5 (114–194) | 154 (114–193) | 143 (111–198) | 0.78 | |
| Scan to groin (minutes) | 118 (91–162) | 125 (97–168) | 109 (90–160.5) | 0.29 | |
ACA, anterior cerebral artery; AF, atrial fibrillation; CHF, congestive heart failure; DWI, diffusion-weighted imaging; HL, hyperlipidemia; HTN, hypertension; ICA, internal carotid artery; IV, intraveneous; LSW, last seen well; MCA, middle cerebral artery; mTICI, modified TICI; MTT, mean transit time; NIHSS, National Institutes of Health Stroke Scale; TICI, thrombolysis in cerebral infarction; tPA, tissue plasminogen activator.
Magnetic Resonance Imaging Feasibility
Of the 138 study patients, MRI was performed in 101 before the intervention. Seventeen patients were CT scanned at an outside hospital or at our emergency department before stroke neurology consultation and transported directly to the interventional suite for treatment, thus leaving 121 patients who presented directly to us. Hence, it was possible to perform acute MRI in 101/121 (83.5%) of cases. A true contraindication to MRI was identified in (15/121) 12.4% of patients: pacemaker (n=7); too medically unstable, moving, or vomiting (n=6); and morbid obesity (n=2). In five cases, there were no true contraindications: one patient had a stroke in-house and was CT scanned to rule out bleed (he had an MRI performed the day before); one had sudden headache, subarachnoid hemorrhage was suspected and CT chosen, but it turned out to be an ischemic stroke; one could not be scanned for technical reasons; and for two patients the reason for choosing CT was not recorded.
Looking at the possible delay of performing MRI, on all the CT-scanned patients there was a scan-to-groin time of 142 minutes (IQR: 90 to 199) and for the MRI selected on 116 minutes (IQR: 91.5 to 158.8) and this was not significantly different (P=0.23).
Angiographic, Clinical, and Safety Outcomes
PostIAT reperfusion defined as an mTICI score of 2b-3 was achieved in 52.2% (72/138) of patients. Consistent with previous studies, the rate of mTICI 2b-3 was increased in cases where stent retrievers were used (82% vs. 40% in nonstent retriever cases, P<0.0001). Applying the more traditional definition of TIMI (Thrombolysis in Myocardial Infarction) score 2 to 3, reperfusion was achieved in 65.9% (91/138).
At 90-day follow-up, 57 (41.3%) patients had a good outcome (90-day mRS 0 to 2). The independent predictors of good outcome were greater reperfusion, lower NIHSS score, the absence of hypertension, and the absence of diabetes.
There was no difference in age (P=0.27), baseline NIHSS (P=0.93), or in the rates of comorbidities between patients who did and did not undergo pretreatment with intraveneous tPA. There was a higher rate of good outcomes among the patients pretreated with intraveneous tPA vs. the intraveneous tPA ineligible patients (47/103=46% vs. 10/35=29%, P=0.12) but this was not statistically significant.
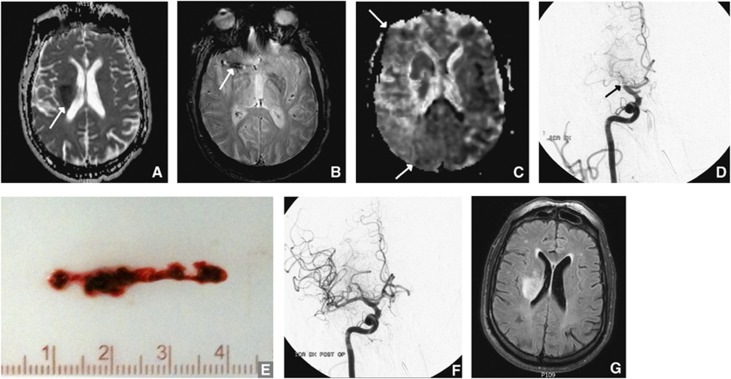
Figure 1 depicts a successful course with a patient who had a small initial DWI and was revascularized with a good outcome.
Figure 1.
Successful treatment. A 70-year-old man with hypertension, diabetes, hyperlipidemia, and paroxysmal atrial fibrillation presented with sudden left-sided hemiparalysis, head and eye deviation to the right and neglect. National Institutes of Health Stroke Scale (NIHSS) was 17. He was not on anticoagulation. Magnetic resonance imaging (MRI) performed 2 hours and 36 minutes after symptom onset revealed a small infarct in the basal ganglia, depicted by restricted diffusion on the apparent diffusion coefficient (ADC) image (A). T2*-weighted imaging (B) showed a clot in the stem of the right middle cerebral artery (MCA). Marked hypoperfusion of the entire MCA territory was evident on the mean transit time (MTT) image (C). The patient was given intraveneous tissue plasminogen activator (tPA) and taken to intraarterial therapy (IAT) immediately without waiting for effect. The right MCA was still occluded on the first run of the digital subtraction angiography (DSA) (D). The clot was successfully removed (E). After thrombectomy, DSA showed the right MCA was now open (F). Recanalization was achieved 5 hours and 18 minutes after symptom onset. At the 24-hour follow-up MRI, the infarct had not grown, but was still in the basal ganglia (G). NIHSS the day after was 7.
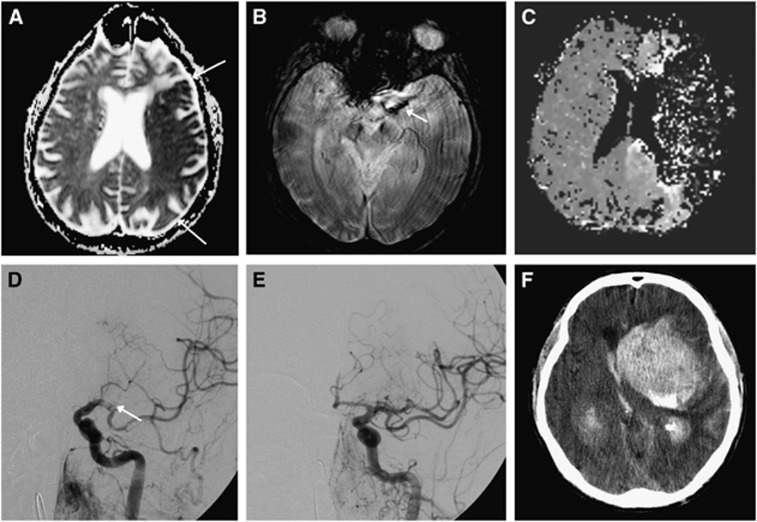
Fourteen (10.1%) patients were dead at 90 days (mRS 6). Six patients died beyond 24 hours from the procedure due to malignant cerebral oedema. Five patients died of pneumonia 12 to 90 days after stroke, and one death was due to a cardiac arrest 33 days after the stroke. One patient died of a large ICH 6 days after successful revascularization, likely related to early anticoagulant therapy. None of these deaths were directly procedure related. The only death within 24 hours of the treatment (i.e., procedure related) was secondary to a large ICH (parenchymal hematoma type 2: hemorrhage >30% of the infarct with mass effect) 2 hours after revascularization (Figure 2).
Figure 2.
Recanalizing into an infarcted area. A 76-year-old man with no vascular risk factors except hyperlipidemia, presented with right-sided weakness, aphasia and eye deviation to the left. National Institutes of Health Stroke Scale (NIHSS) was 21. Magnetic resonance imaging (MRI) was performed only 33 minutes after symptom onset. Despite the fast evaluation, MRI showed a large infarct in the left middle cerebral artery (MCA) territory on the apparent diffusion coefficient (ADC) image (A). A clot was found in the left MCA (B). Time-to-peak map showed artifactual zero signal intensity mapping of the left MCA territory due to severe contrast arrival delay (C). Since the patient was without risk factors and in a good state of health, he was taken for intraarterial therapy (IAT) despite the large infarct. On digital subtraction angiography (DSA), the clot was initially found where the internal carotid artery (ICA) divides into the MCA and the anterior cerebral artery (T-occlusion, not shown). After initial removal, DSA now shows the clot in the left MCA (D). Recanalization (E) was achieved using a stent retriever, clot aspiration, and delivery of 4 mg of intraarterial tissue plasminogen activator (tPA) and the patient was taking to the Intensive Care Unit. Minutes after, the patient lost consciousness. An acute computer tomography (CT) scan (F) showed a large intracerebral hemorrhage. The patient died a few hours later.
Impact of Magnetic Resonance Imaging Findings on Outcomes
The size of the ischemic lesion on DWI was a univariate predictor of good vs. poor outcome, with larger infarcts faring worse (P=0.001, Table 1). In all, 9 of the 101 patients with an acute MRI had a DWI infarct of >70 mL, and only 1 (11.1%) of these had a good outcome (vs. 48.9% for DWI infarct <70 mL; P=0.07). Five (55.6%) patients with DWI volume >70 mL were dead at 90 days (vs. 3.3% for DWI infarct <70 mL; P<0.0001). Across the entire Rankin scale, patients with DWI lesion volume >70 mL had significantly worse outcomes (median mRS 6 (IQR 3 to 6) vs. 3 (IQR 1 to 4) for volume >70 mL vs. <70 mL, respectively; P=0.001). Moreover, the only symptomatic hemorrhage in this series occurred in a patient with a DWI lesion volume of 81 mL (Figure 2). The sensitivity and specificity ranges for selected large DWI lesion volumes are provided in Table 2.
Table 2. Receiver operating characteristics for poor outcome (mRS 3–6) by baseline DWI lesion volume.
| DWI volume (mL) | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 50 | 31 | 87–91 |
| 55 | 22 | 91–93 |
| 60 | 15–20 | 96 |
| 65 | 15–20 | 96 |
| 70 | 2–15 | 98–100 |
DWI, diffusion-weighted imaging; mRS, modified Rankin scale.
Importantly, there was no significant difference in the rate of mTICI 2b-3 (P=0.78) or TIMI 2 to 3 (P=0.79) across the 70-mL threshold. The worse outcomes seen in the >70-mL group occurred despite a nominally younger age (mean 56.6 vs. 64.3 years, P=0.13).
Perfusion imaging was performed on 80 patients. Although patients with worse outcomes had larger volumes of MTT abnormality, this relationship was not statistically significant (Table 1).
Discussion
In a large single-center experience of MRI-based selection for IAT, we have showed that hyperacute MRI is highly feasible. We were able to acquire MRI in the majority (>80%) of patients with a low rate of true contraindications and without introducing longer delay compared with the CT-selected patients. Moreover, we have showed that this advanced imaging technique is highly predictive of subsequent clinical response to IAT, confirming the previously reported impact of DWI lesion volume on outcome.11, 12
Magnetic resonance imaging-based selection has been previously shown to be feasible for treatment decision making in general stroke populations, with rates of acquisition ranging from 88% to 97%.17, 18 We have shown that a similarly high rate can be achieved in a homogeneous population of major stroke patients who are being evaluated for IAT. This is despite the fact that these patients tend to be much sicker (median NIHSS=17 in the present study).
There is a growing literature supporting the importance of core infarct volume for predicting outcome after IAT.19 Diffusion-weighted imaging is widely accepted as the most accurate measure of core infarction in the hyperacute window.20 Yet many centers do not perform this imaging, resulting in little published data supporting its value for IAT selection. Our finding that patients with a good outcome had significantly smaller baseline DWI infarct volumes provides important support for this imaging approach.
Because treatment decision making is binary, a DWI-volume threshold for patient selection is necessary. A natural idea is to exclude patients who have sustained large regions of irreversible injury, for whom treatment provides little to no benefit (i.e., futile) and predisposes the patient to high risk of adverse events such as symptomatic hemorrhage.21 A 70-mL threshold has been proposed as highly specific for poor outcome after IAT.11 Our findings strongly support this idea. Patients in our study with infarcts >70 mL had significantly worse functional outcomes and increased mortality, despite equivalent revascularization rates and nominally younger age. The trend to younger age among the larger infarct group is a likely consequence of a clinical bias toward higher aggressiveness in younger patients.
With respect to symptomatic ICH, we found only one patient with this complication, limiting our ability to identify predictors. This rate of symptomatic ICH is much lower than other studies of IAT, which may be related to the increased safety profile of MRI selection. Notably, the patient with symptomatic ICH in our study had a baseline DWI volume of 81 mL. The idea that it is safer to treat stroke patients selected by MRI is supported by studies which found that patients selected for intraveneous tPA based on MRI findings had significantly lower rates of symptomatic hemorrhage than CT-based selection.17, 22 This was also found in a recent study, where MRI-selected intraveneous tPA-treated patients had lower symptomatic ICH, lower 7-day mortality, and a trend to lower mortality at 90 days.23 Moreover, we had a 90-day mortality rate of 10%, which was half that seen in Interventional Management of Stroke 3, in which patients were selected by CT.8
There has been controversy over the added value of PI over DWI alone for IAT selection.24 DEFUSE 2 tested the value of the perfusion-diffusion mismatch for predicting the clinical benefit of reperfusion. The investigators found that the Target mismatch patients had improved outcomes with reperfusion, while those without a mismatch had worse outcomes with reperfusion. However, in further analysis, it was shown that the only difference in the primary imaging variables between the two groups was in the size of the DWI lesion (P=0.02), not the perfusion lesion.12 This greater importance of DWI is supported by our finding that DWI volume was a significant predictor of outcome in this study, and MTT lesion volume was not. The likely reason is that for terminal ICA and MCA M1 segment occlusions, the ischemic territory is so extensive that virtually all patients will have a mismatch unless the core infarct is prohibitively large.19 In DEFUSE 2, Target mismatch patients had to have a DWI infarct volume of <70 mL. The value of DWI lesion for predicting outcome was also found earlier in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET).25
When it comes to the nonimaging predictors of outcome, we are very much in line with a previous published paper on anterior circulation stroke patients treated with IAT, where young age, low NIHSS, and the absence of diabetes are the most important.26
Our findings support the use of DWI selection criteria for a randomized control trial testing the benefit of IAT. Additionally, future studies should examine whether PI may be useful in IAT-eligible patients with M2 occlusions. Due to the variable size of the territory distal to M2 occlusions, the degree of mismatch may provide useful information.19
Finally, we acknowledge that DWI lesion volume thresholds for decision making may be fluid depending on the clinical context. For example, a threshold for clinical decision making should maximize sensitivity for good outcome to give the patient the greatest possible chance for recovery (e.g., DWI lesion volume <70 mL). However, a threshold for trial selection should maximize power and thus sensitivity and specificity (e.g., DWI lesion volume <50 mL).27 Moreover, we recognize that these thresholds only apply to the definition of good outcome utilized here (i.e., mRS 0 to 2). Although this is the most commonly used end point, it should be kept in mind that patients may still benefit from treatment even if they do not achieve functional independence (e.g., improvement from a Rankin score of 5 to 3).
Conclusion
It is feasible to perform acute MRI in patients with big strokes. Only 12% had a contraindication. The acute DWI is predictive for outcome. Patients with large infarct do poorly and therefore MRI could serve as the best triage tool for IAT, since it is possible to screen out the patients with an invariable bad outcome.
AJY has received research support from Penumbra Inc. and Remedy Pharmaceuticals Inc. Also honorarium from Codman. The remaining authors have no conflict of interest.
References
- The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- The Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial. safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- Saver JL, Hahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Jahan R, Gombein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, Palesch YY, Demchuck AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous tPA versus tPA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AJ, Leslie-Mazwi TM, Jovin TG. Future directions in IAT: better studies, better selection, better timing and better techniques. J Neurointerv Surg. 2013;5:i1–i6. doi: 10.1136/neurintsurg-2013-010741. [DOI] [PubMed] [Google Scholar]
- Hussein HM, Georgiadis AL, Vazquez G, Miley JT, Memon MZ, Mohammad YM, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. Am J Neuroradiol. 2010;31:454–458. doi: 10.3174/ajnr.A2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, González RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and reponse to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta B, Leslie-Mazwi TM, Chandra RV, Chaudhry ZA, Rabinov JD, Hirsch JA, et al. Assessing variability in neurointerventional practice patterns for acute ischemic stroke. J Neurointerv Surg. 2013;5:i52–i57. doi: 10.1136/neurintsurg-2012-010565. [DOI] [PubMed] [Google Scholar]
- Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver J, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnerud A, Emblem KE. A fully automated method for quantitative cerebral hemodynamic analysis using DSC-MRI. J Cereb Blood Flow Metab. 2010;30:1066–1078. doi: 10.1038/jcbfm.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen K, Christensen S, Gyldensted L, Østergaard L. Automatic selection of arterial input function using cluster analysis. Magn Reson Med. 2006;55:524–531. doi: 10.1002/mrm.20759. [DOI] [PubMed] [Google Scholar]
- Köhrmann M, Jütter E, Fiebach JB, Huttner HB, Siebert S, Schwark C, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 2006;6:661–667. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- Sølling C, Ashkanian M, Hjort N, Gyldensted C, Andersen G, Østergaard L. Feasibility and logistics of MRI before thrombolytic treatment. Acta Neurol Scand. 2009;120:143–149. doi: 10.1111/j.1600-0404.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- Morais LT, Leslie-Mazwi TM, Lev MH, Albers GW, Yoo AJ. Imaging-based selection for intra-arterial stroke therapies. J Neurointerv Surg. 2013;5:i13–i20. doi: 10.1136/neurintsurg-2013-010733. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, et al. Evidence-based guidelines: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177–185. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Thomalla G, Fiehler J, Köhrmann M, Molina CA, Neumann-Haefelin T, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows. an analysis of 1210 patients. Stroke. 2007;38:2640–2645. doi: 10.1161/STROKEAHA.107.483255. [DOI] [PubMed] [Google Scholar]
- Gerischer L, Fiebach JB, Scheitz JF, Audebert HJ, Endres M, Nolte CH. Magnetic resonance imaging-based versus computed tomography-based thrombolysis in acute ischemic stroke: comparison of safety and efficacy within a cohort study. Cerebrovasc Dis. 2013;35:250–256. doi: 10.1159/000347071. [DOI] [PubMed] [Google Scholar]
- Simonsen CZ, Andersen G, González RG, Yoo AJ. Selection of patients for intra-arterial therapy. Lancet Neurol. 2013;12:225. doi: 10.1016/S1474-4422(13)70018-8. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimanis A, Jung S, Mono ML, Fischer U, Findling O, Weck A, et al. Endovascular therapy of 623 patients with anterior circulation stroke. Stroke. 2012;43:1052–1057. doi: 10.1161/STROKEAHA.111.639112. [DOI] [PubMed] [Google Scholar]
- Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, et al. Infarct volume is a pivotal biomaker after intra-arterial stroke therapy. Stroke. 2012;43:1323–1330. doi: 10.1161/STROKEAHA.111.639401. [DOI] [PubMed] [Google Scholar]