Abstract
White-matter hyperintensity (WMH) is frequently seen in magnetic resonance imaging (MRI), but the complete physiopathology of WMH remains to be elucidated. In this study, we sought to determine whether there is an association between the maximum brain tissue displacement (maxBTD), as assessed by ultrasound, and the WMH, as observed by MRI. Nine healthy women aged 60 to 85 years underwent ultrasound and MRI assessments. We found a significant negative correlation between maxBTD and WMH (ρ=−0.86, P<0.001), suggesting a link between cerebral hypoperfusion and WMH.
Keywords: brain imaging, cerebral blood flow measurement, leukoaraiosis, pulsatility, ultrasound tissue displacement, white-matter disease
Introduction
White-matter (WM) hyperintensity (WMH) is interpreted as representing lesions or rarefaction of the WM, also referred to as leukoaraiosis. It is frequently observed in T2-weighted magnetic resonance imaging (MRI) images and becomes more common with age.1 However, many studies have reported that WMH is associated with pathologic conditions such as cognitive impairment,2 gait disorders, and depression.3 Some studies have found that decreased cerebral blood flow (CBF) and local or general arteriopathy are associated with the development of WMH.4, 5, 6 However, little is known about the physiologic processes that lead to WMH, possibly because WMH is mainly investigated by MRI, which has certain limitations. For example, MRI is sometimes not well tolerated by patients because it requires them to be confined in a small space. It is also costly and has limited spatio-temporal resolution.
An innovative technique, referred to as ultrasound tissue pulsatility imaging (TPI), has been developed to assess the pulsatility of soft tissues and has been used to characterize brain tissue pulsatility.7, 8, 9 Brain TPI is performed by recording consecutive ultrasound images of the natural movements of the brain in echo-B mode, with a transcranial probe positioned on the temporal window of the skull. Signal acquisition follows classic echography standards, but special signal processing is needed to accurately assess the amplitude of the pulsatile movements of the brain. Pulsatile movements of the brain tissue are mainly caused by CBF; variable blood volume causes the brain to expand and relax over the course of the cardiac cycle,10 and brain TPI is therefore thought to be related to cerebrovascular functioning and CBF regulation.
Tissue pulsatility imaging is a fast, portable, inexpensive, and noninvasive technique that can be applied to subjects in settings that are unsuitable for the use of neuroimaging techniques typically used for CBF assessment. Moreover, unlike the Doppler technique, which assesses blood velocity in only one large vessel, the TPI technique allows the assessment of cerebrovascular reactivity for a large volume of parenchyma (∼100 cm3) in each acquisition, including the reactivity of both large and small arteries in the region of interest (ROI). Tissue pulsatility imaging assesses local CBF and cerebrovascular reactivity more precisely than other neuroimaging techniques. Moreover, TPI is much more suitable for routine use, as setup and acquisition take no >15 minutes.
In this study, we aimed to compare WMH and brain tissue displacement (BTD) in healthy elderly volunteers. We hypothesized that greater WMH would correspond to lower BTD, suggesting that a decreased CBF is associated with greater WMH.
Materials and methods
Subjects
Thirteen healthy volunteers were recruited from the local community by advertisement. All subjects were female and between the ages of 65 and 85 years. Only females were recruited to reduce variability; previous findings indicated significant differences in cerebrovascular impairment between age-matched males and females.11 The exclusion criteria were the presence of a neurologic disorder (stroke and cognitive deficits), suspected dementia (score on the Mini Mental State Examination below 26), any acute disease in the last 3 months, undernutrition or malnutrition (body mass index<21 kg/m2), cardiovascular and/or respiratory disease (hypotension, arrhythmia, and heart failure), use of central analgesics, and contraindication for MRI. Clinical and cognitive assessments were performed by a physician from a memory clinic (to ensure the absence of neurologic and cognitive disorders). Informed consent was obtained from all subjects, and the study protocol was approved by the local human ethical committee. Four of the thirteen participants were excluded because of major intracranial abnormalities found by MRI and poor-quality TPI data (thick temporal bone). Thus, only nine subjects were selected for the study. This study was approved by the Ethics Committee CPP ‘Comité de Protection des Personnes de Tours, Région Centre' and AFSSAPS ‘Agence Française de Sécurité Sanitaire des Produits de Santé' (reference 2009-B91447-80) and was performed in accordance with the Declaration of Helsinki.
Ultrasound Protocol
The ultrasound recordings were performed using an Antares medical scanner (Siemens Healthcare, Paris, France) by a single biophysics technician who was blinded to the MRI results. Transcranial acquisitions were performed with a PX4-1 phased-array transducer (Siemens Healthcare) with a central frequency of 2.5 MHz, a 90° field of view, and 96 × 3 elements (1.5 D). Measurements were performed through the right temporal bone window with the probe positioned perpendicularly to the skull in a mechanical holder to reduce artifacts caused by movement of the operator or the subject. Color Doppler was used to position the ultrasound beam on the middle cerebral artery. With this configuration, we were able to explore the entire right side of the brain in the transverse plane, from the glabella to the opisthocranion. In this work, the ultrasound ROI represents the global ultrasound measurement area in the brain and was chosen between 1.5 and 7 cm to avoid both phase aberrations near the skull and ultrasound attenuation (see Figure 2). All subjects were asked to sit and remain quiet during the recording time. For each subject, the protocol consisted of seven consecutive acquisitions of 6 seconds each, during which imaging data were collected for the ROI. The acquisition frame rate was 30 images/s (total of 180 frames). The acquired data were then downloaded for offline analysis with the MATLAB software (The Mathworks, Inc., Natick, MA, USA). The Siemens Axius Direct Ultrasound Research Interface provided direct access to beam-formed radiofrequency lines, which were used to estimate the displacement of brain tissue.
Brain tissue displacement was estimated using a 1D-intercorrelation method. The maximum of the normalized intercorrelation function was used to estimate the delay between ROIs on ultrasound radiofrequency lines. The correlation coefficient was fixed at 0.7, and the range was 0.7 to 0.99. A kernel size of 4 wavelengths was chosen. Further signal processing by parabolic interpolation was performed to reduce the noise level of the correlation function to 0.8 μm.9, 12 An overlap rate of 80% between spatial kernels was used to improve the axial resolution, resulting in an axial resolution of 0.24 mm. Brain tissue displacement was measured along the ultrasound beam axis. The slow movement caused by respiration has a magnitude on the order of 300 μm, and this movement needs to be filtered out. A bandpass filter was used to focus on displacements related to heart rate; the low cutoff frequency was fixed at 0.6 Hz to eliminate slow movements, such as those induced by respiration, and the high cutoff frequency was fixed at 10 Hz to eliminate rapid displacements. The amplitude of displacement was estimated from an ROI near the middle cerebral artery for each spatial point. The peak-to-peak amplitude was then calculated between the maximum and the minimum displacement. The estimation of maximum BTD (maxBTD) for each subject was computed based on the seven acquisitions. The mean absolute displacement and the standard deviation were then calculated for all subjects.
Magnetic Resonance Imaging Protocol
Once the measurements by ultrasound were performed, the operator placed a mark on the skin of the subject at this level. Then, the MRI was performed with a vitamin E capsule (hyperintense on MRI) positioned at the skin mark. The MRI recordings were performed under the supervision of a senior neuroradiologist, who was unaware of the TPI results, using a 1.5-Tesla General Electric HDx (GE Medical System, Paris, France). Fluid attenuated inversion recuperation T2, T2-weighted spin-echo, and high-resolution T1-weighted MRI 3D volumes were acquired with voxel sizes of 1 × 1 × 5 mm and 1 × 1 × 1.3 mm, respectively. The WMH analysis, including a visual and volumetric analysis, was performed by two neuroradiologists using the MRIcro software (v. 1.37, freeware, courtesy of Chris Rorden)13 and the fluid attenuated inversion recuperation sequence. Three ROIs were located manually in a healthy WM ROI for each subject to obtain a mean intensity. The hyper-signal threshold used to distinguish WMH from healthy WM was more than three times greater than the mean value. Visual analysis of each section allowed us to eliminate gaps volume. The WMH volume was normalized by the total intracranial volume to correct for variations in head size. The relative volume of WMH% is more accurate for the comparison of the leukoaraiosis load among subjects.
Statistical Analyses
The correlation coefficient was estimated using a nonparametric Spearman test. The null hypotheses tested were that the variables were independent. Correlation was considered as significant when the P value was <0.05.
Results
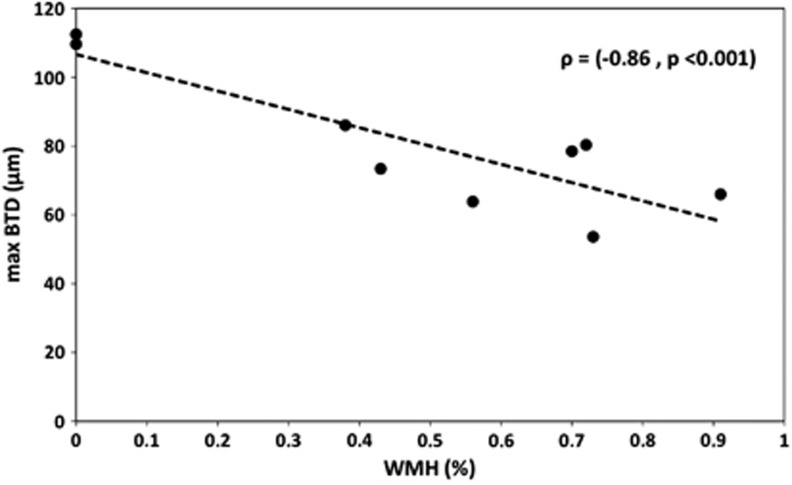
Table 1 shows the age, WMH volume, and the level of BTD for the nine selected subjects. The mean absolute WMH and WMH% volumes were 10.24±6.42 cm3 and 0.49±0.32%, respectively. The mean maxBTD score was 80.42±20 μm.
Table 1. Subject characteristics.
| Subject | Age (years) | Absolute volume WMH (cm3) | Right relative volume WMH (%) | Maximum BTD (μm) |
|---|---|---|---|---|
| 1 | 75 | 15.2 | 0.72 | 80.34 |
| 2 | 81 | 15.9 | 0.91 | 65.98 |
| 3 | 66 | 0.1 | 0 | 109.61 |
| 4 | 67 | 0.4 | 0 | 112.47 |
| 5 | 74 | 13.2 | 0.7 | 78.5 |
| 6 | 73 | 17.2 | 0.73 | 53.62 |
| 7 | 65 | 9.43 | 0.43 | 73.39 |
| 8 | 66 | 13.16 | 0.56 | 63.84 |
| 9 | 68 | 7.57 | 0.38 | 86.05 |
| Mean | 70.56 | 10.24 | 0.49 | 80.42 |
BTD, brain tissue displacement; WMH, white-matter hyperintensity.
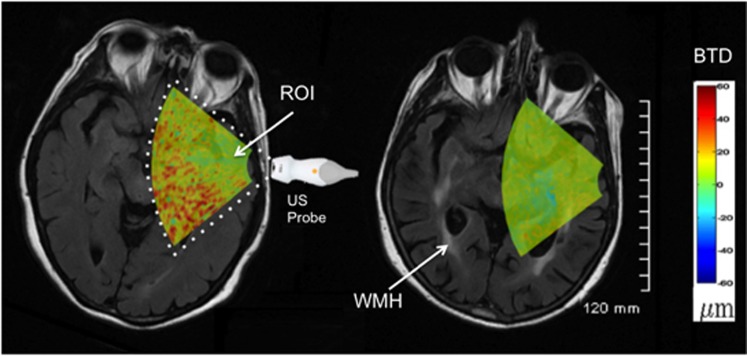
Figure 1 shows the maxBTD as a function of WMH%. A significant negative correlation was found between BTD and WMH% (ρ=−0.86, P<0.001). The mean value of BTD was correlated with WMH% (ρ=−0.72, P<0.001). Ultrasound images for the two extreme cases are shown in Figure 2. In the left image, the subject with the highest BTD (112 μm) had a WMH% volume of 0%. In the right image, the subject with the lowest BTD (53 μm) had a WMH% volume of 0.73%. The two BTD images were estimated at the peak of the systolic cycle.
Figure 1.
The maximum brain tissue displacement (maxBTD) decreased significantly as the severity of white-matter hyperintensity (WMH) increased. Correlation between the maximum BTD (μm) and the relative WMH volume (%).
Figure 2.
Axial images representing the brain tissue displacement (BTD) images overlaid on magnetic resonance (MR) images of subjects 2 and 4, reconstructed in parallel with the ultrasound images. For patient 4 (left image), the relative white-matter hyperintensity (WMH) volume was 0%. For patient 2, who had significant WMH (arrows), the relative WMH volume was 0.91%. The color scale indicating BTD amplitude (−60 to +60 μm) is shown on the far right. ROI, region of interest.
Discussion
Although the anatomopathologic significance of WM changes has not been completely elucidated, a strong association among WMHs, vascular risk factors, and vascular disorders has been proven.3 In elderly subjects, WMHs may be considered as a normal aging phenomenon. However, from the clinical point of view, WMHs have been associated with cognitive, motor, and neuropsychiatric symptoms, and the real impact of the WMH load on brain function remains a research focus. The signal abnormalities observed on MRI represent a morphologic marker of leukoaraiosis, which may reflect the tip of the iceberg. There is a lack of functional biomarkers to measure the real impact of this microangiopathy.
The main result of this study was the significant negative correlation between the maximum magnitude of cerebral tissue pulsatility measured by ultrasound (TPI) and the WMH volume measured by MRI. The results are promising regarding the characterization of subjects with global cerebrovascular changes by TPI. Changes in natural brain pulsatility have been studied previously, and such changes are reported to be related to the quality of cerebral tissue perfusion14 and the regulation of brain activity.7 Other MRI studies have correlated WMH with fibrohyalinotic vessels,15 which are associated with a significant decrease in the CBF observed by ultrasound.5, 6 The significant negative correlation between maxBTD and WMH that we observed could be due to the astrogliosis and fibrohyalinotic vessel changes found in the WMH area. Thus, the maxBTD assessed by ultrasound could represent a new and promising functional biomarker for leukoaraiosis, which is more relevant to the pathologic conditions than MRI for the prognosis of vascular diseases and therapeutic effects.
However, the current study has various limitations. For example, the number of subjects included was small, and data on brain tissue pulsatility were assessed only in the right hemisphere. There is, however, no evidence to suggest that brain tissue movement is different in the two hemispheres, and all the subjects studied were right handed. Despite these limitations, TPI appears to be a promising technique with which to assess brain disorders associated with WMH and any related vascular components. However, this pilot study needs to be replicated with a higher frame rate to improve the time resolution and with neuronavigation to increase the reliability of the measurements. These results will motivate further studies of brain pulsatility in patients with a high volume of WMH (e.g., Alzheimer's, ischemia, and dementia). As shown in Materials and methods in this study, TPI may become a complementary method to MRI. The next step of our study will be the comparison of results from volumetric WMH, TPI, and arterial spin labeling in subjects with Alzheimer's disease. We plan to improve TPI processing to access mechanical parameters, such as brain tissue elasticity, by using intrinsic ultrasound strain imaging.
Acknowledgments
The authors thank Melouka Elkateb-Hachemi PhD for her high quality technical assistance and scientific advice.
The authors declare no conflict of interest.
Footnotes
This work was supported by the ‘Fondation Thérèse et René Planiol pour l'étude du Cerveau' (Tours, France).
References
- Hommet C, Mondon K, Constans T, Beaufils E, Desmidt T, Camus V, et al. Review of cerebral microangiopathy and Alzheimer's disease: relation between white matter hyperintensities and microbleeds. Dement Geriatr Cogn Disord. 2011;32:367–378. doi: 10.1159/000335568. [DOI] [PubMed] [Google Scholar]
- Murray ME, Senjem ML, Petersen RC, Hollman JH, Preboske GM, Weigand S, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JT. Clinical significance of white matter changes. Am J Geriatr Psychiatry. 2013;22:133–137. doi: 10.1016/j.jagp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- Kucewicz JC, Dunmire B, Leotta DF, Panagiotides H, Paun M, Beach KW. Functional tissue pulsatility imaging of the brain during visual stimulation. Ultrasound Med Biol. 2007;33:681–690. doi: 10.1016/j.ultrasmedbio.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucewicz JC, Dunmire B, Giardino ND, Leotta DF, Paun M, Dager SR, et al. Tissue pulsatility imaging of cerebral vasoreactivity during hyperventilation. Ultrasound Med Biol. 2008;34:1200–1208. doi: 10.1016/j.ultrasmedbio.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmidt T, Hachemi ME, Remenieras JP, Lecomte P, Ferreira-Maldent N, Patat F, et al. Ultrasound Brain Tissue Pulsatility is decreased in middle aged and elderly type 2 diabetic patients with depression. Psychiatry Res. 2011;193:63–64. doi: 10.1016/j.pscychresns.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011;8:5. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel DM, Admiraal-Behloul F, ten Dam VH, Olofsen H, Bollen EL, Murray HM, et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63:1699–1701. doi: 10.1212/01.wnl.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- Céspedes I, Huang Y, Ophir J, Spratt S. Methods for estimation of subsample time delays of digitized echo signals. Ultrason Imaging. 1995;17:142–171. doi: 10.1177/016173469501700204. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Greitz D, Wirestam R, Franck A, Nordell B, Thomsen C, Ståhlberg F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. Neuroradiology. 1992;34:370–380. doi: 10.1007/BF00596493. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122:171–185. doi: 10.1007/s00401-011-0851-x. [DOI] [PubMed] [Google Scholar]